Abstract
The ligand for the receptor tyrosine kinase fms-like tyrosine kinase 3 (flt3), also referred to as fetal liver kinase-2 (flk-2), has an important role in hematopoiesis. The flt3 ligand (flt3L) is a growth factor for hematopoietic progenitors and induces hematopoietic progenitor and stem cell mobilization in vivo. In addition, when mice are treated with flt3L immature B cells, natural killer (NK) cells and dendritic cells (DC) are expanded in vivo. To further elucidate the role of flt3L in hematopoiesis, mice lacking flt3L (flt3L−/−) were generated by targeted gene disruption. Leukocyte cellularity was reduced in the bone marrow, peripheral blood, lymph nodes (LN), and spleen. Thymic cellularity, blood hematocrit, and platelet numbers were not affected. Significantly reduced numbers of myeloid and B-lymphoid progenitors were noted in the BM of flt3L−/− mice. In addition a marked deficiency of NK cells in the spleen was noted. DC numbers were also reduced in the spleen, LN, and thymus. Both myeloid-related (CD11c++ CD8−) and lymphoid-related (CD11c++ CD8+) DC numbers were affected. We conclude that flt3L has an important role in the expansion of early hematopoietic progenitors and in the generation of mature peripheral leukocytes.
The continual production of blood cells during an individual's lifetime is mediated by the coordinated effects of a number of cytokines acting on hematopoietic stem and progenitor cells in the bone marrow (BM).1,2 The fms-like tyrosine kinase 3 ligand (flt3L) is 1 of the cytokines that affects the development of multiple hematopoietic lineages.3 These effects are mediated through the binding of flt3L to the flt3 receptor, also referred to as fetal liver kinase-2 (flk-2)4 and STK-1,5 a receptor tyrosine kinase that is expressed on hematopoietic stem and progenitor cells.4 Flt3L is expressed as a membrane-bound protein on the surface of cells and can be proteolytically cleaved to generate a soluble protein. Both the membrane-bound and soluble forms are biologically active.6Although a weak growth stimulator as a single factor in vitro, flt3L synergizes with numerous hematopoietic growth factors to stimulate myeloid progenitors to proliferate and differentiate.7-11Flt3L synergizes with interleukin (IL)-7, c-kit ligand (KL), IL-3, and IL-11 to stimulate B-cell lymphopoiesis in vitro.12-15 Flt3L augments T-cell development from BM-derived precursors cultured in the presence of thymic stroma plus IL-1216 or BM stroma,17 and increases primitive thymus-derived precursor expansion induced in vitro with IL-3 plus IL-6 plus IL-7.18 In this respect, flt3L shares many functional properties with KL, augmenting the response of multipotent and lineage-committed progenitor cells to a variety of cytokines. In addition to the results obtained from in vitro experiments, studies of hematopoietic disorders in humans support the concept that flt3L has an important role in hematopoiesis. Healthy individuals have low levels of circulating flt3L in their plasma.19 However, in the case of hematopoietic disorders that affect the stem cell compartment (pancytopenias), specifically Fanconi anemia and acquired aplastic anemia, soluble flt3L levels are highly elevated. Flt3L may be produced in an effort to compensate for the deficiency in stem cells in these anemias.19 20
When soluble flt3L is administered to mice, hematopoietic progenitors in the BM and spleen are expanded, and potent mobilization of stem and progenitor cells into the peripheral blood (PB) occurs.21The mobilization includes cells with long-term, multilineage reconstitution potential, demonstrating that pluripotent stem cells are mobilized.22 In addition to the effect on stem and progenitor cells of the myeloid lineage, expansion of immature B cells is noted in the BM and spleen.21 Splenomegaly and lymphadenopathy result, and examination of these tissues indicates that administration of flt3L induces a large increase in the number of dendritic cells (DC) in the spleen and lymph nodes (LN) as well as in the PB, lungs, liver, Peyer patches, and thymus.23-25 The flt3L-expanded DC can process and present antigen to naive T lymphocytes in vitro and in vivo.23 Administration of flt3L to tumor-bearing mice results in the generation of specific antitumor T-cell responses, revealing a potent antitumor activity of the protein.26-28 The role of the expanded DC in the generation of this antitumor response is currently under investigation.
Mice lacking the receptor for flt3L (flk-2−/−) exhibit hematologic defects.29 Flk-2−/− mice have reduced numbers of B-cell precursors (pro-B cells) in the BM, though normal numbers of functional B cells are present in the periphery. Transplantation experiments have revealed a defect at the multipotent stem cell level. In competitive repopulation experiments, stem cells from flk-2−/− mice transplanted to irradiated recipients do not effectively reconstitute the hematopoietic system, especially the T-lymphoid lineage.29 The interaction of KL with its receptor c-kit is important for regulation of early events in hematopoiesis. The complete lack of KL or c-kit expression in mice is lethal, but some nonlethal mutations in this cytokine/receptor combination have been described.30 These mice (eg, W/Wv) have severe defects affecting hematopoietic progenitors and erythropoiesis, in addition to nonhematopoietic defects. Mice homozygous for mutations in both c-kit and flk-2 (W/Wv flk-2−/− mice) display reduced hematopoiesis and a life expectancy of 6 weeks.29 It was concluded that flt3L and KL are both required for normal hematopoiesis.
In an effort to further elucidate the role of flt3L in hematopoiesis, we have generated mice lacking flt3L and have found that flt3L−/− mice have a more profound hematologic phenotype than that noted for flk-2−/− mice. Flt3L−/− mice have significantly reduced cellularity in a number of hematopoietic tissues, reduced numbers of myeloid and lymphoid progenitors in the BM, and reduced numbers of DC and NK cells in the lymphoid organs.
Materials and methods
Targeting of the flt3L gene by homologous recombination and generation of flt3L-deficient mice
Genomic clones encoding murine flt3L were isolated from a C57BL/6 genomic library (Stratagene, La Jolla, CA) and mapped by a combination of polymerase chain reaction (PCR) and restriction analyses. A targeting vector was constructed by replacing a 2.8-kb NotI-SpeI fragment encoding exons 1 to 5 (amino acids 1-164) with a PGK-neo cassette. A thymidine kinase cassette (MC-TK) was inserted into the 3′ end of the vector. C57BL/6-derived embryonic stem cells were electroporated with the flt3L targeting construct and selected as described previously.31 Approximately 1/80 G418 and ganciclovir-resistant clones carried an flt3L allele disrupted by homologous recombination as determined by both PCR and genomic Southern blot analyses.
Flt3L-targeted embryonic stem cell clones were injected into day 3.5 BALB/c blastocysts and transferred to day 2.5 pseudopregnant Swiss Webster recipients. Resulting male chimeras were bred to C57BL/6 females and offspring were analyzed for germline transmission of the mutant alleles by PCR and genomic Southern blot analyses. C57BL/6 mice heterozygous for the flt3L mutation (flt3L +/−) were intercrossed to yield mice homozygous for the mutation (flt3L−/−). The lack of flt3L expression in flt3L−/− mice was confirmed by PCR analysis of first strand complementary DNA (cDNA) synthesized according to manufacturer specifications (Amersham Pharmacia Biotech, Piscataway NJ) from spleen and BM total RNA and amplified using the primers 5′-CACCTGACTGTTACTTCAGCC and 5′-CCTGGGCCGAGGCTCTGG, spanning the complete extracellular domain (481 nucleotides) of mature flt3L.6 Amplified products were analyzed by Southern blot analysis using a radiolabeled flt3L cDNA probe. Mouse β-actin reverse transcriptase PCR (RT-PCR) analysis was performed as a control for uniform RNA integrity and first strand synthesis using the primers 5′-AGGTAGTCCGTCAGGTCC and 5′-CGGAGTCCATCACAATGC.
Mice
Flt3L−/− mice, maintained on a C57BL/6 background, were bred at Immunex Corporation (Seattle, WA). C57BL/6 mice were purchased from Taconic Farms Inc (Germantown, NY), housed for a minimum of 1 week, and used as age- and sex-matched controls. DBA/2 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and Swiss Webster mice were purchased from Taconic Farms. Data are presented from mice aged between 5 and 14 weeks. Mice were housed under specific pathogen-free conditions.
Tissues
Peripheral blood was harvested by cardiac puncture and collected into heparinized tubes (Venoject, Fischer Scientific, Pittsburgh, PA). Hematocrits were performed as previously described.21 White blood cell (WBC) counts, blood differentials, and platelet counts were analyzed using a Hemavet Multispecies Hematology Analyzer (CDC Technologies, Oxford, CT). BM cell suspensions were prepared by flushing cold phosphate-buffered saline (PBS) containing 5% fetal bovine serum (FBS) (Intergen, Purchase, NY) through isolated femurs. Cell suspensions were prepared from spleens, LN (2 inguinal plus 2 brachial plus 2 axillary), and thymi by gently breaking up the tissues between frosted glass slides.
In vitro hematopoietic progenitor assays
Colony-forming units–granulocyte-macrophage (CFU-GM), burst-forming units–erythroid (BFU-E), and B-cell colony-forming units (B-CFU) lymphoid assays were performed by plating BM cell suspensions in methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 30% FBS (Intergen) and 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 mmol/L glutamine (JRH Biosciences, Lenexa, KS). Methylcellulose for CFU-GM and BFU-E assays was supplemented with 100 ng/mL recombinant murine (rmu) IL-3, 200 ng/mL rmu KL, plus 2 U/mL recombinant human (rhu) erythropoietin. CFU-GM and BFU-E were scored in the same assay. Methylcellulose for CFU-B lymphoid assays was supplemented with combinations of 50 ng/mL rhu IL-7, 100 ng/mL rhu flt3L, and 200 ng/mL rmu KL. All cytokines were produced and purified at Immunex Corporation except erythropoietin (R & D Systems, Minneapolis, MN). Cells were plated in quadruplicate wells in 24-well plates (Costar, Cambridge, MA) at 0.5 mL/well. Plates were incubated at 37°C, 6.5% CO2. B-lymphoid colonies and myeloid colonies containing more than 50 cells were scored after 7 and 9 days, respectively.
Colony-forming units-spleen13 (CFU-S13) assays
The BM cells were isolated from the femurs of 4 to 5 mice per experiment and pooled. Adult female C57BL/6 mice (9-20 weeks old) served as recipients and were lethally irradiated (1000 rads) using a137Cs source (Mark 1 irradiator, J. J. Shephard and Associates, Glendale, CA) at a rate of 89 to 115 rads/min. BM cells (2.5 × 104) were injected intravenously (IV) in a volume of 0.2 mL PBS, 3 to 4 hours after irradiation (n = 10/group). After 13 days, the mice were killed, the spleens isolated and fixed in Tellyesniczky solution, and the macroscopic colonies were scored.
Monoclonal antibodies and flow cytometry
The following antibodies were used for flow cytometry: anti-CD3 (145-2C11), anti-NK1.1 (PK136), anti-B220 (RA3-6B2), anti-Gr-1 (RB6-8C5), anti-CD11b (M1/70), anti-CD4 (RM4-5), anti-CD8α (53—6.7), anti-CD11c (HL3), anti-IAb (AF6-120.1), (Pharmingen, San Diego, CA), polyclonal goat-anti-IgM, and anti-IgD (SBA 1) (Southern Biotech, Birmingham, AL). Isotype-matched controls used included mouse IgG2a, rat IgG2a, rat IgG2b, and hamster IgG labeled with the appropriate fluorochromes (Pharmingen). Up to 1 × 106 cells per sample were first incubated in FACS buffer (PBS containing 2% FBS and 0.02% sodium azide), containing 2% mouse serum (Biocell, Rancho Dominguez, CA), 2% hamster serum (Jackson Immunoresearch Laboratories, West Grove, PA) and 10 μg/mL anti-CD16/32 (2.4G2) (Immunex) to minimize nonspecific binding. Cells were then labeled with the appropriate antibodies for 30 minutes at 4°C in FACS buffer. Finally propidium iodide (2.5 μg/mL) (Boehringer Mannheim, Indianapolis, IN) was added to exclude dead cells from the analysis. Samples were analyzed on a FACScan (Becton Dickinson, San Jose, CA), or FACSCalibur (Becton Dickinson). Between 20 000 and 100 000 events were collected for analysis. Data were analyzed using Cellquest software (Becton Dickinson).
Isolation of DC
The DC were enriched as previously described.32 Briefly, spleens, thymi, and LN (2 inguinal plus 2 brachial plus 2 axillary nodes) were harvested. Spleens and thymi were infused with 100 U/mL of type III collagenase (Worthington Biochemicals, Lakewood, NJ) and incubated in 400 U/mL of type III collagenase for 30 minutes at 37°C. Intact LN were incubated in 400 U/mL of type III collagenase for 30 minutes at 37°C. Tissues were further dissociated in Ca++-free media (Hanks balanced salt solution [HBSS]) (Life Technologies, Grand Island, NY) containing 10 mmol/L EDTA (Life Technologies), and cells were separated into low- and high-density fractions on a Nycodenz gradient (density 1.080 gm/mL) (Nycomed Pharma, Distributor in the United States, Life Technologies). The low-density cells, enriched for DC, were harvested from the interface. The cells were incubated with anti-CD11c and anti-CD8α and the DC purified on a FACSVantage (Becton Dickinson).
Mixed lymphocyte reaction
The CD4+ T lymphocytes from the LN and spleens of DBA/2 adult female mice were purified as previously described.23One hundred thousand purified CD4+ T cells were incubated in 10% CO2 in air at 37°C with serially diluted purified DC in 96-well round-bottomed plates (ICN Biomedicals, Aurora, OH) in complete Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% FBS (Life Technologies), 50 U/mL penicillin, 50 μg/mL streptomycin and 2 mmol/L l-glutamine (JRH Biosciences), 5 × 10−5 mol/L 2-ME, 0.1 mmol/L nonessential amino acids, and 1 mmol/L sodium pyruvate (Life Technologies). On day 4, 0.5 μCi of tritiated thymidine was added to the wells and after 5 hours the cells were harvested onto glass fiber sheets for counting on a gas-phase β counter.
Cytotoxicity assays
Mice were injected intraperitoneally with PBS or 100 μg of polyinosinic-polycytidylic acid (poly-I:C) (Sigma, St Louis, MO) in PBS and their spleens were isolated the following day. Cell suspensions were prepared and cytotoxicity was measured in a standard 4-hour51Cr-release assay. Yac-1 cells were labeled with Na251CrO4 (100 μCi/3 × 106 cells) for 1 hour at 37°C. Serial dilutions of effector cells were mixed with labeled targets (5 × 104/well) in 96-well round-bottomed plates and centrifuged at 400g for 3 minutes. Assays were performed in RPMI media (Life Technologies) containing 10% FBS (Intergen), 50 U/mL penicillin, 50 μg/mL streptomycin and 2 mmol/Ll-glutamine (JRH Biosciences), 5 × 10−5 mol/L 2-ME, 0.1 mmol/L nonessential amino acids, and 1 mmol/L sodium pyruvate (Life Technologies). In some experiments, labeled P815 target cells were included as a control for NK-specific lysis. Cell-free supernatants were harvested after 4 hours of incubation at 37°C, 5% CO2 using a cell harvester (Skatron, Sterling, VA). Percent specific lysis was calculated as ([experimental release-spontaneous release]/[maximum release-spontaneous release]) × 100.
Statistical analyses
Data were analyzed using a 2-tailed Student t test.
Results
Generation of flt3L−/− mice
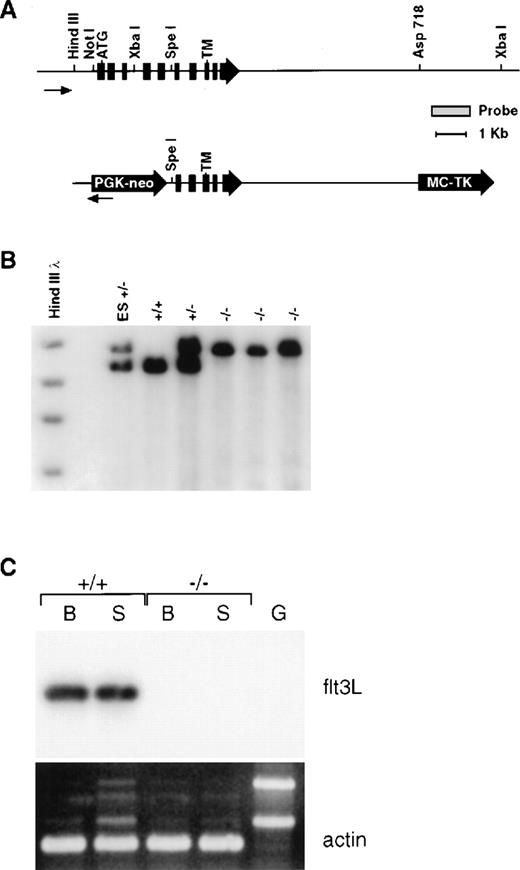
Mice genetically deficient in flt3L (flt3L−/−) were generated by gene targeting using a vector that deletes the majority of the flt3L extracellular domain (Figure 1A). Southern blot analyses using diagnostic restriction digests in conjunction with probes lying beyond the regions of homology contained within the targeting vector confirmed that the flt3L gene was disrupted by homologous recombination (Figure 1B), and a sensitive RT-PCR analysis confirmed the lack of flt3L expression in flt3L−/− mice (Figure 1C). Mice homozygous for the mutation were generated from crosses of heterozygotes at the expected mendelian frequency, displayed no overt phenotype, and bred normally. The flt3L−/− mice used throughout these studies were maintained on a C57BL/6 inbred genetic background.
Generation and molecular characterization of the flt3L mutation.
(A) Schematic diagram of the wild-type flt3L gene and flt3L targeting vector. Exons are depicted in filled boxes. Relevant restriction sites, as well as the probe used for genomic Southern analysis, are shown. The positions of the initiator methionine (ATG) and transmembrane (TM) domain are depicted. (B) Genomic DNA from flt3L-targeted embryonic stem cells as well as representative +/+, +/−, and −/− mice were digested with XbaI and subject to Southern blot analysis using the depicted probe. (C) First strand cDNAs derived from either bone marrow (B) or spleen (S) of +/+ and −/− adult mice and a genomic DNA control (G) were amplified using flt3L specific primers and subjected to Southern blot analysis using a flt3L cDNA probe. Duplicate samples were amplified using mouse β-actin specific primers and visualized by ethidium bromide staining.
Generation and molecular characterization of the flt3L mutation.
(A) Schematic diagram of the wild-type flt3L gene and flt3L targeting vector. Exons are depicted in filled boxes. Relevant restriction sites, as well as the probe used for genomic Southern analysis, are shown. The positions of the initiator methionine (ATG) and transmembrane (TM) domain are depicted. (B) Genomic DNA from flt3L-targeted embryonic stem cells as well as representative +/+, +/−, and −/− mice were digested with XbaI and subject to Southern blot analysis using the depicted probe. (C) First strand cDNAs derived from either bone marrow (B) or spleen (S) of +/+ and −/− adult mice and a genomic DNA control (G) were amplified using flt3L specific primers and subjected to Southern blot analysis using a flt3L cDNA probe. Duplicate samples were amplified using mouse β-actin specific primers and visualized by ethidium bromide staining.
Flt3L−/− mice have reduced numbers of leukocytes in their hematopoietic tissues and PB
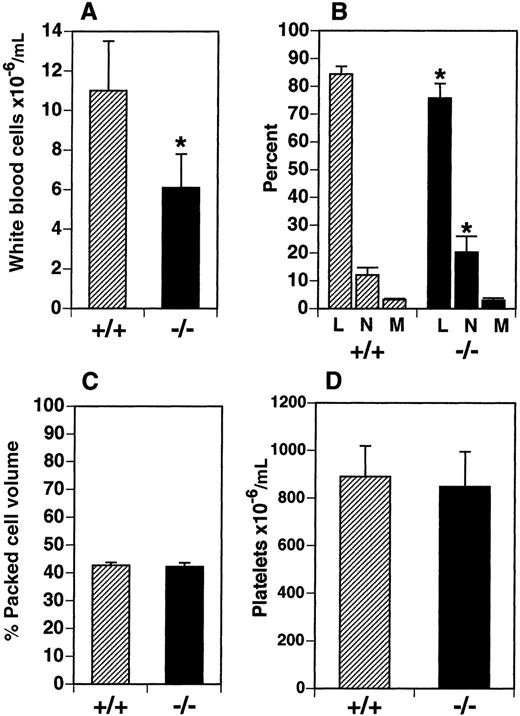
We examined leukocyte cellularity in the PB and a number of hematopoietic tissues in the flt3L−/− mice. Total WBC numbers were reduced by 45% in the PB from a mean of 11.0 × 106 cells/mL in C57BL/6 mice (flt3L+/+) to 6.1 × 106 cells/mL in flt3L−/− mice (Figure 2A). The relative proportion of lymphocytes, neutrophils, and monocytes was examined in the PB of flt3L−/− mice using a Hemavet blood analyzer (n = 8). The proportion of monocytes was unchanged, but the proportion of lymphocytes was significantly reduced from a mean of 84.4% in the flt3L+/+ mice to 75.8% in the flt3L−/− mice. Conversely, the proportion of neutrophils was significantly increased from 12.1% in the flt3L+/+ mice to 20.3% in the flt3L−/− mice (Figure2B). This translates to an absolute lymphocyte count of 9.2 × 106/mL in flt3L+/+ PB compared to 4.6 × 106/mL in flt3L−/− PB, and an absolute neutrophil count of 1.4 × 106/mL in flt3L+/+ PB compared to 1.2 × 106/mL in flt3L−/− PB. The hematocrit (percent packed cell volume) was examined and appeared normal in the flt3L−/− mice (Figure 2C). Platelets were also quantified using the Hemavet blood analyzer. There was no difference in the mean number of platelets in the PB of flt3L−/− mice compared to flt3L+/+ mice (Figure2D). Eosinophils in the PB were quantified and no difference was noted between flt3L−/− and flt3L+/+ mice (flt3L−/−, 4.1 ± 2.6 × 104/mL; flt3L+/+, 2.8 ± 2.5 × 104/ml; n = 8).
Flt3L−/− mice have reduced numbers of leukocytes in the PB.
PB was obtained from flt3L+/+ and flt3L−/− mice by cardiac puncture. (A) WBC counts (n = 8). (B) Blood differentials (n = 8) (L = lymphocytes, n = neutrophils, M = monocytes). (C) Hematocrit (% packed cell volume) (n = 7). (D) Platelet counts (n = 8) were performed as described in “Materials and methods.” Data are presented as the mean ± SD. *P < .0004 for WBC,P = .0011 for percent lymphocytes, and P = .0026 for percent neutrophils.
Flt3L−/− mice have reduced numbers of leukocytes in the PB.
PB was obtained from flt3L+/+ and flt3L−/− mice by cardiac puncture. (A) WBC counts (n = 8). (B) Blood differentials (n = 8) (L = lymphocytes, n = neutrophils, M = monocytes). (C) Hematocrit (% packed cell volume) (n = 7). (D) Platelet counts (n = 8) were performed as described in “Materials and methods.” Data are presented as the mean ± SD. *P < .0004 for WBC,P = .0011 for percent lymphocytes, and P = .0026 for percent neutrophils.
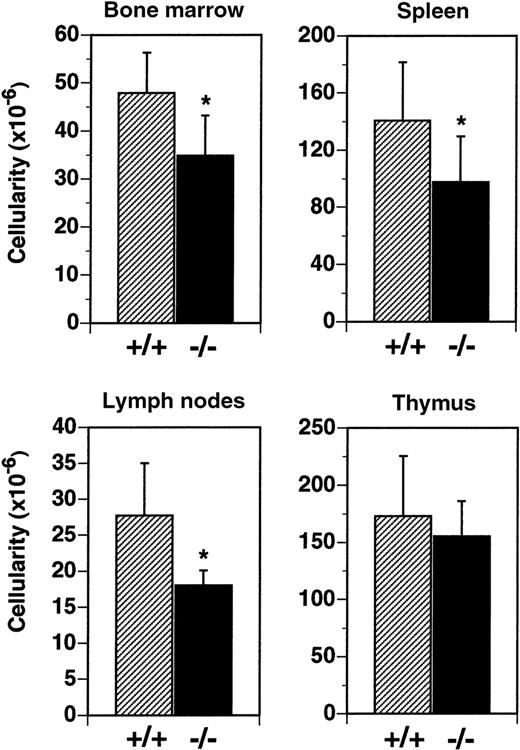
Cellularity of the BM was reduced by 27% in flt3L−/− mice (Figure 3). In flt3L+/+ mice, mean total leukocyte cellularity from 2 femurs was 47.9 × 106cells, compared to a mean of 34.8 × 106 cells from the femurs of flt3L−/− mice. Analysis by flow cytometry revealed that the reduction resulted from a decrease in the number of immature B lymphocytes (B220+, IgM−) and immature myeloid cells (CD11b+, Gr-1+) (data not shown). Spleen cellularity was reduced by 30% from a mean of 140.0 × 106 cells/spleen to 98.0 × 106 cells/spleen in flt3L−/− mice (Figure 3). LN cellularity was reduced by 35% in flt3L−/− mice, from a mean of 27.7 × 106 cells/6 nodes to 18.0 × 106 cells/6 nodes (Figure 3). Analysis of spleen and LN cells by flow cytometry revealed that both T cells and B cells were reduced in number, but that the ratio of T cells (both CD4+ and CD8+) to B cells was unchanged in the flt3L−/− mice when compared to flt3L+/+ mice (data not shown). In contrast flt3L−/− thymus cellularity was not significantly affected (Figure 3). Histochemical analyses of spleen, thymi, and LN revealed no disruption to normal architecture in the tissues of flt3L−/− mice (n = 8, data not shown).
Reduced cellularity in the BM, spleen, and LN of flt3L−/− mice.
BM was isolated from the 2 femurs of each mouse (n = 25 mice) and cellularity determined. Spleens (n = 14), LN (n = 7), and thymi (n = 11) were isolated, single-cell suspensions prepared, and cell counts were performed. LN counts represent cellularity from 6 LN per mouse (2 inguinal nodes plus 2 brachial nodes plus 2 axillary nodes). Data are presented as the mean ± SD. *P < .0001 for BM, P = .0046 for spleen, and P = .0053 for LN.
Reduced cellularity in the BM, spleen, and LN of flt3L−/− mice.
BM was isolated from the 2 femurs of each mouse (n = 25 mice) and cellularity determined. Spleens (n = 14), LN (n = 7), and thymi (n = 11) were isolated, single-cell suspensions prepared, and cell counts were performed. LN counts represent cellularity from 6 LN per mouse (2 inguinal nodes plus 2 brachial nodes plus 2 axillary nodes). Data are presented as the mean ± SD. *P < .0001 for BM, P = .0046 for spleen, and P = .0053 for LN.
We conclude that targeted disruption of the flt3L gene results in reduced leukocyte cellularity in primary hematopoietic tissue, secondary lymphoid organs, and the PB. Thymic cellularity, blood hematocrit, and platelet numbers were not affected. Although leukocyte cellularity was significantly reduced in the flt3L−/− mice, these animals do not appear to suffer an increased frequency of infection, and their lifespan in a specific pathogen-free animal facility appears to be equivalent to flt3L+/+ mice (data not shown).
Reduced numbers of hematopoietic myeloid and lymphoid progenitors in the BM of flt3L−/− mice
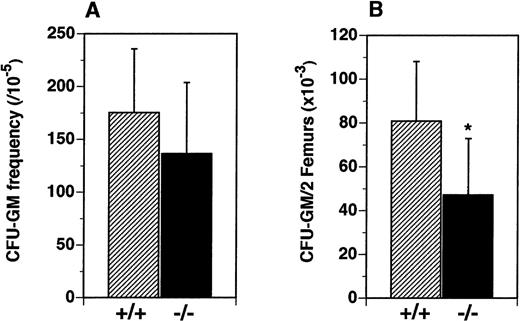
We hypothesized that the reduced numbers of leukocytes observed in the periphery of flt3L−/− mice resulted from reduced numbers of lymphoid and myeloid hematopoietic progenitors in the BM. The frequency and numbers of myeloid precursors were examined in vitro and in vivo. Although the frequency of precursors for the myeloid lineage (CFU-GM) in the BM was not significantly different (Figure 4A), the absolute number of CFU-GM was reduced from a mean of 80.8 × 103/2 femurs in the flt3L+/+ mice to a mean of 47.2 × 103/2 femurs in the flt3L−/− mice (Figure 4B).
Reduced numbers of CFU-GM precursors in the BM of flt3L−/− mice.
BM cells were cultured in methylcellulose in the presence of rmu IL-3 plus rmu KL plus rhu EPO (see “Materials and methods”). (A) Frequency of clonal CFU-GM progenitors. (B) Absolute number of CFU-GM per 2 femurs (n = 16 mice). Data were pooled from 4 experiments and are presented as the mean ± SD. *P = .0012.
Reduced numbers of CFU-GM precursors in the BM of flt3L−/− mice.
BM cells were cultured in methylcellulose in the presence of rmu IL-3 plus rmu KL plus rhu EPO (see “Materials and methods”). (A) Frequency of clonal CFU-GM progenitors. (B) Absolute number of CFU-GM per 2 femurs (n = 16 mice). Data were pooled from 4 experiments and are presented as the mean ± SD. *P = .0012.
In addition to CFU-GM, the frequency and total number of multipotent CFU-S13 progenitors in the femurs were determined. BM cells from flt3L+/+ and flt3L−/− mice were injected IV into lethally irradiated C57BL/6 recipients, and after 13 days, the spleens were isolated, fixed in Tellyesniczky solution, and the macroscopic spleen colonies were scored (Table 1). The frequency of CFU-S13 progenitors in the BM was not significantly different in the flt3L+/+ and the flt3L−/− mice; however, the absolute number of CFU-S13 progenitors in the BM of flt3L−/− mice was reduced 39% from a mean of 9777 CFU-S13 progenitors per 2 femurs in the flt3L+/+ mice to a mean of 5951 CFU-S13 progenitors per 2 femurs in the flt3L−/− mice.
Reduced numbers of CFU-S13 precursors in the BM of flt3L−/− mice
| Experiment number . | BM cellularity (×106/2 femurs) . | CFU-S13 frequency (/105) . | Total CFU-S13 (per 2 femurs) . | |||
|---|---|---|---|---|---|---|
| +/+ . | −/− . | +/+ . | −/− . | +/+ . | −/− . | |
| 1 | 46.4 | 28.8 | 22.8 | 19.4 | 10 579 | 5587 |
| 2 | 47.3 | 30.0 | 19.4 | 20.2 | 9176 | 6060 |
| 3 | 42.7 | 26.3 | 22.8 | 23.6 | 9576 | 6207 |
| Mean ± SD | 45.5 ± 2.4 | 28.4 ± 1.9 | 21.7 ± 2.0 | 21.1 ± 2.2 | 9777 ± 723 | 5951 ± 324 |
| Experiment number . | BM cellularity (×106/2 femurs) . | CFU-S13 frequency (/105) . | Total CFU-S13 (per 2 femurs) . | |||
|---|---|---|---|---|---|---|
| +/+ . | −/− . | +/+ . | −/− . | +/+ . | −/− . | |
| 1 | 46.4 | 28.8 | 22.8 | 19.4 | 10 579 | 5587 |
| 2 | 47.3 | 30.0 | 19.4 | 20.2 | 9176 | 6060 |
| 3 | 42.7 | 26.3 | 22.8 | 23.6 | 9576 | 6207 |
| Mean ± SD | 45.5 ± 2.4 | 28.4 ± 1.9 | 21.7 ± 2.0 | 21.1 ± 2.2 | 9777 ± 723 | 5951 ± 324 |
BM cells were isolated from the femurs of flt3L+/+ and flt3L−/− adult mice (n = 4-5/experiment), and pooled for each group. Groups of 10 lethally irradiated C57BL/6 mice were injected intravenously with 2.5 × 104 BM cells. After 13 days, the mice were sacrificed, the spleens isolated and fixed in Tellyesniczky solution. In each experiment, macroscopic colonies (≥1.0 mm in diameter) on the spleens were scored by 2 individuals, and the results averaged. Data are presented as the mean ± SD from the 3 experiments. BM cellularity: P = .0007; CFU-S13 frequency: not significantly different; Total CFU-S13 per 2 femurs:P = .0011.
The numbers of BFU-E were scored as a measure of the number of erythroid progenitors in the BM. The number of BFU-E per 2 femurs was slightly reduced in the BM of flt3L−/− mice but did not reach significance (5.8 ± 3.0 × 103 in flt3L+/+ BM compared to 4.3 ± 2.8 × 103 in flt3L−/− BM; n = 12).
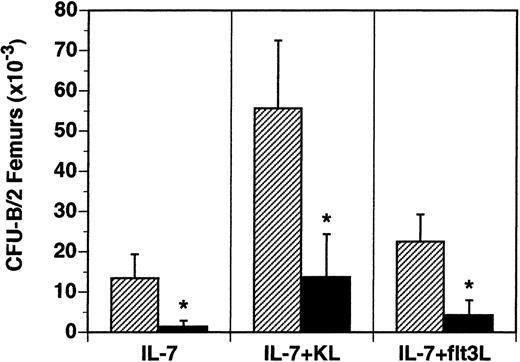
The B-lymphocyte precursors (B-CFU) present in the BM were also examined in vitro. B-cell precursors responsive to IL-7 alone were reduced 90% from 13.5 × 103/2 femurs in flt3L+/+ mice to 1.4 × 103/2 femurs in flt3L−/− mice (Figure 5). The frequency of IL-7-responsive precursors was reduced from 28/1 × 105 BM cells in flt3L+/+ mice to 4/1 × 105 BM cells in flt3L−/− mice. B-lymphocyte precursors responsive to the cytokine combinations IL-7 plus KL or IL-7 plus flt3L were reduced 81% and 75%, respectively (Figure 5). Unlike the myeloid precursors, which were reduced in absolute number in the BM, but not in frequency, the absolute numbers and the frequency of B-cell precursors were significantly reduced in the BM of flt3L−/− mice.
Reduced numbers of B-lymphoid progenitors in the BM of flt3L−/− mice.
BM was isolated from the femurs of flt3L+/+ and flt3L−/− mice and cultured in methylcellulose supplemented with rhu IL-7 alone or combined with rmu KL or rhu flt3L to determine the frequency of CFU-B progenitors. Data are presented as the absolute number of progenitors per 2 femurs (n = 8 mice). Data were pooled from 2 experiments and are presented as the mean ± SD. *P < .0001.
Reduced numbers of B-lymphoid progenitors in the BM of flt3L−/− mice.
BM was isolated from the femurs of flt3L+/+ and flt3L−/− mice and cultured in methylcellulose supplemented with rhu IL-7 alone or combined with rmu KL or rhu flt3L to determine the frequency of CFU-B progenitors. Data are presented as the absolute number of progenitors per 2 femurs (n = 8 mice). Data were pooled from 2 experiments and are presented as the mean ± SD. *P < .0001.
Because the number of B-cell precursors in the BM was severely reduced, we examined the peripheral B-lymphocyte populations to determine whether these were affected qualitatively or quantitatively. B cells present in the spleen and LN expressed normal levels of surface IgM and IgD (data not shown), and normal levels of circulating IgA, IgG1, IgG2a, IgG2b, IgG3, and IgE were present in the serum (n = 7 mice, data not shown). However, significantly increased levels of IgM were noted in the serum of flt3L−/− mice. The mean concentration of serum IgM in flt3L+/+ mice was 63 ±12 μg/mL, as compared to 178 ± 66 μg/mL in flt3L−/− mice. Flt3L−/− mice were immunized with trinitrophenol-conjugated keyhole limpet hemocyanin (TNP-KLH) in alum, and 3 weeks later rechallenged with TNP-KLH. Concentrations of various immunoglobulin isotypes were measured in the serum after the primary and secondary challenge. No differences in the concentration of TNP-specific IgA, IgG1, IgG2a, IgG2b, IgG3, IgE, or IgM were noted between flt3L−/− and flt3L+/+ mice (data not shown). In addition, the spleens of immunized animals were examined for the presence of peanut agglutinin-positive germinal centers and no differences were noted in the number formed in the spleens of flt3L−/− mice compared to flt3L+/+ mice (data not shown). These results indicate that although the numbers of B-cell precursors present in the BM of flt3L−/− mice are severely reduced, peripheral B cells are present and appear to function normally in response to immunization with a T-cell-dependent immunization protocol.
Flt3L−/− mice have reduced numbers of DC
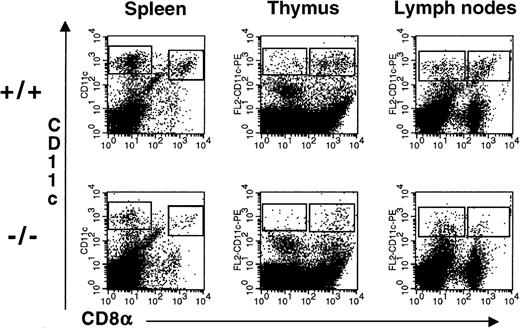
Administration of flt3L to mice for 9 to 10 days induces a dramatic expansion of DC in the PB as well as in multiple tissues23 suggesting a role for flt3L in the development of DC. We examined the spleens, thymi, and LN of flt3L−/− mice for the presence of DC. To enrich for DC, the tissues were treated with collagenase and the resulting cell suspension was centrifuged over Nycodenz (1.080 g/mL) to enrich for low-density cells. Cells were incubated with antibodies to CD11c and CD8α. Myeloid-related DC were identified as low-density cells expressing high levels of CD11c and lacking expression of CD8α, whereas lymphoid-related DC were identified as cells expressing high levels of CD11c and CD8α. All CD11cbright cells expressed high levels of major histocompatibility complex class II (IAb) (data not shown). Representative examples from the spleen, thymus, and LN are presented in Figure 6. The absolute numbers of CD8α− and CD8α+ DC in each tissue are presented in Table 2. The numbers of myeloid-related and lymphoid-related DC were reduced in the spleen, thymus, and LN of flt3L−/− mice. In some experiments the number of DC present in the LN of flt3L−/− mice was below the level of detection (0.1 × 104/6 LN) (Table 2).
Reduced numbers of DC present in the lymphoid tissues of flt3L−/− mice.
DC were enriched from the spleen, thymus, and LN (n = 6/mouse) by first treating the tissues with collagenase followed by centrifugation over Nycodenz to enrich for low-density cells. Cells were incubated with antibodies against CD11c and CD8α. A representative example of 3 to 5 separate experiments is shown in which tissues from 3 to 8 mice were pooled (see Table 2).
Reduced numbers of DC present in the lymphoid tissues of flt3L−/− mice.
DC were enriched from the spleen, thymus, and LN (n = 6/mouse) by first treating the tissues with collagenase followed by centrifugation over Nycodenz to enrich for low-density cells. Cells were incubated with antibodies against CD11c and CD8α. A representative example of 3 to 5 separate experiments is shown in which tissues from 3 to 8 mice were pooled (see Table 2).
Reduced numbers of DC in the spleen, thymus, and LN of flt3L−/− mice
| . | flt3L+/+ CD11c++ CD8α− . | flt3L−/− CD11c++ CD8α− . | Fold reduction . | flt3L+/+ CD11c++ CD8α+ . | Flt3L−/− CD11c++ CD8α+ . | Fold reduction . |
|---|---|---|---|---|---|---|
| Spleen* | ||||||
| Experiment 1 (n = 3) | 17.8 | 1.6 | 11.1 | 10.0 | 0.7 | 14.3 |
| Experiment 2 (n = 4) | 17.5 | 1.9 | 9.2 | 4.7 | 0.4 | 11.8 |
| Experiment 3 (n = 3) | 12.8 | 3.1 | 4.1 | 5.1 | 0.8 | 6.4 |
| Thymus† | ||||||
| Experiment 1 (n = 5) | 6.9 | 0.9 | 7.7 | 9.0 | 1.2 | 7.5 |
| Experiment 2 (n = 8) | 8.0 | 0.9 | 8.9 | 8.7 | 1.7 | 5.1 |
| Experiment 3 (n = 4) | 12.2 | 4.6 | 2.7 | 11.7 | 2.4 | 4.9 |
| Experiment 4 (n = 3) | 3.9 | 1.4 | 2.8 | 4.9 | 0.8 | 6.1 |
| LN† | ||||||
| Experiment 1 (n = 5) | 4.2 | 1.5 | 2.8 | 5.5 | 0.6 | 9.2 |
| Experiment 2 (n = 8) | 1.6 | 0.2 | 8.0 | 1.2 | 0.3 | 4.0 |
| Experiment 3 (n = 4) | 1.9 | <0.1 | 1.2 | <0.1 | ||
| Experiment 4 (n = 5) | 1.7 | <0.1 | 1.3 | <0.1 | ||
| Experiment 5 (n = 7) | 2.6 | 0.2 | 13.0 | 1.8 | <0.1 |
| . | flt3L+/+ CD11c++ CD8α− . | flt3L−/− CD11c++ CD8α− . | Fold reduction . | flt3L+/+ CD11c++ CD8α+ . | Flt3L−/− CD11c++ CD8α+ . | Fold reduction . |
|---|---|---|---|---|---|---|
| Spleen* | ||||||
| Experiment 1 (n = 3) | 17.8 | 1.6 | 11.1 | 10.0 | 0.7 | 14.3 |
| Experiment 2 (n = 4) | 17.5 | 1.9 | 9.2 | 4.7 | 0.4 | 11.8 |
| Experiment 3 (n = 3) | 12.8 | 3.1 | 4.1 | 5.1 | 0.8 | 6.4 |
| Thymus† | ||||||
| Experiment 1 (n = 5) | 6.9 | 0.9 | 7.7 | 9.0 | 1.2 | 7.5 |
| Experiment 2 (n = 8) | 8.0 | 0.9 | 8.9 | 8.7 | 1.7 | 5.1 |
| Experiment 3 (n = 4) | 12.2 | 4.6 | 2.7 | 11.7 | 2.4 | 4.9 |
| Experiment 4 (n = 3) | 3.9 | 1.4 | 2.8 | 4.9 | 0.8 | 6.1 |
| LN† | ||||||
| Experiment 1 (n = 5) | 4.2 | 1.5 | 2.8 | 5.5 | 0.6 | 9.2 |
| Experiment 2 (n = 8) | 1.6 | 0.2 | 8.0 | 1.2 | 0.3 | 4.0 |
| Experiment 3 (n = 4) | 1.9 | <0.1 | 1.2 | <0.1 | ||
| Experiment 4 (n = 5) | 1.7 | <0.1 | 1.3 | <0.1 | ||
| Experiment 5 (n = 7) | 2.6 | 0.2 | 13.0 | 1.8 | <0.1 |
DC were enriched from the spleen, the thymus, and 6 LN (2 inguinal plus 2 brachial plus 2 axillary) as described in “Materials and methods.” Tissues were digested with collagenase, and the low-density cells were isolated by centrifugation on a Nycodenz gradient. The proportion of myeloid-related DC (CD11c++, CD8α−) and lymphoid-related DC (CD11c++, CD8α+) were analyzed by flow cytometry. The data are presented as the absolute number of DC per spleen, per thymus, or per 6 LN, from 3 to 5 experiments in which tissues from 3 to 8 mice were pooled for analysis.
×105.
×104.
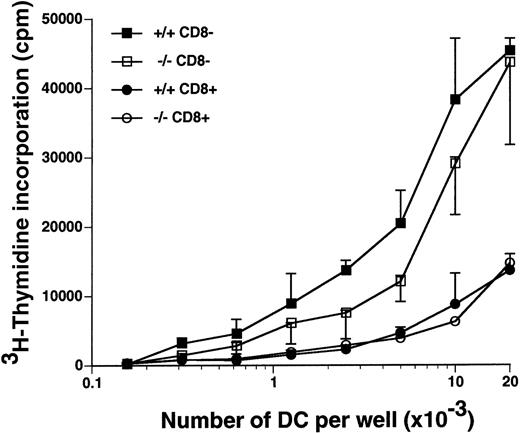
The CD8α+ DC and the CD8α− DC from the spleens of flt3L−/− and flt3L+/+ mice were purified by cell sorting, and their ability to stimulate proliferation of allogeneic naive T cells was examined in a mixed lymphocyte reaction (MLR) (Figure 7). The CD8α− DC from the flt3L−/− mice were equivalent to those isolated from flt3L+/+ mice at stimulating allogeneic T-cell proliferation (Figure 7). The CD8α+ DC were less efficient than the CD8α− DC at stimulating allogeneic T-cell proliferation; however, no difference between the CD8α+ DC from the flt3L+/+ and the flt3L−/− mice was noted. We conclude that the number of splenic DC is markedly reduced in flt3L−/− mice, but the DC that are present are able to efficiently stimulate naive allogeneic T-cell proliferation in vitro.
Splenic CD8+ DC and CD8− DC from flt3L−/− mice stimulate allogeneic T-cell proliferation in an MLR.
Splenic CD8α+ DC and CD8α− DC from flt3L+/+ and flt3L−/− mice were purified by flow cytometry (see “Materials and methods”), and cultured in titrating numbers with 1 × 105 purified allogeneic naive CD4+ T cells isolated from the spleen and LN of DBA/2 mice. T-cell proliferation as measured by uptake of tritiated thymidine was determined on day 4; ▪ indicates flt3L+/+ CD8α−DC; □, flt3L−/− CD8α− DC; •, flt3L+/+ CD8α+ DC; ○, flt3L−/− CD8α+ DC. Data are presented as the mean ± SD of triplicate counts. The experiment is representative of 3 separate experiments.
Splenic CD8+ DC and CD8− DC from flt3L−/− mice stimulate allogeneic T-cell proliferation in an MLR.
Splenic CD8α+ DC and CD8α− DC from flt3L+/+ and flt3L−/− mice were purified by flow cytometry (see “Materials and methods”), and cultured in titrating numbers with 1 × 105 purified allogeneic naive CD4+ T cells isolated from the spleen and LN of DBA/2 mice. T-cell proliferation as measured by uptake of tritiated thymidine was determined on day 4; ▪ indicates flt3L+/+ CD8α−DC; □, flt3L−/− CD8α− DC; •, flt3L+/+ CD8α+ DC; ○, flt3L−/− CD8α+ DC. Data are presented as the mean ± SD of triplicate counts. The experiment is representative of 3 separate experiments.
Flt3L−/− mice are deficient in NK cells
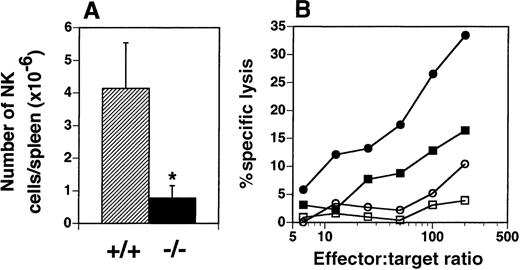
The presence of NK cells (NK1.1high, CD3−) in the spleen was examined by flow cytometry and yielded an unexpected finding. The number of NK cells in the spleens of flt3L−/− mice was dramatically reduced from a mean of 4.14 × 106 cells in the spleens of flt3L+/+ mice (range 2.31-6.37 × 106) to a mean of 0.78 × 106 (range 0.39-1.64 × 106) in the spleens of flt3L−/− mice (Figure 8A). The number of natural T (NT) cells (NK1.1low, CD3low) present in the spleens of flt3L−/− mice did not appear to be affected (data not shown). Administration of poly-I:C to mice induces quiescent NK cells to become activated, which can be quantified by their ability to lyse Yac-1 lymphoma cells. Flt3L+/+ and flt3L−/− mice were injected intraperitoneally with poly-I:C and the following day their spleens were isolated. NK cytotoxicity was measured by culturing splenocytes with51Cr-labeled Yac-1 cells. Poly-I:C treatment stimulated NK cell activity in flt3L+/+ mice (Figure 8B). Poly-I:C treatment of flt3L−/− mice stimulated a small increase in the percent specific lysis of Yac-1 cells, compared to the activity observed from flt3L−/− splenocytes from mice treated with PBS. However the level of killing was below the level of background killing observed with splenocytes from flt3L+/+ mice treated with PBS. An example of the levels of specific lysis observed is presented in Figure 8B. We conclude that targeted disruption of the flt3L gene affects development of NK cells.
NK cells are deficient in the spleens of flt3L−/− mice.
(A) The number of NK1.1++ CD3− NK cells present in the spleens of flt3L+/+ and flt3L−/− mice (n = 14) was calculated from the percentage of NK cells detected by flow cytometry combined with splenic cellularity. Data are presented as the mean ± SD. *P < .0001. (B) Mice were injected intraperitoneally with either PBS or 100 μg poly-I:C. The following day spleens were harvested and a cell suspension prepared (effector cells). Serial dilutions of effector cells were cultured for 4 hours with 51Cr-labeled Yac-1 lymphoma cells (targets). Percent specific lysis of the target cells was estimated as described in “Materials and methods”; • indicates flt3L+/+, poly-I:C treatment; ▪, flt3L+/+, PBS treatment; ○, flt3L−/−, poly-I:C treatment; □, flt3L−/−, PBS treatment. Data shown are representative of data generated from 18 mice.
NK cells are deficient in the spleens of flt3L−/− mice.
(A) The number of NK1.1++ CD3− NK cells present in the spleens of flt3L+/+ and flt3L−/− mice (n = 14) was calculated from the percentage of NK cells detected by flow cytometry combined with splenic cellularity. Data are presented as the mean ± SD. *P < .0001. (B) Mice were injected intraperitoneally with either PBS or 100 μg poly-I:C. The following day spleens were harvested and a cell suspension prepared (effector cells). Serial dilutions of effector cells were cultured for 4 hours with 51Cr-labeled Yac-1 lymphoma cells (targets). Percent specific lysis of the target cells was estimated as described in “Materials and methods”; • indicates flt3L+/+, poly-I:C treatment; ▪, flt3L+/+, PBS treatment; ○, flt3L−/−, poly-I:C treatment; □, flt3L−/−, PBS treatment. Data shown are representative of data generated from 18 mice.
Discussion
We have generated mice deficient for the gene encoding the hematopoietic growth factor flt3L. Although these mice are viable, breed normally, and appear healthy, at least when maintained in a specific pathogen-free facility, we have observed a number of deficiencies in hematopoietic lineages. Specifically, leukocyte cellularity was significantly reduced in the PB, BM, spleen, and LN and the numbers of DC and NK cells were dramatically reduced.
Examination of the PB revealed a decreased proportion of lymphocytes and a corresponding increase in the proportion of neutrophils. The proportion of monocytes was not changed (Figure 2B). Conversely, erythrocyte and platelet production was not affected in the flt3L−/− mice (Figure 2C and D). In addition to reduced leukocyte cellularity, decreased numbers of hematopoietic progenitors were present in the BM (Figures 4 and 5, Table 1). The absolute numbers of myeloid progenitors were reduced, but the frequency of these cells was not different from that observed in flt3L+/+ mice (Figure 4, Table1). Similar observations have been made in W/Wvflk-2−/− mice.29 These mice, which lack flk-2 and have a mutated c-kit receptor, have severely reduced hematopoiesis, including reduced absolute numbers of progenitors in the BM, but the frequency of the myeloid progenitors is normal. This implies that a mechanism to maintain normal myeloid progenitor frequencies in vivo exists, which does not involve flt3L or KL. In contrast to the myeloid lineage, both the absolute number and the frequency of B lymphoid progenitors in the BM were reduced in the BM of flt3L−/− mice, implicating a critical role for flt3L in B lymphopoiesis (Figure 5). The reduced numbers of lymphoid and myeloid progenitors in the BM of flt3L−/− mice are consistent with the numerous observations that flt3L synergizes with myeloid and lymphoid cytokines to augment expansion of these progenitors.3 Interestingly, mice with mutations in KL (Sl/Sld) have reduced numbers of hematopoietic progenitors in their BM, along with reduced BM cellularity, as described here for the flt3L−/− mice, but, unlike flt3L−/− mice, peripheral leukocyte numbers are close to normal.30
The reduced numbers of DC noted in the spleens, LN, and thymus of flt3L−/− mice (Figure 6, Table 2) support our hypothesis that flt3L is an important cytokine in the generation of DC. This hypothesis was based on our previous observations that flt3L administration to mice for 9 to 10 days induces a profound expansion of DC in multiple tissues.23 Analysis of DC from the spleens, LN, and thymus of flt3L−/− mice revealed a marked reduction in both myeloid-related and lymphoid-related DC. However, there were detectable numbers of DC in the flt3L−/− mice, and on purification the splenic DC were as efficient at stimulating naive allogeneic T-cell proliferation in an MLR as DC isolated from the flt3L+/+ mice (Figure 7). As has previously been described,33 the lymphoid-related DC were less effective than the myeloid-related DC at stimulating CD4+ T-cell proliferation. It remains to be determined whether the reduced numbers of DC will have an effect on infectious agent or tumor cell challenge in vivo. In vitro studies show that the addition of flt3L to a combination of cytokines can augment the generation and expansion of myeloid-related34,35 and lymphoid-related DC.36A number of other cytokines have been implicated in DC development including GM-CSF, tumor necrosis factor-α (TNFα), and transforming growth factor-β1 (TGFβ1). DC can be generated in vitro with GM-CSF alone37 or TNFα plus GM-CSF,38-40 but mice lacking GM-CSF do not have reduced numbers of lymphoid tissue DC.41 Mice deficient in TGFβ1 lack Langerhans DC, which reside in the epithelia, implying a critical role for TGFβ1 in the development of this subset of DC.42 One or more of these cytokines may substitute for the lack of flt3L to generate the residual DC present in flt3L−/− mice.
The flt3L−/− mice have a marked reduction in NK cells in the spleen (Figure 8A) demonstrating that flt3L is critical for NK cell generation. NK numbers were reduced 5.3-fold and this resulted in an almost complete loss in the ability of poly-I:C-stimulated flt3L−/− splenocytes to lyse Yac-1 lymphoma targets (Figure8B). Administration of flt3L to C57BL/6 mice results in increased numbers of NK cells in numerous tissues,43 and administration of flt3L to severe combined immunodeficient (SCID) mice (which lack T and B cells) also expands splenic NK cells (D.H.L., R.E.M., and K.B. unpublished observation). These flt3L-expanded NK cells appear to be functional because SCID mice inoculated with fibrosarcoma cells and treated with flt3L exhibit slower tumor growth26 and this effect can be abrogated if the mice are treated in vivo with an anti-NK cell antibody (PK136) (D.H.L. and R.E.M., unpublished observation).
A number of reports have demonstrated that flt3L has a role in the expansion of immature B cells that develop in the BM.13-15Flt3 receptor expression has been detected in the earliest B-cell progenitors, the pre-progenitor B cells, and as development progresses, flt3 receptor expression is down-regulated.44 The significant reduction in BM-derived B-cell progenitors we noted in the flt3L−/− mice is similar to that described for the flk-2−/− mice.29 However, preliminary immunization experiments with TNP-KLH plus alum (T-cell-dependent antibody response) indicated that flt3L−/− mice generated TNP-specific antibodies at levels similar to flt3L+/+ mice. Normal B-cell function was also indicated by the presence of normal levels of circulating polyclonal immunoglobulins in the serum of unchallenged flt3L−/− mice, with the exception of IgM, which was elevated (2.8-fold increase). The significance of this is unclear. Peripheral B cells in the spleen and LN expressed normal levels of cell-surface IgM (data not shown), and after immunization no differences in serum IgM concentrations were noted.
Thymic cellularity in the flt3L−/− mice was unaffected. Flt3L is expressed in the thymus45 and primitive thymocytes express the flt3 receptor4 implying a role for flt3L in thymocyte development. However, like the flt3L−/− mice described here, the flk-2−/− mice have normal thymic cellularity, though reduced numbers of primitive thymocytes are noted in neonatal mice.29 Although thymic cellularity was normal, the absolute number of T cells present in the lymphoid tissues was reduced. Factors produced by other circulating cells, which are reduced in the flt3L−/−mice, may be required to maintain normal numbers of circulating T cells.
There is no evidence that there are other ligands for the flt3 receptor, nor is there any evidence that flt3L binds to any other protein besides the flt3 receptor. Thus it would be expected that mice carrying targeted disruptions in either the ligand or the receptor gene would have an identical phenotype. It is therefore not clear why such differences are seen between the flt3L−/− mice described here and the flk-2−/− mice previously described.29 We have observed that 10 days of flt3L administration to flt3L−/− mice partially restores DC and NK numbers (data not shown), confirming that these defects are due specifically to the flt3L mutation. The DC and NK lineages were not discussed in the manuscript describing the flk-2−/− mice, so it remains possible that there are defects in these lineages in the flk-2−/− mice. However, the decreased leukocyte cellularities in the PB and hematopoietic tissues, as well as decreased numbers of myeloid precursors, were not observed in the flk-2−/− mice, though the more primitive stem cells, as assayed by competitive repopulation, are deficient. The possibility exists that the differences are strain dependent, because the flk-2−/− mice were created on a 129 background, and the flt3L−/− mice were created on a C57BL/6 background. Strain-dependent phenotypes have been described for gene knock-out mice. Epidermal growth factor receptor knock-out mice have varying phenotypes and life expectancies depending on the strain in which the mutation is introduced.46-48
We conclude that flt3L is a multipotent cytokine with effects on the expansion of hematopoietic progenitors of both the myeloid and lymphoid lineage. Flt3L appears to be particularly important for the generation of B-cell precursors, splenic NK cells, and lymphoid tissue DC because these cell types were the most severely reduced in flt3L−/− mice.
Acknowledgments
The authors acknowledge the expert editorial assistance of Anne Aumell, the contributions of the animal facility technical staff and Joann Schuh for reading the histology slides. In addition we thank Doug Williams for helpful discussions.
Reprints:Hilary McKenna, Immunobiology Department, Immunex Corporation, 51 University St, Seattle, WA 98101; e-mail:mckenna@immunex.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal