Abstract
Patients with Ph+ chronic myelogenous leukemia who relapse after a first allogeneic stem cell transplant still have a possibility of long-term survival. To assess the value of the individual therapeutic options, the factors predicting outcome should be identified. We investigated data from 500 patients who relapsed before July 1996; follow-up was updated during 1998. The actuarial survival from relapse was 34.2% (95% confidence interval [CI]: 29.9%-38.5%) at 5 years and 23.4% (95% CI: 18.9%-27.9%) at 10 years. Survival after relapse was significantly related to 5 factors: time from diagnosis to transplant (< 2 years vs ≥ 2 years), disease phase at transplant (first chronic phase vs other), disease stage at relapse (cytogenetic or chronic phase vs advanced phase), time from transplant to relapse (< 1 year vs ≥ 1 year), and donor type (HLA-identical sibling vs volunteer unrelated donor). The effects of individual adverse risk factors were cumulative: The probability of survival at 10 years decreased stepwise from 42% (0 factors), 32% (1 factor), 14% (2 factors), 3% (3 factors), to 0% (4 or 5 factors). Novel strategies for high-risk patients are warranted. We conclude that these 5 factors should be taken into account when comparing results of salvage therapies in patients with Ph+ chronic myeloid leukemia relapsing after allogeneic stem cell transplant.
Allogeneic blood or marrow stem cells transplantation (SCT) from an HLA-identical sibling is the treatment of choice for younger patients with chronic myeloid leukemia (CML). With the use of standard conditioning regimens and graft-versus-host disease (GvHD) prophylaxis, more than 50% of patients are alive and well with no sign of disease more than 10 years from transplant.1Transplant-related mortality and relapse remain the major obstacles to success. Relapse occurs in about 20% of patients transplanted in first chronic phase (CP) with unmanipulated marrow cells2,3; the risk increases to more than 50% for patients transplanted at a later stage of the disease or those transplanted in first CP with a T-cell depleted marrow.1-8
Not all patients who relapse will die as a consequence of disease recurrence. Immune modulation to achieve a graft-versus-leukemia effect, standard therapy for CML, or second allogeneic SCT have all been used with variable degrees of success. Thus, some patients may regain complete remission of the disease following withdrawal of immunosuppression,9,10 donor lymphocyte infusion (DLI),11 treatment with α-interferon (α-IFN),12,13 or a second allogeneic SCT.14-16 Features of both the patient and the disease influence the efficacy of these salvage treatments. In a previous retrospective study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT), based mostly on patients who relapsed before 1990, survival after relapse was significantly affected by the disease phase at relapse, time from SCT to relapse, and patient gender.17 In addition, treatment with α-IFN was associated with better survival.17 During the 1990s, durable restoration of a graft-versus-leukemia effect by DLI was documented and DLI has become the treatment of choice for early relapse.11,18-29 The availability of such an effective strategy and the ability to monitor patients for molecular evidence of relapse has also prompted a reappraisal of T-cell depletion as the prophylactic measure for GvHD.30 Because of such changes in the approach to relapse, we wished to reevaluate the prognostic factors after relapse, not only by updating the 130 patients included in our previous study,17 but also by including 370 new cases primarily relapsing after 1989. These data have identified a number of prognostic factors and allowed us to generate different risk groups for outcome after relapse from an allogeneic SCT for CML.
Patients and methods
Study design
As of June 1996, 500 Ph+ CML patients in the EBMT registry, who had relapsed after an allogeneic SCT from an HLA-identical sibling donor or from an HLA-matched unrelated donor, were identified. During 1998, additional specific data on disease features at relapse, treatments after relapse, disease progression, and survival were retrospectively collected from the individual transplant teams. The monitoring practices after transplant were not specifically reported for each patient, but the participating centers aimed to perform a cytogenetic evaluation every 3-4 months during the first 2 years and at least once a year later. As of December 1998, the available data were collected and analyzed. These data include all the patients reported in the previous EBMT study,17 which analyzed 130 relapses observed as of January 1992 in 17 EBMT centers, together with 120 more recent patients from the same 17 EBMT centers, and 250 patients reported by 62 other contributing EBMT centers. Sixty-nine percent of our patients received transplants before 1990, 30% in the period 1990-1993, and 1% in 1994.
Definition of relapse
Relapse was defined as the appearance, after engraftment with full donor chimerism, either of Ph+ metaphases on cytogenetics performed on bone marrow cells or of hematological signs of the disease that were subsequently confirmed by cytogenetics. Relapse was categorized as cytogenetic relapse when no hematological and clinical signs of the disease appeared within 30 days from the first demonstration of Ph+ metaphases. Relapse was categorized as hematological relapse if the hematological or clinical signs preceded cytogenetic reappearance of Ph+ metaphases or were observed within 30 days from cytogenetic analysis. The phase of the disease at time of the first SCT and at time of hematological relapse was defined according to the criteria of the International Blood and Marrow Transplantation Registry.4
Treatment categories
Four major treatment options were encountered in this retrospective analysis: chemotherapy, α-IFN, DLI, and second transplant. Chemotherapy includes both single-agent chemotherapy and intensive multidrug therapy. The doses and schedules for chemotherapy, α-IFN, and DLI were not specified. A second transplant was defined as the reinfusion of donor cells following a myeloablative conditioning regimen. Any reinfusion of donor cells without myeloablation was defined as DLI. Because the sequence of treatments as well as the response to each specific treatment could not be assessed retrospectively, the cumulative therapy received from the first diagnosis of relapse to the last follow-up was utilized to distinguish groups of similarly treated patients. Because molecular evidence of relapse was not a criterion for eligibility for this study, no patient, for whom salvage therapy was available, was treated before the first evidence of relapse at either cytogenetic or hematological level.
Prognostic factors
The following features thought to be potential prognostic factors for survival were included in the analysis: interval from diagnosis to first transplant (< 1 year vs ≥ 1 year; < 2 years vs ≥ 2 years); disease phase at transplant (first CP vs more advanced phases [APs]); donor type (HLA-identical sibling vs volunteer unrelated); patient gender, recipient/donor gender combination (male/female vs others); T-cell depletion vs T-cell replete, acute-GvHD before relapse (no vs yes; grade 0-I vs grade ≥ II), chronic-GvHD before relapse (no vs yes); patient age at relapse (< 35 years vs ≥ 35 years); interval from transplant to relapse (< 1 year vs ≥ 1 year); disease phase at relapse (cytogenetic relapse vs hematological relapse in CP vs hematological relapse in AP); and date of relapse (< January 1, 1990, vs ≥ January 1, 1990).
Statistical analysis
The association between variables was analyzed by the chi-square test, with the appropriate degrees of freedom. All tests were 2-sided, and to adjust for multiple comparisons P ≤ .001 was considered to indicate statistical significance. Survival was calculated from date of first cytogenetic or hematological evidence of relapse to death or to last follow-up. Actuarial curves were computed according to the Kaplan-Meier method and compared by the 2-sided log rank test. A Cox proportional-hazards model was constructed to detect independent predictors of death.31 As since 1990, therapy with DLI has become available for patients relapsing after allogeneic transplantation, the date of relapse (< January 1, 1990 vs ≥ January 1, 1990) was included in the analysis of prognostic factors together with factors having a P < .05 in the univariate analysis.
Results
Patient characteristics and pattern of relapse
The majority (59%) of patients had been transplanted in first CP from an HLA-identical sibling donor (46% of these were transplanted within 1 year from diagnosis, 27% in the second year, and 27% thereafter). However, relapses after volunteer unrelated donor (VUD) transplants and transplants performed in more APs were also included. Frequently patients had features known to be associated with a higher risk of relapse after allogeneic transplant for CML (ie, T-cell depletion, 46%; grade 0-I acute GvHD, 74%; no chronic GvHD, 55%). About half (51%) of all relapses occurred during the first year posttransplant; only a few (< 5%) were observed beyond 5 years from transplant. A significant proportion (43%) of the relapses occurred in the 1990s, and these patients were more frequently treated with DLI than those relapsing before 1990 (31% vs 10%, P < .001). By contrast, we observed a reduction in the number of patients treated by second transplant before and after 1990 (26% vs 10%,P < .001) (Table 1).
Patient characteristics and pattern of relapse
| Patient characteristics . | Total (%) n = 500 . | Pattern of relapse . | χ2P value . | |||
|---|---|---|---|---|---|---|
| CYT (%) n = 187 . | HCP (%) n = 121 . | HAP (%) n = 136 . | UNK (%) N = 56 . | |||
| Donor type | ||||||
| SIB | 93 | 95 | 94 | 93 | 88 | >.10 |
| VUD | 7 | 5 | 6 | 7 | 12 | |
| Interval from Dx to SCT | ||||||
| <2 years | 69 | 74 | 67 | 65 | 77 | >.10 |
| ≥2 years | 31 | 26 | 33 | 35 | 23 | |
| Disease phase at SCT | ||||||
| 1st CP | 63 | 77 | 79 | 33 | 55 | <.001 |
| >1st CP | 37 | 23 | 21 | 67 | 43 | |
| Patient sex | ||||||
| Male | 59 | 56 | 59 | 65 | 60 | >.10 |
| Female | 41 | 44 | 41 | 35 | 40 | |
| R/D gender combination | ||||||
| Male/female | 20 | 22 | 16 | 21 | 16 | >.10 |
| Other | 80 | 79 | 84 | 79 | 74 | |
| T-cell–depleted SCT | ||||||
| Yes | 46 | 62 | 50 | 29 | 21 | <.001 |
| No | 41 | 34 | 31 | 55 | 54 | |
| NA | 13 | 4 | 19 | 16 | 25 | |
| Acute GvHD* | ||||||
| No | 47 | 52 | 51 | 41 | 41 | >.10 |
| Yes | 49 | 47 | 46 | 57 | 32 | |
| NA | 4 | 1 | 3 | 2 | 27 | |
| Grade of acute GvHD* | ||||||
| 0 | 47 | 52 | 51 | 41 | 32 | >.10 |
| I | 28 | 26 | 27 | 30 | 29 | |
| ≥II | 21 | 21 | 19 | 27 | 12 | |
| Chronic GvHD* | ||||||
| No | 63 | 64 | 67 | 63 | 54 | >.10 |
| Yes | 28 | 33 | 27 | 27 | 19 | |
| NA | 9 | 3 | 6 | 10 | 27 | |
| Patient age at relapse | ||||||
| <35 yr | 45 | 42 | 36 | 50 | 57 | >.10 |
| ≥35 yr | 55 | 58 | 64 | 50 | 43 | |
| Interval from SCT to relapse | ||||||
| <1 yr | 51 | 54 | 30 | 63 | 55 | <.001 |
| ≥1 yr | 49 | 46 | 70 | 37 | 45 | |
| Date of relapse | ||||||
| <1/1/90 | 57 | 65 | 47 | 57 | 46 | >.10 |
| ≥1/1/90 | 43 | 35 | 53 | 43 | 54 | |
| Treatment after relapse | ||||||
| CHT alone | 17 | 6 | 19 | 36 | 2 | |
| α-IFN ± CHT | 14 | 21 | 14 | 10 | 2 | |
| DLI ± other | 20 | 25 | 33 | 9 | 0 | |
| SCT ± α-IFN ± CHT | 19 | 21 | 25 | 15 | 5 | |
| Other | 2 | 4 | 2 | 2 | 0 | |
| No therapy | 12 | 15 | 4 | 18 | 4 | |
| NA | 16 | 8 | 3 | 10 | 87 | |
| Patient characteristics . | Total (%) n = 500 . | Pattern of relapse . | χ2P value . | |||
|---|---|---|---|---|---|---|
| CYT (%) n = 187 . | HCP (%) n = 121 . | HAP (%) n = 136 . | UNK (%) N = 56 . | |||
| Donor type | ||||||
| SIB | 93 | 95 | 94 | 93 | 88 | >.10 |
| VUD | 7 | 5 | 6 | 7 | 12 | |
| Interval from Dx to SCT | ||||||
| <2 years | 69 | 74 | 67 | 65 | 77 | >.10 |
| ≥2 years | 31 | 26 | 33 | 35 | 23 | |
| Disease phase at SCT | ||||||
| 1st CP | 63 | 77 | 79 | 33 | 55 | <.001 |
| >1st CP | 37 | 23 | 21 | 67 | 43 | |
| Patient sex | ||||||
| Male | 59 | 56 | 59 | 65 | 60 | >.10 |
| Female | 41 | 44 | 41 | 35 | 40 | |
| R/D gender combination | ||||||
| Male/female | 20 | 22 | 16 | 21 | 16 | >.10 |
| Other | 80 | 79 | 84 | 79 | 74 | |
| T-cell–depleted SCT | ||||||
| Yes | 46 | 62 | 50 | 29 | 21 | <.001 |
| No | 41 | 34 | 31 | 55 | 54 | |
| NA | 13 | 4 | 19 | 16 | 25 | |
| Acute GvHD* | ||||||
| No | 47 | 52 | 51 | 41 | 41 | >.10 |
| Yes | 49 | 47 | 46 | 57 | 32 | |
| NA | 4 | 1 | 3 | 2 | 27 | |
| Grade of acute GvHD* | ||||||
| 0 | 47 | 52 | 51 | 41 | 32 | >.10 |
| I | 28 | 26 | 27 | 30 | 29 | |
| ≥II | 21 | 21 | 19 | 27 | 12 | |
| Chronic GvHD* | ||||||
| No | 63 | 64 | 67 | 63 | 54 | >.10 |
| Yes | 28 | 33 | 27 | 27 | 19 | |
| NA | 9 | 3 | 6 | 10 | 27 | |
| Patient age at relapse | ||||||
| <35 yr | 45 | 42 | 36 | 50 | 57 | >.10 |
| ≥35 yr | 55 | 58 | 64 | 50 | 43 | |
| Interval from SCT to relapse | ||||||
| <1 yr | 51 | 54 | 30 | 63 | 55 | <.001 |
| ≥1 yr | 49 | 46 | 70 | 37 | 45 | |
| Date of relapse | ||||||
| <1/1/90 | 57 | 65 | 47 | 57 | 46 | >.10 |
| ≥1/1/90 | 43 | 35 | 53 | 43 | 54 | |
| Treatment after relapse | ||||||
| CHT alone | 17 | 6 | 19 | 36 | 2 | |
| α-IFN ± CHT | 14 | 21 | 14 | 10 | 2 | |
| DLI ± other | 20 | 25 | 33 | 9 | 0 | |
| SCT ± α-IFN ± CHT | 19 | 21 | 25 | 15 | 5 | |
| Other | 2 | 4 | 2 | 2 | 0 | |
| No therapy | 12 | 15 | 4 | 18 | 4 | |
| NA | 16 | 8 | 3 | 10 | 87 | |
CYT indicates cytogenetic relapse; HCP, hematologic relapse in chronic phase; HAP, hematologic relapse in advanced phase (ie, accelerated phase or blastic phase); UNK, unknown; Dx, initial diagnosis; SIB, HLA-identical sibling; VUD, volunteer unrelated donor; SCT, stem cell transplantation; CP, chronic phase; R/D, recipient/donor; NA, not applicable or unavailable; GvHD, graft-versus-host disease; CHT, chemotherapy; α-IFN, α-interferon; DLI, donor lymphocyte infusion.
Before the date of relapse.
The disease stage at relapse was available in 444 patients: 42% had a cytogenetic relapse, 27% had a hematological relapse in CP, and 31% had a hematological relapse in AP. Hematological relapse in AP occurred less frequently in patients transplanted in first CP of the disease (P < .001), in those who relapsed more than 1 year after transplant (P = .002), and in patients who received a T-cell– depleted transplant (P < .001). Hematological relapse in AP occurred within 6 months from SCT in 41% of the cases, from 6 to 12 months in 22%, from 12 to 24 months in 18%, and later than 24 months from SCT in 19%. The correlation of T-cell depletion with a relapse in AP was more marked for the 255 patients transplanted in first CP (P < .001) than for the 138 patients transplanted in more APs (P = .11). There was no significant correlation of disease stage at relapse with previous acute and/or chronic GvHD.
The majority (72%) of patients were treated after relapse. Chemotherapy alone was given in 85 patients: 11 cytogenetic relapses, 23 relapses in CP, 49 in AP, and 2 with unknown patterns of relapse. Seventy-one patients received α-IFN with or without chemotherapy: 39 cytogenetic, 17 CP, 14 AP, and 1 with an unknown pattern of relapse. Ninety-nine patients received DLI with or without other treatments (including 11 patients treated with DLI followed by a second transplant): 47 cytogenetic relapses, 40 relapses in CP, and 12 in AP. Second transplant not preceded by DLI was given in 41 patients with cytogenetic relapse, 30 with hematologic relapse in CP, and 21 in AP. Other treatments were applied in < 1% of the cases. In 12% of patients, including 27 cytogenetic relapses, 5 relapses in CP, and 13 in AP, no treatment was given after relapse. The treatment modality was unknown in 80 patients, and in 48 of them, the disease stage at time of relapse was also unknown.
Overall outcome
Sixty-four percent of patients with cytogenetic relapses progressed to hematological relapse at a median of 8 months (range 1-97), and 50% of hematological relapses in CP progressed to a more AP (accelerated or blastic) at a median of 12 months (range 2-65). Overall, 347 patients have died, mostly with progressive disease, and 153 are alive at a median follow-up of 6.7 years from relapse (range 0.2-11.8 years). The actuarial survival from relapse is 34.2% (95% confidence interval [CI]: 29.9%-38.5%) at 5 years and 23.4% (95% CI: 18.9%-27.9%) at 10 years.
The cytogenetic status of the disease at the last follow-up was available in 444 cases. The number of patients in cytogenetic remission at the last follow-up among survivors in the various treatment groups was as follows: 1 of 4 patients treated with chemotherapy alone, 11 of 17 patients treated with α-IFN with or without chemotherapy, 45 of 49 patients treated with DLI with or without other treatments, and 30 of 32 patients treated with a second transplant without previous DLI. Some patients died while in cytogenetic remission: 16 were treated with DLI with or without other treatments and 28 were treated with a second transplant without previous DLI.
Factors associated with survival after relapse
Five factors (ie, donor type, interval from diagnosis to transplant, disease phase at transplant, interval from transplant to relapse, and disease stage at relapse) were significantly correlated to survival from relapse (Table 2). Relapses after a transplant from a VUD have a worse survival compared with those occurring after transplant from an HLA-identical sibling donor (Figure1A). Survival after relapse is better in patients transplanted within 2 years from diagnosis than in patients transplanted later (Figure 1B). Patients who relapsed after transplantation in first CP did better than those who were transplanted in a more AP of the disease (Figure 1C). The outcome of patients who relapsed early after transplant was poorer than those who relapsed more than 1 year after transplant (Figure 1D). Survival after relapse was particularly poor in patients developing a sudden hematological relapse in AP (Figure 1E). Survival after hematological relapse in CP (median, 4.7 years; 49% alive at 5 years) was not statistically different from that after cytogenetic relapse (median, 5.3 years; 51% alive at 5 years). Therefore, these 2 categories of disease stage at relapse were combined for the multivariate analysis.
Features of 500 patients and survival from relapse
| Features . | Total no. . | Survival from relapse . | Log-rank Pvalue . | |
|---|---|---|---|---|
| Median years . | At 5 y (%) . | |||
| Interval from Dx to SCT | ||||
| <2 yr | 345 | 2.7 | 40 | =.001 |
| ≥2 yr | 155 | 1.7 | 23 | |
| Disease phase at SCT | ||||
| 1st CP | 315 | 4.3 | 46 | <.001 |
| >1st CP | 184 | 0.4 | 15 | |
| Donor type | ||||
| SIB | 466 | 2.4 | 36 | <.001 |
| VUD | 34 | 0.4 | 14 | |
| Patient sex | ||||
| Male | 297 | 2.1 | 32 | >.10 |
| Female | 201 | 2.3 | 37 | |
| R/D gender combination | ||||
| Male/female | 98 | 2.5 | 37 | >.10 |
| Other | 391 | 2.1 | 34 | |
| T-cell depleted SCT | ||||
| Yes | 228 | 2.7 | 37 | >.10 |
| No | 207 | 1.5 | 36 | |
| Acute GvHD* | ||||
| No | 233 | 2.5 | 36 | >.10 |
| Yes | 246 | 1.7 | 36 | |
| Grade of acute GvHD* | ||||
| 0 | 233 | 2.5 | 35 | >.10 |
| I | 139 | 2.0 | 34 | |
| ≥II | 107 | 1.5 | 38 | |
| Chronic GvHD* | ||||
| No | 277 | 2.9 | 39 | >.10 |
| Yes | 138 | 2.7 | 42 | |
| Patient age at relapse | ||||
| <35 yr | 223 | 1.7 | 35 | >.10 |
| ≥35 yr | 277 | 2.1 | 34 | |
| Interval from SCT to relapse | ||||
| <1 yr | 254 | 0.6 | 23 | <.001 |
| ≥1 yr | 246 | 4.3 | 57 | |
| Disease stage at relapse | ||||
| CYT or HCP | 308 | 4.4 | 47 | <.001 |
| HAP | 136 | 0.4 | 7 | |
| Date of relapse | ||||
| <1/1/90 | 283 | 2.5 | 35 | >.10 |
| ≥1/1/90 | 217 | 1.7 | 34 | |
| Total | 500 | 2.1 | 34 | |
| Features . | Total no. . | Survival from relapse . | Log-rank Pvalue . | |
|---|---|---|---|---|
| Median years . | At 5 y (%) . | |||
| Interval from Dx to SCT | ||||
| <2 yr | 345 | 2.7 | 40 | =.001 |
| ≥2 yr | 155 | 1.7 | 23 | |
| Disease phase at SCT | ||||
| 1st CP | 315 | 4.3 | 46 | <.001 |
| >1st CP | 184 | 0.4 | 15 | |
| Donor type | ||||
| SIB | 466 | 2.4 | 36 | <.001 |
| VUD | 34 | 0.4 | 14 | |
| Patient sex | ||||
| Male | 297 | 2.1 | 32 | >.10 |
| Female | 201 | 2.3 | 37 | |
| R/D gender combination | ||||
| Male/female | 98 | 2.5 | 37 | >.10 |
| Other | 391 | 2.1 | 34 | |
| T-cell depleted SCT | ||||
| Yes | 228 | 2.7 | 37 | >.10 |
| No | 207 | 1.5 | 36 | |
| Acute GvHD* | ||||
| No | 233 | 2.5 | 36 | >.10 |
| Yes | 246 | 1.7 | 36 | |
| Grade of acute GvHD* | ||||
| 0 | 233 | 2.5 | 35 | >.10 |
| I | 139 | 2.0 | 34 | |
| ≥II | 107 | 1.5 | 38 | |
| Chronic GvHD* | ||||
| No | 277 | 2.9 | 39 | >.10 |
| Yes | 138 | 2.7 | 42 | |
| Patient age at relapse | ||||
| <35 yr | 223 | 1.7 | 35 | >.10 |
| ≥35 yr | 277 | 2.1 | 34 | |
| Interval from SCT to relapse | ||||
| <1 yr | 254 | 0.6 | 23 | <.001 |
| ≥1 yr | 246 | 4.3 | 57 | |
| Disease stage at relapse | ||||
| CYT or HCP | 308 | 4.4 | 47 | <.001 |
| HAP | 136 | 0.4 | 7 | |
| Date of relapse | ||||
| <1/1/90 | 283 | 2.5 | 35 | >.10 |
| ≥1/1/90 | 217 | 1.7 | 34 | |
| Total | 500 | 2.1 | 34 | |
Dx indicates initial diagnosis; SCT, stem cell transplantation; CP, chronic phase; SIB, HLA-identical sibling; VUD, volunteer unrelated donor; R/D, recipient/donor; GvHD, graft-versus-host disease; CYT, cytogenetic relapse; HCP, hematologic relapse in CP; HAP, hematologic relapse in advanced phase (ie, accelerated phase or blastic phase).
Before the date of relapse.
Actuarial survival from relapse according to prognostic features.
(A) Donor type (SIB, HLA-identical sibling; VUD, volunteer unrelated donor); (B) interval from diagnosis to transplant; (C) disease phase at transplant (CP, chronic phase); (D) time to relapse; (E) disease stage at relapse (CYT, cytogenetic relapse; HCP, hematologic relapse in chronic phase; HAP, hematologic relapse in advanced phase [ie, accelerated phase or blastic phase]).
Actuarial survival from relapse according to prognostic features.
(A) Donor type (SIB, HLA-identical sibling; VUD, volunteer unrelated donor); (B) interval from diagnosis to transplant; (C) disease phase at transplant (CP, chronic phase); (D) time to relapse; (E) disease stage at relapse (CYT, cytogenetic relapse; HCP, hematologic relapse in chronic phase; HAP, hematologic relapse in advanced phase [ie, accelerated phase or blastic phase]).
The following features did not appear to influence survival after relapse: patient age, patient gender, recipient/donor gender combination, T-cell depletion, previous acute and/or chronic GvHD, date of relapse (Table 2). All 5 prognostic factors identified in univariate analysis were assessed in 89% of patients (444 of 500): all of them contributed significantly in a Cox regression analysis (Table3). The number of patients relapsing after a VUD transplant is rather limited in this cohort: 27 of 444. Their prognostic features are worse compared with patients relapsing after transplants from an HLA-identical sibling donor. For this reason, the multivariate analysis, reported in Table 3, was also performed after exclusion of the patients relapsing after a VUD transplant. This analysis showed all 4 remaining variables being significant with similar hazard rates and confidence intervals.
Cox regression analysis of prognostic factors for survival after relapse
| Factor . | RR . | 95% CI . |
|---|---|---|
| Donor type | ||
| HLA-identical sibling | 1 | |
| Volunteer unrelated | 1.58 | 1.07-2.30 |
| Disease phase at SCT | ||
| 1st CP | 1 | |
| >1st CP | 2.03 | 1.60-2.56 |
| Interval from Dx to SCT | ||
| <2 yr | 1 | |
| ≥2 yr | 1.40 | 1.11-1.76 |
| Interval from SCT to relapse | ||
| ≥1 yr | 1 | |
| <1 yr | 2.28 | 1.82-2.87 |
| Disease stage at relapse | ||
| CYT or HCP | 1 | |
| HAP | 3.08 | 2.41-3.88 |
| Factor . | RR . | 95% CI . |
|---|---|---|
| Donor type | ||
| HLA-identical sibling | 1 | |
| Volunteer unrelated | 1.58 | 1.07-2.30 |
| Disease phase at SCT | ||
| 1st CP | 1 | |
| >1st CP | 2.03 | 1.60-2.56 |
| Interval from Dx to SCT | ||
| <2 yr | 1 | |
| ≥2 yr | 1.40 | 1.11-1.76 |
| Interval from SCT to relapse | ||
| ≥1 yr | 1 | |
| <1 yr | 2.28 | 1.82-2.87 |
| Disease stage at relapse | ||
| CYT or HCP | 1 | |
| HAP | 3.08 | 2.41-3.88 |
RR indicates risk ratio; CI, confidence interval; Dx, diagnosis; SCT, stem cell transplantation; CP, chronic phase; CYT, cytogenetic relapse; HCP, hematologic relapse in CP; HAP, hematologic relapse in advanced phase (ie, accelerated phase or blastic phase).
Risk score and treatment
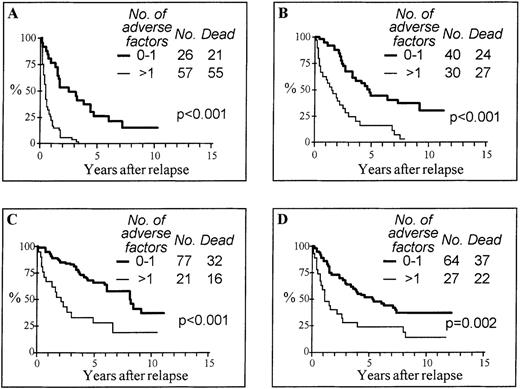
On the basis of the multivariate analysis, 5 risk groups were assessable according to the cumulative number of adverse features: 101 (23%) patients had no adverse feature, 148 (33%) had 1 adverse feature, 85 (19%) had 2, 78 (18%) had 3, and 32 (7%) had more than 3 adverse features (30 with 4 and 2 with 5). The actuarial survival curves in these 5 risk groups are shown in Figure2. This marked difference in survival, depending on risk factors, was observed in all treatment categories (Figure 3). In each treatment group, patients with 0 or 1 adverse feature had a significantly better survival compared with patients with 2 or more adverse factors. In the group treated with DLI with or without other treatments (Figure 3C), the difference in survival between the risk groups is also statistically significant with a P < .001 by censoring 11 patients at the date of second SCT (not shown).
Actuarial survival from relapse according to the cumulative number of adverse features.
Adverse features include interval from diagnosis to transplant 2 years or more, volunteer unrelated donor, transplant not in first chronic phase, relapse within 1 year from transplant, and advanced phase at relapse.
Actuarial survival from relapse according to the cumulative number of adverse features.
Adverse features include interval from diagnosis to transplant 2 years or more, volunteer unrelated donor, transplant not in first chronic phase, relapse within 1 year from transplant, and advanced phase at relapse.
Actuarial survival from relapse according to the cumulative number of adverse features in treatment groups.
Adverse features include interval from diagnosis to transplant 2 years or more, volunteer unrelated donor, transplant not in first chronic phase, relapse within 1 year from transplant, and advanced phase at relapse. (A) Chemotherapy alone; (B) α-interferon ± chemotherapy; (C) donor lymphocyte infusion ± other (11 patients were treated with donor lymphocyte infusion followed by a second SCT); (D) second transplant ± α-interferon ± chemotherapy.
Actuarial survival from relapse according to the cumulative number of adverse features in treatment groups.
Adverse features include interval from diagnosis to transplant 2 years or more, volunteer unrelated donor, transplant not in first chronic phase, relapse within 1 year from transplant, and advanced phase at relapse. (A) Chemotherapy alone; (B) α-interferon ± chemotherapy; (C) donor lymphocyte infusion ± other (11 patients were treated with donor lymphocyte infusion followed by a second SCT); (D) second transplant ± α-interferon ± chemotherapy.
Discussion
Patients with Ph+ CML who relapse after an allogeneic transplant may still achieve a prolonged survival.12,14 27 We have shown, in a large series of patients treated at 79 EBMT centers, that the actuarial curve tends to plateau, indicating that control of the disease and possible cure are a reasonable objective of the currently available salvage treatments for more than 20% of patients. However, survival after relapse is strictly related to 5 risk factors (donor type, interval from diagnosis to SCT, disease phase at SCT, interval from SCT to relapse, and disease stage at relapse), and patients at different risk may be easily identified by the cumulative number of adverse features at relapse. The majority of our patients with a cytogenetic relapse evolved to hematological relapse within 12 months. Moreover, patients with hematological relapse tend to evolve rapidly into a blastic phase that was the main cause of death after relapse in our series. Therefore, factors predictive of survival after relapse also indicate the risk of disease progression to a fatal blastic phase. We acknowledge that the prognostic value of the disease stage at relapse should be interpreted with some caution since this variable could also be related to the methods of disease monitoring after transplant, a factor which could not be evaluated in sufficient detail in such a large, retrospective, multicenter study. However, we expect that cases that could have been detected at an earlier phase of relapse are a minority of patients who experienced late relapse in AP. Despite these limitations, we included the disease stage at relapse in the multivariate analysis, believing that the main and “new” message of our study is that survival after relapse is related to 4 factors: donor type, interval from diagnosis to SCT, disease stage at SCT, and interval from SCT to relapse. These factors retain statistical significance in a multivariate analysis that includes the disease phase at relapse, a factor whose prognostic value was also confirmed by the Cox model. This observation should encourage accurate monitoring of disease after transplant particularly in patients with more risk factors since it is possible that their outcome might improve with salvage therapy given at an earlier stage of the relapse.
In contrast to a previous observation,17 patients with cytogenetic relapse had a similar outcome to those who relapsed with CP disease. However, while in cytogenetic relapses, the survival reported in the present analysis is comparable to that observed in the previous study (51% vs 52% at 5 years, respectively), a better survival has been now reported for patients with hematological relapse in CP (49% vs 30% at 5 years, respectively). Multiple factors must be considered when comparing these 2 retrospective studies. We have shown that the date of relapse correlated with both the use of T-cell depletion and treatment with DLI. In our previous report, 66% of the cases had received T-cell–depleted grafts and none of 130 cases had been treated with DLI.17 In contrast, the present study includes a higher proportion of relapses after an unmanipulated transplant (41%), and 20% of all cases were treated with DLI. The definition of disease stage at relapse was identical in the 2 studies, and the median follow-ups are also comparable. Therefore, the efficacy of therapy with DLI in patients with hematological relapse in CP might account for the improved outcome for this subgroup of patients. This interpretation is further substantiated by the observation that not only is DLI an effective salvage therapy for hematological relapse in CP, but also that its efficacy is greater in patients relapsing without previous signs of GvHD, such as those relapsing after a T-cell–depleted transplant, than in patients with previous GvHD.27 Another new observation of our study is the significant association of the disease phase at relapse with T-cell depletion, particularly in patients transplanted in first CP. This correlation suggests that the increased rate of relapse in T-cell–depleted patients is associated with more relapses in patients with biologically favorable disease that might otherwise have been cured with a T-cell replete transplant.
In our retrospective study we could not determine the sequence and the response to each specific salvage treatment and were, therefore, unable to compare the efficacy of treatments after relapse. However, the great majority of surviving patients treated with either DLI or a second transplant remain in cytogenetic remission, whereas this is the case for only a few patients surviving without such treatments. By contrast, fatalities in cytogenetic remission were observed only among patients treated with either DLI or a second transplant, indicating that these treatments, which are effective in inducing durable cytogenetic remissions, are still accompanied with a significant treatment-related mortality. The question of whether the overall outcome may be improved by the achievement of a remission of the disease at a cytogenetic or molecular level is unanswered from our present study. However, we have observed that the risk of death after relapse may be effectively assessed in each of the 4 major treatment groups comparing patients with 0-1 adverse factors to those with 2 or more factors. Our study suggests that novel treatment strategies should be found to treat patients with 2 or more adverse features at relapse. This group represents more than one third of our cases and < 10% of them will be alive at 10 years. These prognostic factors should be reported in studies of salvage therapy of relapse after an allogeneic transplantation for Ph+ CML.
Lymphocytes from sibling and unrelated donors have been found to be equally effective in treating relapse of CML after allogeneic SCT.32 Our model that includes donor type as a risk factor should be then validated in a group of patients receiving similar treatment. We should also note that our study relies on standard cytogenetics and clinical observation to detect relapse after transplant. Molecular monitoring for disease recurrence is now able to identify patients at high risk of cytogenetic or hematologic relapse.33-37 However, there are no data regarding prognostic factors for survival after relapse in such patients. New risk factors may emerge when patients are treated at an earlier stage of relapse.
Acknowledgments
The authors are extremely grateful to Anja van Biezen and Nelleke Tazelaar for their assistance in collecting and analyzing data and to the following transplant centers who reported patients in this study (person responsible, department, center, city, and state or country; listed by decreasing number of cases in this study): J. Apperley, Dept. of Hematology, Hammersmith Hospital, London, UK-England; A. Bacigalupo, Dept. of Hematology, Ospedale San Martino, Genova, Italy; W. Arcese, Hematology, Univ. “La Sapienza,” Rome, Italy; D. Bunjes, Abt. Innere Medizin III, Universität Ulm, Ulm, Germany; T. deWitte, Div. of Hematology, University Hospital, Nijmegen, The Netherlands; A. Devergie, Dept. of Hematology-BMT, Hôpital St. Louis, Paris, France; A. Gratwohl, Dept. of Hematology, Kantonsspital, Basel, Switzerland; E. Carreras, Dept. of Hematology, Hospital Clinic, Barcelona, Spain; H.-J. Kolb, Med. Klinik III, Klinikum Grosshadern, Munchen, Germany; H. G. Prentice, Dept. of Hematology, Royal Free Hospital, Hampstead, London, UK-England; G. Bandini, Inst. of Hematology, Hospital San Orsola, Bologna, Italy; P. Ljungman, Dept. of Hematology, Huddinge Univ. Hospital, Huddinge, Sweden; J.-Y. Cahn, Service d'Hematologie, Hospital Jean Minjoz, Besancon, France; A. Ferrant, Dept. of Haematology, Clinique Univ. St. Luc, Brussels, Belgium; I. Franklin, Dept. of Medicine, Glasgow Royal Infirmary, Glasgow, UK-Scotland; G. Lucarelli, Dept. of Hematology, Pesaro Hospital, Pesaro, Italy; J. J. Cornelissen, Dept. of Hematology, AZR/DDHK, Rotterdam, The Netherlands; B. Chapuis, Div. d'Hematologie, Hospital Cantonal Univ., Geneva, Switzerland; M. A. Boogaerts, Dept. of Hematology, Univ. Hospital Gasthuisberg, Leuven, Belgium; B. Rio, Service d'Hematologie, Hotel Dieu, Paris, France; C. Cordonnier, Sve d'Hematologie, Hôpital Henri Mondor, Creteil, France; P. J. Gravett, Dept. of Hematology, The London Clinic, London, UK-England; A. Iriondo, Hospital Univ., “Marques de Valdecilla,” Santander, Spain; G. Torlontano, Dept. of Hematology, Ospedale Civile, Pescara, Italy; J. M. Vossen, BMT Centre Leiden, Leiden University Hospital, Leiden, The Netherlands; F. Aversa, Dept. of Hematology, Univ. of Perugia, Perugia, Italy; A. H. Goldstone, Dept. of Hematology, Univ. College L Hospital, London, UK-England; S. McCann, Dept. of Hematology, St. James Hospital Trinity C, Dublin, Ireland; G. Lambertenghi Deliliers, Ospedale Maggiore di Milano, IRCCS, Milano, Italy; J. P. Jouet, Service de Maladies du Sang, Hôpital Claude Huriez, Lille, France; N. Jacobsen, BMT Unit Dept. of Hematology L 4042, Copenhagen, Denmark; L. F. Verdonck, Dept. of Hematology, AZU, Utrecht, The Netherlands; J. J. Sotto, Dept. of Hematology, Hôpital A. Michallon, Grenoble, France; W. Scheinder, Klinik für Hamatologie, H. H. Universität, Dusseldorf, Germany; D. Blaise, Institut Paoli Calmettes, Marseille, France; R. E. Clark, Royal Liverpool University Hospital, Dept. Hematology, Liverpool, UK-England; A. C. Parker, Dept. of Hematology, Western General Hospital, Edinburgh, UK-Scotland; N. Schmitz, BMT Unit/Dept. of Internal Medicine II, C-A. Univ., Kiel, Germany; B. Sallerfors, Dept. of Hematology, University Hospital, Lund, Sweden; I. Majolino, Div. di Ematol. e Unità Trapianti, Ospedale V. Cervello, Palermo, Italy; K. Paloczi, National Institute of Hematology and Immunology, BMT Unit, Budapest, Hungary; Y. Beguin, Dept. of Hematology, University of Liege, Liege, Belgium; J. Gmur, Dept. of Medicine, University Hospital, Zurich, Switzerland; N. C. Gorin, Dept. of Hematology, Hôpital St. Antoine, Paris, France; M. Aglietta, Dept. of Hematology, University Hospital, Torino, Italy; J. Sierra, Clinical Hematology Div, Hospital Santa Creu i Sant Pau, Barcelona, Spain; V. Leblond, Pitie-Salpetriere, Paris, France; D. W. Milligan, Dept. of Hematology, Heartlands Hospital, Birmingham, UK-England; N. Petti, Dept. of Hematology, Ospedale S. Camillo, Rome, Italy; R. Cairoli, Div. di Ematologia, Ospedale di Niguarda, Milano, Italy; B. Hertenstein, Dept. of Hematology/Oncology, Med. School, Hannover, Germany; M. Abecasis, Inst. Portugues Oncologia, BMT Unit, Lisboa, Portugal; J. F. Murray, Dept. of Hematology, Univ. Hospital NHS Trust, Birmingham, UK-England; A. Fassas, Hematology Dept/BMT Unit, George P. General, Exokhi (Thessaloniki), Greece; R. Marcus, Dept. of Hematology, Addenbrookes Hospital, Cambridge, UK-England; R. Schots, Dept. of Med. Oncology/Hematology, Uni H VUB, Brussels, Belgium; L. Feldman, Unidad de Transplante de Medula Osea, Antatida H, Buenos Aires, Argentina; D. Niederwieser, Dept. Internal Medicine, University of Innsbruck, Innsbruck, Austria; D. Bron, Experimental Hematology, Institut Jules Bordet, Brussels, Belgium; R. Powles, Leukemia Myeloma Units, Royal Marsden H., Sutton, UK-England; K. Remes, Turku Univ, Central Hospital, Turku, Finland; H. T. Greinix, AKH Vienna, Klinik fur Innere Medizin I, Vienna, Austria; S. A. Evensen, Dept. of Medicine, Rikshospitalet, Oslo, Norway; P. Bordigoni, Unite de Transpl. Medullaire, Hôpital d'Enfant, Nancy, France; D. Guyotat, Service d'Hematologie Clinique, Hôpital Nord, Saint Etienne, France; J. L. Harousseau, Dept. of Hematology, Hotel Dieu, Nantes, France; S. Slavin, Dept. of BMT, Hadassah Univ Hospital, Jerusalem, Israel; V. Runde, Dept. of BMT, University Hospital, Essen, Germany; J. Reiffers, CHR Bordeaux, Hôpital du Haut Leveque, Pessac, France; G. Dini, Institute G. Gaslini, Genova, Italy; E. P. Alessandrino, Dept. of Hem/BMT Unit, Policlinico San Matteo, Pavia, Italy; K. Ozerkan, Dept. of Hem/BMT Unit, Univ. H. Hacettepe, Ankara, Turkey; A. Bosi, BMT Unit, Dept. of Hematology, Osp. di Careggi, Firenze, Italy; T. Ruutu, Dept. of Medicine, University Central Hospital, Helsinki, Finland; A. M. Will, Royal Manchester Children's Hospital, Pendleburry, UK-England; F. Locatelli, Pediatric Clinic, Univ. of Pavia, IRCCS, Pavia, Italy; H. Koc, Dept. of Hematology/Oncology, Univ. Ibni Sina, Ankara, Turkey; N. Harhalakis, Div. of Hematology, BMT Unit, Evangelismos H, Athens, Greece; and J. Wachowiak, Dept. of Hematology, K. Marcinkowski Univ., Poznan, Poland.
Supported in part by Sezione di Roma della Associazione Italiano contro le Leucemie (ROMAIL).
Reprints:Cesare Guglielmi, Cattedra di Ematologia, Università “La Sapienza,” via Benevento 6, 00161 Roma, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Actuarial survival from relapse according to prognostic features. / (A) Donor type (SIB, HLA-identical sibling; VUD, volunteer unrelated donor); (B) interval from diagnosis to transplant; (C) disease phase at transplant (CP, chronic phase); (D) time to relapse; (E) disease stage at relapse (CYT, cytogenetic relapse; HCP, hematologic relapse in chronic phase; HAP, hematologic relapse in advanced phase [ie, accelerated phase or blastic phase]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3328/6/m_bloo01128001x.jpeg?Expires=1765026686&Signature=VR~yElC7A4Wg8jW3yckpXQIMnJMnqEy~pCg5O5MLYP-2Bmoxb~PZANSNlIRCNFCjrOIV3szK3v6tZMYxV~NlgGkyxF3OYuiK~y~cKT2t~6Ps7hJu~BFDOsFy0GYQZJJu49RyP8zRgMTIApTKkHflczpkbvhQAPWyX9vkQGSYhOKyalY07mlnLGcRPdRUd0h1dLQqp3fO3RgP59vZlnEpbdGCwwvmo-YnkAwYJ~7QkOMjPHWR37YRvjoOSVYRkZ9s3viY3aZle1ZZlhDbQJYeOvtVHPQGxVG34~5-8sLrW6hVQP4o~NLmP0ZIppqZYS4nzJ9esMg2qEPXZZAiJksRog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal