Abstract
Glanzmann thrombasthenia is an inherited bleeding disorder characterized by qualitative or quantitative defects of the platelet-specific integrin, IIbβ3. As a result, IIbβ3 cannot be activated and cannot bind to fibrinogen, leading to a loss of platelet aggregation. Thrombasthenia is clinically characterized by mucocutaneous hemorrhage with episodes of intracranial and gastrointestinal bleeding. To develop methods for gene therapy of Glanzmann thrombasthenia, a murine leukemia virus (MuLV)-derived vector, −889PlA2β3, was transduced into peripheral blood CD34+ cells from 2 patients with thrombasthenia with defects in the β3 gene. The human IIb promoter was used in this vector to drive megakaryocyte-targeted expression of the wild-type β3 subunit. Proviral DNA and IIbβ3 biosynthesis were detected after in vitro differentiation of transduced thrombasthenic CD34+ cells with megakaryocyte growth and development factor. Flow cytometric analysis of transduced patient samples indicated that 19% of megakaryocyte progeny expressed IIbβ3 on the surface at 34% of normal receptor levels. Treatment of transduced megakaryocytes with a combination of agonists including epinephrine and the thrombin receptor-activating peptide induced the IIbβ3 complex to form an activated conformation capable of binding fibrinogen as measured by PAC-1 antibody binding. Transduced cells retracted a fibrin clot in vitro similar to megakaryocytes derived from a normal nonthrombasthenic individual. These results demonstrate ex vivo phenotypic correction of Glanzmann thrombasthenia and support the potential use of hematopoietic CD34+ cells as targets for IIb promoter-driven MuLV vectors for gene therapy of platelet disorders.
Glanzmann thrombasthenia is a rare, autosomal recessive bleeding disorder characterized by an absence or dysfunction of the platelet receptor for fibrinogen, integrin αIIbβ3(glycoproteins [GP] IIb-IIIa). To date, about 50 distinct mutations associated with Glanzmann thrombasthenia have been localized with relatively equal occurrence on either the αIIb or β3 gene.1 As in many genetic disorders, the molecular abnormalities range from major deletions and inversions to single point mutations.1Although the percentage of abnormal αIIbβ3 expressed on the platelet surface may vary with the type of defect, all thrombasthenic platelets are functionally indistinguishable as characterized by the failure of defective αIIbβ3 to bind fibrinogen resulting in the absence of platelet aggregation after stimulation by physiologic agonists.
Clinically, Glanzmann thrombasthenia is characterized by irregular bleeding from mucous membranes with easy bruising, epistaxis, gingival bleeding, and menorrhagia, which usually appears at an early age and recurs throughout the individual's life.2 These individuals occasionally experience severe intracranial or gastrointestinal bleeding that may result in death. Platelet transfusions are used to treat severe cases of bleeding associated with Glanzmann thrombasthenia, although many patients become refractory to transfusions.
Glanzmann thrombasthenia occurs at a low rate internationally, but certain geographically restricted groups have a high carrier rate for thrombasthenia including Iraqi Jews, distinct Arab populations of the Middle East, French gypsies, and individuals from southern India.2 Approximately 2.3% of the 270 000 Iraqi Jews living in Israel are carriers for thrombasthenia.3Individuals of Arabic decent also have a prevalence for this disorder, with more than 13 patients identified from 5 kindreds. The discovery of the molecular genetic defects in relatively large, “high-risk” populations has influenced the development of rapid DNA-based diagnostic assays,4 which provides opportunities for genetic counseling and family planning for suspected carriers of the abnormal αIIb or β3 gene, and could also help to identify prospective candidates for gene therapy.
The CD34+ hematopoietic cells are potential targets for gene therapy strategies because these cells can be safely collected from the body, genetically modified, and reinfused into the patient.5,6 This cell population has the capacity to generate an engrafting cell mass capable of establishing hematopoiesis with progeny cells of multilineages expressing the transferred gene for the life span of the graft recipient. The use of CD34+cells in vitro to develop a strategy for gene therapy of platelet disorders was facilitated by the discovery of techniques7that induce pluripotent CD34+ progenitor cells to proliferate and differentiate into megakaryocytes in vitro and in vivo using c-Mpl-ligand or megakaryocyte growth and development factor (MGDF),8 flk2/flt3 ligand, interleukin (IL)-3, IL-6, IL-11, and stem cell factor (SCF). Among other effects, MGDF plays a direct role in increasing the transcriptional activity of the integrinαIIb gene in megakaryocytes9; therefore, an αIIb promoter-directed expression system may be activated by MGDF within transduced CD34+ cells. Thus, CD34+cells can be transduced with genes driven by the αIIb promoter and induced to form megakaryocytes that can be examined for targeted proviral gene expression.
This study examined the use of peripheral blood CD34+ cells from patients with thrombasthenia as a model system for gene therapy of disorders affecting platelets. This investigation used the murine leukemia virus (MuLV)-derived vector, −889PlA2β3, in which the transcription of β3 is controlled by an 889-nucleotide fragment of the human αIIb promoter. We previously used this vector to direct megakaryocyte-specific expression of the platelet alloantigen 2 (PlA2) form of β3 in human cell lines and expression of the proviral αIIbβ3 complex in megakaryocyte progeny of transduced CD34+ cells derived from a normal (nonthrombasthenic) individual.10Alloantigens were used in that study to allow us to distinguish the biosynthesis of proviral (PlA2) β3 from endogenous (PlA1) β3 in the normal human cells. The present study extends the use of the −889PlA2β3 vector to examine expression of the integrin αIIbβ3 complex on the surface of megakaryocytes following transduction and MGDF-induced differentiation of thrombasthenic CD34+ cells. Functional studies demonstrated that transduced thrombasthenic cells were capable of agonist-induced αIIbβ3 activation and retraction of a fibrin clot indicating ex vivo phenotypic correction of Glanzmann thrombasthenia. These results indicate that ex vivo gene transfer of a megakaryocyte-targeted gene expression system into peripheral blood CD34+ cells, followed by reinfusion of transduced cells, may be appropriate for gene therapy for disorders of platelets.
Patients, materials, and methods
Patients
Two patients with type I Glanzmann thrombasthenia, RSΔ9β3,11 and EAY115Cβ3, who is a member of a previously reported kindred,12 volunteered for the study. Both have lifelong bleeding episodes characterized by prominent mucous membrane bleeding and bleeding secondary to surgery or trauma. In each case, the diagnosis of thrombasthenia was established by a prolonged bleeding time, the failure of platelets to aggregate with physiologic agonists (adenosine diphosphate [ADP], epinephrine, collagen, and thrombin), and the failure of platelets to retract a fibrin clot. Platelets from each subject contained less than 5% detectable αIIbβ3, which is consistent with type I Glanzmann thrombasthenia. Distinct defects in β3 have been defined at the molecular level: patient E.A. has a novel nucleotide substitution in β3 resulting in a single amino acid substitution of tyrosine to cysteine at residue 115 (Y115C), and patient R.S. has a previously reported single nucleotide substitution in β3 that affects the splice-donor site of exon 9 resulting in the deletion of 45 amino acids (Δ9). All of these studies have been conducted with patient consent and approval by the human rights committees of Johns Hopkins University and the University of North Carolina and conducted according to the principles expressed in the Declaration of Helsinki.
Antibodies and reagents
Polyclonal antibodies specific for αIIb and β3 and a monoclonal antibody that recognizes an epitope on β3, AP3,13 were gifts from Dr Peter J. Newman (Blood Research Institute, Milwaukee, WI). The fluorescein isothiocyante (FITC)-conjugated monoclonal antibody, PAC-1, which recognizes an epitope on the activated αIIbβ3 complex was purchased from Becton Dickinson (San Jose, CA). The monoclonal antibody, AP2,14 which recognizes an epitope on the αIIbβ3 complex, was provided by Dr Robert R. Montgomery (Blood Research Institute, Milwaukee, WI). The monoclonal antibody, 6D1,15 which recognizes an epitope on glycoprotein (GP)Ib was provided by Dr Barry Coller (Mt Sinai School of Medicine, New York, NY). Phycoerythrin (PE)-conjugated anti-GPIbα antibody (mouse antihuman CD42b) and isotype standards (PE-IgG, FITC-IgM) were purchased from PharMingen (San Diego, CA). ADP was purchased from Fisher (Pittsburgh, PA), and epinephrine was from Bio/Data Corp (Horsham, PA). Thrombin receptor-activating peptide (TRAP) and an Arg-Gly-Asp–containing peptide (GRGDW) were synthesized at the Blood Research Institute Core Facility (Milwaukee, WI).
Retroviral construct p-889PlA2β3
As previously described,10 a fragment of the αIIb promoter beginning at nucleotide −889 was used to drive transcription of complementary DNA (cDNA) encoding the platelet alloantigen 2 (PlA2) form of β3 (provided by Dr Peter J. Newman, Blood Research Institute, Milwaukee, WI).16 The −889PlA2β3 DNA cassette was positioned within the MuLV-derived retroviral vector, pHIT-SIN, which encodes a 3′ long terminal repeat (LTR) sequence (provided by Dr Estuardo Aguilar-Cordova, Baylor College of Medicine, Houston, TX)17 lacking the viral enhancer/promoter so that the αIIb promoter could be used to promote megakaryocyte-targeted gene transcription.
Retrovirus production
Human 293 cells were transiently transfected on 10-cm plates with 15 μg each of pCI-GPZ, pCI-VSV-G helper plasmids, and p-889PlA2β3 using the Calcium Transfection System (Life Technologies, Gaithersburg, MD).10 Media containing retrovirus was concentrated 500-fold and resuspended in Iscove's modified Dulbecco's Eagle medium (IMDM). Viral preps were stored at −80°C until needed. Replication competent virus was not detected in −889PlA2β3 viral preparations using extended marker rescue assays as previously described.18
Selection of CD34+ cells from peripheral blood
Peripheral blood collection was performed after obtaining written informed consent from adult Glanzmann thrombasthenic volunteers enrolled in a protocol approved by the University of North Carolina and Johns Hopkins University Committees on the Protection of the Rights of Human Subjects. Subjects were given granulocyte colony-stimulating factor (Amgen, Thousand Oaks, CA) at 10 μg/kg/d subcutaneously for 4 days and peripheral blood cell collection was performed on day 5 using a COBE Spectra Blood Cell Separator. CD34+ cells were immunoselected from the apheresis product on an Isolex 300i Magnetic Cell Separator (Nexell Therapeutics, Irvine, CA, distribution through Baxter Healthcare) as previously described.19 Cell yields from both patients were approximately 600 million total nucleated cells, with a final recovery of 150 million CD34+ cells (85% CD34+ purity). Selected cells were suspended in X-VIVO 10 (Biowhittaker, Walkersville, MD) containing 1% (w/v) human serum albumin, frozen in 10% (v/v) DMSO at 5 × 106 cells/mL, and stored in liquid nitrogen.
Transduction of CD34+ cells
Human CD34+ cells were transduced as previously described.10 Briefly, cells were prestimulated in IMDM containing 20% fetal bovine serum (FBS), 10 U/mL recombinant human (rh) IL-3, 100 U/mL rhIL-6, 30 U/mL recombinant murine SCF (Genetics Institute, Cambridge, MA) and 10 ng/mL flk2/flt3 ligand (R&D Systems, Minneapolis, MN) for 48 hours at 37°C in 5% CO2. Cells were transduced at 5 × 105 per well of a sterile, 24-well nontissue culture-treated plate (Falcon-Becton Dickinson, Franklin Lakes, NJ) coated with 20 μg/cm2RetroNectin20,21 (Takara Shuzo, Otsu, Shiga, Japan) with an estimated multiplicity of infection of 10 retrovirus (−889PlA2β3) per cell in IMDM plus 20% FCS and rhIL-3, rhIL-6, SCF, and flk2/flt3 ligand. Fresh viral supernatant was added after 2 hours. This procedure was repeated 1 time 24 hours later. Twenty-four hours after the final transduction, megakaryocyte formation was induced similar to a previously described method.7 Cells were resuspended at 5 × 105/mL in IMDM containing 10% platelet poor plasma and rhIL-3, rhIL-6, SCF, flk2/flt3 ligand plus 100 ng/mL rhIL-11 (Genetics Institute) and 100 ng/mL rhMGDF (Amgen) for up to 17 days. Cells were collected, washed twice in phosphate-buffered saline (PBS), and solubilized in 1 mL of lysis buffer and stored at −80°C.
Detection of proviral DNA in transduced cells by polymerase chain reaction
Cells (1.2 × 105) were split into 3 aliquots and DNA was amplified in 3 separate polymerase chain reaction (PCR) reactions: (1) amplification of αIIb genomic DNA from nucleotides 13 703 to 14 064 (361 base product)22 was performed as a positive control to detect genomic DNA from untransduced and transduced samples; (2) PCR of plasmid vector backbone DNA (P−889PlA2β3) was performed using sense primer 5′-TGACTGGTGAGTACTCAACC-3′ from nucleotide 1861 to 1880 and antisense primer 5′-TTCACACCGCATACAGGTGGC-3′, which consisted of nucleotides 2323 to 2303 (462 base product) outside of the region packaged into retroviral capsids as a negative control that demonstrated retroviral plasmid DNA was not transfected into the cells during transduction; and (3) plasmid vector (P−889PlA2β3) sequence packaged into retroviral capsids was amplified by PCR using sense primer “OL-psi-1” 5′-TTGAACCTCCTCGTTCGAC-3′ beginning 111 nucleotides upstream of −889PlA2β3 DNA cassette and antisense primer 5′-ACTCCTCCTCCGTCTTGAGCC-3′, which consisted of nucleotides −572 to −592 of the αIIb promoter (428 base product) to detect proviral DNA in transduced samples. The PCR products were separated by electrophoresis on a 2.0% agarose gel and visualized by ethidium bromide staining.
Immunoprecipitation analysis
Immunoprecipitaion analysis was performed as previously described.10 Precleared lysates were immunoprecipitated for 1 hour at 25°C with AP2 coupled to Affi-gel Hz (Bio-Rad, Hercules, CA). Immunoprecipitates were electrophoresed on a 7% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel under nonreducing conditions and proteins were transferred to Immobilon-P (Millipore, Bedford, MA) at 200 mA for 3 hours and blocked for 12 hours at 4°C in 10% FBS in TBS-Tween. Protein was detected using rabbit polyclonal antibodies specific for αIIb (5 μg/mL) and β3 (4 μg/mL) and a peroxidase-conjugated F(ab′)2 fragment donkey antirabbit IgG (H+L) (Jackson ImmunoResearch, West Grove, PA) at a 1:20 000 dilution followed by chemiluminescence detection and exposure to autoradiography film from 1 second to 10 minutes.
Indirect immunofluorescence
Transduced and untransduced cells (5 × 105) were blocked for 15 minutes in 2% bovine serum albumin (BSA) and 0.1 mmol/L Ca/Mg in PBS and incubated with 5 μg AP2 for 20 minutes at 25°C, then treated with PE-conjugated F(ab′)2 donkey antimouse secondary antibody (Jackson ImmunoResearch) for 20 minutes on ice. Cells were resuspended in 200 μL of 2% formaldehyde, 0.2% glutaraldehyde in PBS and fixed to single wells of a 24-well tissue culture-treated plate for 15 minutes at 25°C while centrifuging at 230g. Positively staining cells were detected and photographed using a Zeiss Axiovert 10 fluorescence microscope at × 320 magnification.
Flow cytometric analysis
Transduced and untransduced cells (1.5 × 105) were blocked for 15 minutes in 2% BSA and 0.1 mmol/L Ca/Mg in PBS; incubated with 2.5 μg of AP2, AP3, 6D1, mouse IgG for 20 minutes at 25°C; and treated with PE-conjugated F(ab′)2donkey antimouse secondary antibody (Jackson ImmunoResearch) for 20 minutes on ice. Cells were resuspended in 200 μL of 1% paraformaldehyde in PBS and analyzed on a FACScan flow cytometer (Becton Dickinson) using CellQuest software. A minimum of 2 × 103 cells exhibiting light scattering properties of megakaryocytes were used for the analysis. Identification of megakaryocytes in this cell population was further confirmed with antibodies directed against glycoprotein GPIbα. Megakaryocytes expressing αIIbβ3 were determined using samples stained with AP2 and the secondary antibody described above. Cells expressing β3 were determined using samples stained with AP3 and secondary antibody. Negative populations of cells were determined using unreactive isotype-specific antibodies as a control for background staining. Fluorescence contours are shown as 50% log density plots. The efficiency of −889PlA2β3 to transduce CD34+ cells was calculated by comparing the percent of the cell population that expressed the αIIbβ3 complex following transduction with untransduced and normal nonthrombasthenic megakaryocytes under identical culture conditions. The mean fluorescence intensity of AP2 and AP3 staining was measured at saturating antibody levels13 14 to determine receptor expression level and estimate receptor density on the cell samples.
Agonist-induced IIbβ3 activation
Culture cells were harvested for physiologic studies of αIIbβ3 function 8 to 13 days after transduction. Cells (1.5 × 106/mL) were incubated with PE-GPIbα and FITC-PAC1 (5 μg/mL) antibodies in modified Tyrode buffer containing 1 mmol/L CaCl2, 1 mmol/L MgCl2, 50 μmol/L each of TRAP, ADP, and epinephrine for 15 minutes at 25°C. FITC-PAC1 binding was monitored in the FL1 channel of the flow cytometer on the gated subset of cells that expressed GPIbα (FL2). The specificity of FITC-PAC1 binding was determined by co-incubation of samples with a blocking peptide containing Arg-Gly-Asp (GRGDW, 2.5 mmol/L). Samples were diluted 10-fold with buffer and examined using flow cytometry and 2-color analysis performed as described above.
Clot retraction
Clot retraction assays were performed using a slightly modified version of a previously described method.23 Ten to 14 days after transduction, cultured cells (1.5 × 106/mL) were resuspended in serum-free IMDM containing 60 μg/mL human fibrinogen in a standard aggregometry tube. Clot formation was initiated by the addition of 2.5 U/mL thrombin. Tubes were incubated at 37°C for up to 12 hours and photographed.
Results
Formation of the IIbβ3 complex following transduction of thrombasthenic CD34+ cells
Patients R.S.11 and E.A.12 have type I Glanzmann thrombasthenia due to defects in β3 associated with undetectable surface expression of the αIIbβ3complex, failure of platelets to bind fibrinogen, absence of platelet-platelet aggregates, and inability to retract a fibrin clot. Patient R.S. has a previously reported single nucleotide substitution that affects the splice-donor site of exon 9 of β3resulting in the deletion of 45 amino acids (RSΔ9β3), and patient E.A. has a novel nucleotide substitution of β3 resulting in a single amino acid substitution of tyrosine to cysteine at residue 115 (EAY115Cβ3). To assess the feasibility of human gene therapy of Glanzmann thrombasthenia, granulocyte colony-stimulating factor mobilized, peripheral blood CD34+ cells were collected from each patient and transduced with MuLV-derived vector −889PlA2β3. The transduced CD34+ cells were subjected to in vitro expansion and differentiation with IL-3, IL-6, IL-11, flk2/flt3 ligand, SCF, and MGDF, and examined for the presence of proviral DNA by PCR analysis. After 10 days of cytokine treatment, successful transduction of CD34+ cells from RSΔ9β3 and EAY115Cβ3 was indicated by the detection of proviral DNA by PCR in −889PlA2β3-transduced cells but not in untransduced cells (not shown, see “Materials and methods”).
Immunoblot analysis was performed to determine if the αIIbβ3 complex was synthesized in megakaryocyte progeny of −889PlA2β3-transduced CD34+ cells from RSΔ9β3 and EAY115Cβ3. On days 10 and 15 after differentiation, respectively, the αIIbβ3 receptor was immunoprecipitated from cellular lysates with an αIIbβ3 complex-specific monoclonal antibody (AP2) and detected using a mixture of well-characterized polyclonal antibodies specific for the αIIb and β3 integrin subunits. Both αIIb (Mr = 145 000) and β3(Mr = 95 000) were detected in the β3-transduced cells (Figure1) indicating that there was proviral-derived, αIIb promoter-directed synthesis of PlA2β3 resulting in formation of the αIIbβ3 complex. The αIIbβ3 complex was not detected in untransduced thrombasthenic CD34+ cells (Figure 1) nor in cells transduced with a similar MuLV-derived vector (−889nLacz), which encoded the β-galactosidase gene in place of PlA2β3 (not shown).
Immunoprecipitation analysis of −889PlA2β3 transduced CD34+cells from patient RS▵9β3 and EAY115Cβ3.
Cells (5 × 105) were collected 10 and 15 days, respectively, after transduction and detergent lysates were immunoprecipitated with 10 μg of an αIIbβ3 complex-specific antibody (AP2). The complexed proteins were separated on a 7% SDS-PAGE gel under nonreducing conditions. Immunoanalysis using polyclonal antibodies to αIIb and β3 followed by chemiluminescence detection showed that transduction with the −889PlA2β3 vector resulted in the synthesis of detectable levels of β3and αIIb (arrows on right), whereas untransduced samples did not have detectable protein. Molecular mass markers are in kilodaltons (left). Additional bands appearing equally in each lane are nonspecific background resulting from chemiluminescence detection using a murine monoclonal antibody for immunoprecipitation and rabbit polyclonal antibodies and a horseradish peroxidase-conjugated donkey antirabbit antibody for analysis.
Immunoprecipitation analysis of −889PlA2β3 transduced CD34+cells from patient RS▵9β3 and EAY115Cβ3.
Cells (5 × 105) were collected 10 and 15 days, respectively, after transduction and detergent lysates were immunoprecipitated with 10 μg of an αIIbβ3 complex-specific antibody (AP2). The complexed proteins were separated on a 7% SDS-PAGE gel under nonreducing conditions. Immunoanalysis using polyclonal antibodies to αIIb and β3 followed by chemiluminescence detection showed that transduction with the −889PlA2β3 vector resulted in the synthesis of detectable levels of β3and αIIb (arrows on right), whereas untransduced samples did not have detectable protein. Molecular mass markers are in kilodaltons (left). Additional bands appearing equally in each lane are nonspecific background resulting from chemiluminescence detection using a murine monoclonal antibody for immunoprecipitation and rabbit polyclonal antibodies and a horseradish peroxidase-conjugated donkey antirabbit antibody for analysis.
Surface expression of IIbβ3 on transduced thrombasthenic megakaryocytes
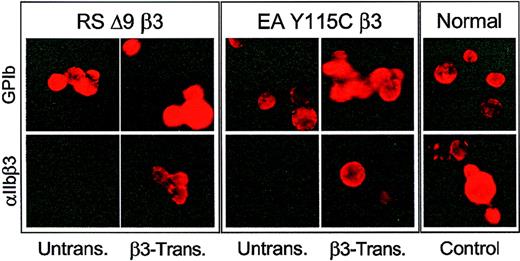
Indirect immunofluorescence was performed to examine expression of αIIbβ3 on the surface of megakaryocytes following −889PlA2β3 transduction of CD34+ cells from RSΔ9β3 and EAY115Cβ3. Five to 10 days after ex vivo expansion and differentiation of CD34+ cells, megakaryocytes were identified in cell cultures by detection of the megakaryocyte-specific glycoprotein (GP)Ib using the monoclonal antibody 6D1. GPIb was detected on the surface of megakaryocytes in untransduced thrombasthenic, transduced thrombasthenic, and normal (nonthrombasthenic) samples (Figure 2, top row), and cells transduced with −889nLacz (not shown). Only megakaryocytes derived from −889PlA2β3- transduced CD34+ cells expressed αIIbβ3 receptors on the cell surface. This expression was qualitatively similar to megakaryocyte progeny of CD34+ cells from a normal nonthrombasthenic individual as demonstrated by detectable AP2 staining (Figure 2, bottom row).
Indirect immunofluorescence analysis of thrombasthenic CD34+ cells transduced with −889PlA2β3.
The RSΔ9β3 and EAY115Cβ3CD34+ cells were transduced with −889PlA2β3, induced to form megakaryocytes ex vivo, and then examined by indirect immunofluorescence analysis for αIIbβ3surface expression. Cells were blocked in 2% BSA, and incubated with 5 μg monoclonal antibody 6D1 that recognizes megakaryocyte-specific glycoprotein (GP)Ib (top panels) or 5 μg AP2, which recognizes αIIbβ3 (bottom panels), and detected with a PE-conjugated F(ab′)2 donkey antimouse secondary antibody. Five to 10 days after transduction, megakaryocytes were present in untransduced and β3-transduced cell cultures as demonstrated with the anti-GPIb antibody; however, only β3-transduced cells demonstrated detectable αIIbβ3 complex on the surface of derived megakaryocytes similar to cultured megakaryocytes from a normal individual (control). There are at least 3 cells in each field of untransduced thrombasthenic cells stained with AP2 for αIIbβ3.
Indirect immunofluorescence analysis of thrombasthenic CD34+ cells transduced with −889PlA2β3.
The RSΔ9β3 and EAY115Cβ3CD34+ cells were transduced with −889PlA2β3, induced to form megakaryocytes ex vivo, and then examined by indirect immunofluorescence analysis for αIIbβ3surface expression. Cells were blocked in 2% BSA, and incubated with 5 μg monoclonal antibody 6D1 that recognizes megakaryocyte-specific glycoprotein (GP)Ib (top panels) or 5 μg AP2, which recognizes αIIbβ3 (bottom panels), and detected with a PE-conjugated F(ab′)2 donkey antimouse secondary antibody. Five to 10 days after transduction, megakaryocytes were present in untransduced and β3-transduced cell cultures as demonstrated with the anti-GPIb antibody; however, only β3-transduced cells demonstrated detectable αIIbβ3 complex on the surface of derived megakaryocytes similar to cultured megakaryocytes from a normal individual (control). There are at least 3 cells in each field of untransduced thrombasthenic cells stained with AP2 for αIIbβ3.
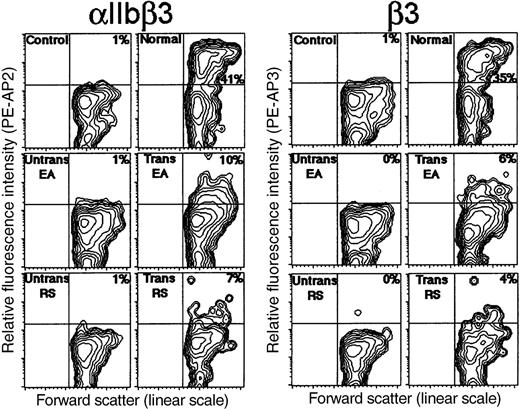
To quantitate the efficiency of transduction and estimate αIIbβ3 receptor density on megakaryocytes, flow cytometric analysis was performed following transduction of patient CD34+ cells with −889PlA2β3. After day 9 of ex vivo cellular expansion and differentiation, typically 38% of the large cells from each culture sample differentiated to megakaryocytes expressing GPIb (not shown). In Figure 3, megakaryocytes that expressed αIIbβ3 on the surface were identified on contour plots as the cells that emitted a high fluorescence intensity with AP2 at saturating antibody levels.14 An unreactive isotype-specific antibody was used as a control for background staining of normal and patient cells. Normal nonthrombasthenic CD34+ cells were induced to form megakaryocytes with detectable αIIbβ3 in 41% of the cell population (Figure 3), whereas untransduced CD34+cells from EAY115Cβ3 and RSΔ9β3 showed no detectable αIIbβ3 expression above background levels (Figure 3). Megakaryocytes expressing αIIbβ3 were detected in 10% and 7% of the transduced cell population from EAY115Cβ3 and RSΔ9β3, respectively (Figure 3). This indicates that approximately 19% of patient megakaryocytes were transduced with −889PlA2β3, when the percent of megakaryocytes expressing αIIbβ3 in the transduced cell populations were compared with untransduced and normal nonthrombasthenic cultures (Figure 3). Transduced patient megakaryocytes had a mean fluorescence intensity of AP2 staining that was 34% of αIIbβ3 receptor expression level on normal (nonthrombasthenic)-derived megakaryocytes indicating a reduced receptor density on transduced patient cells (Figure 3). Flow cytometric analysis using a monoclonal antibody (AP3) specific for the β3 subunit demonstrated β3 expression on approximately 5% of transduced patient cells at 28% of normal receptor density (Figure 3). Because the percent of the hematopoietic population expressing β3 (which normally pairs with αV on monocytes and lymphocytes as well as megakaryocytes) is nearly identical with data obtained for αIIbβ3 expression on transduced patient cells, these results suggest that the αIIb promoter directed megakaryocyte-specific expression of the β3 subunit.
Flow cytometric analysis following −889PlA2β3 transduction of thrombasthenic CD34+ cells.
Untransduced and transduced cells were induced to form megakaryocytes for 9 days ex vivo, and then examined by flow cytometric analysis for surface expression of the β3 subunit. Shown in the first set of panels are megakaryocytes expressing αIIbβ3 as detected with complex-specific antibody AP2 and a PE-conjugated F(ab′)2 donkey antimouse secondary antibody. The second set of panels are cells expressing the β3 subunit as detected with monoclonal antibody, AP3, and secondary antibody. Fluorescence contour plots are presented for normal nonthrombasthenic cells (upper right), untransduced and transduced cells from EAY115Cβ3 (middle panels), and untransduced and transduced cells from RSΔ9β3 (lower panels). The x-axis depicts cell size as measured with forward scatter on a linear scale, and the y-axis is relative fluorescence intensity of PE-AP2 or PE-AP3. Megakaryocytes that expressed αIIbβ3 on the cell surface were detected in the upper right quadrant as were cells that expressed β3. Normal and patient cells incubated with an isotype nonspecific antibody and secondary antibody were presented as controls for background staining (upper left panels).
Flow cytometric analysis following −889PlA2β3 transduction of thrombasthenic CD34+ cells.
Untransduced and transduced cells were induced to form megakaryocytes for 9 days ex vivo, and then examined by flow cytometric analysis for surface expression of the β3 subunit. Shown in the first set of panels are megakaryocytes expressing αIIbβ3 as detected with complex-specific antibody AP2 and a PE-conjugated F(ab′)2 donkey antimouse secondary antibody. The second set of panels are cells expressing the β3 subunit as detected with monoclonal antibody, AP3, and secondary antibody. Fluorescence contour plots are presented for normal nonthrombasthenic cells (upper right), untransduced and transduced cells from EAY115Cβ3 (middle panels), and untransduced and transduced cells from RSΔ9β3 (lower panels). The x-axis depicts cell size as measured with forward scatter on a linear scale, and the y-axis is relative fluorescence intensity of PE-AP2 or PE-AP3. Megakaryocytes that expressed αIIbβ3 on the cell surface were detected in the upper right quadrant as were cells that expressed β3. Normal and patient cells incubated with an isotype nonspecific antibody and secondary antibody were presented as controls for background staining (upper left panels).
IIbβ3 signaling in β3-transduced thrombasthenic megakaryocytes
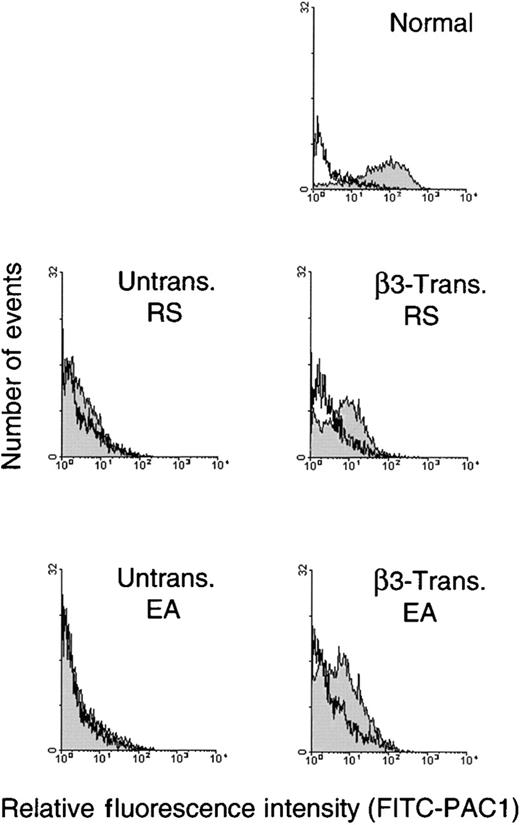
Inside-out signaling in β3-transduced cells was measured by performing agonist-dependent binding assays with the fibrinogen mimetic antibody, PAC1, to determine if αIIbβ3 could be induced to form an activated conformation capable of binding fibrinogen. Activation of αIIbβ3 was measured 9 days after ex vivo cellular expansion and differentiation using megakaryocytes selected with a PE-GPIbα antibody following stimulation of cells with TRAP, ADP, and epinephrine. Results of these studies are shown in Figure4 and demonstrate that in the presence of agonist, β3-transduced megakaryocytes bound FITC-PAC1 at a peak value that was on the average 10-fold above levels from untransduced patient cells. Similar to Figure 3, β3-transduced megakaryocytes bound antibody at a reduced fluorescence intensity that was approximately 9% of the peak value level on normal control megakaryocytes (Figure 4). FITC-PAC1 was inhibited from binding β3-transduced and normal control megakaryocytes in the presence of an Arg-Gly-Asp–containing peptide that is known to block the specific-binding of αIIbβ3to PAC1 and fibrinogen (Figure 4). In contrast, untransduced thrombasthenic megakaryocytes did not bind FITC-PAC1 in the presence or absence of the inhibitor peptide (Figure 4). These results indicate that αIIbβ3 expressed on β3-transduced megakaryocytes functions similar to the integrin complex on normal nonthrombasthenic megakaryocytes because excitatory agonists stimulated PAC1 binding and an inhibitory peptide blocked antibody recognition.
Analysis of agonist induced activation of IIbβ3 on β3-transduced thrombasthenic megakaryocytes.
Cultured cells were harvested for physiologic studies of αIIbβ3 function at 9 days after transduction with −889PlA2β3. Untransduced and β3-transduced cells (1.5 × 106/mL) were incubated with PE-GPIbα and FITC-PAC1 antibodies in modified Tyrode buffer containing TRAP, ADP, and epinephrine agonists. Binding of the αIIbβ3 activation-sensitive antibody, FITC-PAC1, was monitored in the FL1 channel of the flow cytometer on the gated subset of megakaryocytes that expressed GPIbα (FL2). In each panel, FITC-PAC1 binding was measured in the absence (shaded histograms) and presence (unshaded histograms) of an Arg-Gly-Asp–containing peptide (GRGDW) that blocks FITC-PAC1 binding specifically to activated αIIbβ3. The β3-transduced megakaryocytes from patients R.S. and E.A. bound FITC-PAC1 at a fluorescence intensity peak value of 13 and 7, respectively, which is on average 10-fold higher than the FITC-PAC1 peak value of 1 for untransduced megakaryocytes from R.S. and E.A. (shaded). The β3-transduced samples from R.S. and E.A. bound FITC-PAC1 at a fluorescence intensity peak value that was approximately 9% of the peak value of 110 for normal nonthrombasthenic megakaryocytes (shaded top). In the presence of the GRGDW peptide, FITC-PAC1 did not bind to megakaryocytes from β3-transduced, untransduced, or normal samples as demonstrated with fluorescence intensity peak value of 1 for each sample (unshaded) and β3-transduced megakaryocytes from R.S. that had a peak value of 2.
Analysis of agonist induced activation of IIbβ3 on β3-transduced thrombasthenic megakaryocytes.
Cultured cells were harvested for physiologic studies of αIIbβ3 function at 9 days after transduction with −889PlA2β3. Untransduced and β3-transduced cells (1.5 × 106/mL) were incubated with PE-GPIbα and FITC-PAC1 antibodies in modified Tyrode buffer containing TRAP, ADP, and epinephrine agonists. Binding of the αIIbβ3 activation-sensitive antibody, FITC-PAC1, was monitored in the FL1 channel of the flow cytometer on the gated subset of megakaryocytes that expressed GPIbα (FL2). In each panel, FITC-PAC1 binding was measured in the absence (shaded histograms) and presence (unshaded histograms) of an Arg-Gly-Asp–containing peptide (GRGDW) that blocks FITC-PAC1 binding specifically to activated αIIbβ3. The β3-transduced megakaryocytes from patients R.S. and E.A. bound FITC-PAC1 at a fluorescence intensity peak value of 13 and 7, respectively, which is on average 10-fold higher than the FITC-PAC1 peak value of 1 for untransduced megakaryocytes from R.S. and E.A. (shaded). The β3-transduced samples from R.S. and E.A. bound FITC-PAC1 at a fluorescence intensity peak value that was approximately 9% of the peak value of 110 for normal nonthrombasthenic megakaryocytes (shaded top). In the presence of the GRGDW peptide, FITC-PAC1 did not bind to megakaryocytes from β3-transduced, untransduced, or normal samples as demonstrated with fluorescence intensity peak value of 1 for each sample (unshaded) and β3-transduced megakaryocytes from R.S. that had a peak value of 2.
Transduced thrombasthenic cells retract a fibrin clot
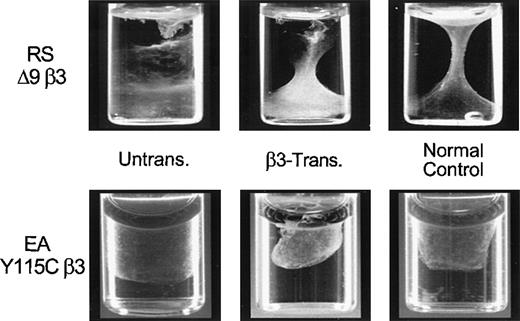
To further identify the function of the expressed αIIbβ3, CD34+ cells from RSΔ9β3 and EAY115Cβ3 were transduced with −889PlA2β3, treated with cytokines for 10 days to form megakaryocytes, and examined for the ability to retract a fibrin clot (Figure 5). The −889PlA2β3 transduced cells mediated clot retraction (middle panels) that was similar to cultured CD34+ cells from a nonthrombasthenic individual (normal control, right panels), whereas untransduced thrombasthenic cells were unable to retract a fibrin clot (left panels). The data suggest that the expressed αIIbβ3 receptors are functional in mediating clot retraction, implying an ex vivo correction of the Glanzmann thrombasthenia phenotype.
Fibrin clot retraction assay following −889PlA2β3 transduction of thrombasthenic CD34+ cells.
The RSΔ9β3 and EAY115Cβ3CD34+ cells were transduced with −889PlA2β3, induced for 10 to 14 days to form megakaryocytes in vitro, and then examined for the ability to retract a fibrin clot. Cells (1.5 × 106/mL) were resuspended in IMDM containing 60 μg/mL human fibrinogen in a standard aggregometry tube. Clot formation was initiated by the addition of 2.5 U/mL thrombin. Tubes were incubated at 37°C for up to 12 hours and photographed. The β3-transduced cells were able to mediate clot retraction in vitro similar to the nonthrombasthenic cells (normal control), whereas untransduced patient samples were not able to retract a fibrin clot. A normal control was included for the time each patient sample was assayed.
Fibrin clot retraction assay following −889PlA2β3 transduction of thrombasthenic CD34+ cells.
The RSΔ9β3 and EAY115Cβ3CD34+ cells were transduced with −889PlA2β3, induced for 10 to 14 days to form megakaryocytes in vitro, and then examined for the ability to retract a fibrin clot. Cells (1.5 × 106/mL) were resuspended in IMDM containing 60 μg/mL human fibrinogen in a standard aggregometry tube. Clot formation was initiated by the addition of 2.5 U/mL thrombin. Tubes were incubated at 37°C for up to 12 hours and photographed. The β3-transduced cells were able to mediate clot retraction in vitro similar to the nonthrombasthenic cells (normal control), whereas untransduced patient samples were not able to retract a fibrin clot. A normal control was included for the time each patient sample was assayed.
Discussion
The results of this investigation demonstrate successful transduction of peripheral blood CD34+ cells from 2 patients with Glanzmann thrombasthenia using the MuLV-derived vector, −889PlA2β3. Integrin β3subunit synthesis, αIIbβ3 complex formation, and surface expression were demonstrated on transduced megakaryocytes derived from thrombasthenic patients R.S. and E.A. Although FACS analysis indicated a subnormal αIIbβ3 receptor density on the surface of megakaryocytes, these −889PlA2β3 transduced cells demonstrated function by mediating retraction of a fibrin clot and binding the αIIbβ3 activation-dependent PAC1 antibody on cellular stimulation with agonists, which is consistent with the ex vivo correction of the Glanzmann thrombasthenic phenotype. Proplatelet formation was observed in transduced cell culture, but the quantity of platelets were too few to test aggregation. Our in vitro results show that despite suboptimal transduction efficiency of CD34+cells and reduced αIIbβ3 receptor density on patient megakaryocytes, there is correction of Glanzmann thrombasthenia. This is consistent with the fact that genetic carriers for Glanzmann thrombasthenia (about 50% normal αIIbβ3 receptor density levels) are disease free with normal platelet aggregation and clot retraction. We have previously observed measurable platelet aggregation and retraction of a fibrin clot using a mixture of 10% normal platelets with 90% thrombasthenic platelets in vitro (unpublished data). Thus, we might expect to see in vivo correction of thrombasthenia with expression of αIIbβ3 on less than 20% of transduced megakaryocytes as demonstrated in vitro; however, if transduced progenitor cells were delivered to patients in the absence of total marrow ablation, the actual percentage of corrected megakaryocytes could be greatly reduced in the total cell population. Nevertheless, our data suggest that compared to uncorrected platelets, a circulating population of platelets derived from β3-transduced megakaryocytes have an increased potential to aggregate at the site of vascular injury due to the expression of αIIbβ3 that can be induced by agonist to become activated and bind fibrinogen.
One key aspect of this work is the use of a fragment of the αIIb promoter to target gene expression to human megakaryocytes. Regulatory elements of the αIIb promoter necessary for high level, megakaryocyte-specific gene transcription have been localized to the first 800 nucleotides of the human αIIb promoter using cell lines,24-27 transfected rat primary cells,28and transgenic mice.29,30 Megakaryocyte progenitor cells express GATA and Ets factors that bind within this region to induce a high level of transcription of the αIIbgene,31 while as yet undefined factors that play a role in restricting transcription to developing megakaryocytes have been localized between nucleotides −80 and −130 of the αIIb gene.28,32,33 An MGDF-responsive element has also been recently identified that increases the transcriptional activity of the integrin αIIbgene in megakaryocytes.9 Investigations with transgenic mouse models have demonstrated megakaryocyte-targeted transcription in vivo when the transcriptional activation of an 800-nucleotide fragment of the human αIIb promoter directed expression of the thymidine kinase gene in multipotent hematopoietic cells leading to sustained expression in megakaryocyte progeny and down-regulated expression during erythroid and myeloid lineage differentiation.29,30We have recently observed supporting results when the MuLV-derived expression vector, −889PlA2β3, selectively targeted expression of β3 to transduced promegakaryocyte cell lines, and a similarly controlled construct, −889nLacZ, preferentially directed β-galactosidase activity to megakaryocytes following transduction and MGDF-induced differentiation of human CD34+ cells.10 The current investigation demonstrates the use of an 889 nucleotide fragment of the human αIIb promoter for targeted gene expression to correct diseased human megakaryocytes following transduction and MGDF induced differentiation of thrombasthenic hematopoietic CD34+cells. We detected levels of integrin β3 expression nearly equal to αIIbβ3 by flow cytometric analysis suggesting megakaryocyte-specific expression of the transgene in transduced patient cells. The cumulative data indicate that the MuLV-derived αIIb promoter expression system used in this report has the capacity to direct expression of the β3 gene with selective, MGDF-inducible expression in megakaryocyte progeny suggesting this system may be ideal for gene therapy for platelet disorders such as Glanzmann thrombasthenia.
Two potential challenges arise with our proposal to use the MuLV construct, −889PlA2β3, which expresses the rare PlA2 alloantigen form of β3 for genetic correction of thrombasthenia. First, individuals have become refractory to infused donor platelets due to the production of antibodies against mismatched alloantigenic determinants of β3 resulting in clearance of the transfused platelets34; therefore, epitopes of β3 may have to be identically matched to result in acceptance of corrected platelets as an additional requirement for gene therapy of thrombasthenia. We speculate that further experimentation with different platelet alloantigens of β3 for gene therapy of Glanzmann thrombasthenia may help us overcome some principal problems of transfusion medicine concerning antigen tolerance and correction of platelet refractoriness. This could ultimately lead to a definitive strategy that will pose as a viable alternative to the current treatment of platelet transfusions for bleeding episodes.35Second, individuals suffering from acute coronary thrombosis have been noted with an increased prevalence for the presence of the PlA2 allelic isoform of β3 on their platelets36 37; alternatively, the PlA1 form of β3 may be used for gene therapy of Glanzmann thrombasthenia. These results indicate that expression of different PlA1 or PlA2 forms of β3 in transduced thrombasthenic cells may lead to changes in the ability of platelets to activate and aggregate.
The results of this investigation suggest that transduction of altered forms of integrin subunits into CD34+ cells of thrombasthenics may allow elucidation of events important for integrin expression and intracellular signaling in primary human hematopoietic cells. Cultured cell lines transfected with recombinant αIIbβ3 subunits have been traditionally utilized to identify key steps that take place during integrin biosynthesis,38-41 intracellular signaling,23,42 and receptor activation.43Although these studies have advanced our understanding concerning fundamental concepts of integrin biology, data suggest that β1, β2, and β3 integrins may behave differently in transfected cells compared to natural host cells due to the influence of cell type-specific cytosolic proteins in governing signal transduction.44-46 Information gained from our study may inspire new strategies for investigating integrin-mediated events via megakaryocyte-targeted gene expression during megakaryocytopoiesis of primary human hematopoietic cells.
The αIIb promoter controlled expression system, −889PlA2β3, was developed to treat thrombasthenics with β3 defects; however, this system may be used for gene therapy of other molecular genetic defects of platelets. The system could potentially be used for gene therapy of thrombasthenics with αIIb defects or individuals with defects characterized in (GP)Ib-V-IX complex with Bernard Soulier syndrome, platelet-type von Willebrand disease, and pseudo von Willebrand disease.47 Other diseases may also benefit from the expression of novel proteins in megakaryocytes, because this study suggests that a platelet could potentially deliver other therapeutic agents to the site of a vascular injury during a primary hemostasis response.
Acknowledgments
We would like to thank Suzanne Lyman for her technical assistance with clot retraction assays in this study and Dr Susan Gidwitz for her technical advice. We thank Dr Julie Oliver (Duke University, Durham, NC) for technical assistance with flow cytometry and Dr Thomas F. Tedder for use of the flow cytometry facilities at Duke University Medical Center. We thank Drs Masamichi Shiraga and Sanford Shattil (Scripps Research Institute, La Jolla, CA) for technical advice for megakaryocyte activation assays.
Supported by grants HL-45100 and HL-58931 (G.C.W.) from the National Institutes of Health and by an American Heart Association (North Carolina Affiliate) Postdoctoral Fellowship Award NC-95-FW-63 (D.A.W.).
Reprints:Gilbert C. White II, Division of Hematology-Oncology, University of North Carolina, 932 Mary Ellen Jones, 231H/CB#7035, Chapel Hill, NC 27599; e-mail:gcwhite@med.unc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal