Abstract
Endostatin, which corresponds to the C-terminal fragment of collagen XVIII, is a potent inhibitor of angiogenesis. Fibroblast growth factor-2 (FGF-2)–induced angiogenesis in the chicken chorioallantoic membrane was inhibited by endostatin, but not by an endostatin mutant R158/270A, lacking heparin-binding ability. Endostatin was internalized by endothelial cells, but not by mouse fibroblasts. Treatment of murine brain endothelial (IBE) cells with endostatin reduced the proportion of cells in S phase, whereas growth-arrested IBE cells in collagen gels treated with endostatin displayed enhanced tubular morphogenesis. IBE cells overexpressing Shb, an adaptor protein implicated in angiostatin-induced apoptosis, displayed elevated apoptosis and decreased tubular morphogenesis in collagen gels in response to endostatin when added together with FGF-2. Induction of apoptosis was dependent on the heparin-binding ability of endostatin and the expression of Shb with a functional Src homology 2 (SH2)-domain. Endostatin treatment for 10 minutes or 24 hours induced tyrosine phosphorylation of Shb and formation of multiprotein complexes. An Shb SH2 domain fusion protein precipitated a 125-kd phosphotyrosyl protein in endostatin-treated cells. The 125-kd component either contained intrinsic tyrosine kinase activity or occurred in complex with a tyrosine kinase. In conclusion, our data show that endostatin induces tyrosine kinase activity and enhanced apoptosis in FGF-treated endothelial cells.
Angiogenesis, formation of new capillaries from preexisting vessels, is a prerequisite for many physiological processes, including embryonic development, wound healing, and the female reproductive functions.1 On the other hand, a number of pathologic conditions such as cancer, rheumatoid arthritis, and other chronic inflammatory diseases are characterized by excessive angiogenesis.2 The concept that progression of these diseases may be halted by inhibiting the endothelial cell compartment has raised considerable interest, and a series of antiangiogenic substances have been described.3 Interestingly, many of these are fragments of naturally occurring proteins. Thus, a 29-kd fragment of fibronectin,4 a 16-kd fragment of prolactin,5 and a 38-kd fragment of plasminogen6 (angiostatin) have been shown to inhibit angiogenes in in vivo tumor models.
O'Reilly et al7 purified a potent angiogenesis inhibitor, termed endostatin, from medium conditioned by a murine hemangioendothelioma cell line. Endostatin corresponds to a 20-kd fragment derived from the carboxy-terminal noncollagenous NC1 domain of collagen α1 (XVIII),8,9 which is present in the basement membrane zones around blood vessels.10 Boehm et al11 showed that cyclic treatment with endostatin in an insoluble form, possibly acting via slow release, efficiently eradicated a number of different model tumors in mice. The tumors were reduced to the size of a small nodule, where the tumor remained dormant after the cessation of the treatment. Recently, soluble recombinant endostatin produced in yeast was used to treat renal cell cancer in nude mice, which resulted in the arrest but not the shrinking of the tumor.12 Furthermore, adenovirus-mediated expression of endostatin has been shown to lead to the inhibition of endothelial cell growth, indicating that gene therapy could be a useful approach in, for example, tumor treatment.13
Structural analyses of murine endostatin by x-ray crystallography showed a compact globular folding, with arginine-rich clusters exposed on the surface of the molecule,14 mediating the binding of endostatin to heparin/heparan sulfate proteoglycans,15 and a core structure related to the carbohydrate-recognition domain of C-type lectins.14 Furthermore, human and murine endostatin were shown to bind Zn2+ at their amino termini, possibly of importance for the processing of the inactive collagen XVIII precursor.16 Boehm et al17 reported Zn2+-binding to endostatin at a 1:1 mmol/L ratio, and implicated Zn2+ in the antiangiogenic effect of endostatin during progression of Lewis lung carcinoma. Endostatin is localized in adult basement membranes and frequently in elastic fibers and microfibrils, and in the skin, brain, and vascular basement membrane in the developing embryo.15 18
The molecular mechanisms of endostatin in inhibition of tumor growth are not yet clear. Endostatin was recently shown to inhibit proliferation and migration of endothelial cells.12,19,20Moreover, endostatin treatment was shown to induce a block in cell cycle progression and apoptosis of cells.20 21
In this paper, these data are confirmed and extended in that we show that endostatin induces tyrosine kinase activity and the formation of multiprotein signaling complexes in endothelial cells. One component identified in these complexes is the Shb adaptor protein,22that previously has been implicated in apoptosis.23-25 Shb is tyrosine phosphorylated in response to nerve growth factor treatment of PC12 cells26 and is known to participate in multiprotein signaling complexes in CD3-stimulated Jurkat cells.27Structurally, Shb consists of a proline-rich amino-terminus, a central phosphotyrosine-binding (PTB) domain and a carboxy-terminal Src homology 2 (SH2) domain.22,27 We have previously shown that cells overexpressing Shb respond with increased apoptosis on treatment with the angiogenesis inhibitor angiostatin.24 Here we show that overexpression of Shb augments endostatin-induced apoptosis, whereas this response is counteracted by the expression of a Shb SH2-domain mutant. Endostatin-induced inhibition of angiogenesis in the chorioallantoic membrane assay, activation of tyrosine kinase activity, and Shb-mediated apoptosis of endothelial cells correlated with the heparin-binding ability of endostatin.
Materials and methods
Tissue culture
Murine brain endothelial cells (IBE)28 were cultured on gelatin-coated dishes in Ham's F12, 10% fetal calf serum (FCS), and 20 U/mL interferon γ (IFN-γ, Peprotech, Rocky Hill, NJ) at 33°C. Parental and fibroblast growth factor (FGF) receptor-1 (FGFR-1) transfected porcine aortic endothelial (PAE)29 were cultured in Ham's F12, 10% FCS.
Generation of murine brain endothelial cells overexpressing wild-type and R522K-mutant Shb
Wild-type or R522K-mutant (inactivation of the SH2 domain27) Shb cDNA were inserted into the pBABE vector30 and used to generate retroviruses. IBE cells were infected with retroviruses containing vector, wild-type Shb, or Shb R522K, and clones were isolated after selection in the presence of 5 μg/mL puromycin. Overexpression of wild-type or R522K Shb was verified by Western blot analysis of cell lysates from different clones, and the Shb and Shb R522K clones displayed approximately 5-fold increased levels of Shb (L. Lu and M. Welsh, unpublished data).
Expression, purification, and biotinylation of endostatin
The preparation of recombinant mouse endostatin in human 293-Epstein-Barr virus-associated nuclear antigen (EBNA) cells has been described.15 This endostatin is highly soluble and contains 1.02 zinc atoms per molecule as shown by atomic emission spectroscopy. Expression vectors for 2 different double mutations of endostatin were made by fusion polymerase chain reaction (PCR), following standard protocols, and used for production of mutant proteins as described.15 Mutant R158/270A showed a strongly reduced heparin-binding affinity (displacement at 0.11 mol/L NaCl) when compared with the wild-type (0.35 mol/L NaCl). Mutant H134A/D136A endostatin displayed a reduced zinc content (0.07 atoms per molecule). A full description of the mutants is given elsewhere.31 The purified endostatin in ammonium acetate buffer was dried, redissolved in 4 mol/L GuHCl in phosphate-buffered saline (PBS), and applied on a Fast Desalting column (Amersham Pharmacia Biotech, Uppsala, Sweden), equilibrated, and eluted in PBS. Biotinylation was performed by incubation of endostatin with biotinamidocaproic acid 3-sulfo-N-hydroxy-succinimide ester (100:25 w/w) in PBS for 60 minutes. The reaction mixture was applied onto a Superdex 75 (Amersham Pharmaca Biotech) column and eluted with PBS. MALDI-MS analysis of the protein fraction showed that the major fraction contained 1 biotin molecule per endostatin molecule.
Chorioallantoic membrane assay
Angiogenesis in the chicken chorioallantoic membrane (CAM) was performed as described.31 Fertilized chick embryos were preincubated for 10 days at 38°C/70% humidity. A hole was drilled over the air sac at the end of the egg and an avascular zone was identified on the CAM. A second hole was made over the CAM, which was separated from the shell by applying vacuum to the first hole. A 1 × 1-cm window in the shell was made to expose the CAM. Samples were prepared using filter disks (Whatman, Clifton, NJ), saturated with 3 mg/mL cortisone acetate (Sigma, St Louis, MO), and soaked in buffer (40 μL for each filter) with or without FGF-2 (Boehringer Mannheim, Mannheim, Germany; 0.2 μg for each filter) and endostatin or endostatin mutants (3 μg for each filter). The windows were sealed with tape and the eggs were incubated for 3 more days. The membrane was cut around the disks, which was turned upside down and inspected using a light microscope (Nikon Eclipse TE 300; Nikon, Tokyo, Japan; magnification 2.5 or 4).
Tube formation
Parental IBE and IBE/Shb cells cultured sparsely on gelatin-coated culture plastic were washed and fresh Ham's F12/0.25% bovine serum albumin (BSA) was added. After 24 hours, the cells were trypsinized and resuspended in fresh medium with 0.25% BSA, with or without endostatin at a final concentration of 0.7 μg/mL or FGF-2 (Boehringer Mannheim) at 5 ng/mL, and seeded on a collagen gel. The collagen gel was prepared by mixing collagen I (Vitrogen, Palo Alto, CA), 10 × Ham's F12 medium and neutralizing buffer (260 mmol/L NaCO3, 200 mmol/L HEPES, and 50 mmol/L NaOH) at an 8:1:1 ratio. The mixture was pipetted into 24-well plates (250 μL per well). After solidification, serum- and IFN-γ-starved IBE cells were seeded in the absence and presence of FGF-2 and endostatin, as indicated. After 2 hours, a second collagen layer was added and incubation continued at 33°C. Tube formation was analyzed with a Nikon Eclipse TE 300 microscope and photographed using a SPOT 2 digital camera (Diagnostic Instruments, Sterling Heights, MI).
Endostatin internalization
IBE cells or Swiss 3T3 cells were trypsinized and seeded on fibronectin-coated (IBE) or uncoated (Swiss 3T3) 8-chamber microscope slides (NUNC, Als Roskilde, Denmark). After 24 hours, the medium was changed to F12/0.25% BSA and 5 ng/mL FGF-2. Another 24 hours later, 10 μg/mL biotinylated endostatin was added, and at indicated time points, the cells were put on ice, washed twice in PBS, and fixed in 3% paraformaldehyde for 10 minutes. After 3 washes in PBS, the cells were permeabilized in −20°C acetone for 5 minutes. The acetone was removed, the slides were air-dried, and blocked in PBS with 10% FCS for 1 hour at 37°C. Bound endostatin was detected using Alexa 488-conjugated avidin (Molecular Probes, Eugene, OR) for 45 minutes. Cells were washed 5 times in PBS and, after adding “Slow fade light” (Molecular Probes, Eugene, OR) antifade and coverslip, the preparations were examined with a Nikon Eclipse TE 300 microscope and photographed using a SPOT 2 digital camera (Diagnostic Instruments).
In vitro kinase assay
IBE/Shb R522K cells or PAE cells, either parental or overexpressing FGFR-1, were seeded on 6-cm dishes in Ham's F12 medium, 10% FCS. After 24 hours, the cells were washed twice in serum-free medium and cultured for 24 hours in F12/0.25% BSA. Cells were stimulated with growth factors and endostatin for 10 minutes, put on ice, washed with ice-cold Na3VO4 in Tris-buffered saline and lysed in Nonidet P-40 (NP-40) lysis buffer (1% NP-40, 20 mmol/L HEPES pH 7.5, 150 mmol/L NaCl, 10% glycerol, 300 μmol/L Na3VO4, 1% aprotinin, and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF) for 10 minutes. The cells were scraped and lysates centrifuged at 18 000 × g for 13 minutes at 4°C. The supernatants were incubated with antibodies against phosphotyrosine (4G10; Upstate Biotechnology, Lake Placid, NY) on ice for 2 hours and with immobilized protein A (Immunosorb; EC Diagnostics, Uppsala, Sweden) for another 30 minutes at 4°C. Alternatively, lysates were incubated with a GST-Shb SH2 fusion protein,22 coupled to glutathione-Sepharose (Amersham Pharmacia Biotech, Uppsala, Sweden). Beads were washed 3 times in lysis buffer and 2 times in kinase buffer (0.05% Triton X-100, 20 mmol/L HEPES, 10 mmol/L MgCl2, 2 mmol/L MnCl2, 0.185 MBq (5 μCi) [γ-32P]ATP was added per sample. Samples were incubated 10 minutes at room temperature and heated in sample buffer (8% SDS, 0.4 mol/L Tris-HCl, pH 8.0, 1 mol/L sucrose, 10 mmol/L EDTA, 0.02% bromophenol blue, 4% β-mercaptoethanol), followed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in 9% gels. The gel was fixed in 2.5% glutaraldehyde for 10 minutes, incubated at 55°C in 1 mol/L KOH, rinsed, dried, and analyzed using a BioImager (Fujifilm, Tokyo, Japan).
Immunoprecipitation, glutathione-S-transferase–fusion protein precipitation, and immunoblotting
IBE cells, IBE/ShbR552K cells, or PAE/FGFR-1 cells in 75-cm2 flasks were starved over night in Ham's F12, supplemented with 0.25% BSA, followed by treatment with or without FGF-2 (100 ng/mL) and endostatin or endostatin mutants (1 μg/mL) for 10 minutes or 24 hours at 37°C. The cells were washed with ice-cold PBS containing 100 μmol/L Na3VO4 and lysed for 10 minutes on ice in 0.5% Triton X-100, 20 mmol/L Tris HCl, pH 7.5, 0.15 mol/L NaCl, 1 mmol/L EDTA, 0.1 mmol/L Na3PO4, 1% aprotinin, 2 mmol/L PMSF, 0.05 mmol/L leupeptin, and 20 μmol/L N-acetyl-leu-leu-norleucinal. Cleared lysates were incubated with 10 μg/ mL affinity-purified Shb antibody,23 and protein A-Sepharose, or the glutathione-S-transferase (GST)-Shb SH2 domain fusion protein, and glutathione-Sepharose. Samples were heated in sample buffer and separated by SDS-PAGE. For immunoblotting, proteins were electrophoretically transferred onto nitrocellulose membranes (Hybond-C extra; Amersham Pharmacia Biotech). The membranes were blocked in PBS-T (0.2% Tween-20 in PBS) containing 5% BSA, incubated with antiphosphotyrosine antibodies (4G10) or Shb antiserum for 1 hour, followed by washing in PBS. An appropriate secondary antibody was incubated with the membranes for another hour, and after washing in PBS, the immunoreactive proteins were visualized by an enhanced chemiluminescence detection system, based on a protocol described earlier.32
Transferase-mediated dUTP nick end labeling assay
IBE cells were seeded on fibronectin-coated plastic dishes and incubated for 24 hours. The cells were washed and starved for 24 hours in F12/0.25% BSA. Then, fresh F12/0.25% BSA was added and the cells were incubated in the presence or absence of 1 μg/mL endostatin and 5 ng/mL FGF-2 as indicated at 33°C for 48 hours, harvested, fixed, and prepared according to the In situ Cell Death Detection kit, Fluorescein (Boehringer Mannheim), a terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) kit, with fluorescein-conjugated dUTP. Briefly, the cells were fixed in 4% paraformaldehyde, washed twice in PBS, permeabilized in 0.1% Triton-X-100 and 0.1% sodium citrate, washed twice in PBS and incubated 1 hour at 37°C in the presence of the fluorescein-conjugated dUTP. The cells were washed once in PBS and analyzed with a flow cytometer (FACScalibur; Becton Dickinson, Franklin Lakes, NJ), using a 488-nm laser for excitation. Data for light scattering and green fluorescence were collected.
Annexin assay
IBE cells were seeded and starved as for the TUNEL assay, and incubated with and without 1 μg/mL endostatin and 5 ng/mL FGF-2 at 33°C for 24 hours. The cells were prepared according to the Annexin-V-FLUOS Kit (Boehringer Mannheim). Briefly, the cells were trypsinized, and resuspended in fluorescein-conjugated Annexin-V and 2.5 μg/mL propidium iodide, incubated for 10 minutes and analyzed with a flow cytometer (FACScalibur), with 488-nm excitation and collecting light scatter, green, and red fluorescence. Apoptotic cells were defined as cells with enhanced Annexin-V fluorescence simultaneously exhibiting normal propidium iodide staining. The frequency of necrotic cells (with strongly elevated propidium iodide staining) showed no differences between the experimental conditions.
Cell cycle analysis
IBE cells were seeded and starved as described under TUNEL assay and then incubated with and without 1 μg/mL endostatin and 5 ng/mL FGF-2 as indicated at 33°C for 24 hours. The cells were harvested, fixed in −20°C ethanol for 10 minutes and washed twice in PBS. RNAse A (1 mg/mL) and propidium iodide (25 μg/mL) were added. The samples were incubated for 1 hour at 37°C and analyzed with a flow cytometer (FACScalibur), with 488-nm excitation and collecting light scatter and red fluorescence.
Results
Angiogenesis in the CAM is inhibited by endostatin
Angiogenesis in the chicken CAM was induced by treatment with FGF-2 for 3 days on day 10 embryos. Coincubation of FGF-2 with a 10-fold molar excess of endostatin led to an efficient inhibition of angiogenesis in this assay (Figure 1). The effects of mutated forms of endostatin were analyzed by CAM angiogenesis. The H134A, D136A mutant, which does not bind Zn2+,31 inhibited FGF-2–induced angiogenesis to the same extent as wild-type endostatin (Figure 1). In contrast, the R158/270A mutant, which lacks heparin-binding ability31failed to suppress FGF-2–induced angiogenesis in the CAM.
Angiogenesis in the CAM is inhibited by endostatin dependent on its heparin-binding ability.
Ten-day chick embryos were incubated with filter disks saturated with buffer alone, or with FGF-2 with or without wild-type and mutated endostatins (ES). The effect of the additions on CAM angiogenesis was analyzed after 3 days of incubation, by excising the filter and microscopical examination. The R158/270A mutant lacks heparin-binding ability and the H134A, D136A mutant lacks Zn2+-binding ability.
Angiogenesis in the CAM is inhibited by endostatin dependent on its heparin-binding ability.
Ten-day chick embryos were incubated with filter disks saturated with buffer alone, or with FGF-2 with or without wild-type and mutated endostatins (ES). The effect of the additions on CAM angiogenesis was analyzed after 3 days of incubation, by excising the filter and microscopical examination. The R158/270A mutant lacks heparin-binding ability and the H134A, D136A mutant lacks Zn2+-binding ability.
Endostatin regulates cell cycle progression in endothelial cells
IBE cells treated with or without FGF-2 and endostatin were analyzed for DNA content by flow cytometry. After an initial 24-hour serum deprivation, cells were cultured for 24 hours in the presence or absence of additions indicated in Table 1. The addition of FGF-2 increased the proportion of cells in the S-phase in the cell cycle, compared with control cultures. This was evidenced by a significantly increased S/G1 ratio. The addition of endostatin had no effect when added alone, whereas it decreased the S/G1 ratio when added together with FGF-2, compared with FGF-2 alone. This suggests that endostatin reduces the proportion of endothelial cells actively synthesizing DNA after FGF-2 stimulation.
Effect of endostatin on the cell cycle profile of murine brain endothelial cells
| Culture conditions . | G1 . | S . | G2/M . | S/G1 . |
|---|---|---|---|---|
| No addition | 50.7 ± 2.8 | 16.7 ± 1.6 | 33.2 ± 3.3 | 0.33 ± 0.03 |
| Endostatin (1 μg/mL) | 52.5 ± 3.1 | 17.4 ± 1.0 | 30.6 ± 2.1 | 0.34 ± 0.04 |
| FGF-2 (5 ng/mL) | 47.1 ± 1.8 | 20.5 ± 2.6 | 32.6 ± 0.6 | 0.44 ± 0.07 |
| Endostatin + FGF-2 | 45.2 ± 1.3 | 16.6 ± 2.0* | 38.8 ± 1.1 | 0.37 ± 0.06* |
| FCS | 41.1 ± 4.7 | 21.4 ± 2.6 | 37.3 ± 1.9 | 0.54 ± 0.12 |
| Culture conditions . | G1 . | S . | G2/M . | S/G1 . |
|---|---|---|---|---|
| No addition | 50.7 ± 2.8 | 16.7 ± 1.6 | 33.2 ± 3.3 | 0.33 ± 0.03 |
| Endostatin (1 μg/mL) | 52.5 ± 3.1 | 17.4 ± 1.0 | 30.6 ± 2.1 | 0.34 ± 0.04 |
| FGF-2 (5 ng/mL) | 47.1 ± 1.8 | 20.5 ± 2.6 | 32.6 ± 0.6 | 0.44 ± 0.07 |
| Endostatin + FGF-2 | 45.2 ± 1.3 | 16.6 ± 2.0* | 38.8 ± 1.1 | 0.37 ± 0.06* |
| FCS | 41.1 ± 4.7 | 21.4 ± 2.6 | 37.3 ± 1.9 | 0.54 ± 0.12 |
FGF-2 = Fibroblast growth factor-2; FCS = fetal calf serum.
Murine brain endothelial (IBE) cells were cultured for 24 hours as indicated after which the percentages of cells in the different phases of the cell cycle were determined by flow cytometry. The S/G1 ratios are also given. Means ± SEM are given for 3 different experiments.
denotes P < .05, compared with the corresponding FGF-2 value.
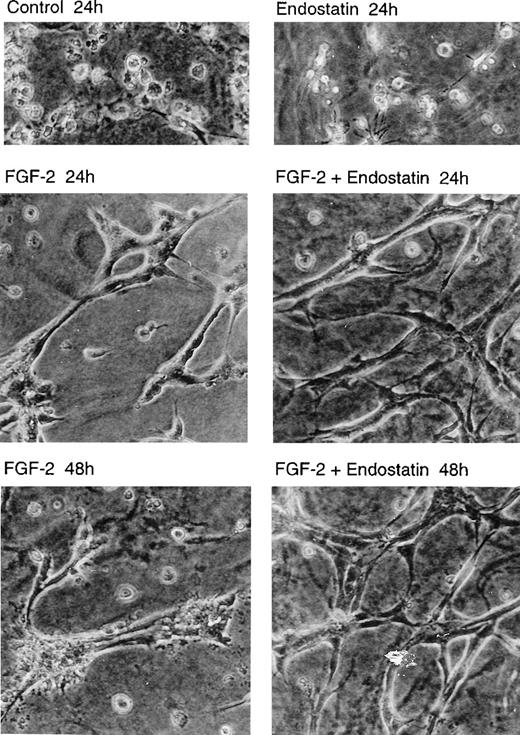
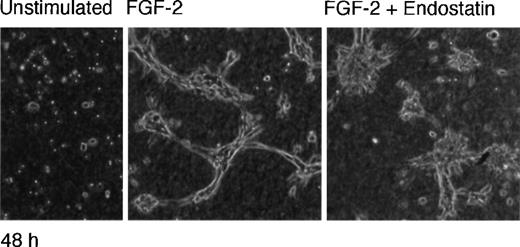
Tubular morphogenesis of endothelial cells is supported by endostatin
IBE cells were seeded on solidified collagen in the absence or presence of FGF-2 and endostatin as indicated, and a second layer of collagen was added on top. The cultures were incubated 24 or 48 hours and inspected by light microscopy (Figure2). The cells maintained in the absence of FGF-2 underwent apoptosis after 24 hours as previously reported,33 regardless of whether they were exposed to endostatin or not. In contrast, FGF-2 treatment led to tubular morphogenesis, with branching structures (Figure 2). We have previously shown that IBE-cell DNA synthesis ceases under these conditions.33 In the combined presence of FGF-2 and endostatin for 24 hours, the tubular structures were slender and contained an increased number of branch points, reminiscent of active angiogenesis. Incubation with FGF-2 alone for 48 hours led to a collapse of the tubular structures, with evident signs of cell death. This was in contrast to cultures incubated with FGF-2 and endostatin for 48 hours, in which the cells remained in good condition, forming a complex pattern of anastomosing and branching slender tubes (Figure 2). These data indicate that collagen-cultured, cell-cycle arrested IBE cells are not adversely affected by endostatin, and that endostatin in fact stabilized the tubular structures.
Tubular morphogenesis of murine brain endothelial cells is supported by endostatin.
IBE cells were cultured between 2 layers of collagen gels, in Ham's F12, 0.25 mg/mL BSA. The cultures were incubated in the absence of additions (control) or in the presence of 1 μg/mL of endostatin and 5 ng/mL of FGF-2 alone or in combination for 24 or 48 hours. The cultures were analyzed by light microscopy and photographed (×20 objective).
Tubular morphogenesis of murine brain endothelial cells is supported by endostatin.
IBE cells were cultured between 2 layers of collagen gels, in Ham's F12, 0.25 mg/mL BSA. The cultures were incubated in the absence of additions (control) or in the presence of 1 μg/mL of endostatin and 5 ng/mL of FGF-2 alone or in combination for 24 or 48 hours. The cultures were analyzed by light microscopy and photographed (×20 objective).
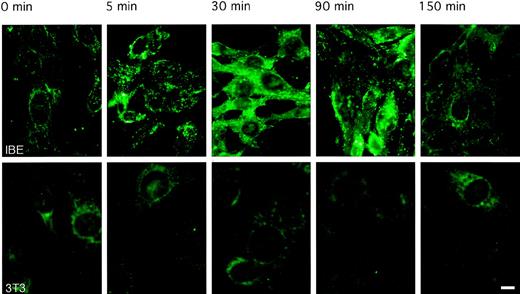
Internalization of endostatin
To follow the pattern of internalization of endostatin, IBE cells were incubated with 10 μg/mL biotinylated endostatin for different periods at 37°C. Cell-associated endostatin was detected after the fixation of cells, by use of Alexa 488-conjugated avidin. As seen in Figure 3, 5 minutes of incubation at 37°C led to a punctate staining of endostatin, indicating clustering at the cell surface. By 30 minutes of incubation, endostatin was taken up and distributed evenly in the cell. By 90 minutes of incubation, staining was considerably weaker, and by 150 minutes, cells were no longer stained, indicating degradation and clearing of endostatin. In contrast, there was no uptake of endostatin in similarly treated mouse Swiss 3T3 fibroblasts (Figure 3). These data are compatible with an active uptake of endostatin by endothelial cells, possibly mediated by a cell surface receptor.
Rapid internalization and degradation of endostatin.
IBE cells or Swiss 3T3 cells were incubated in the absence (control) or presence of 10 μg/mL biotinylated endostatin for different periods from 5 minutes to 150 minutes. Binding was identified using Alexa 488-labeled avidin l that was added to the control and endostatin-incubated samples, which subsequently were analyzed microscopically (Bar, 10 μM). Upper panels show IBE cells cultures; lower panels show Swiss 3T3 cell cultures.
Rapid internalization and degradation of endostatin.
IBE cells or Swiss 3T3 cells were incubated in the absence (control) or presence of 10 μg/mL biotinylated endostatin for different periods from 5 minutes to 150 minutes. Binding was identified using Alexa 488-labeled avidin l that was added to the control and endostatin-incubated samples, which subsequently were analyzed microscopically (Bar, 10 μM). Upper panels show IBE cells cultures; lower panels show Swiss 3T3 cell cultures.
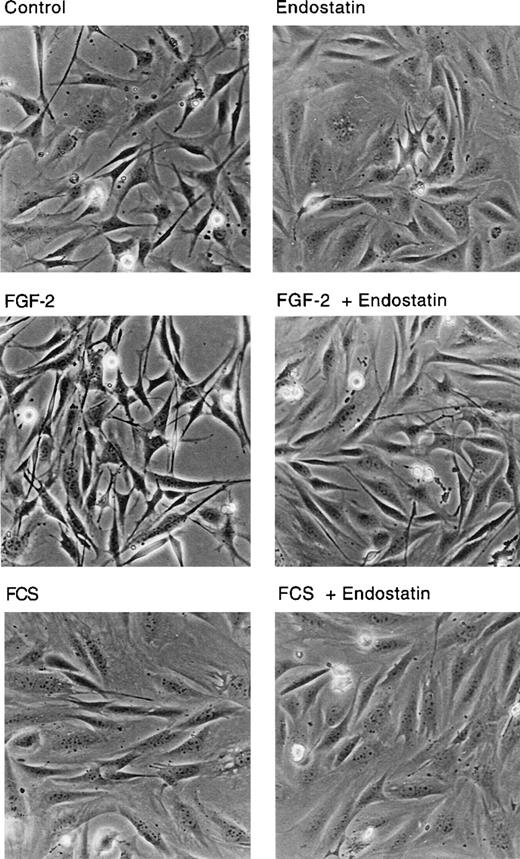
IBE cells that had internalized endostatin displayed a different cell shape, compared with control cells. Figure4 shows that control IBE cells, incubated in BSA-containing medium were polygonal and contained long extensions. The cells in FGF-2-treated cultures were generally elongated, with long, crossing processes, typical for growth factor-treated cells. Treatment of BSA or FGF-2 cultures with endostatin for 24 hours led to a change in cell shape, with flatter, more spread cells that lacked extensions. Indeed, treatment of IBE cells with endostatin led to a morphology very similar to that of serum-treated cells (Figure 4).
Cell shape change in endostatin-treated cells.
IBE cells cultured on fibronectin-coated dishes were treated with 1 μg/mL endostatin, 5 ng/mL FGF-2, and 10% FCS in different combinations as indicated for 24 hours. The cultures were analyzed by light microscopy and photographed.
Cell shape change in endostatin-treated cells.
IBE cells cultured on fibronectin-coated dishes were treated with 1 μg/mL endostatin, 5 ng/mL FGF-2, and 10% FCS in different combinations as indicated for 24 hours. The cultures were analyzed by light microscopy and photographed.
Endostatin-induced apoptosis in murine brain endothelial cells
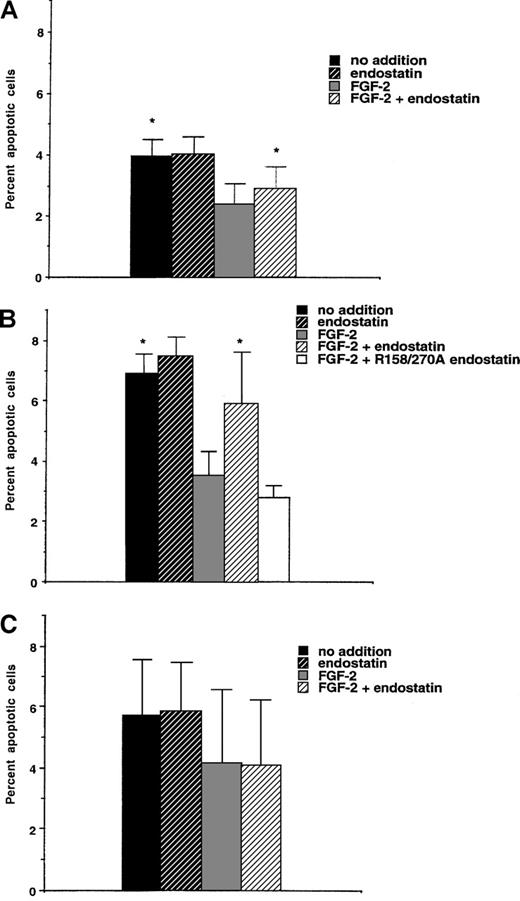
The antiangiogenic effects of endostatin may partly be the consequence of endothelial cell apoptosis.20,21,24 To determine whether endostatin causes apoptosis of IBE cells, labeling using Annexin-V was performed after 24 hours of incubation in the presence of endostatin (Figure 5A). Annexin-labeling detects early plasma membrane changes connected with the apoptotic process.34 The addition of FGF-2 to the IBE cell cultures reduced the rate of apoptosis, compared with untreated cells. Endostatin alone had no effect, but when added together with FGF-2, endostatin caused a slight (24% ± 8%) but significant increase of apoptosis (Figure 5A). IBE cells maintained for 48 hours in the presence of FGF-2 and in the absence or presence of endostatin were also examined. Under these conditions, endostatin induced a relative increase in the rate of apoptosis of 51% ± 12% (n = 4,P < .05) as assessed by the TUNEL-technique35(data not shown).
Endostatin-induced apoptosis is dependent on the adaptor molecule Shb.
IBE infected with vector-containing retrovirus (A) or retrovirus encoding Shb (B) or an Shb SH2-domain mutant R522K (C) were analyzed with regard to proportion of Annexin V-stained cells by flow cytometry. * denotes P < .05 when tested against FGF-2 alone using a paired Student t test.
Endostatin-induced apoptosis is dependent on the adaptor molecule Shb.
IBE infected with vector-containing retrovirus (A) or retrovirus encoding Shb (B) or an Shb SH2-domain mutant R522K (C) were analyzed with regard to proportion of Annexin V-stained cells by flow cytometry. * denotes P < .05 when tested against FGF-2 alone using a paired Student t test.
We have previously reported that angiostatin-induced apoptosis is elevated by overexpression of the Shb adaptor protein and decided to examine the effect of Shb overexpression on endostatin-treated IBE cells. Five-fold overexpression of Shb in IBE cells (L. Lu and M. Welsh, unpublished data) led to an increase in the basal level of Annexin V-positive cells after a 24-hour incubation period (Figure 5B), compared with the vector-transfected IBE cells. FGF-2 caused a significant inhibition of apoptosis under these conditions. Again, endostatin alone had little effect, but augmented the rate of apoptosis when added in the presence of FGF-2. The increase of apoptosis in the combined presence of FGF-2 and endostatin was 66% ± 20% above that in the FGF-2–treated IBE/Shb cells. Thus, Shb overexpressing IBE cells exhibit an exaggerated response to endostatin, compared with the control cells. The R158/270A heparin-binding defective endostatin mutant did not mediate increased apoptosis when added in the presence of FGF-2 (Figure 5B).
Tubular morphogenesis of IBE cells overexpressing Shb in response to endostatin
As endostatin-induced apoptosis was augmented in Shb-overexpressing IBE cells, we decided to investigate tubular morphogenesis of these cells when grown in a collagen matrix. IBE/Shb cell cultures treated for 48 hours with FGF-2 contained tubular structures (Figure6). In contrast, and unlike the pattern in the parental IBE cells, IBE/Shb cell cultures treated with FGF-2 and endostatin showed deteriorated tubular morphogenesis and contained numerous clusters of cells lacking distinct morphologic features (Figure 6). This suggests that the expression of Shb plays a role for the effect of endostatin on in vitro tube formation of IBE cells.
Decreased tubular morphogenesis of IBE cells overexpressing Shb in response to endostatin.
IBE/Shb cells were grown in collagen as in Figure 2 for 48 hours in the absence of FGF-2, presence of 5 ng/mL FGF-2, or presence of FGF-2 and 1 μg/mL endostatin, as indicated. Scoring of the endostatin-induced decrease in tubular morphogenesis (defined as tubular structures with a diameter of 1 cell and a length of more than 7 cells) led to the estimation that endostatin diminished the number of tubular structures by 54% ± 1.9% (n = 3, P < .01) in the IBE/Shb cells.
Decreased tubular morphogenesis of IBE cells overexpressing Shb in response to endostatin.
IBE/Shb cells were grown in collagen as in Figure 2 for 48 hours in the absence of FGF-2, presence of 5 ng/mL FGF-2, or presence of FGF-2 and 1 μg/mL endostatin, as indicated. Scoring of the endostatin-induced decrease in tubular morphogenesis (defined as tubular structures with a diameter of 1 cell and a length of more than 7 cells) led to the estimation that endostatin diminished the number of tubular structures by 54% ± 1.9% (n = 3, P < .01) in the IBE/Shb cells.
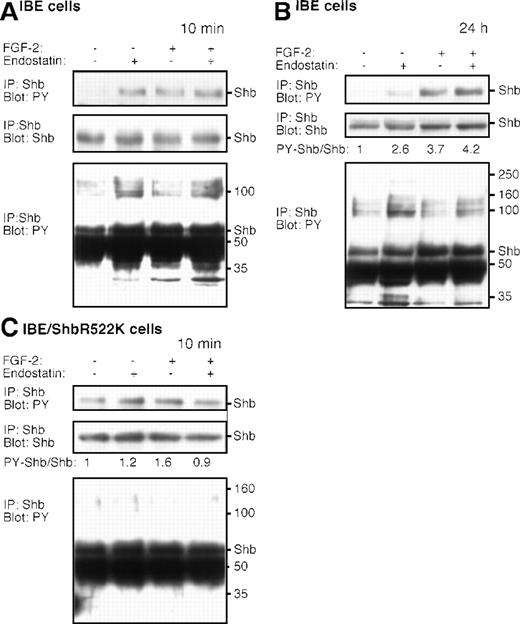
Endostatin-induced Shb-signaling in murine brain endothelial cells
To examine the potential effects of endostatin on Shb-signaling, IBE cells were treated for 10 minutes with or without FGF-2 and endostatin. Phosphotyrosine immunoblotting of Shb immunoprecipitated from endostatin-treated IBE cells showed that 10 minutes of treatment with endostatin alone induced Shb tyrosine phosphorylation (Figure7A, top panel). Furthermore, Shb occurred in complex with a number of tyrosine phosphorylated molecules, eg, of 100, 35, and 32 kd (Figure 7A, lower panel). Longer exposure of the blot showed endostatin-induced tyrosine phosphorylation of additional proteins of 200 and 160 kd (data not shown). Treatment with FGF-2 also increased Shb tyrosine phosphorylation and coimmunoprecipitation of the 32-kd component (Figure 7A). Exposure to the combination of FGF-2 and endostatin resulted in Shb tyrosine phosphorylation and coimmunoprecipitation of both the 35- and 32-kd components (Figure 7A).
Endostatin-treatment induces signal transduction in IBE cells.
IBE cells were incubated with (+) or without (−) FGF-2 and endostatin for 10 minutes (A, C) or 24 hours (B). The lower panels in (A) and (B) represent longer exposures of the immunoblot shown in the top panel. In (C), R522KShb-IBE cells were stimulated with endostatin and/or FGF-2. Cells were lysed and immunoprecipitated (IP) using antiserum against Shb, followed by SDS-PAGE and immunoblotting using the antiphosphotyrosine antibodies 4G10 or the anti-Shb antiserum, as indicated. The ratios of densitometric recordings of Shb and tyrosine phosphorylated Shb are provided in the figure, because the total amounts of Shb varied slightly between the different lanes in the (B) and (C) panels. Molecular masses of marker proteins run in parallel and the migration of Shb are indicated to the right in the panels.
Endostatin-treatment induces signal transduction in IBE cells.
IBE cells were incubated with (+) or without (−) FGF-2 and endostatin for 10 minutes (A, C) or 24 hours (B). The lower panels in (A) and (B) represent longer exposures of the immunoblot shown in the top panel. In (C), R522KShb-IBE cells were stimulated with endostatin and/or FGF-2. Cells were lysed and immunoprecipitated (IP) using antiserum against Shb, followed by SDS-PAGE and immunoblotting using the antiphosphotyrosine antibodies 4G10 or the anti-Shb antiserum, as indicated. The ratios of densitometric recordings of Shb and tyrosine phosphorylated Shb are provided in the figure, because the total amounts of Shb varied slightly between the different lanes in the (B) and (C) panels. Molecular masses of marker proteins run in parallel and the migration of Shb are indicated to the right in the panels.
A similar analysis was performed on IBE cells treated for 24hours. Figure 7B, top panel, shows an elevated level of tyrosine phosphorylated Shb in cells treated for 24 hours with endostatin, albeit to a lesser extent than after the 10-minute stimulation. Shb was in complex with a similar spectrum of tyrosine phosphorylated molecules, as in the short-term–treated IBE cells. In cells treated for 24 hours with FGF-2, or FGF-2 and endostatin, Shb also exhibited an elevated degree of tyrosine phosphorylation, whereas the tyrosine phosphorylation of proteins in complex with Shb was reduced and similar to the basal levels of untreated IBE cells.
To assess to what extent endostatin-induced signaling through Shb required a functional SH2 domain, IBE/Shb R522K cells were analyzed (Figure 7C). The anti-Shb antiserum immunoprecipitates both the endogenously expressed Shb and the transfected R522K Shb. When related to the amount of Shb protein present in the immunoprecipitations, the basal Shb tyrosine phosphorylation was similar in the parental IBE cells and in the IBE/Shb R522K cells. Tyrosine phosphorylation of R522K Shb is likely to be the consequence of non-SH2 domain (proline-rich or PTB domains) interactions between Shb and tyrosine kinases. Endostatin and FGF-2 treatment failed to significantly increase the degree of Shb phosphorylation further. Likewise, no signs of complex formation between Shb and other phosphotyrosyl proteins could be detected after exposure of IBE/Shb R522K cells to endostatin or FGF-2 (Figure 7C). The data thus suggest that endostatin and FGF-2 signaling through Shb requires a functional Shb SH2 domain.
Endostatin-induced signal transduction in endothelial cells
To identify potential Shb SH2 domain-binding proteins of relevance for endostatin signaling, precipitation using GST-Shb SH2 domain fusion protein was performed after endostatin stimulation. Because it is less likely that the SH2 domain of endogenously expressed Shb is blocking SH2 domain-binding sites in the IBE/Shb R522K cells, SH2 domain pull-down using a GST-Shb SH2 domain fusion protein was performed using these cells. As seen in Figure 8A, GST-Shb SH2, but not GST alone, specifically retained a component of approximately 125 kd. The binding or tyrosine phosphorylation of this component was increased by stimulation for 10 minutes with endostatin or FGF-2. This protein may be present in the cluster of bands immunoprecipitated with the Shb antibody in Figure 7A and B.
Endostatin-induced tyrosine kinase activity in complex with Shb SH2 domain.
(A) IBE or PAE/FGFR-1 cells were incubated with (+) or without (−) FGF-2 and endostatin for 10 minutes. Cell lysates were incubated with the GST Shb SH2 domain fusion protein, and associated proteins were analyzed by SDS-PAGE and immunblotting with phosphotyrosine antibodies (blot PY) or by incubation in the presence of γ32P]ATP (kinase assay). The migration rate of the Shb SH2-associated protein of 125 kd is indicated to the right. (B) PAE cells or Swiss 3T3 cells were incubated with indicated concentrations of endostatin and processed for immunoprecipitation using antiphosphotyrosine antibodies 4G10 (IP PY) and in vitro kinase assay. PAE cells treated with the endostatin mutant R158/270A were analyzed similarly. (C) PAE cells were incubated with (+) or without (−) FGF-2 and endostatin for 10 minutes, lysed, and similar protein amounts from the different samples were separated by SDS-PAGE, transferred to nitrocellulose and blotted using antiphosphotyrosine antibodies (4G10). Migration rates of marker proteins are indicated to the right. Phosphotyrosyl proteins induced by endostatin are indicated by *.
Endostatin-induced tyrosine kinase activity in complex with Shb SH2 domain.
(A) IBE or PAE/FGFR-1 cells were incubated with (+) or without (−) FGF-2 and endostatin for 10 minutes. Cell lysates were incubated with the GST Shb SH2 domain fusion protein, and associated proteins were analyzed by SDS-PAGE and immunblotting with phosphotyrosine antibodies (blot PY) or by incubation in the presence of γ32P]ATP (kinase assay). The migration rate of the Shb SH2-associated protein of 125 kd is indicated to the right. (B) PAE cells or Swiss 3T3 cells were incubated with indicated concentrations of endostatin and processed for immunoprecipitation using antiphosphotyrosine antibodies 4G10 (IP PY) and in vitro kinase assay. PAE cells treated with the endostatin mutant R158/270A were analyzed similarly. (C) PAE cells were incubated with (+) or without (−) FGF-2 and endostatin for 10 minutes, lysed, and similar protein amounts from the different samples were separated by SDS-PAGE, transferred to nitrocellulose and blotted using antiphosphotyrosine antibodies (4G10). Migration rates of marker proteins are indicated to the right. Phosphotyrosyl proteins induced by endostatin are indicated by *.
The 125-kd component was analyzed for intrinsic or associated kinase activity by the GST-Shb SH2 protein pull-down from IBE/Shb R522K cells, followed by incubation in the presence of [γ-32P]ATP, and analysis by SDS-PAGE. As seen in Figure 8A, lower panel, 32P was incorporated into the 125-kd protein, in cells treated for 10 minutes with endostatin alone, as well as in cells treated with FGF-2, or with a combination of the two. The requirement of the Shb SH2 domain for endostatin-induced apoptosis (Figure 5C) and increased tyrosine phosphorylation of Shb (Figure 7C), together with the endostatin-dependent interaction between the Shb SH2 domain and the 125-kd phosphotyrosyl protein, raises the possibility that this protein is of relevance for the effects of endostatin in IBE cells.
We examined whether endostatin-treated PAE/FGFR-1 cells also contained the Shb SH2-binding 125-kd protein (Figure 8A, right). Again, the GST-Shb SH2, but not GST alone, bound a 125-kd protein after 10 minutes of endostatin treatment. In vitro kinase assay in the presence of [γ-32P]ATP showed that the protein associating with the Shb SH2 domain contained intrinsic or associated kinase activity (lower panel).
Parental PAE cells were further examined for endostatin-induced kinase activity. Antiphosphotyrosine antibodies were used for immunoprecipitation from PAE cells treated for 10 minutes with different concentrations of endostatin, or with the endostatin mutant R158/270A. The samples were processed for in vitro kinase assay in the presence of [γ-32P]ATP, followed by SDS-PAGE. As seen in Figure 8B, endostatin treatment increased the32P-incorporation into a cluster of proteins of about 125 kd, possibly corresponding to the Shb SH2-associated phosphotyrosyl protein identified above. This induction of kinase activity was not seen in endostatin-treated Swiss 3T3 cells, or in PAE cells treated with the endostatin mutant R158/270A (Figure 8B).
Phosphotyrosine immunoblots of cell lysates from PAE/FGFR-1 cells treated with endostatin, FGF-2 or a combination of the two, showed induction of a spectrum of tyrosine phosphorylated components with the different treatments (Figure 8C). Treatment with endostatin alone appeared to induce a subset of phosphotyrosyl proteins (indicated by * in Figure 8C), which was different from that observed after treatment with FGF-2. Notably, FGF-2–stimulation induced tyrosine phosphorylation of 160-kd proteins, indicating that endostatin does not exert its effects by activating the FGF receptor.
Discussion
In this paper, we show that treatment of FGF-2–induced angiogenesis in the chicken CAM is efficiently inhibited by endostatin purified from 293 cells. The specificity of this effect was demonstrated by the lack of inhibition when treating the FGF-2–stimulated CAM with an endostatin mutant, R158/270A, in which 2 of 4 arginine residues in a region that constitutes a major heparin-binding site have been replaced with alanine residues.31 Mutation of H134 and D136 to alanine residues eliminates Zn2+-binding.31 Because this mutant retained the capacity to inhibit angiogenesis in the CAM assay, we conclude that Zn2+-binding is not required for this particular function of endostatin. Our data therefore indicate that the ability to bind heparin, but not Zn2+, is an important factor in the antiangiogenic action of endostatin.
The antiangiogenic effects of endostatin appear to involve inhibition of cell growth mediated via both reduced G1/S phase transition and increased apoptosis20,21 (Table 1, Figure5). It should be noted that although the effect was small, it would be cumulative. Our data show that overexpression of Shb with a functional SH2 domain can augment the apoptotic response to endostatin when added together with FGF-2. Likewise, tubular morphogenesis was reduced in the Shb-overexpressing cells but not in the parental IBE cells, in agreement with the possibility of an involvement of Shb in the antiangiogenic effects of endostatin. Our data do not exclude the significant contribution of other signal transduction molecules to these effects. To elucidate signaling pathways responsible for the action of endostatin, tyrosine kinase activity in response to endostatin was examined. The data show rapid endostatin-induced tyrosine kinase activity in endothelial cells, but not in mouse fibroblasts, in agreement with the specificity of endostatin for endothelial cells reported by O'Reilly et al.7 Treatment with endostatin alone induced Shb tyrosine phosphorylation and the formation of multiprotein complexes. Although both FGF-2 and endostatin induced tyrosine phosphorylation of Shb, the composition of the multiprotein complexes found associated with Shb varied between the 2 conditions of stimulation. Such differences may be of relevance for the cellular responses to the 2 ligands; FGF-2 treatment mediated reduction in apoptosis, whereas endostatin antagonized this effect. Furthermore, the Shb SH2 domain specifically interacted with a kinase-active, or kinase-associated, 125-kd protein in an endostatin- and FGF-2–dependent manner. Kinase activity was not induced by the endostatin mutant R158/270A. Thus, heparin-binding ability appears to be a prerequisite for kinase activation by endostatin, possibly by facilitating activation directly or indirectly of a cell surface expressed or cytoplasmic tyrosine kinase.
Recent data show that endostatin does not compete with the binding of FGF-2 to human tissues.36 The fact that FGF-2–binding, but not endostatin-binding, to the human tissues could be removed by heparitinase digestion36 indicates clear distinctions in the heparin/heparan sulfate requirement of FGF-2 and endostatin. Moreover, several observations in this study suggest that FGFR-1 signaling is not affected by endostatin treatment. Endostatin did not prevent FGF-2–induced tube formation in the parental IBE cells (Figure2), or tyrosine phosphorylation of Shb and a 160-kd phosphotyrosyl protein observed in the PAE cell lysates (Figure 8C). Thus, it appears that endostatin exerts its effects via activation of specific signaling pathway(s), and we favor the notion that this occurs primarily via activation of a tyrosine kinase. This kinase may in turn activate phosphatases or other regulatory signaling proteins, which may interfere with FGF effects on endothelial cells. The endostatin-activated kinase remains to be identified. It does not appear to correspond to Src cytoplasmic tyrosine kinases, focal adhesion kinase (FAK), or to Abl tyrosine kinase, based on immunoblotting analyses (data not shown). In repeated assays, endostatin-coated plastic wells failed to mediate attachment of various endothelial cells (T. Sasaki and R. Timpl, unpublished data). This suggests that endostatin does not bind to integrins and that it does not act by disrupting integrin–extracellular matrix interactions. Furthermore, endostatin does not appear to act via FGF receptors. Although tyrosine phosphorylation of Shb was induced both by FGF-2 and by endostatin, the number of and molecular masses of phosphotyrosyl proteins induced were distinct for the 2 conditions.
Angiostatin,6 which is a fragment of plasminogen, was found to activate FAK in endothelial cells, resulting in an increased apoptosis. The effect was accentuated by overexpression of Shb.24 Angiostatin binds to a cell surface–exposed ATP synthase37; it is unclear at this point whether the ATP synthase couples to FAK and Shb. We have not been able to further analyze and compare angiostatin- and endostatin-induced signal transduction, but our present data indicate that these angiogenesis-inhibitors have different modes of action, although Shb may be involved in regulation of the apoptotic response to both inhibitors. In a recent report from the Hanahan and Folkman laboratories,38 endostatin and angiostatin were shown to act in a synergistic manner in arresting tumor expansion in an insulinoma model tumor in mouse, further emphasizing the different modes of action of these inhibitors.
Taken together, our data indicate that endostatin binds to the endothelial cell surface via heparan-sulfated proteoglycans, and thereby directly or indirectly induces tyrosine kinase activity, which may lead to apoptosis, dependent on the proliferative state of the cells.
Supported by grants from The Swedish Medical Research Council (31x-10822) to M.W.; from The Swedish Cancer Foundation (project no. 3820-B98-03XAC), The Novo Nordisk Foundation, The Göran Gustavsson Foundation to L.C-W.; and by an EC grant BI04-CT96-0537 to R.T. J.D. was supported by Pharmacia & Upjohn and by the Swedish Research Council for Engineering Sciences. M.W. and L.C.W. contributed equally to this study.
Reprints:L. Claesson-Welsh, Department of Genetics and Pathology, Rudbeck Laboratory, S-751 85 Uppsala, Sweden; e-mail:lena.welsh@genpat.uu.se.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 8. Endostatin-induced tyrosine kinase activity in complex with Shb SH2 domain. / (A) IBE or PAE/FGFR-1 cells were incubated with (+) or without (−) FGF-2 and endostatin for 10 minutes. Cell lysates were incubated with the GST Shb SH2 domain fusion protein, and associated proteins were analyzed by SDS-PAGE and immunblotting with phosphotyrosine antibodies (blot PY) or by incubation in the presence of γ32P]ATP (kinase assay). The migration rate of the Shb SH2-associated protein of 125 kd is indicated to the right. (B) PAE cells or Swiss 3T3 cells were incubated with indicated concentrations of endostatin and processed for immunoprecipitation using antiphosphotyrosine antibodies 4G10 (IP PY) and in vitro kinase assay. PAE cells treated with the endostatin mutant R158/270A were analyzed similarly. (C) PAE cells were incubated with (+) or without (−) FGF-2 and endostatin for 10 minutes, lysed, and similar protein amounts from the different samples were separated by SDS-PAGE, transferred to nitrocellulose and blotted using antiphosphotyrosine antibodies (4G10). Migration rates of marker proteins are indicated to the right. Phosphotyrosyl proteins induced by endostatin are indicated by *.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3403/6/m_bloo01107008w.jpeg?Expires=1769239374&Signature=znhPlDbpG7XNti85BWXmk8OvHlOt5Rs~igzf583mu8QdTYwJGSjipSDcEScc03vZzYMte5efvVy7cX7OOjVanZ2hWz4iFM1i2xGugG8HXkIXdLy7FFF7l8as03kGSTPI02hmXLS1JaK~P288iT4fyZOYZAnCjZ-lCj7tg19QYbJ8A3Ms-bDwHmLsonL9OtCfYfUd~Wu8uabq9xbZQe5U1Q~Ff7--HktxRUoHr0P5GFUj0OsRCyp6pbeiaTVeD9teGTdd-OJlqRN92MPaix1UD5QFacq59yu7KGezbGvwVREtM~DSQOgtOx-ogeGP9UYj-0V6COtSsFPgylTgqSLteA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal