The ability to microinject small, nonadherent blood cells with macromolecules promises to open a number of new avenues of research and of potential therapy. The recent paper by Davis et al1 demonstrates both the need for the development of such techniques and the difficulty in so doing. Davis et al used “glass needles” (or micropipettes) to inject material into small cells by stabbing the cells and applying a pulse of pressure to eject the material (nucleic acid) from the micropipette. Glass-based microinjection is not new, and although the authors referred to papers in the 1970s,2,3 the technique is much older, with applications dating back at least to the 1920s.4 Not surprisingly for technology nearly 80 years old, there are limitations with the glass “stab injection” method. There are two fundamental problems that have limited the usefulness of this approach to blood cell research.
The first problem is the need for the cell to be firmly immobilized to withstand the stabbing action of the micropipette. Davis et al1 elegantly overcame this problem by temporarily immobilizing the cells while injection was occurring. Although it may be questioned whether a procedure for firmly adhering the cells which resulted in cell shape change and micropodia formation was neutral to the cell physiology, it at least permitted the microinjection to be performed. The second problem is the damage caused to the cell by the insertion of the glass micropipette through the plasma membrane and into the cytosol. There are two types of cellular damage that occur during microinjection, one associated with the breaking into the cell (mechanical damage to the cell surface) and the other associated with the presence of the glass in the cytosol (protein denaturation and coagulation on the glass). Although the plasma membrane may readily “seal” after microinjection, the effect of the glass on the cytosol can be less tolerable. While limited cytosolic damage can be tolerated by cells, the percentage of damage inflicted by this “glass-stab injection” increases as the size of the cell decreases. Thus a large cell of diameter 100 μm may tolerate the insult of microinjection if only 0.01% of its volume experiences the damage. But with the same injection process on a cell which is 1/10th this size (ie, with a spherical diameter of 10 μm, having a volume 1/1000th that of the 100-μm cell), the damage resulting from microinjecting the smaller cell will be 1000 times greater (ie, 10% of its volume will experience the effect of microinjection). Even with a micropipette of 0.2-μm tip diameter (as opposed to a “standard” 0.5 μm), the improvement in reducing glass-volume-related damage is only a factor of 2.5. It can thus be appreciated why Davis et al1 must be congratulated on achieving such “good” survival rates as 50%, with only 10% to 20% of cells showing immediate signs of damage from microinjection.
But there is now an alternative procedure for microinjecting nucleic acid, proteins, and other membrane impermeant molecules into small cells, a method that overcomes the two major problems with traditional microinjection. The new method does not require the cells to be firmly adhered to a substrate, and the glass micropipette does not penetrate the cell. This procedure has been used with a number of cell types, including blood cells, and has been demonstrated in detail with human neutrophils.5 The new approach is based on a modification of the standard microinjection procedure and is called SLAM (simple lipid-assisted microinjection). In essence, the tip of the glass micropipette is coated with a lipid bilayer to produce a “soft” non-cell–penetrating tip.5,6 On contact between the lipid bilayers at the tip of the micropipette and the cell membrane, fusion between the lipid bilayers occurs, and an aqueous channel is formed between the inside of the micropipette and the cytosol. Material inside the micropipette therefore gains access to the inside of the blood cell either by diffusion or by gentle ejection with low pressures.5 Removal of the SLAM pipette permits the lipid bilayers to reform. There is no need for the cell to be firmly attached to a substrate because there is no sudden movement of the micropipette. There is no mechanical damage to the plasma membrane, and because the glass of the SLAM-pipette does not enter the cell cytosol, no glass-induced damage occurs inside the cell. Not only does this procedure result in extremely high cell survival (at least 80% to 90%), but it can be used on cells that have hitherto been impossible to microinject.
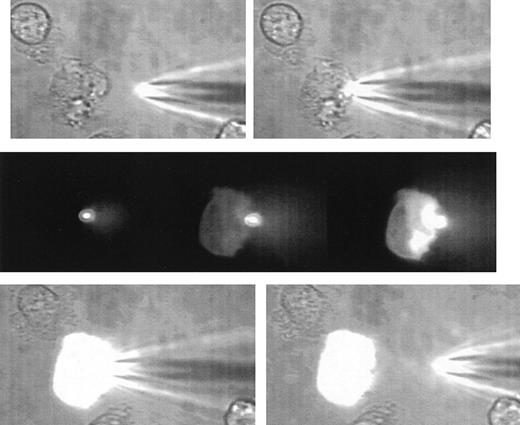
A comparison between the stab injection and the SLAM injection methods was made recently by Carmichael7 by considering the methods from the cell's point of view (if it has one). The stab injection when scaled up to human size is likened to a 40-mph advancing basketball that must be stopped precisely to achieve its goal. But with SLAM injection a gentle touch (to ensure contact between the two lipid bilayers) is all that is required. The cells do not need to adhere to a surface, although a loose adherence is useful to avoid the cell being moved by currents in the medium as the micropipette advances. The health of SLAM-injected neutrophils is demonstrable by their ability to maintain normal cytosolic-free Ca2+concentration,8 and the lack of damage can be shown by choosing to microinject neutrophils with forming cytosolic ruffling extensions that are very responsive to damage and that retract and round up during attempts at standard stab injection. The Figure shows such a SLAM injection in which the neutrophil maintains its shape and the ruffling of the membrane persists.
A sequence of images illustrating the gentle nature of SLAM-injection.
The uppermost 2 images show a human neutrophil that is loosely adhered and ruffling at the left-hand edge and at the beginning of the microinjection process. The SLAM injector contains lucifer yellow as a fluorescent (non-protein–binding) marker. The middle panel shows 3 fluorescent images (left) at contact between the SLAM-injector tip (in focus and brightly glowing) and then 8 seconds (middle) and 14 seconds (right) later. The transfer of fluorescent material into the cell can be seen. There is no indication of cell damage or response to the SLAM injection as evidenced by cell-shape change or recoiling of the ruffled membrane. The lowest panel shows the phase-contrast images at the end of SLAM injection, when a high level of uniform intracellular fluorescence is seen. (See Hallett and Laffafian9 for a movie of other SLAM injections.)
A sequence of images illustrating the gentle nature of SLAM-injection.
The uppermost 2 images show a human neutrophil that is loosely adhered and ruffling at the left-hand edge and at the beginning of the microinjection process. The SLAM injector contains lucifer yellow as a fluorescent (non-protein–binding) marker. The middle panel shows 3 fluorescent images (left) at contact between the SLAM-injector tip (in focus and brightly glowing) and then 8 seconds (middle) and 14 seconds (right) later. The transfer of fluorescent material into the cell can be seen. There is no indication of cell damage or response to the SLAM injection as evidenced by cell-shape change or recoiling of the ruffled membrane. The lowest panel shows the phase-contrast images at the end of SLAM injection, when a high level of uniform intracellular fluorescence is seen. (See Hallett and Laffafian9 for a movie of other SLAM injections.)
An additional bonus resulting from the SLAM technique is the ability to modify and modulate the composition of the plasma membrane. If bioactive lipids are included in the slam coating, they are transferred to the cell membrane. Also, it may be possible to incorporate membrane-associated proteins into the SLAM bilayer for its transfer to the cell membrane (with its orientation defined by which side of the bilayer in which is incorporated). Thus the development of new techniques for microinjection (and manipulation of plasma membrane) of blood cells promises to permit a new strategy for the investigation of blood cell physiology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal