Abstract
Heme arginate infusions blunt the symptoms of patients with acute intermittent porphyria without evidence of the vascular or thrombotic side effects reported for hematin. To provide a rationale for heme arginate's safety, the present study examined the effects of various ferriporphyrins to sensitize human endothelial cells to free radical injury and to induce heme oxygenase and ferritin expression. Heme arginate, unlike hematin, did not amplify oxidant-induced cytotoxicity mediated by hydrogen peroxide (5.3 ± 2.4 versus 62.3 ± 5.3% 51Cr release,P < .0001) or by activated neutrophils (14.4 ± 2.9 versus 41.1 ± 6.0%, P < .0001). Nevertheless, heme arginate efficiently entered endothelial cells similarly to hematin, since both markedly induced heme oxygenase mRNA (more than 20-fold increase) and enzyme activity. Even with efficient permeation, endothelial cell ferritin content was only minimally increased by heme arginate compared with a 10-fold induction by hematin; presumably less free iron was derived from heme arginate despite up-regulation of heme oxygenase. Hematin is potentially vasculopathic by its marked catalysis of oxidation of low-density lipoprotein (LDL) to endothelial-toxic moieties. Heme arginate was significantly less catalytic. Heme arginate–conditioned LDL was less than half as cytotoxic to endothelial cells as hematin-conditioned LDL (P < .004). It is concluded that heme arginate may be less vasculotoxic than hematin since it is an effective heme oxygenase gene regulator but a less efficient free-radical catalyst.
Acute hepatic porphyrias, a failure of iron–protoporphyrin IX(heme) biosynthesis and accumulation of porphyrin precursors,1-3 cause a myriad of disabling symptoms and may even be fatal.4 Therapeutically, infusion of hematin has proven efficacious by correcting heme metabolism5-9 but has been accompanied by considerable toxicities, including vasculitis and coagulopathy.8,10-17Finnish investigators recently successfully introduced heme arginate in the treatment of acute hepatic porphyrias without evidence of vasculopathy or thrombotic side effects.18-23
Iron is a marked potentiator of oxidant damage in diverse systems and plays a substantial role in phagocyte-mediated vascular injury.24-26 For example, prolonged preexposure of endothelium to the iron chelator deferoxamine prevented activated polymorphonuclear cell (PMN)– or hydrogen peroxide (H2O2)–mediated endothelial cell damage.24 We have shown that one critical feature of highly damaging iron to endothelium is permeation of the metal into cells.27 Chelation of iron by lipophilic chelators, such as 8-hydroxyquinoline, results in the accumulation of catalytically active lipophilic iron chelates in endothelial lipid compartments; endothelium pretreated with 8-hydroxyquinoline-iron chelate was exquisitely sensitive to both endogenous and exogenous oxidant stress. A physiologically ubiquitous hydrophobic iron chelate, heme, readily concentrated within the hydrophobic milieu of intact endothelium and greatly amplified reactive oxygen species–mediated cytotoxicity.28 The plasma heme-binding protein, hemopexin, attenuated sensitization of endothelium by affecting heme-iron uptake and catalytic activity.28-30 Purified hemopexin, when preincubated with heme for 15 minutes in stoichiometric amounts, completely abrogated heme-augmented endothelial oxidant injury.28 Furthermore, heme served as a catalyst for the oxidation of low-density lipoprotein (LDL) from native LDL; the toxic moieties of oxidized LDL could, in turn, mediate endothelial activation and damage.31
Our previous studies have demonstrated that endothelial cells respond to chronic heme exposure by up-regulating heme oxygenase and ferritin synthesis.32 The latter was critically important in providing cytoprotection against further iron-driven oxidant damage. Heme oxygenase is a heme-degrading enzyme that opens the porphyrin ring producing biliverdin and carbon monoxide and releasing iron.33,34 Three genes encode for 3 isoenzymes for heme oxygenase.35 Heme oxygenase-1, identified as the 32.8-kd stress protein,36 is the form transcriptionally inducible by a variety of agents, such as heme, oxidants, and cytokines.34,36-41 Heme oxygenase-2 is constitutively active as a metabolizer of heme. The function of heme oxygenase-3 is still under investigation. Ferritin is the major intracellular depot of nonmetabolic iron. This multimeric protein consists of 24 subunits of 2 types (heavy chain and light chain) and has a very high capacity for storing iron (up to 4500 mol of iron per mol of ferritin).42,43 The heavy chain of ferritin manifests ferroxidase activity.43,44 The proportion of heavy and light subunits depends on the iron status of the cell or tissue and varies among organs and species.42 43
We hypothesize that heme arginate's lack of vascular side effects in vivo may be due to its inefficient catalyses of oxidant-mediatedendothelial cell injury. In order to test whether various heme compounds used clinically in the treatment of porphyria are vasculotoxic in vitro, we examined the effects of ferriporphyrins with different hydrophobic properties on oxidant susceptibility of endothelial cells or LDL. We also tested ferriporphyrin's ability to induce the synthesis of heme oxygenase and ferritin in endothelium.
Materials and methods
Reagents
Medium 199, fetal calf serum (FCS) and Hank's balanced salt solution (HBSS) were purchased from Life Technology (Rockville, MD); dispase II was from Gibco (Grand Island, NY); and hydroxyethyl starch was from Du Pont (Wilmington, DE). 51CrO4 (as the sodium salt) was purchased from Amersham Corp (Piscataway, NJ). Iron deuteroporphyrin IX, iron deuteroporphyrin IX,2,4-bis-glycol, iron deuteroporphyrin IX,2,4-bis-sulfonate, and iron coproporphyrin III were synthesized by Porphyrin Products (Logan, UT). Heme arginate was obtained from Dr Falk Pharma, GmbH (Freiburg, Germany), and Huhtamäki Oy Pharmaceuticals (Helsinki, Finland). For the preparation of hematin stock solutions (1 and 5 mmol/L), hemin was dissolved in the dark in NaOH 20 mmol/L and used within 1 hour after preparation. Subsequent centrifugation of hematin solutions (1 mmol/L and 5 mmol/L) at 12 000g for 10 minutes resulted in losses of less than 0.5% and 5% of the heme content, respectively. All other reagents used were purchased from Sigma (St Louis, MO) unless otherwise specified.
Endothelial cell isolation and culture
As in our previous studies,45 human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical veins. Human aortic endothelial cells and human dermal microvascular endothelial cells were grown as described.32 Endothelial cells were identified by their morphology, the presence of von Willebrand factor, and the ability to take up acetylated LDL. The cell cultures were studied within 48 hours of reaching confluence.
Preparation of human neutrophils
As described previously,28 PMNs were isolated from blood of human volunteers after informed consent (following the guidelines of the Commitee on the Use of Human Subjects in Research of the University of Minnesota and Medical University of Debrecen).
Endothelial cell cytotoxicity assays
Confluent endothelial cell monolayers grown in 2-cm2wells were radiolabeled with 2 μCi of [51Cr]Na2CrO4 in cell culture medium overnight. The monolayers were washed 3 times with HBSS and exposed to H2O2 (100 μmol/L) or phorbol myristate acetate (PMA) (100 ng/mL)–activated PMNs (2:1 PMN:endothelial cell ratio) in HBSS for 2 hours. Spontaneous 51Cr release was less than 10%. Endothelial cell cytotoxicity mediated by oxidation of LDL (200 μg/mL) was measured at 4 hours, and the spontaneous 51Cr release was below 15%. Samples and cells were processed in the dark. Specific cytotoxicity values were calculated as described previously.28
Preparation of human LDL
Plasma LDL was prepared from EDTA (1 mg/mL)–anticoagulated venous blood of healthy volunteers who had fasted overnight. LDL was isolated from plasma by rate zonal density gradient ultracentrifugation after a 2000g centrifugation of blood at 4°C for 20 minutes as previously described.31 Blood, plasma, and LDL samples were processed in subdued light on ice. LDL fraction was assessed for purity by means of agarose electrophoresis and used in the experiments within 24 hours of isolation. The LDL cholesterol was measured enzymatically on a Synchron CX5 autoanalyzer (Beckman Instruments, Brea, CA), and LDL protein concentration was determined by BCA protein assay (Pierce, Rockford, IL).
Oxidation of LDL
Oxidation of LDL (200 μg/mL protein) was catalyzed by hematin (5 μmol/L) or heme arginate (5 μmol/L), and the lipid peroxidation was monitored by measuring the formation of conjugated dienes, lipid hydroperoxides and thiobarbituric acid–reactive substances. LDL oxidation was promoted by the presence of H2O2 (50 μmol/L). Lipid hydroperoxide in LDL was detected by means of the ferrous oxidation in xylenol orange (FOX) assay.46 Briefly, 50 μL of LDL samples or cumene hydroperoxide as standard peroxide were added to 450 μL of reaction mixture containing ferrous sulfate (250 μmol/L), xylenol orange (100 μmol/L), sorbitol (100 mmol/L), and sulfuric acid (25 mmol/L). The formation of Fe3+-xylenol orange complex was followed spectrophotometrically at 560 nm for 30 minutes at room temperature. The thiobarbituric-acid colorimetric assay and the measurement of conjugated dienes were performed as described previously.31The kinetics of lipid peroxidation of LDL was characterized by the length of initiation phase (lag time) in minutes, the maximal velocity of propagation phase (Vmax) in milliabsorbance units/minute, and the time period passing until the maximal velocity of propagation phase (ΔT at Vmax) in minutes.47 Samples were processed in the dark. For cytotoxicity assays and induction of heme oxygenase gene, the LDL was incubated with either hematin or heme arginate in the presence of H2O2 for 45 minutes at 37°C prior to the endothelial cell experiments.
Heme determinations
At the end of incubations of HUVECs with heme solutions, the cell monolayers grown in 24-well tissue-culture plates were washed 3 times with 1 mL of HBSS, and the endothelial cells removed with 250 μL of concentrated formic acid. The heme content of the formic acid–solubilized HUVECs was determined spectrophotometrically at 398 nm with the use of an extinction coefficient of 1.5 × 105mol/L−1 · Cm−1.28
LDL-associated heme was measured spectrophotometrically as a heme-pyridine complex. Pyridine (0.35 mL) and 0.15 mL of 1.0 N NaOH were added to 1.25 mL of 200 μg/mL LDL samples. After vortexing, the samples were divided into 2 equal parts. The first was oxidized by 25 μL of 3 mmol/L K3Fe(CN)6, and the second was reduced by 1 mg of dithionite. The absorbances were measured at 541 and 557 nm, respectively, with the use of the oxidized samples as blanks. For calculation of the results, the differences between optical densities at 541 and 557 nm were used, with the extinction coefficient of 2.07 × 104mol/L−1 · Cm−1.31Apolipoprotein B-100 was measured by the Unimate 3 ApoB immunoturbidometric assay (F. Hoffmann-La Roche AG, Basel, Switzerland) to calculate the number of molecules of heme per LDL particle.
Heme oxygenase enzyme activity assay
Heme oxygenase enzyme activity was measured by bilirubin and carbon monoxide generation.32 HUVECs grown in 10-cm–diameter tissue-culture dishes were treated with control media, hematin (10 μmol/L), iron deuteroporphyrin IX (10 μmol/L), iron deuteroporphyrin IX,2,4-bis-glycol (10 μmol/L), iron deuteroporphyrin IX,2,4-bis-sulfonate (10 μmol/L), and iron coproporphyrin III (10 μmol/L) (all the ferriporphyrins were dissolved in NaOH [20 mmol/L] and were added to media or buffer with the final pH 7.4), or heme arginate (10 μmol/L) (dissolved in media or buffer with final pH 7.4) for 60 minutes. In separate experiments, endothelial cells were exposed to LDL (50 μg/mL protein) alone, LDL conditioned by hematin (1.25 μmol/L), and H2O2 (6.25 μmol/L), or LDL conditioned by heme arginate (1.25 μmol/L) and H2O2 (6.25 μmol/L) for 60 minutes followed by replacement of the test solutions with complete media for 8 hours. Samples and cells were processed in the dark. The endothelial cell monolayers were washed, scraped with a rubber policeman, and centrifuged at 1000g for 10 minutes at 4°C. The pellet was suspended in MgCl2 (2 mmol/L) phosphate (100 mmol/L) buffer (pH 7.4), frozen and thawed 3 times, and sonicated on ice prior to centrifugation at 18 800g for 15 minutes at 4°C. The supernatant was added to the reaction mixture (400 μL) containing glucose 6-phosphate (2 mmol/L), glucose 6-phosphate dehydrogenase (0.2 units), hemin (20 μmol/L), and rat liver cytosol (2 mg protein) for bilirubin generation. In some experiments, heme arginate (20 μmol/L) was used as a substrate for heme oxygenase. The assays were started by the addition of NADPH (0.8 mmol/L). After incubation for 60 minutes at 37°C in the dark, the formed bilirubin was extracted with chloroform and a Δ optical density of 464 to 530 nm was measured (extinction coefficient, 40 mmol/L−1 · Cm−1 for bilirubin), or the carbon monoxide was determined with the use of gas chromatography. Heme oxygenase enzyme activity is expressed as picomole of bilirubin formed per milligram of cell protein per 60 minutes or as microliter of carbon monoxide formed per milligram of cell protein per 60 minutes.
Ferritin assay
Endothelial cell ferritin content was measured in cells grown in 6-well tissue-culture plates treated with control media or various ferriporphyrins (10 μmol/L), as indicated, for 60 minutes; the culture media were then replaced with ferriporphyrin-free medium for 15 hours. Samples and cells were processed in the dark. HUVECs' ferritin content was measured after the cells were washed 3 times with HBSS (Ca and Mg free), solubilized with 1% Triton X-100, 0.5% Nonidet P-40 in Tris-HCl (10 mmol/L) buffer (pH 7.2) containing EDTA (5 mmol/L) and phenylmethylsulfonyl fluoride, and centrifuged at 10 000g for 10 minutes at 4°C. The supernatant was analyzed for ferritin with the use of the Stratus fluorometric enzyme immunoassay system.32 The results are expressed as nanogram of ferritin per milligram of cell protein. The protein content of the endothelial cell monolayers was determined by means of BCA protein assay.
Heme oxygenase and ferritin messenger RNA analysis
Heme oxygenase messenger RNA (mRNA) and H- and L-chain ferritin content were analyzed in HUVEC cultured in 10-cm–diameter tissue-culture dishes after endothelial cells were exposed to test solutions, as described for the measurement of heme oxygenase enzyme activity above, for 1 hour and then replaced with complete media for an additional 4 hours. Samples and cells were processed in the dark. Endothelial cell RNA was isolated by the RNAzol method (TEL-TEST, Inc, Friendswood, TX). The RNA transferred to nylon membranes was then hybridized at 42°C with 32P-labeled complementary DNA (cDNA) probes synthesized by random primed DNA labeling for human heme oxygenase32,36 and H ferritin and L ferritin.32 48 Autoradiograms were quantified by computer-assisted videodensitometry and expressed as arbitrary OD units.
Statistical analysis
Data are expressed as means ± SE The Student t test was employed for comparisons.
Results
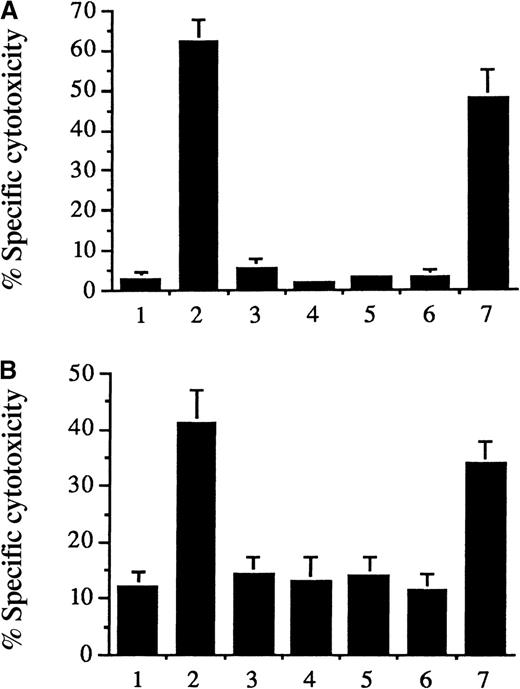
As shown in Figure 1 (and reported previously in another context28), 1-hour exposure of cultured endothelial cells to hematin (5 μmol/L) markedly aggravated H2O2 (Figure 1A, second bar) or phorbol-stimulated polymorphonuclear oxidant–mediated cytotoxicity (Figure 1B, second bar). Substitution of vinyl side chains of heme with hydrogen does not alter the hydrophobicity of the resultant ferriporphyrin, iron deuteroporphyrin IX; accordingly, hypersusceptibility was similarly provoked (seventh bars). In contrast, if water solubility of heme is conferred associatively with the arginate counterion (heme arginate) (5 μmol/L) (third bars)49 or the vinyl side chains of heme are substituted by sulfonate, propionate, or glycol leading to hydrophilic ferriporphyrins (iron deuteroporphyrin IX,2,4-bis-sulfonate [5 μmol/L] [fourth bars], iron coproporphyrin III [5 μmol/L] [fifth bars], and iron deuteroporphyrin IX,2,4-bis-glycol [5 μmol/L] [sixth bars]), these ferriporphyrins failed to sensitize cells to H2O2or activated polymorphonuclear leukocytes. This was not HUVEC-specific since the same results were observed when the target cells were human aortic and human dermal microvascular endothelial cells instead of HUVECs (data not shown). We asked: could hematin sensitize endothelial cells to oxidative challenge in the presence of plasma? After all, plasma is enriched with binding proteins, such as albumin and hemopexin, known to inhibit heme-mediated cell damage.28-30 Exposure of HUVECs to hematin (600 μmol/L in whole human plasma) (plasma concentration attained in heme arginate– or hematin-treated porphyria patients) synergized cellular oxidant damage with added H2O2 (100 μmol/L) (36.8 ± 5.1% [hematin plus H2O2] versus 4.3 ± 3.8% [H2O2 alone]51Cr release; P = .001), with an optimal hematin-exposure duration of 60 minutes. In contrast, cytotoxicity studies showed little added toxicity to HUVECs incubated with heme arginate (600 μmol/L) (heme arginate plus H2O2) in plasma for 60 minutes and then challenged with H2O2 (100 μmol/L) (14.8 ± 5.9% versus 4.3 ± 4.1% [H2O2 alone] 51Cr release; not significant).
Exposure of endothelial cells to iron deuteroporphyrin IX, hematin, heme arginate, and other substances.
Iron deuteroporphyrin IX, like hematin, but unlike heme arginate, iron deuteroporphyrin IX,2,4-bis-sulfonate, iron coproporphyrin III, or iron deuteroporphyrin IX,2,4-bis-glycol, sensitized endothelial cells to H2O2 and activated PMN-mediated cytolysis. Confluent 51Cr-labeled human umbilical vein endothelial cells grown in 24-well (2 cm2/well) tissue-culture plates were incubated with medium 199 alone (first bars), 5 μmol/L hematin (second bars), 5 μmol/L heme arginate (third bars), 5 μmol/L iron deuteroporphyrin IX,2,4-bis-sulfonate (fourth bars), 5 μmol/L iron coproporphyrin III (fifth bars), 5 μmol/L iron deuteroporphyrin IX,2,4-bis-glycol (sixth bars), or 5 μmol/l iron deuteroporphyrin IX (seventh bars) in 500 μL of media 199 for 60 minutes. After removal of solutions, the cells were washed with HBSS and exposed for 2 hours to (A) 100 μmol/L H2O2or (B) PMA (100 ng/mL–activated neutrophils (2:1 PMN:endothelial cell ratio). Results represent the percentage of specific cytotoxicity (mean ± SE) of at least 3 experiments performed in duplicate.
Exposure of endothelial cells to iron deuteroporphyrin IX, hematin, heme arginate, and other substances.
Iron deuteroporphyrin IX, like hematin, but unlike heme arginate, iron deuteroporphyrin IX,2,4-bis-sulfonate, iron coproporphyrin III, or iron deuteroporphyrin IX,2,4-bis-glycol, sensitized endothelial cells to H2O2 and activated PMN-mediated cytolysis. Confluent 51Cr-labeled human umbilical vein endothelial cells grown in 24-well (2 cm2/well) tissue-culture plates were incubated with medium 199 alone (first bars), 5 μmol/L hematin (second bars), 5 μmol/L heme arginate (third bars), 5 μmol/L iron deuteroporphyrin IX,2,4-bis-sulfonate (fourth bars), 5 μmol/L iron coproporphyrin III (fifth bars), 5 μmol/L iron deuteroporphyrin IX,2,4-bis-glycol (sixth bars), or 5 μmol/l iron deuteroporphyrin IX (seventh bars) in 500 μL of media 199 for 60 minutes. After removal of solutions, the cells were washed with HBSS and exposed for 2 hours to (A) 100 μmol/L H2O2or (B) PMA (100 ng/mL–activated neutrophils (2:1 PMN:endothelial cell ratio). Results represent the percentage of specific cytotoxicity (mean ± SE) of at least 3 experiments performed in duplicate.
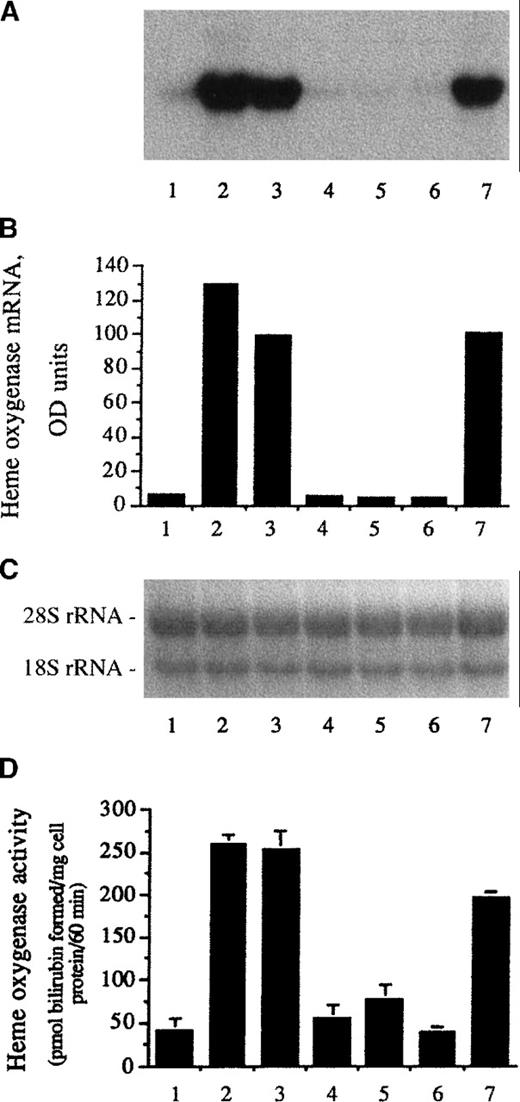
Since incubation of endothelial cells with heme increased the expression of heme oxygenase,32 45 we tested whether other ferriporphyrins could act as inducers of heme oxygenase mRNA and enzyme activity. As demonstrated in Figure 2, heme arginate (third lane) and iron deuteroporphyrin IX (seventh lane) induced heme oxygenase mRNA to a similar degree as hematin (second lane). Accompanying this mRNA induction, expression of heme oxygenase enzyme activity was markedly enhanced in endothelial cells exposed to heme arginate (Figure 2D, third bar) or iron deuteroporphyrin IX (Figure 2D, seventh bar). Iron deuteroporphyrin IX,2,4-bis-sulfonate (fourth lanes and bars), iron coproporphyrin III (fifth lanes and bars), and iron deuteroporphyrin IX,2,4-bis-glycol (sixth lanes and bars) did not alter endothelial heme oxygenase mRNA level and enzyme activity. Heme arginate did not provoke oxidant-mediated endothelial damage but entered cells similarly to hematin and iron deuteroporphyrin IX, since mRNA level and enzyme activity for heme oxygenase were markedly induced in HUVECs. In support, 1-hour exposure of HUVECs to heme arginate (5 μmol/L) in FCS free media increased endothelial cell heme content to an extent similar to what was observed after hematin treatment (5 μmol/L) (from 68 ± 18 pmol heme per milligram endothelial cell protein to 5.04 ± 0.6 and 3.94 ± 0.91 nmol heme per milligram endothelial cell protein, respectively). Comparable heme uptake can be obtained in the presence of human plasma although at 2 orders of magnitude greater concentration for both heme arginate and hematin. One-hour exposure of HUVECs to heme arginate (600 μmol/L) or hematin (600 μmol/L) in human plasma enhanced endothelial cell heme content to 1.24 ± 0.12 nmol heme per milligram protein and 1.16 ± 0.21 nmol heme per milligram endothelial cell protein, respectively. The heme content of cytosol was below levels of detection in these experiments. Thus, accurate cytosolic penetration of the ferriporphyrins could not be determined. Similarly to endothelium, lipoproteins also compete for heme added to plasma. When plasma LDL was incubated with equimolar concentrations of heme arginate and hematin for 2 hours at 37°C, 1.06% and 1.54% of the added heme became associated with LDL particles, respectively.
Heme oxygenase induction by heme analogues.
(A) For heme oxygenase mRNA Northern analysis, confluent human umbilical vein endothelial cells cultured in 10-cm tissue-culture dishes were incubated with medium 199 alone (first lane), 10 μmol/L hematin (second lane), 10 μmol/L heme arginate (third lane), 10 μmol/L iron deuteroporphyrin IX,2,4-bis-sulfonate (fourth lane), 10 μmol/L iron coproporphyrin III (fifth lane), 10 μmol/L iron deuteroporphyrin IX,2,4-bis-glycol (sixth lane), or 10 μmol/L iron deuteroporphyrin IX (seventh lane) in media 199 for 60 minutes followed by an additional 4 hours' incubation with hematin and heme analogue-free medium. RNA was isolated, electrophoresed, blotted, and hybridized with a 32P-labeled heme oxygenase cDNA probe. (B) Densitometry tracings of heme oxygenase mRNA bands expressed as arbitrary OD units. (C) The corresponding 28S and 18S ribosomal RNA of the Northern blot in (A). (D) Heme oxygenase enzyme activity (picomolar of bilirubin formed per milligram of cell protein per 60 minutes) measured at 8 hours after exposure of endothelial cell monolayers to the same compounds as above. Results represent the enzyme activity (mean ± SE) of at least 3 experiments done in duplicate.
Heme oxygenase induction by heme analogues.
(A) For heme oxygenase mRNA Northern analysis, confluent human umbilical vein endothelial cells cultured in 10-cm tissue-culture dishes were incubated with medium 199 alone (first lane), 10 μmol/L hematin (second lane), 10 μmol/L heme arginate (third lane), 10 μmol/L iron deuteroporphyrin IX,2,4-bis-sulfonate (fourth lane), 10 μmol/L iron coproporphyrin III (fifth lane), 10 μmol/L iron deuteroporphyrin IX,2,4-bis-glycol (sixth lane), or 10 μmol/L iron deuteroporphyrin IX (seventh lane) in media 199 for 60 minutes followed by an additional 4 hours' incubation with hematin and heme analogue-free medium. RNA was isolated, electrophoresed, blotted, and hybridized with a 32P-labeled heme oxygenase cDNA probe. (B) Densitometry tracings of heme oxygenase mRNA bands expressed as arbitrary OD units. (C) The corresponding 28S and 18S ribosomal RNA of the Northern blot in (A). (D) Heme oxygenase enzyme activity (picomolar of bilirubin formed per milligram of cell protein per 60 minutes) measured at 8 hours after exposure of endothelial cell monolayers to the same compounds as above. Results represent the enzyme activity (mean ± SE) of at least 3 experiments done in duplicate.
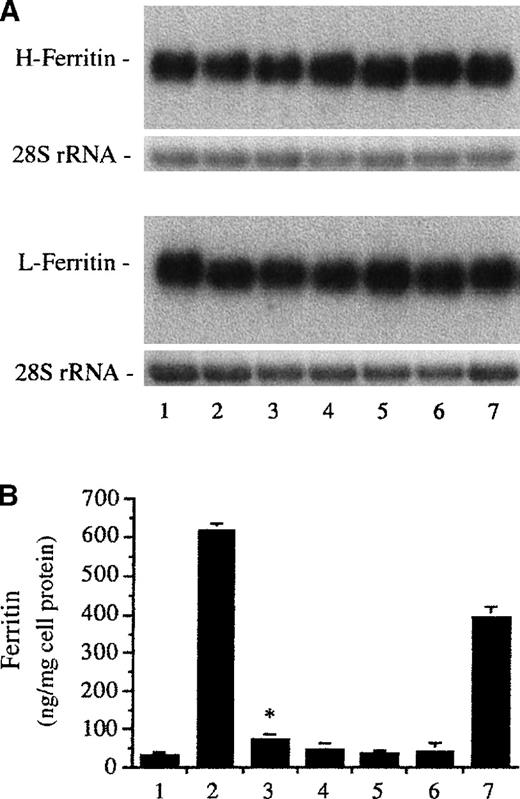
The hematin induction of the heme oxygenase gene was accompanied by increased ferritin synthesis in endothelial cells.32,45Therefore, we examined whether endothelial cell ferritin content was affected by other ferriporphyrins. Like hematin (Figure3B, second bar), iron deuteroporphyrin IX strikingly increased the ferritin level in endothelial cells (Figure3B, seventh bar). In contrast, heme arginate enhanced endothelial cell ferritin content to much less an extent (Figure 3B, third bar). Heavy- and light-chain ferritin mRNA levels were not affected by heme arginate and iron deuteroporphyrin IX (Figure 3A, third and seventh lanes), concordant with previous studies42,48,50 51and demonstrating that iron-mediated regulation of ferritin synthesis occurs at the posttranscriptional/translational level. Treatment of cells with iron deuteroporphyrin IX,2,4-bis-sulfonate (fourth lanes and bar), iron coproporphyrin III (fifth lanes and bar), and iron deuteroporphyrin IX,2,4-bis-glycol (sixth lanes and bar) did not affect mRNA levels for H and L ferritin chains and ferritin content in endothelium.
Effect of ferriporphyrins on ferritin expression.
Human umbilical vein endothelial cell monolayers were treated with medium 199 alone (first lanes and bar) or equimolar (10 μmol/L), various ferriporphyrins, including hematin (second lanes and bar), heme arginate (third lanes and bar), iron deuteroporphyrin IX,2,4-bis-sulfonate (fourth lanes and bar), iron coproporphyrin III (fifth lanes and bar), iron deuteroporphyrin IX,2,4-bis-glycol (sixth lanes and bar), or iron deuteroporphyrin IX (seventh lanes and bar), in media 199 for 60 minutes followed by additional incubations with ferriporphyrin free medium. (A) Northern blot analysis of mRNA for heavy-chain (H) and light-chain (L) ferritin at 4 hours after treatment of endothelial cells. Upper Panel: After transfer and hybridization, the H-ferritin and L-ferritin mRNAs are recognized by 32P-labeled cDNA probes. Lower Panel: Equal quantities of 28S rRNA were shown in the ethidium bromide–stained agarose gel. (B) Ferritin content at 16 hours after treatment of endothelium was measured for the same groups as in (A). Results are expressed as nanograms of ferritin per milligram of endothelial cell protein and represent mean ± SE of at least 3 experiments performed in duplicate. P < .004 heme arginate (third bar) versus control (first bar).
Effect of ferriporphyrins on ferritin expression.
Human umbilical vein endothelial cell monolayers were treated with medium 199 alone (first lanes and bar) or equimolar (10 μmol/L), various ferriporphyrins, including hematin (second lanes and bar), heme arginate (third lanes and bar), iron deuteroporphyrin IX,2,4-bis-sulfonate (fourth lanes and bar), iron coproporphyrin III (fifth lanes and bar), iron deuteroporphyrin IX,2,4-bis-glycol (sixth lanes and bar), or iron deuteroporphyrin IX (seventh lanes and bar), in media 199 for 60 minutes followed by additional incubations with ferriporphyrin free medium. (A) Northern blot analysis of mRNA for heavy-chain (H) and light-chain (L) ferritin at 4 hours after treatment of endothelial cells. Upper Panel: After transfer and hybridization, the H-ferritin and L-ferritin mRNAs are recognized by 32P-labeled cDNA probes. Lower Panel: Equal quantities of 28S rRNA were shown in the ethidium bromide–stained agarose gel. (B) Ferritin content at 16 hours after treatment of endothelium was measured for the same groups as in (A). Results are expressed as nanograms of ferritin per milligram of endothelial cell protein and represent mean ± SE of at least 3 experiments performed in duplicate. P < .004 heme arginate (third bar) versus control (first bar).
Conversion of heme by heme oxygenase to carbon monoxide, biliverdin, and bilirubin is accompanied by the release of iron from the porphyrin ring, which can drive the synthesis of ferritin.48 To assess the degradation of the porphyrin ring in endothelial cells, we measured the generation of bilirubin and carbon monoxide. Since human endothelial cells have very low levels of biliverdin reductase activity (data not shown), we used rat liver cytosol (a rich source of biliverdin reductase) to reduce biliverdin to bilirubin for the heme oxygenase enzyme activity assay.32,45 52 When heme arginate (20 μmol/L) was used as a substrate for heme oxygenase, less bilirubin (79.7 ± 12.8 versus 259.1 ± 22.1 pmol bilirubin formed per milligram of cell protein per 60 minutes) and carbon monoxide (7.6 ± 1.2 versus 22.0 ± 4.2 μL × 10−3 carbon monoxide formed per milligram of cell protein per 60 minutes) were generated in isolated endothelial cell microsomes compared with what happened when hematin (20 μmol/L) was used as a substrate.
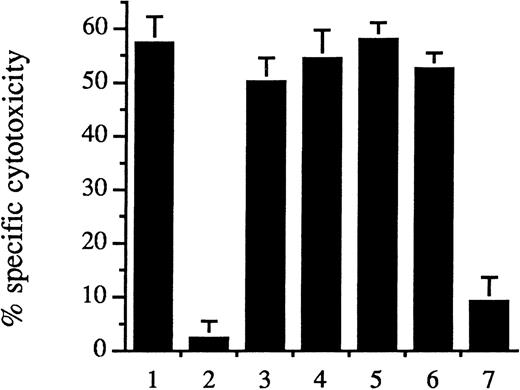
We have previously demonstrated that intracellular ferritin protects endothelium from oxidant-mediated cytolysis and does so in a dose-responsive manner.32 The induction of ferritin synthesis by pretreatment of endothelial cells with hematin (10 μmol/L) for 1 hour followed by a further incubation for 15 hours provided resistance to the very damaging combination of newly added hematin (5 μmol/L) plus H2O2 (100 μmol/L) (Figure4, second bar). Prolonged preincubation of cells with iron deuteroporphyrin IX (Figure 4, seventh bar) attenuated cytotoxicity derived from hematin plus H2O2 (Figure 4, first bar). In contrast, endothelial cells challenged by hematin plus H2O2 were not protected from cytolysis after pretreatment for 16 hours with heme arginate (Figure 4, third bar), iron deuteroporphyrin IX, 2,4-bis-sulfonate (fourth bar), iron coproporphyrin III (fifth bar), or iron deuteroporphyrin IX,2,4-bis-glycol (sixth bar).
Pretreatment of endothelium.
Hematin and iron deuteroporphyrin IX pretreatment of endothelium provided cell protection against hematin/H2O2–mediated lysis. Human umbilical vein endothelial cells were pretreated with control medium (first bar) or 10 μmol/L of ferriporphyrins, consisting of hematin (second bar), heme arginate (third bar), iron deuteroporphyrin IX,2,4-bis-sulfonate (fourth bar), iron coproporphyrin III (fifth bar), iron deuteroporphyrin IX,2,4-bis-glycol (sixth bar), or iron deuteroporphyrin IX (seventh bar), for 60 minutes, and the culture media were then replaced with ferriporphyrin-free medium for 15 hours. Endothelial cell oxidant stress was then provided by exposure of the cells to hematin (5 μmol/L) for 60 minutes followed by H2O2 (100 μmol/L) for 2 hours. Results are the specific cytotoxicity (mean ± SE) of 3 experiments performed in duplicate.
Pretreatment of endothelium.
Hematin and iron deuteroporphyrin IX pretreatment of endothelium provided cell protection against hematin/H2O2–mediated lysis. Human umbilical vein endothelial cells were pretreated with control medium (first bar) or 10 μmol/L of ferriporphyrins, consisting of hematin (second bar), heme arginate (third bar), iron deuteroporphyrin IX,2,4-bis-sulfonate (fourth bar), iron coproporphyrin III (fifth bar), iron deuteroporphyrin IX,2,4-bis-glycol (sixth bar), or iron deuteroporphyrin IX (seventh bar), for 60 minutes, and the culture media were then replaced with ferriporphyrin-free medium for 15 hours. Endothelial cell oxidant stress was then provided by exposure of the cells to hematin (5 μmol/L) for 60 minutes followed by H2O2 (100 μmol/L) for 2 hours. Results are the specific cytotoxicity (mean ± SE) of 3 experiments performed in duplicate.
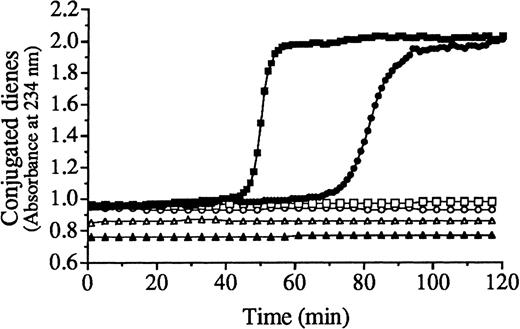
Oxidative damage to endothelial cells can also be mediated by oxidized LDL.31 Heme-catalyzed lipid peroxidation of LDL was monitored by the formation of conjugated dienes, lipid hydroperoxides, and thiobarbituric acid–reactive substances (Figure5; Table 1). The kinetics of lipid peroxidation of LDL mediated by heme arginate/H2O2 (Figure 5, closed circles) differed from that mediated by hematin/H2O2(closed squares). Measurement of LDL-conjugated diene formation demonstrated that the lag time and ΔT at Vmax were longer (74 versus 48 minutes and 82 versus 51 minutes) and Vmax was slower (112.7 versus 66.6 milliabsorbance units per minute) for LDL exposed to heme arginate/H2O2 (closed circles) compared with LDL exposed to hematin/H2O2 (closed squares) (Figure 5). In the absence of H2O2, the kinetics of LDL oxidation by heme arginate alone (open circles) or hematin alone (open squares) was characterized by a lag phase lasting more than 3 hours. During long-term incubations, hematin alone enhanced the lipid peroxidation of LDL more than heme arginate alone. As shown in Table 1, addition of hematin to LDL led to increased formation of conjugated dienes, lipid hydroperoxides, and thiobarbituric acid–reactive substances by 18 hours of incubation while with heme arginate similar oxidation required 36 hours. In these respects, hematin-catalyzed oxidation of LDL was approximately twice as active as heme arginate–mediated oxidation of LDL. Since the number of heme molecules associated with LDL particles was the same in LDL exposed to hematin in serum free solution as in LDL exposed to heme arginate (12.7 ± 0.4 versus 12.1 ± 0.9 heme molecules per LDL particle), the more efficient free-radical catalysis in LDL by hematin could not be attributed to quantitative characteristics in these experiments (Figure 5; Table 1). Potentially relevant to in vivo vascular damage was the fact that oxidative modification of LDL mediated by heme was cytotoxic to endothelium. The cytotoxicity of heme arginate/H2O2–conditioned LDL to endothelial cells was significantly less than endothelial cell cytotoxicity evoked by LDL conditioned with hematin/H2O2 (Figure6) (P < .004).
Kinetics of lipid peroxidation of LDL catalyzed by hematin or heme arginate.
The LDL (200 μg/mL protein) was exposed to hematin (5 μmol/L) (closed squares) or heme arginate (5 μmol/L) (closed circles) in the presence of H2O2 (75 μmol/L), hematin alone (5 μmol/L) (open squares), heme arginate alone (5 μmol/L) (open circles), and H2O2 alone (75 μmol/L) (open triangles). Closed triangles represent native LDL alone. The lipid peroxidation was monitored spectrophotometrically at 234 nm at 37 °C for 120 minutes to assess the formation of conjugated dienes.
Kinetics of lipid peroxidation of LDL catalyzed by hematin or heme arginate.
The LDL (200 μg/mL protein) was exposed to hematin (5 μmol/L) (closed squares) or heme arginate (5 μmol/L) (closed circles) in the presence of H2O2 (75 μmol/L), hematin alone (5 μmol/L) (open squares), heme arginate alone (5 μmol/L) (open circles), and H2O2 alone (75 μmol/L) (open triangles). Closed triangles represent native LDL alone. The lipid peroxidation was monitored spectrophotometrically at 234 nm at 37 °C for 120 minutes to assess the formation of conjugated dienes.
TBARS, total lipid hydroperoxide and conjugated diene formation in a low density lipoprotein exposed to hematin or heme arginate
| Parameter . | 0 h . | 18 h . | 36 h . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LDL . | LDL + Hematin . | LDL + HA . | LDL . | LDL + Hematin . | LDL + HA . | LDL . | LDL + Hematin . | LDL + HA . | |
| TBARS (nmol/mg LDL) | 0.07 ± 0.01 | 0.10 ± 0.02 | 0.10 ± 0.01 | 0.09 ± 0.03 | 10.48 ± 1.27 | 0.25 ± 0.09 | 0.12 ± 0.03 | 8.69 ± 1.61 | 11.07 ± 2.08 |
| Total LOOH (nmol/mg LDL) | 9.5 ± 0.2 | 9.0 ± 0.7 | 9.8 ± 0.1 | 8.0 ± 0.2 | 140.7 ± 2.5 | 9.7 ± 0.2 | 7.5 ± 1.8 | 156.9 ± 8.2 | 165.1 ± 15.6 |
| Conjugated dienes (Absorbance at 234 nm) | 0.245 ± 0.004 | 0.276 ± 0.006 | 0.289 ± 0.001 | 0.260 ± 0.002 | 1.058 ± 0.002 | 0.282 ± 0.003 | 0.258 ± 0.007 | 1.120 ± 0.011 | 1.150 ± 0.047 |
| Parameter . | 0 h . | 18 h . | 36 h . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LDL . | LDL + Hematin . | LDL + HA . | LDL . | LDL + Hematin . | LDL + HA . | LDL . | LDL + Hematin . | LDL + HA . | |
| TBARS (nmol/mg LDL) | 0.07 ± 0.01 | 0.10 ± 0.02 | 0.10 ± 0.01 | 0.09 ± 0.03 | 10.48 ± 1.27 | 0.25 ± 0.09 | 0.12 ± 0.03 | 8.69 ± 1.61 | 11.07 ± 2.08 |
| Total LOOH (nmol/mg LDL) | 9.5 ± 0.2 | 9.0 ± 0.7 | 9.8 ± 0.1 | 8.0 ± 0.2 | 140.7 ± 2.5 | 9.7 ± 0.2 | 7.5 ± 1.8 | 156.9 ± 8.2 | 165.1 ± 15.6 |
| Conjugated dienes (Absorbance at 234 nm) | 0.245 ± 0.004 | 0.276 ± 0.006 | 0.289 ± 0.001 | 0.260 ± 0.002 | 1.058 ± 0.002 | 0.282 ± 0.003 | 0.258 ± 0.007 | 1.120 ± 0.011 | 1.150 ± 0.047 |
Low density lipoprotein (20 mg/dL protein) in NaCl (150)-HEPES (10) buffer (pH 7.4) was exposed to hematin (5 μM) or heme arginate (HA) (5 μM) for the indicated time periods in air. Lipid peroxidation was analyzed by measuring thiobarbituric acid-reactive substances (TBARS), total lipid hydroperoxide (LOOH), and conjugated dienes as described under “Materials and methods.” Results represent mean ± SE of 3 experiments performed in duplicate.
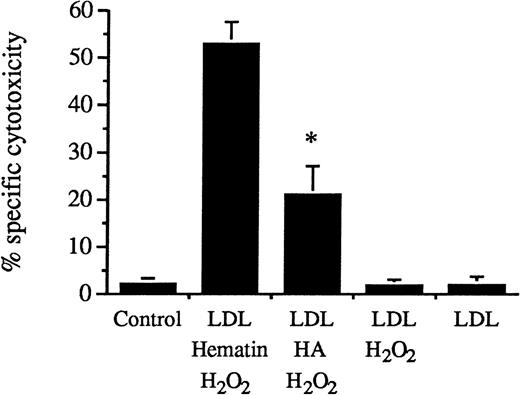
Heme arginate–conditioned and hematin–conditioned LDL.
Heme arginate–conditioned LDL induced less endothelial cell lysis than hematin–conditioned LDL. Human umbilical vein endothelial cells were incubated with LDL (200 μg/mL protein) treated with hematin (5 μmol/L) plus H2O2 (75 μmol/L), LDL treated with heme arginate (HA) (5 μmol/L) plus H2O2, and LDL treated with H2O2 alone in HBSS for 4 hours. Endothelial cells were also exposed to native LDL alone, and for control cells, HBSS was free of lipoprotein. Results were the percentage of specific cytotoxicity (mean ± SE) of 3 experiments performed in duplicate. P < .004 versus LDL treated with hematin plus H2O2.
Heme arginate–conditioned and hematin–conditioned LDL.
Heme arginate–conditioned LDL induced less endothelial cell lysis than hematin–conditioned LDL. Human umbilical vein endothelial cells were incubated with LDL (200 μg/mL protein) treated with hematin (5 μmol/L) plus H2O2 (75 μmol/L), LDL treated with heme arginate (HA) (5 μmol/L) plus H2O2, and LDL treated with H2O2 alone in HBSS for 4 hours. Endothelial cells were also exposed to native LDL alone, and for control cells, HBSS was free of lipoprotein. Results were the percentage of specific cytotoxicity (mean ± SE) of 3 experiments performed in duplicate. P < .004 versus LDL treated with hematin plus H2O2.
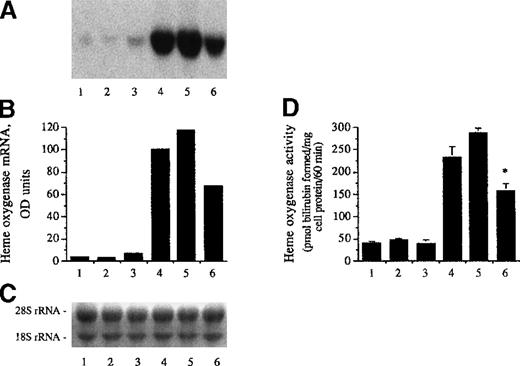
The up-regulation of heme oxygenase mRNA and enzyme activity in endothelium was shown to occur after sublethal stress of oxidatively modified LDL.41 We probed for expression of heme oxygenase as a marker for oxidative stress in endothelial cells mediated by hematin- or heme arginate–modified LDL. Exposure of endothelium to LDL conditioned with hematin/H2O2 for 1 hour resulted in a marked cellular expression of heme oxygenase mRNA and enzyme activity (Figure 7, fifth lanes and bars). In contrast, native LDL (second lanes and bars) or LDL exposed to H2O2 (third lanes and bars) did not affect mRNA level and enzyme activity for heme oxygenase in endothelial cells. Induction of heme oxygenase was also observed when endothelial monolayers were exposed to LDL conditioned with heme arginate/H2O2 (sixth lanes and bars), albeit heme arginate/H2O2–conditioned LDL was a significantly weaker inducer (P < .001) compared with hematin/H2O2–conditioned LDL. In these studies, the induction of heme oxygenase mRNA and enzyme activity by hematin (10 μmol/L) alone served as a positive control (fourth lanes and bars). In experiments in which oxidation of LDL was catalyzed by hematin or heme arginate, heme per se could not be ascribed as the cause of the induction of heme oxygenase mRNA and enzyme activity, since in the course of catalyzing the oxidation of LDL, heme itself undergoes degradation.31,47 Treatment of LDL with antioxidants prior to its exposure to hematin/H2O2 prevented the oxidation of lipoproteins and the induction of both mRNA and enzyme activity for heme oxygenase, in spite of the fact that heme content of LDL remained high.41 In support, 1.25 μmol/L of butylated hydroxytoluene or 12.5 μmol/L of alpha tocopherol added to LDL (50 μg/mL) prior to its exposure to heme arginate/H2O2 also abrogated the induction of heme oxygenase enzyme activity (36.8 ± 8.2 and 32.5 ± 8.4 versus 298.3 ± 17.7 pmol of bilirubin formed per milligram of cell protein per 60 minutes, respectively), which was accompanied by prevention of lipid peroxidation of LDL; 93% of the added hemes remained LDL associated. Heme arginate alone (87.5 nmol/L) (7% of the added heme) did not enhance heme oxygenase enzyme activity in HUVECs (32.8 ± 8.4 versus 39.5 ± 5.4 pmol of bilirubin formed per milligram of cell protein per 60 minutes). Thus, in these studies, oxidation of LDL per se could be ascribed as the cause for the induction of heme oxygenase in endothelial cells.
LDL conditioned with heme arginate or hematin.
Heme arginate–conditioned LDL was a weaker inducer for heme oxygenase than hematin-conditioned LDL. (A) For heme oxygenase mRNA analysis, human umbilical vein endothelial cell monolayers were incubated for 1 hour with LDL (50 μg/mL protein) alone (second lane), LDL treated with H2O2 (6.25 μmol/L) (third lane), LDL treated with hematin (1.25 μmol/L) plus H2O2 (6.25 μmol/L) (fifth lane), and LDL treated with heme arginate (1.25 μmol/L) plus H2O2 (sixth lane) in HBSS, and then replaced with medium for 4 hours. For negative control (first lane), HBSS was free of lipoprotein, and for positive control (fourth lane), cells were exposed to hematin alone (10 μmol/L) for 1 hour followed by a 4-hour incubation with culture medium. RNA was isolated, electrophoresed, blotted, and hybridized with a 32P-labeled heme oxygenase cDNA probe. (B) Autoradiograph was quantified by videodensitometry and expressed as arbitrary OD units. (C) Ethidium bromide–stained nylon membrane with the corresponding 18S and 28S rRNA for the above autoradiogram. (D) Heme oxygenase enzyme activity (picomole of bilirubin formed per milligram of cell protein per 60 minutes) was measured at 8 hours after exposure of endothelium to the same test solutions as for Northern blot in (A). Results represent enzyme activity (mean ± SE) of at least 3 experiments done in duplicate.P < .001 versus bar 5.
LDL conditioned with heme arginate or hematin.
Heme arginate–conditioned LDL was a weaker inducer for heme oxygenase than hematin-conditioned LDL. (A) For heme oxygenase mRNA analysis, human umbilical vein endothelial cell monolayers were incubated for 1 hour with LDL (50 μg/mL protein) alone (second lane), LDL treated with H2O2 (6.25 μmol/L) (third lane), LDL treated with hematin (1.25 μmol/L) plus H2O2 (6.25 μmol/L) (fifth lane), and LDL treated with heme arginate (1.25 μmol/L) plus H2O2 (sixth lane) in HBSS, and then replaced with medium for 4 hours. For negative control (first lane), HBSS was free of lipoprotein, and for positive control (fourth lane), cells were exposed to hematin alone (10 μmol/L) for 1 hour followed by a 4-hour incubation with culture medium. RNA was isolated, electrophoresed, blotted, and hybridized with a 32P-labeled heme oxygenase cDNA probe. (B) Autoradiograph was quantified by videodensitometry and expressed as arbitrary OD units. (C) Ethidium bromide–stained nylon membrane with the corresponding 18S and 28S rRNA for the above autoradiogram. (D) Heme oxygenase enzyme activity (picomole of bilirubin formed per milligram of cell protein per 60 minutes) was measured at 8 hours after exposure of endothelium to the same test solutions as for Northern blot in (A). Results represent enzyme activity (mean ± SE) of at least 3 experiments done in duplicate.P < .001 versus bar 5.
Discussion
In acute life-threatening porphyric attacks, hematin has been successfully used 5-9 although there may develop harmful effects on the vasculature, such as vasculitis, thrombosis, and disseminated intravascular coagulation.8,10-17 Heme arginate treatment for acute porphyric attacks has been very effective without evidence of vascular side effects.18-23 In our studies, heme arginate, unlike hematin, did not amplify hydrogen peroxide or activated polymorphonuclear leukocyte–mediated endothelial cell cytotoxicity. Nonpermeant heme analogues, iron deuteroporphyrin IX,2,4-bis-sulfonate, iron coproporphyrin III, and iron deuteroporphyrin IX,2,4-bis-glycol, also failed to sensitize endothelial cells to oxidants. In contrast, brief exposure of endothelium to the lipid-soluble ferriporphyrin iron deuteroporphyrin IX sensitized cells to oxidant injury mediated by hydrogen peroxide or activated neutrophils.
Heme can also threaten vascular endothelial cell integrity by its ability to catalyze the oxidation of LDL.31 LDL oxidized by heme is extremely cytotoxic to endothelial cells. The kinetics of LDL lipid peroxidation mediated by heme arginate in the presence of H2O2 was characterized by a longer lag phase and ΔT at Vmax as well as a slower propagation phase compared with hematin-mediated lipid peroxidation of LDL as judged by conjugated diene formation. The results of several independent assays for LDL oxidation—production of thiobarbituric acid–reactive substances, conjugated diene formation, and lipid hydroperoxide generation—stimulated by hematin alone or heme arginate alone all support the conclusion that heme arginate promoted LDL oxidation less efficiently. Accordingly, heme arginate/H2O2–conditioned LDL was less cytotoxic to cultured human endothelial cells than hematin/H2O2–conditioned LDL.
Heme arginate did not provoke oxidant-mediated endothelial damage but nevertheless entered endothelial cells similarly to hematin and iron deuteroporphyrin IX; heme oxygenase mRNA level and enzyme activity were markedly increased in cells exposed to heme arginate as in hematin- or iron deuteroporphyrin IX–treated cells (Figure 2). This substantial induction of heme oxygenase gene was accompanied by increased endothelial ferritin synthesis. In spite of the similar enhancement of heme oxygenase mRNA and enzyme activity, heme arginate only doubled endothelial cell ferritin content, while in endothelium exposed to hematin and iron deuteroporphyrin IX, ferritin increased 20-fold and 13-fold, respectively. To assess differences in degradation of the porphyrin ring, heme arginate or hematin was used as a substrate for heme oxygenase. We measured the generation of bilirubin and carbon monoxide by using isolated microsomes from endothelium as a source of heme oxygenase. Less bilirubin and carbon monoxide were generated when heme arginate was used as a substrate for heme oxygenase than when hematin was used. The lower level of iron release from the heme arginate porphyrin ring may explain the blunted ferritin response. Heavy- and light-chain ferritin mRNA levels were not altered by heme arginate, hematin, or iron deuteroporphyrin IX, concordant with previous studies42,43,48,50 51 and demonstrating that in these cell systems, iron-mediated regulation of ferritin synthesis occurred primarily at the posttranscriptional/translational level.
Prolonged incubation of endothelial cells with heme rendered them remarkably resistant to oxidant challenge via the induction of heme oxygenase and ferritin.32 The ultimate cytoprotectant against iron-driven oxidant injury was identified as ferritin32 in a wide range of experimental conditions. Such protection arises from the ability of ferritin to sequester intracellular iron and/or to exhibit ferroxidase activity. Endothelial cells, when incubated with iron deuteroporphyrin IX, were also markedly induced to increase heme oxygenase mRNA and enzyme activity (Figure 2) as well as their ferritin content (Figure 3). The remarkably increased synthesis of heme oxygenase and ferritin proteins were associated with resistance to oxidative stress imposed by the highly damaging combination of hematin and H2O2 (Figure 4). However, no associated cytoprotection against iron-driven oxidant injury was afforded if only a high level of heme oxygenase enzyme activity was present without a substantial increase in ferritin content, as seen after endothelial cells are exposed to heme arginate (Figure 4, third bar). Nonpermeant ferriporphyrins, iron deuteroporphyrin IX,2,4-bis-sulfonate, iron coproporphyrin III, and iron deuteroporphyrin IX,2,4-bis-glycol, were noninducers for heme oxygenase and ferritin in endothelium and did not provide cytoprotection against oxidant damage.
Catabolism of heme by heme oxygenase may rid the cells of a membrane-permeant form of iron, but the resultant nonheme iron would represent a potential hazard unless sequestered by ferritin.32 The cytoprotective nature of ferritin against iron-driven oxidative stress is attributable to high sequestering capacity for inorganic iron and ferroxidase activity of its H chain.32 The cytoprotectant role of heme oxygenase and ferritin has been confirmed in various models.59-63 A study by Vile et al64 demonstrated the adaptive role of ferritin in oxidative stress in human skin fibroblast; Van Lenten et al26 showed that intracellular ferritin abolishes iron-induced LDL modification; and Lin et al53 described the protective effects of the H ferritin chain in human leukemia cells, confirming the antioxidant role of ferritin. Increased coexpression of heme oxygenase and ferritin can be observed in humans and in animal models.52,54-65 A recent study from our laboratory has revealed that ferritin is highly expressed in endothelial cells of human atherosclerotic lesions54 while Wang et al55 have shown that there is increased expression of heme oxygenase-1 in atherosclerotic endothelium. In vivo, induction of heme oxygenase and ferritin protects rats against rhabdomyolysis-induced renal failure.56 Heme oxygenase can be important for cardiac xenograft survival in rats, as hearts from heme oxygenase-1 knockout mice were rapidly rejected.57 Heme oxygenase- 2–deficient mice were more sensitive to hyperoxia-induced oxidative lung injury with the absence of ferritin induction.58 Heme oxygenase-1 deficiency in a human was accompanied by fragmentation hemolysis and increased oxidant sensitivity of lymphoblastoid cell line derived from the patient.59 Conversion of heme by heme oxygenase to biliverdin and bilirubin was demonstrated to protect neurons against oxidative stress.60,61 Induction of heme oxygenase was associated with inhibition of monocyte chemotaxis induced by oxidized LDL.62 In fact, it has been shown that bilirubin is an important antioxidant.63 In addition to bilirubin, the carbon monoxide produced during the degradation of heme has attracted a great deal of interest on its potential function in regulating vascular tone via guanylate cyclase.66 Despite the fact that heme oxygenase and ferritin can provide cytoprotection against heme-catalyzed injury in many ways, our previous studies and those presented here emphasize the paramount role of iron sequestration by ferritin in these cell systems.
The induction of heme oxygenase can be a response to oxidative modification of LDL in endothelial cells.41 Heme oxygenase mRNA and enzyme activity were increased after cells were exposed to either hematin/H2O2–conditioned LDL or heme arginate/H2O2–conditioned LDL (Figure 7). That heme arginate promoted LDL oxidation less efficiently than heme was confirmed by the finding that heme arginate/H2O2–conditioned LDL was a significantly weaker inducer for heme oxygenase mRNA and enzyme activity than hematin/H2O2–conditioned LDL.
We conclude that heme arginate, in contrast to hematin, was an inefficient free-radical catalyst in endothelial cells and a less potent catalyst for the oxidative modification of LDL particles, features possibly explaining the safety of heme arginate treatment for porphyria. The beneficial effect of exogenous heme for patients with acute hepatic porphyria may be due to repletion of the intrahepatic heme pool, which restores heme proteins, and to negative feedback reducing the overall porphyrin synthesis through inhibition of hepatic delta-aminolevulinic acid synthase.1-9,67 The theoretical concern with the administration of heme is that induction of heme oxygenase increases the catabolism of heme, depriving the cell of essential heme. Indeed, coadministration of tin protoporphyrin, a competitive inhibitor for heme oxygenase,68 prolonged the biochemical remission produced by heme arginate in acute hepatic porphyria.69 In our study, heme arginate acted as a gene regulator for heme oxygenase in endothelial cells as effectively as hematin, but endothelial cell heme oxygenase degraded porphyrin rings of heme arginate less than the use of hematin did. Possibly the arginine moieties protect and stabilize the porphyrin ring.
Hematin-induced coagulopathy and thrombocytopenia have been reported11,12,17; hematin inhibits coagulation and enhances platelet aggregation. In contrast, heme arginate treatment does not alter hemostasis.70 71 Our findings may help to explain why heme arginate can replenish cellular heme deficiency in a nonvasculopathic form.
Acknowledgment
We thank Dr Claus A. Pierach (Abbott Northwestern Hospital, Minneapolis, MN) for helpful discussions.
Supported in part by National Institutes of Health grant HL-55552; Hungarian government grants OTKA-T029558/021023, ETT-1161361996, and FKFP-06171999; and the Cecil J. Watson Research Laboratory (J.B.).
Reprints:Gregory M. Vercellotti, Box 293, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: verce001@maroon.tc.umn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal