Abstract
The hemolytic uremic syndrome (HUS) is the most common cause of acute renal failure in children. The role of a verocytotoxin (VT)-producing Escherichia coli has been strongly implicated in the epidemic form of HUS. Although direct toxicity of VT on glomerular endothelial cells has been demonstrated, it remained still unclear how the VT is transported from the intestine to the target organs. In this study we demonstrate that VT, when incubated in whole blood, binds rapidly and completely to human polymorphonuclear leukocytes (PMNs) and not to other components of blood. Binding studies with125I-VT-1 showed a single class of binding sites on freshly isolated, nonstimulated human PMNs. TheKd of VT-binding to PMNs was 10-8 mol/L, 100-fold less than that of the VT-receptor globotriaosylceramide. On incubation of VT-preloaded PMNs with human glomerular microvascular endothelial cells (GMVECs), transfer of VT-1 to the endothelial cells occurred. Incubation of nonstimulated GMVECs with VT-preloaded PMNs, but not with PMNs or VT-1 alone, caused inhibition of protein synthesis and cell death. Our data are in concert with a role of PMNs in the transfer of VT from the intestine to the kidney endothelium. This transfer occurs by selective binding to a specific receptor on PMNs and subsequent passing of the ligand VT to the VT-receptor on GMVECs, which causes cell damage. This new mechanism further underpins the important role of PMNs in HUS.
The hemolytic uremic syndrome (HUS) is the most frequent cause of acute renal failure in children. The traditional diagnostic criteria for this syndrome include hemolytic anemia with fragmented erythrocytes, thrombocytopenia, and renal failure.1 The endothelium of kidney arterioles and glomeruli plays a central role in the pathogenesis of HUS. Histopathological studies of the kidney of HUS patients show characteristic lesions, consisting of swelling and detachment of the endothelial cells of glomeruli and deposits of fibrin in glomeruli and arterioles.2 3 In severe cases, the cell damage is not limited to the kidney but other organs, such as brain and pancreas, are also involved.
The epidemic form of HUS, or (D+) HUS, occurs mostly following a prodromal phase of bloody diarrhea. In 90% of the cases with (D+) HUS, an infection with a verocytotoxin (VT)-producing Escherichia coli is strongly implicated.2-6 Strains of the VT-producing E coli associated with HUS can produce VT-1 or VT-2, or both. The structure of the VT is formed by a biologically active A subunit and 5 B subunits by which the toxin binds to specific glycolipid receptors. The VT-producing E coli is transmitted by contaminated food or water or from person to person. After ingestion, the E coli binds to specific receptors to the intestinal wall, and VT enters the circulation via a still unknown mechanism.3,7,8 VT is transported to the target organs and can bind specifically to its receptor globotriaosylceramide, also called Gb3. This receptor has been demonstrated in human renal tissue and in human endothelial cells.9,10 After binding to Gb3, the active subunit of the VT enters the cell and causes inhibition of protein synthesis.11-16
The route of transport of the VT from intestine to the kidney or other target organs is not solved yet. Although epidemiological studies have pointed to a role of VT in (D+) HUS, no VT has been encountered thus far in the plasma of patients with HUS. In vitro experiments showed that VT could bind to erythrocytes, depending on the P-blood group glycolipids, that are structurally related to the known VT receptor Gb3. It has been reported that VT can bind to the P1 phenotype (Pk, P1, P2 antigens) and, to a lesser extent, to the P2 phenotype (Pk and P antigens) but not to the P phenotype (lacking antigens).17-20 In vitro experiments also showed binding to activated human monocytes.21 Other possible candidates for transporting the VT are platelets22 and lipoproteins.23 24 In this study we evaluate which fractions of the blood contribute to VT binding and transfer. Our data suggest that polymorphonuclear leukocytes (PMNs) are responsible for transporting the VT in blood and that the receptor responsible for binding VT to PMNs is different from that found on endothelial cells. Furthermore, we demonstrate that VT is transferred from PMNs to human glomerular endothelial cells.
Materials and methods
Materials
Purified VT-1 was kindly provided by Dr M. A. Karmali (Toronto, ON, Canada). Ficoll was purchased from Pharmacia (Uppsula, Sweden). Plastic-coated silica gel F1500 thin-layer chromatography (TLC) plates were obtained from Schleicher and Schuell (Dassel, Germany). A standard mixture of pure neutral glycolipids was obtained from Biocarb AB (Lund, Sweden). VT-1 labeled with fluorescein isothiocyanate (VT-FITC) was kindly donated by Dr Lingwood (Hospital for Sick Children, Toronto, ON, Canada). Flow-activated cell sorter (FACS) lysing solution was purchased from Becton Dickinson Immunocytometry Systems (San Jose, CA). CD13-PE, CD14-PE, and CD45-TRITC were purchased from DAKO (Glostrup, Denmark). All other reagents were of analytical grade or as described previously.21,25 26
FACS analysis and immunohistological study of whole blood
VT-FITC was used to determine binding of VT in whole blood. Blood was obtained from 10 different healthy donors, 8 adults and 2 children (aged 10 and 6 years), and blood group of the adults was determined according to established procedures. Two donors had blood group O, 4 blood group A, and 2 had blood group B. There were 2 donors with the P1 blood group. Blood was immediately put on ice; 100 μL of blood was incubated for 20 minutes on ice with VT-FITC, followed by addition of FACS lysing solution to remove erythrocytes. The solution was centrifuged at 200g for 5 minutes at 4°C. The cells were washed twice with phosphate-buffered saline (PBS) containing 1% albumin. Cells were resuspended in 500 μL of a 0.5% paraformaldehyde solution for fixation. Flow cytometry was used to determine binding of VT-FITC. Fluorescence was measured in a histogram, using a log scale. The different cell types were characterized with the use of monoclonal antibodies; CD13-PE and CD16-PE for PMNs, CD14-PE for monocytes, and CD45-TRITC for lymphocytes. To exclude nonspecific binding, whole blood was previously incubated with unlabeled VT for 20 minutes, followed by incubation with VT-FITC. The same procedure as above was used for direct immunofluorescence studies. Briefly, cells were incubated for 20 minutes with VT-FITC and a monoclonal antibody to indicate the cell type. Cells were washed twice with PBS and were then resuspended in 500 μL of 0.5% paraformaldehyde. Cells were centrifuged at 200g. Subsequently, cells were analyzed by a Zeiss fluorescence microscope (Aksioscope) with standard FITC filter (09) and PE-filter (014) (excluding the possibility of interference of FITC and PE-staining), for VT-FITC, CD13-PE, CD14-PE, and CD45-TRITC staining, respectively.
Isolation of PMNs
Twenty milliliters of EDTA or heparin blood of 8 healthy adult donors and of 2 children was obtained for the isolation of PMNs. Blood was directly put on ice. Blood was mixed with 15 mL of PBS and put into a 50-mL tube. The blood was underlayed using Ficoll 1.077 g/mL. The cells were centrifuged 20 minutes at 200g at 4°C in a Sorvall centrifuge. The pellet contained PMNs and erythrocytes. Erythrocytes were lysed using ammonium chloride or FACS lysing solution, and PMNs were washed twice with PBS containing 1% bovine serum albumin (BSA). PMNs were resuspended in PBS or RPMI medium containing 1% of human serum and stored at 4°C for < 1 hour until use. The population PMNs was more than 95% pure as measured by an H3-analyser (Technicon, Bayer).
Flow cytometric analysis after isolation of different cell types
Pooled PMNs as described above were used. Blood was mixed with PBS and cells were separated using Ficoll. The interphase was collected for studying binding to lymphocytes and monocytes separately. The pellet contained PMNs and erythrocytes. The pellet was resuspended in 20 mL PBS. Subsequently, Ficoll 1.077 g/mL was used to separate PMNs from erythrocytes. To make sure that the different cell populations were more than 95% pure, cell populations were analyzed by an H3-analyzer. Different cell types were incubated with 0.5 μL VT-FITC (1 mg/mL) on ice for 20 minutes. Cells were then washed twice with PBS/1%BSA and centrifuged at 200g for 5 minutes. Cells were resuspended in 500 μL of 0.5% paraformaldehyde. Binding of VT-FITC was measured using flow cytometry. Experiments were repeated for the different cell types, using an incubation time of 3 hours.
Results were confirmed by using direct immunofluorescence and incubation of the different cell populations separately with125I-VT-1 (see below).
Binding of VT-FITC to lipoproteins
To examine the binding between VT and lipoproteins, lipoproteins were isolated by ultracentrifugation and a precipitation method.27 Very low-density lipoproteins (VLDLs), high-density lipoproteins (HDLs), or low-density lipoproteins (LDLs) were incubated with VT-FITC during 3 hours on ice. After the incubation, lipoprotein-depleted serum was added. The solution was mixed thoroughly and incubated for 10 minutes. Subsequently, a precipitation solution was added, followed by centrifugation. After centrifugation, binding of VT-FITC was determined by fluorescence spectrometry. Experiments were repeated, using an incubation time of 1 hour or 20 minutes to determine whether binding of VT to lipoproteins occurred after a similar incubation period as needed for PMNs.
Binding of 125I-VT-1 to human PMNs
VT-1 was labeled with Na-125I according to the Iodogen procedure.28 After isolation of PMNs from EDTA blood, PMNs were washed twice with PBS and were then resuspended in Hanks balanced salt solution (HBSS) containing 1% human serum albumin (Central Laboratory of the Red Cross, Amsterdam, The Netherlands) at 0°C. Subsequently, 0.5 × 106 PMNs/250 μL were incubated for 3 hours with 125I-VT-1 in different concentrations, ranging from 0.3 up to 70 nmol/L. To determine nonspecific binding, unlabeled VT-1 in 25-fold excess was added parallel with 125I-VT-1. After incubation, the free fraction of 125I-VT-1 was separated from the fraction of125I-VT-1 bound to the PMNs, using Ficoll 1.077 g/mL. PMNs were then washed with Hanks balanced salt solution/1% human serum albumin and centrifuged at 200g for 10 minutes. Cell-associated125I-VT-1 was determined in a gamma-counter. All determinations were done in duplicate. Binding data were analyzed, using the method of Scatchard.29
Thin layer chromatography of neutral glycolipids extracted from PMNs
PMNs were isolated as described before. They were washed twice with PBS and subsequently resuspended in 0.5 mL of PBS. Next, glycolipids of the cells were extracted and separated as described by Lingwood et al.30 For the extraction of neutral glycolipids, 1 mol/L NaCl was used instead of water to receive maximal yield. After separation of the neutral glycolipids, the TLC plate was coated with polyisobutylmetacrylate, blocked overnight with 1%PBS/Tween and incubated with 125I-VT-1 in 1% albumin and 0.05% Tween-20 in PBS.26 After washing, the binding of125I-VT-1 was analyzed, using a Fuji BAS 1000 phosphor-imager.
Human glomerular microvascular endothelial cells
Human glomerular microvascular endothelial cells (GMVECs) were isolated and cultured on gelatin-coated dishes as described by van Setten et al.26 Cells were characterized by indirect immunofluorescence microscopy, using antibodies against von Willebrand factor, PECAM-1, and VE-cadherin. No immunoreactivity was observed with antigens against α-smooth muscle actin or cytokeratin 20, indicating that there was no contamination with mesangial or epithelial cells. GMVECs were cultured in 24-well plates to perform experiments and used between passage 6 and 10. They were used 5 days after reaching confluence and stimulated for 24 hours with 10 ng/mL tumor necrosis factor α (TNF-α), if indicated.
Transfer of VT from PMNs to endothelium in vitro
After isolation, PMNs were washed twice with PBS and were then resuspended in medium M199 containing 20% fetal calf serum. PMNs were incubated with a monoclonal antibody, CD16-PE, to exclude the possibility that PMNs interfere with FACS analysis. Subsequently, different amounts of PMNs, varying between 0.5 and 2.0 × 106 PMNs were incubated with 0.5 μL VT-FITC on ice during 30 minutes. After that, PMNs were washed twice with medium M199. Nonstimulated GMVECs and TNF-α–stimulated GMVECs were incubated on ice for 4 hours with 0.5-2.0 × 106PMNs loaded with VT-FITC. As control, GMVECs were also incubated alone with VT-FITC, PMNs, or with medium containing 10% fetal calf serum. After the incubation period, GMVECs were washed 3 times with M199. GMVECs were detached with trypsin treatment and centrifuged for 5 minutes at 200g at 4°C. Cells were washed with PBS, then resuspended in 500 μL 0.5% paraformaldehyde, and transferred into a tube suitable for FACS analysis.
Measurement of protein synthesis
GMVECs were cultured in 24-well plates and were used 5 days after reaching confluence. GMVECs stimulated by TNF-α (10 ng/mL) and nonstimulated GMVECs were incubated with 106 PMNs at 37°C for 24 hours. PMNs were preloaded with VT in different concentrations, ranging from 0.1 nmol/L to 10 nmol/L VT and washed 2 times before adding to the GMVECs. PMNs not preincubated with VT were used as control. Protein synthesis was determined by assaying the incorporation of 3H-leucine in newly synthesized proteins as described previously.26
Statistics
All results of measuring protein synthesis are expressed as mean ± SEM. Changes with respect to control values were analyzed by using an unpaired Student t test. Values in which P ≤ .05 were regarded as significant.
Results
Binding of VT in blood
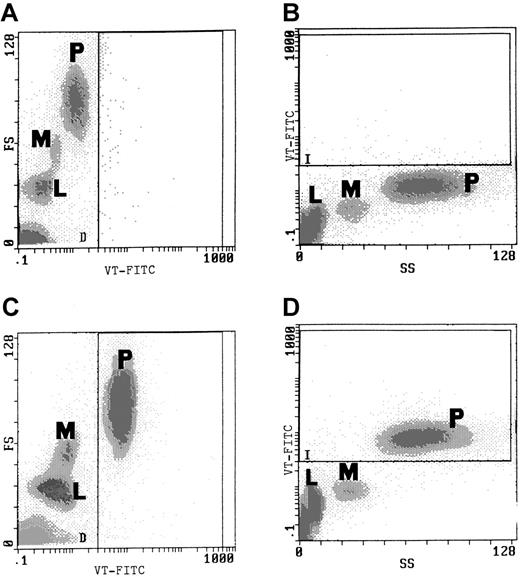
Incubation of whole blood with VT-FITC after 20 minutes showed almost complete binding of VT to PMNs (Figure1C and 1D), independent of blood group or age of the donor. Binding was completely blocked by pre-incubation of blood with unlabeled VT-1 for 20 minutes (data not shown). More than 90% of PMNs were positive, and all other cell types were negative. Control blood, incubated with an immunoglobulin (Ig)G antibody conjugated with FITC, showed no staining (Figure 1A and 1B). Selective binding of VT-FITC was also demonstrated by direct immunofluorescence studies (Figure 2). Only PMNs bound VT-FITC.
Binding of VT-FITC to PMNs in whole blood was demonstrated by flow cytometric analysis.
(A, B) Flow cytometric analysis of control blood (incubated with IgG-FITC alone) before addition of VT-FITC. (C, D) Analysis after 20-minute incubation with 0.5 μL VT-FITC (1 mg/mL) exclusive binding to PMNs was found. L indicates lymphocytes; M, monocytes; P, PMNs. The experiment is representative for duplicate determinations in 10 experiments with blood of different donors.
Binding of VT-FITC to PMNs in whole blood was demonstrated by flow cytometric analysis.
(A, B) Flow cytometric analysis of control blood (incubated with IgG-FITC alone) before addition of VT-FITC. (C, D) Analysis after 20-minute incubation with 0.5 μL VT-FITC (1 mg/mL) exclusive binding to PMNs was found. L indicates lymphocytes; M, monocytes; P, PMNs. The experiment is representative for duplicate determinations in 10 experiments with blood of different donors.
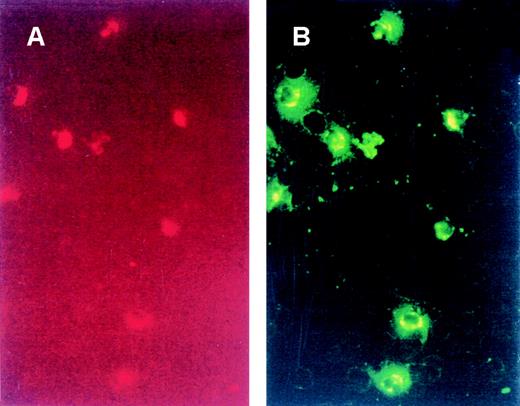
Direct immunofluorescence of binding of VT-FITC to PMNs.
Whole blood was incubated with CD13-PE (A), a specific marker for PMNs, and with VT-FITC (B) on ice for 20 minutes. PMNs were the cells that had bound VT-FITC. No other components of blood were positive for binding VT-FITC.
Direct immunofluorescence of binding of VT-FITC to PMNs.
Whole blood was incubated with CD13-PE (A), a specific marker for PMNs, and with VT-FITC (B) on ice for 20 minutes. PMNs were the cells that had bound VT-FITC. No other components of blood were positive for binding VT-FITC.
To substantiate that PMNs were the only cells binding VT in blood, VT-FITC binding to purified erythrocytes, monocytes, and platelets was also studied. Erythrocytes were incubated, and binding was analyzed using FACS analysis and direct immunofluorescence. No binding of VT-FITC to erythrocytes was observed independent of the P blood group (8 donors). No significant binding of VT-FITC to nonstimulated monocytes was observed (6 donors). Lymphocytes and thrombocytes (3 different donors) also did not bind VT-FITC in our conditions.
Lingwood23 24 suggested that lipoproteins may bind glycolipids, such as Gb3, and that VT may be cotransported by lipoproteins in a piggyback way. LDL and HDL preparations were incubated separately with VT-FITC on ice for 3 hours. No significant binding was observed. This experiment was repeated with incubation times of 20 minutes and 1 hour. Again, no binding was found (data not shown). Similarly, no binding of 125I-VT to these preparations was found. From these data, we conclude that VT only significantly binds to PMNs in whole blood and not to other components, such as erythrocytes, lymphocytes, monocytes, and lipoproteins.
Analysis VT binding to PMNs
To evaluate whether high-affinity binding sites were involved in the binding of VT to PMNs, the binding of125I-VT-1 to PMNs was determined. Figure3A shows that the binding of125I-VT-1 is saturable and specific. After incubation with a 25-fold excess of unlabeled VT-1, binding with 125I-VT-1 decreased more than 95%. Scatchard plot analysis (Figure 3B) showed that nonstimulated PMNs have 2.1 × 105 binding sites with a high affinity for VT (Kd = 10−8 mol/L). This affinity is 100-fold less than what we and others10 26consistently found for VT-1 binding to Gb3 in human vein and GMVECs. To confirm that VT remained exposed on the surface, 125I-VT-1 was bound to PMNs (30 minutes, 37°C), and, after 3 washings, the VT-loaded PMNs were incubated for 2.5 hours at 37°C in medium supplemented with 10% fetal calf serum and 100 U/mL aprotinin. After removal of the serum-containing medium, treatment of these PMNs for 10 minutes by trypsin/EDTA released 95% of the125I-VT-1 associated with the cells. This indicates that the VT-1 remained available at the cell surface.
PMNs of 2 different donors were used to determine binding of 125I-VT-1 to PMNs.
(A) PMNs were incubated with increasing concentrations of125I-VT-1 (0.3 to 70 nmol/L) at 4°C for 3 hours (▴). Nonspecific binding (•) was determined in the presence of 25-fold excess of unlabeled VT-1. (B) Shows the result of Scatchard plot analysis.
PMNs of 2 different donors were used to determine binding of 125I-VT-1 to PMNs.
(A) PMNs were incubated with increasing concentrations of125I-VT-1 (0.3 to 70 nmol/L) at 4°C for 3 hours (▴). Nonspecific binding (•) was determined in the presence of 25-fold excess of unlabeled VT-1. (B) Shows the result of Scatchard plot analysis.
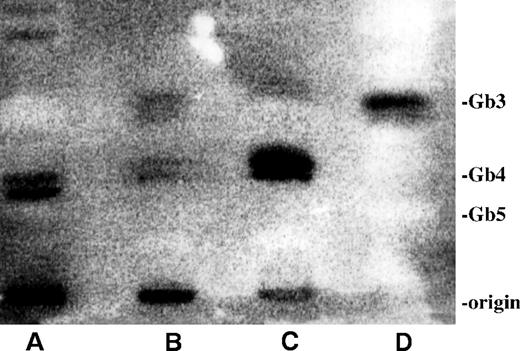
To investigate whether Gb3 or a different neutral glycolipid was responsible for binding of VT, thin layer chromatography (TLC) of neutral glycolipids extracted from nonstimulated PMNs was performed and compared with those of TNF-α–stimulated GMVECs and LPS-stimulated monocytes. Thin layer chromatograms were incubated with radiolabeled VT-1 and washed thoroughly. The bound125I-VT-1 was detected with the use of a phosphor-imager (Figure 4). 125I-VT-1 strongly bound to the Gb3 in the standard neutral glycolipid preparation (lane D) and the TNF-α–treated neutral glycolipid extract of GMVECs (lane B). Two other bands binding 125I-VT-1 just below the globotetraosylceramide (Gb4) were found in glycolipid extractions of monocytes (lane C) and in smaller amounts in those of GMVECs. Nonstimulated PMNs showed 2 small bands with a Rf value just below the Gb4 but distinct from that observed for monocytes. This pattern was consistently found with 4 different neutral glycolipid extracts of PMNs obtained from 4 different donors.
125I-VT-1 binding to neutral glycolipid extracts from human PMNs, GMVECs, and monocytes.
Glycolipids were extracted and separated as described in “Materials and methods.” Binding of 125I-VT-1 was visualized, using a phosphor-imager. (A) Glycolipid extract of 30 million PMNs of 1 representative donor. (B) Neutral glycolipid extraction of TNF-α–treated GMVECs. (C) Glycolipid extract of monocytes stimulated with LPS (1 ng/mL). (D) Standard mixture of neutral glycolipids, 2 μg of each glycolipid. Standard of neutral glycolipids was stained with orcinol. (Gb3, globotriaosylceramide; Gb4, globotetraosylceramide; and Gb5, Forssman pentasaccharide).
125I-VT-1 binding to neutral glycolipid extracts from human PMNs, GMVECs, and monocytes.
Glycolipids were extracted and separated as described in “Materials and methods.” Binding of 125I-VT-1 was visualized, using a phosphor-imager. (A) Glycolipid extract of 30 million PMNs of 1 representative donor. (B) Neutral glycolipid extraction of TNF-α–treated GMVECs. (C) Glycolipid extract of monocytes stimulated with LPS (1 ng/mL). (D) Standard mixture of neutral glycolipids, 2 μg of each glycolipid. Standard of neutral glycolipids was stained with orcinol. (Gb3, globotriaosylceramide; Gb4, globotetraosylceramide; and Gb5, Forssman pentasaccharide).
Transfer of VT from PMNs to GMVECs
To investigate if VT-1 could be transferred from PMNs to GMVECs, we incubated nonstimulated and TNF-α-stimulated GMVECs with PMNs that had bound VT-FITC at 0°C.
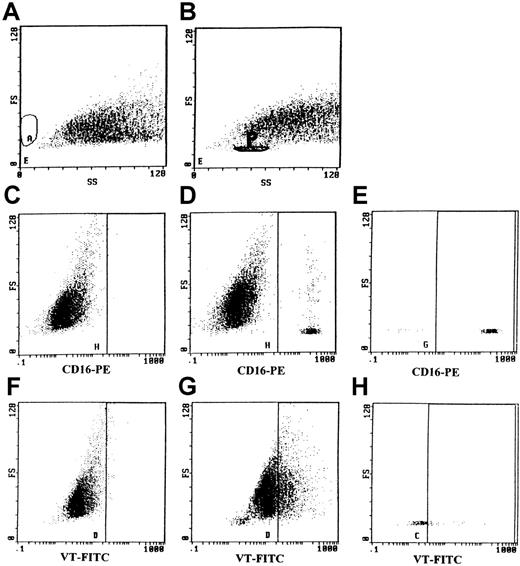
PMNs were previously incubated on ice with CD16-PE, a specific monoclonal antibody for PMNs, to prevent interference of PMNs by FACS analysis. After an incubation time of 3 hours on ice, GMVECs were washed carefully 3 times with PBS. Subsequently, GMVECs were detached and analyzed by flow cytometry. No PMNs were retained by nonstimulated GMVECs (as revealed by FACS analysis after staining with CD16-PE; Figure 5A and 5C). GMVECs were negative for CD16-PE staining (Figure 5C). However, TNF-α–stimulated GMVECs retained a small percentage of the PMNs added (ranging between 2.8% and 3.6%) (Figure 5B). The population of PMNs was gated (indicated by the area P in Figure 5B), which allowed simultaneous analysis of PMNs and GMVECs. The population of gated PMNs was positive for CD-16-PE (Figure 5E) and distinct from the CD-16-PE–negative GMVECs (Figure5D). The differentiation between PMNs and GMVECs by gating permitted us to investigate if a transfer of VT-FITC from PMNs to GMVECs had occurred. After the 3-hour incubation period, 30% of the stimulated GMVECs had bound VT-FITC (Figure 5G). Nonstimulated GMVECs did not bind any VT-FITC (Figure 5F). When IgG-FITC was incubated with GMVECs, no binding occurred. This excludes nonspecific binding of FITC to these cells. Ligand passing of VT-FITC from PMNs to GMVECs was further confirmed by the fact that no VT-FITC could be demonstrated on PMNs that were removed after the 3-hour incubation period on GMVECs on ice. Furthermore, PMNs that were CD16-PE positive and still adhering to endothelial cells (2.8%-3.6% of total PMNs added) were negative for VT-FITC binding (Figure 5H). No uptake of VT-FITC by PMNs occurred during the incubation period.
The transfer of VT-1-FITC bound to PMNs to human GMVECs was studied by flow cytometry.
Panels A, C, and F: FACS analysis of nonstimulated GMVECs incubated for 4 hours with PMNs loaded with VT-FITC on ice. (A) Forward scatter and side scatter of nonstimulated GMVECs are shown. (C) No positive staining for CD16-PE was observed, indicating that all PMNs were removed by washing the monolayers. (F) Nonstimulated GMVECs did not bind any VT-FITC after incubation with VT-FITC–loaded PMNs. Panels B, D, E, G, and H: TNF-α–stimulated GMVECs retained 2.8%-3.6% of PMNs (present in the gated area P in panel B) after 4 hours of incubation and washing of the monolayers. (D, E) PMNs were distinguished from GMVEC using CD16-PE; panel D represents all cells, whereas panel E reflects the gated area. (G) TNF-α–treated GMVECs incubated with VT-FITC-loaded PMNs were able to bind 30%-50% of VT-FITC after the incubation period of 4 hours (all cells minus the gated area P). (H) PMNs showed no positive staining for VT-FITC, indicating that ligand passing of VT-FITC from PMNs to GMVECs had occurred.
The transfer of VT-1-FITC bound to PMNs to human GMVECs was studied by flow cytometry.
Panels A, C, and F: FACS analysis of nonstimulated GMVECs incubated for 4 hours with PMNs loaded with VT-FITC on ice. (A) Forward scatter and side scatter of nonstimulated GMVECs are shown. (C) No positive staining for CD16-PE was observed, indicating that all PMNs were removed by washing the monolayers. (F) Nonstimulated GMVECs did not bind any VT-FITC after incubation with VT-FITC–loaded PMNs. Panels B, D, E, G, and H: TNF-α–stimulated GMVECs retained 2.8%-3.6% of PMNs (present in the gated area P in panel B) after 4 hours of incubation and washing of the monolayers. (D, E) PMNs were distinguished from GMVEC using CD16-PE; panel D represents all cells, whereas panel E reflects the gated area. (G) TNF-α–treated GMVECs incubated with VT-FITC-loaded PMNs were able to bind 30%-50% of VT-FITC after the incubation period of 4 hours (all cells minus the gated area P). (H) PMNs showed no positive staining for VT-FITC, indicating that ligand passing of VT-FITC from PMNs to GMVECs had occurred.
GMVEC damage by ligand passing of VT from PMNs
Similar experiments were performed at 37°C. Unlabeled VT-1 bound to PMNs was used to study whether the transfer of VT-1 from PMNs to GMVECs caused a biological effect. In TNF-α–stimulated GMVECs, both VT-binding PMNs and VT alone caused severe cytotoxity during a 24-hour period, whereas PMNs alone had no effect (Figure6). The exposure of nonstimulated GMVECs to VT-binding PMNs also caused considerable cell death (30%-40%), whereas exposure of VT-1 or PMNs alone had no effect. Comparable toxicities were observed when the VT-binding PMNs were washed 3 times and first incubated for 2.5 hours at 37°C (to mimic a circulation time before reaching the target tissue) before they were washed again and transferred to the endothelial cells (data not shown).
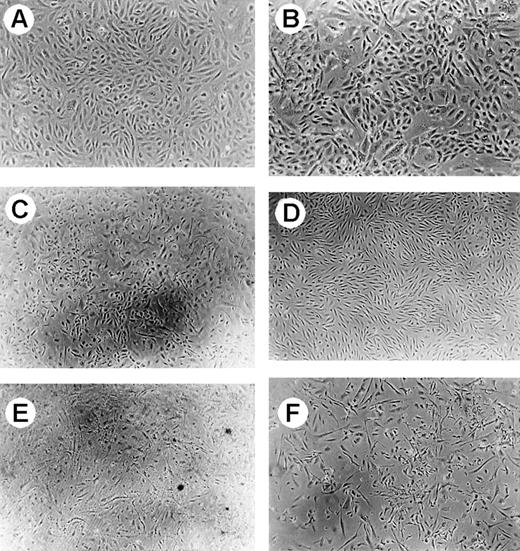
Phase-contrast microscopy of human GMVECs.
Magnification × 50, except for (B) (magnification × 100). (A) Represents control GMVECs. (B) Shows the result of GMVECs incubated with PMNs for 24 hours. No change of morphology was observed. When cells were incubated with PMNs loaded with VT-1, cell death was observed after 24-hour incubation (C). (D) Represents control TNF-α–treated GMVECs. The effect of PMNs loaded with VT-1 was also studied on TNF-α–treated GMVECs (E) and compared with the effect of VT-1 on TNF-α–treated GMVECs (F). In both conditions, equal amounts of cell death were observed.
Phase-contrast microscopy of human GMVECs.
Magnification × 50, except for (B) (magnification × 100). (A) Represents control GMVECs. (B) Shows the result of GMVECs incubated with PMNs for 24 hours. No change of morphology was observed. When cells were incubated with PMNs loaded with VT-1, cell death was observed after 24-hour incubation (C). (D) Represents control TNF-α–treated GMVECs. The effect of PMNs loaded with VT-1 was also studied on TNF-α–treated GMVECs (E) and compared with the effect of VT-1 on TNF-α–treated GMVECs (F). In both conditions, equal amounts of cell death were observed.
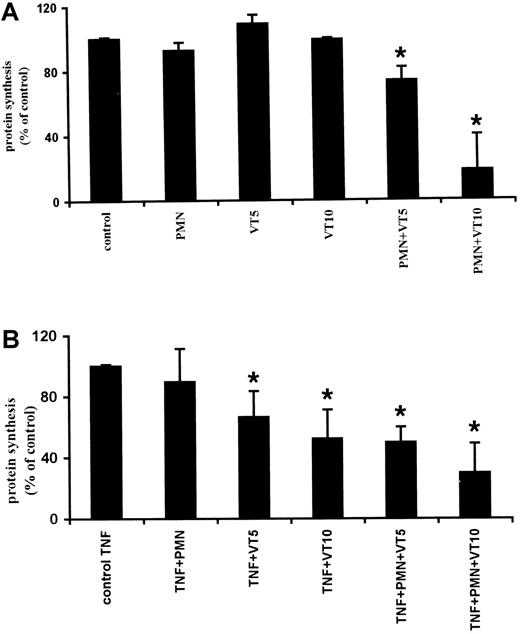
Because VT exerts its cytotoxic effects by inhibiting protein synthesis, we quantified the effect of VT-preloaded PMNs on the viability of GMVECs by determining the overall protein synthesis (from the incorporation of 3H-leucine in newly synthesized proteins). No effect on protein synthesis was observed when stimulated or nonstimulated GMVECs were incubated with PMNs. However, when nonstimulated and TNF-α–stimulated GMVECs were incubated with VT-preloaded PMNs, inhibition of protein synthesis (30% ± 15% and 60% ± 25%, respectively) was observed (Figure7).
Measurement of protein synthesis by incorporation of3H-leucine in newly synthesized proteins.
(A) Nonstimulated GMVECs incubated with VT-1 loaded PMNs showed during 24-hour incubation a reduction of protein synthesis. (B) GMVECs prestimulated for 24 hours with TNF-α (10 ng/mL) showed strong inhibition during incubation with VT-1 alone or after incubation with VT-1 preloaded PMNs. Similar results were obtained from 5 different donors for GMVECs. Results are expressed as ± SEM. Statistical analysis was performed, using unpaired Studentt test. P values smaller than .05 were considered to be significant. * P < 0.05 as compared to nonstimulated GMVECs (A) and TNF-α–prestimulated GMVECs (B).
Measurement of protein synthesis by incorporation of3H-leucine in newly synthesized proteins.
(A) Nonstimulated GMVECs incubated with VT-1 loaded PMNs showed during 24-hour incubation a reduction of protein synthesis. (B) GMVECs prestimulated for 24 hours with TNF-α (10 ng/mL) showed strong inhibition during incubation with VT-1 alone or after incubation with VT-1 preloaded PMNs. Similar results were obtained from 5 different donors for GMVECs. Results are expressed as ± SEM. Statistical analysis was performed, using unpaired Studentt test. P values smaller than .05 were considered to be significant. * P < 0.05 as compared to nonstimulated GMVECs (A) and TNF-α–prestimulated GMVECs (B).
Discussion
In this study, we demonstrate that VT-1 binds almost exclusively to nonstimulated PMNs in whole blood. Only 20 minutes of incubation was sufficient to bind VT-1 to PMNs for more than 90%. No binding to other components of blood was observed, not even when incubation time was prolonged to 3 hours. The Kd of the high-affinity receptor for VT on PMNs is 100-fold less than that found for the functional receptor for VT, Gb3, on GMVECs. In line with this difference in affinity of VT, we found transfer of VT from PMNs to GMVECs and subsequent inhibition of protein synthesis and cell death.
Several suggestions have been made to explain the transfer of VT from the intestine to the kidney. Bizan et al18 described that erythrocytes could bind VT, depending on their P blood group phenotype. Robson et al19 as well as Taylor et al20suggested that there was an association between the outcome of the (D+) HUS and P blood group. However, other investigators could not demonstrate a protective effect of the P1 blood group in HUS. We also could not find VT binding to erythrocytes in whole blood of the 2 donors with P1 blood group in our study. However, we cannot exclude yet that local variations exist in P1 blood group-binding proteins causing VT-binding in some people. Cooling et al22 indicated that, in particular, small and older platelets, obtained by apheresis, contain small amounts of Gb3 and can bind VT. In a whole blood environment, we did not observe any significant binding of 125I-VT-1 and VT-FITC to platelets (8 subjects studied). Therefore, the relative contribution of platelets to the transport of VT in plasma appears insignificant as compared with PMNs. Lingwood23,24 suggested that lipoproteins might be responsible for transporting VT. However, in the present study, we could not find binding of VT to human LDL, VLDL, or HDL, thus excluding this possibility. In line with our previous observation that purified human monocytes only bind VT significantly after activation by LPS,21 we found no VT binding to monocytes in whole blood. On the contrary, VT binding occurred exclusively to PMNs, independent of blood groups or age of the donor. These data lead to the conclusion that PMNs are a good candidate for transporting VT in the systemic circulation to target organs in adults as well as in children.
The rapid binding of VT to PMNs can explain why VT is usually not detectable in blood plasma.31 After binding the VT, PMNs are able to transport the VT to target organs and transfer the VT to endothelial cells in vitro. Stimulating the GMVECs with inflammatory mediators, such as TNF-α or LPS, induces the VT receptor Gb3 on the cell surface.26 32 As expected, transfer of VT-1 from PMNs to endothelium increased when GMVECs were previously stimulated with TNF-α. We demonstrate in this study that one type of binding site is involved in VT-1 binding on PMNs and that this binding site is different from the Gb3 receptor found on endothelial cells. It is uncertain whether the VT receptor on PMNs represents a glycolipid, because VT-1 binding to PMNs was highly sensitive to trypsin treatment. In contrast to the classical VT receptor, Gb3, the VT receptor found on PMNs does not result in VT internalization. The lowerKd for the receptor on PMNs than for Gb3 allows transfer of VT from PMNs to endothelial cells. Within 24 hours, this transfer was accompanied by biological effects, especially inhibition of protein synthesis and cell death. Interestingly, we observed that such biological effects not only affected cells that were prestimulated with TNF-α but also in nonstimulated GMVECs that were incubated with VT-binding PMNs. These data indicate that PMNs not only can bind VT in the circulation but also can transfer it to target cells that express the VT-receptor Gb3.
That PMNs may play a seminal role in pathogenesis of HUS has been suggested many times. First, the number of PMNs is elevated in HUS. It has been suggested that the number of PMNs is a predictive factor for the outcome of the disease.33-35 In addition, Taylor and colleagues36,37 showed that there was an increased number of PMNs in autopsy material of diarrhea-associated patients with HUS. Furthermore, Fitzpatrick et al38,39 described that PMNs of HUS patients were activated and that, in HUS patients, levels of elastase and IL-8 were elevated. These results were confirmed by other investigators,40 and they suggested that PMNs may damage the endothelium through release of the intracellular components, such as elastase.38,39,41 Finally, Morigi et al42 described that VT-1 increased PMNs adhesion to the endothelium under flow conditions by up-regulating adhesive proteins. The binding of PMNs to the endothelium was reduced by blocking of E-selectin, ICAM-1, and VCAM-1 with respective antibodies. The adhesion of PMNs to the endothelium was enhanced by pre-exposure of the endothelial cells by TNF-α. These investigators found that VT-1 was able to inhibit the process of rolling that normally precedes adhesion of cells to the endothelium. In line with these findings is that PMNs of HUS patients adhere more avidly to endothelial cells than PMNs of healthy control subjects.41,43 All these results together indicate that PMNs play an important role in pathogenesis of HUS. Our data show a new and crucial aspect of the involvement of PMNs in (D+) HUS, namely the specific binding and transport of VT to PMNs in whole blood. Additional studies are needed to evaluate whether the binding of VT to PMNs causes a metabolic effect in PMNs themselves. In this respect, it is of interest to note that, in a recent study,44 the induction of superoxide production in PMNs by Shiga toxin-1 was described.
In conclusion, our study demonstrates an additional role of PMNs in the pathogenesis of HUS and strongly suggests that PMNs are the cells that transport the VT from intestine to endothelium. This transport is facilitated by a receptor that has a 100-fold lower affinity than the high-affinity receptor (Gb3) that is expressed on GMVECs after exposure to TNF-α. PMNs loaded with VT display a direct cytotoxic effect to the endothelium of the kidney in vitro by inhibition of protein synthesis. The occurrence of VT-1 binding to PMNs in vivo and ligand passing of VT and PMNs has to be demonstrated in future studies. We believe that this observation is important in understanding the pathogenesis of (D+) HUS and opens perspectives for future treatment.
Supported by grant 97.1645 from the Dutch Kidney Foundation.
Reprints:Victor W. M. van Hinsbergh, Gaubius Laboratory, TNO-PG, Zer-nikedreef 9, PO Box 2215, 2301 CE Leiden, The Netherlands; e-mail: VWM.vanHinsbergh@pg.tno.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal