Abstract
We report 2 new mutations identified in 3 patients and characterized by the markedly decreased affinity of von Willebrand factor (vWF) for factor VIII (FVIII). Patients 2 and 3, who have a typical type 2N phenotype, were found to be compound heterozygous for Arg91Gln and Cys25Tyr or Cys95Phe, respectively. Patient 1, who is the first cousin of patient 2, had an FVIII binding defect of vWF, low levels of vWF, and multimeric impairment. She was found to be compound heterozygous for the mutations Cys25Tyr and a stop codon (D93ter) in exon 4. Transient expression of recombinant vWF (rvWF) containing either Cys25Tyr or Cys95Phe mutations resulted in mutated rvWF with markedly reduced FVIII binding ability, multimeric structure impairment, and a significant decrease in the vWF expression level. Moreover, the use of anti-vWF monoclonal antibodies that inhibit the FVIII binding showed that these 2 mutations likely induce a conformational change in the D′ domain. These results show that the native conformation of the D′ domain of vWF is not only required for FVIII binding but also for normal multimerization and optimal secretion.
von Willebrand factor (vWF) is a large, multimeric glycoprotein synthesized by megakaryocytes and endothelial cells in a precursor form, named pre-pro-vWF, consisting of a 22-amino acid (aa) signal peptide, a 741-aa propeptide, and a 2050-aa mature vWF subunit.1 After several processing steps, including dimerization, glycosylation, sulfation, multimerization, and proteolytic cleavage of propeptide,2 vWF is secreted and circulates in plasma as a series of high molecular weight (HMW) multimers composed of disulfide-linked mature subunits that range from 500 to more than 15 000 kd.3 vWF plays 2 main hemostatic roles: it mediates platelet adhesion to the subendothelium and platelet aggregation at the site of damaged vessel walls, and it serves as carrier protein for factor VIII (FVIII).4 Defects of vWF result in von Willebrand disease (vWD), an autosomal bleeding disorder classified into 3 main types.5 Type 1 refers to a partial quantitative deficiency of vWF and is often dominant, whereas type 3, inherited in a recessive manner, is characterized by extremely low levels of or undetectable vWF. Type 2 includes all qualitative deficiencies of vWF and is subdivided into 4 subtypes. Among type 2 vWD, type 2N is inherited in a recessive manner and is initially characterized by a normal multimeric pattern and normal platelet-dependent function but a markedly decreased affinity of vWF for FVIII, leading to a secondary deficiency in plasma FVIII levels.5 6
Formation of a noncovalent complex with vWF is required for the normal survival of FVIII in the blood circulation.7,8 The association between FVIII and vWF shows characteristics of both electrostatic and hydrophobic interactions.9 Binding domains within each protein have been identified. The light chain of FVIII contains 2 distinct regions that associate with vWF. One is an acidic cluster in the amino terminus of A3 domain (aa 1670-1684),10-13 and the other is localized in the C2 domain (aa 2173-2332).14,15 On vWF, the FVIII binding site resides within the tryptic fragment consisting of the first 272 aa of the mature subunit.16,17 This part of vWF, named SpIII-T4, contains 24 cysteine residues, all of which are involved in intrasubunit disulfide bonds required for the functional conformation of this domain.16 Furthermore, the epitopes of anti-vWF monoclonal antibodies (mAbs), which block FVIII binding to vWF, have been mapped to aa residues in position 2-53,1851-60,19 and 78-96.20 These data, suggesting that the FVIII binding site consists of discontinuous sequences, were reinforced by the identification of molecular gene defects found in patients with type 2N vWD. To date, several missense mutations reported in the database (Ginsburg and Sadler21; Internet Web pagehttp://mmg2.im.med.umich.edu/vWF) have been localized on aa sequences previously defined as epitopes of mAbs, which inhibit FVIII/vWF interaction. Affected persons with normal vWF levels were generally found to be either homozygous or compound heterozygous for type 2N vWD mutations, in agreement with the recessive inheritance pattern of this variant disease. Another group of patients is characterized by moderately decreased vWF levels, whereas FVIII levels are disproportionately low. Such patients are compound heterozygous for type 2N and type 3 vWD mutations localized on separate alleles.6
In this paper, 2 new candidate mutations affecting cysteine residues 25 and 95 in the mature vWF subunit were identified in the vWF gene of 3 patients classified as type 2N vWD. These mutations were characterized by expression in COS 7 cells of the corresponding mutated rvWF (Tyr25rvWF and Phe95rvWF). In addition to the FVIII/vWF interaction defect, both mutations gave rise to a secretion defect and to an abnormal multimeric pattern of rvWF. The use of mAbs directed to the N-terminal part of vWF aroused suspicion of conformational change, which was emphasized by the detection of free sulfhydryl groups.
Materials and methods
Reagents
Thermo Sequenase Radiolabeled Terminator Cycle Sequencing kit, shrimp alkaline phosphatase, exonuclease I, radioactive chemicals, and streptavidin–peroxidase were purchased from Amersham (Cleveland, OH). Restriction enzymes were obtained from Eurogentec (Seraing, Belgium). Transformer site-directed mutagenesis kit was purchased from Clontech (Palo Alto, CA). Protein A Sepharose CL-4B and NAP5 columns were obtained from Pharmacia–LKB (Uppsala, Sweden). Biotinamidocaproate N-hydroxysuccinimide ester (BAC-NHS) was purchased from Sigma (St. Louis, MO). EZ-link biotin-HPDP and BCA protein reagent were obtained from Pierce (Rockford, IL). All cell culture reagents were from Life Technologies (Cergy Pontoise, France). Phenylmethylsulfonyl fluoride (PMSF) and N-ethylmaleimide (NEM) were from Merck (Darmstadt, Germany) and Serva (Heidelberg, Germany), respectively.
Routine coagulation tests
The determination of the Ivy bleeding time, FVIII coagulant activity (FVIII:C), FVIII antigen (FVIII:Ag), and vWF antigen (vWF:Ag) have been described previously.22 The multimeric structure of vWF was analyzed by sodium dodecyl sulfate (SDS)-1.5% agarose gel electrophoresis.22 Ristocetin cofactor activity (vWF:RCo) was measured using commercial lyophilized platelets sensitized with ristocetin (Behringwerke, Marburg, Germany).
Molecular genetic studies
Genomic DNA was extracted from peripheral blood leukocytes, and exons 18 to 23 of vWF gene were amplified by polymerase chain reaction (PCR) as described previously.23 After treatment by shrimp alkaline phosphatase and exonuclease I (according to the manufacturer's instructions) to remove any remaining dNTPs and primers, the PCR products were directly sequenced using the Thermo Sequenase Radiolabeled Terminator Cycle Sequencing kit (Amersham).
In patients 1 and 2, all the exons of the vWF gene were screened using a modified multiplex single-stranded conformation polymorphism (manuscript in preparation). Briefly, separate amplifications of each of the 52 exons were performed, then pools of 3 independent amplified exons were set before labeling at 72°C for 3 minutes in the presence of [α-33P] dATP and 2 μmol cold dCTP, dGTP, and dTTP. The resultant labeled multiplexes were heat denaturated in the presence of 50% formamide, run on a 0.5 × MDE nondenaturing sequencing gel for 16 hours at 700 V, and visualized by overnight autoradiography.
Restriction enzyme analysis
The nucleotide substitution in exon 20 at codon 854 of the vWF cDNA destroys an MspI site. PCR-amplified exon 20 was digested withMspI, according to the manufacturer's recommendation, then electrophoresed in agarose gel. The fragment resulting from the loss of an MspI restriction site was cut from the gel and sequenced as described above.
Plasmid constructions
The previously described plasmid pSVvWF, containing the full-length cDNA of human vWF, has been used as a template for the construction of the mutant plasmids. Plasmids pSV25Tyr, pSV95Phe, and pSV91Gln were derived from pSVvWF by introducing a G→A transition at nucleotide (nt) 2363, a G→T transversion at nt 2573, and a G→A transition at nt 2561, respectively, using the Transformer site-directed mutagenesis kit (Clontech). The oligonucleotide primers used for mutagenesis are listed in Table 1. The primers 25A, 95A, and 91B were used to introduce the desired mutation into pSVvWF, and the primer NheI-O was used to destroy the unique, previously created NheI restriction site of the plasmid for the purpose of selection. Clones containing the desired mutation were digested by HindIII and NotI, and the resultant fragment was cloned into pSVvWF treated with the same restriction enzymes. The oligonucleotide sequence between HindIII (nt 2235) and NotI (nt 2880) restriction sites of all constructs was verified by sequencing as described above.
Primers used for site-directed mutagenesis
| Primer . | Sequence (5′ → 3′)* . | Location Exon/Nucleotide† . |
|---|---|---|
| 25A | GGG CTC GAG TAT ACC AAA ACG | 18/2353-2373 |
| 95A | GAA GTG GAA CTT CAC AGA CCA TG | 20/2562-2584 |
| 91B | TGT CGG GAC CAG AAG TGG AAC | 20/2551-2571 |
| NheI-O | AGT TTC CCA GCT TCT TAT TTT GAT G | 29/5101-5125 |
| Primer . | Sequence (5′ → 3′)* . | Location Exon/Nucleotide† . |
|---|---|---|
| 25A | GGG CTC GAG TAT ACC AAA ACG | 18/2353-2373 |
| 95A | GAA GTG GAA CTT CAC AGA CCA TG | 20/2562-2584 |
| 91B | TGT CGG GAC CAG AAG TGG AAC | 20/2551-2571 |
| NheI-O | AGT TTC CCA GCT TCT TAT TTT GAT G | 29/5101-5125 |
Underlined nucleotides correspond to the substitutions.
Nucleotides are numbered starting from A of the initiating Met codon of exon 2.27
Expression of recombinant vWF
COS-7 cells were transiently transfected with plasmids pSVvWF (wild-type) and pSV25Tyr, pSV91Gln, and pSV95Phe (mutants) using the diethylaminoethyl-dextran method and culture conditions previously described.24 Forty hours after transfection, cells were incubated in serum-free Dulbecco's modified Eagle's medium for 72 hours. Then cell-conditioned media were collected into a final concentration of 5 mmol/L EDTA and 2 mmol/L PMSF. Expression levels in conditioned media were measured by enzyme linked immunosorbent assay (ELISA).25
Monoclonal antibodies
All mAbs were purified from ascites fluid by chromatography on protein A Sepharose CL-4B according to the manufacturer's instructions. Directed to the A2 domain of FVIII heavy chain (aa 372-740), mAb 52H7 was biotinylated and used to detect bound FVIII in the FVIII binding assay. Biotinylation of mAb 52H7 was performed using BAC–NHS as follows: purified IgG at a concentration of 2 mg/mL in Tris-buffered saline (Tris 25 mmol/L, NaCl 150 mmol/L, pH 7.35) was gently mixed with 100-fold molar excess of freshly prepared biotinylation reagent. The reaction was allowed to proceed for 2.5 hours at 22°C and was followed by gel filtration on a NAP5 column equilibrated in Tris-buffered saline. Fractions containing the biotinylated IgG were pooled and stored at 4°C.
Directed toward the COOH-terminal part of vWF subunit (aa 1366-2050), mAb 9311A2 reacts with all multimeric forms and does not inhibit any function of vWF. mAbs 32B12 and 31H3 against the NH2-terminal part of vWF subunit (aa 51-60 and 66-76, respectively) have been described in detail previously.19mAb 418 is a potent inhibitor of FVIII/vWF interaction, and its epitope has been mapped to aa 2-53 of mature vWF subunit.18
FVIII binding assay
The binding of FVIII to vWF was assayed as previously described26 with minor modifications. Briefly, increasing amounts of vWF were immobilized by binding to anti-vWF mAb 9311A2-coated microplates. Recombinant FVIII (Baxter Healthcare, Glendale, CA) was incubated with the immobilized vWF, and the bound rFVIII was then measured by incubating for 1 hour at 37°C successively 100 μL biotinylated mAb 52H7 and 100 μL streptavidin peroxidase conjugate (1:1000 dilution). Peroxidase activity was determined using O-phenylenediamine substrate. The absorbance at 492 nm was then read using a Vmax microplate reader (Molecular Devices, Menlo Park, CA). After washing, the relative amount of immobilized vWF in each well was also measured by ELISA using peroxidase-conjugated mAb 333A9 (aa 911-1365) as a detector and a pool of normal plasma as a standard.
Comparative recognition of vWF by mAbs
The ability of mAbs to capture wild-type (WT) or mutant rvWF was examined by ELISA as previously described.26Absorbance at 492 nm was converted to mU/mL vWF using the standard curve generated for each capture mAb using dilutions of a pool of normal plasma.
Detection of free cysteine residues
EZ-link biotin-HPDP (Pierce) is a biotinylation reagent that reacts specifically with thiol groups. Biotinylation of rvWF was carried out according to the manufacturer's instructions with minor modifications. Conditioned medium (0.5 mL) was applied to a NAP5 column equilibrated in phosphate-buffered saline (PBS)–EDTA (20 mmol/L phosphate buffer, pH 7.4, 150 mmol/L NaCl, 1 mmol/L EDTA), and the amount of recovered proteins was quantified using the BCA protein assay reagent. On a part of the recovered proteins, 3 mol/L guanidine HCl was added and incubated for 30 minutes before biotin–HPDP was added. Biotin–HPDP was added at 0.1 mmol/mg protein, and biotinylation was performed by incubation for 90 minutes at room temperature. The excess of reagent was removed by gel filtration onto a NAP5 column. Serial dilutions of eluate analyzed in duplicate were incubated for 1 hour at 37°C in microtiter wells coated with anti-vWF polyclonal antibodies. Wells were then washed in PBS-T, and the amount of immobilized rvWF was detected by anti-vWF peroxidase-conjugated polyclonal antibodies, whereas biotin was detected by streptavidin–peroxidase (1:1000 dilution). After additional washing with PBS-T, O-phenylenediamine substrate was added to the wells, and the reaction was stopped by the addition of 1 mol/L H2SO4.
Results
Case report and phenotypic analysis
Three patients from 2 unrelated families were referred to us for mild to moderate bleeding disorders. Routine biologic data are summarized in Table 2. Patient 1, a woman born in 1972 who has a moderate mucosal bleeding, shows a prolonged bleeding time and decreased levels of plasma vWF associated with disproportionately lower FVIII levels. The patient's mother and sister, who are asymptomatic, have normal FVIII levels (98 and 66 U/dL, respectively) but a borderline (50 U/dL) and slightly decreased (42 U/dL) plasma vWF level, respectively. Patient 2, a woman born in 1977 and the first cousin of patient 1, had mild bleeding after tonsillectomy and adenoidectomy. She has normal bleeding time and normal vWF levels but moderately decreased FVIII levels. Patient 3, a woman born in 1929, has had a lifelong history of bleeding. She had epistaxis, excessive bleeding after tooth extraction, and was treated in 1980 with intermediate-purity FVIII concentrate for hysterectomy. In 1990, she was found seropositive for hepatitis C markers. She was treated with vWF concentrate (facteur Willebrand-LFB) for liver biopsy in 1996 and for mastectomy in 1999. The current biologic data of this patient indicate normal bleeding time and normal plasma vWF levels but significantly decreased FVIII levels. However, 20 years ago, the newly diagnosed FVIII deficiency was associated with borderline plasma vWF levels (54 U/dL vWF:Ag; 50 U/dL vWF:RCo).
Routine biologic data and DNA analyses of patients
| Patients . | Bleeding Time (min) . | FVIII:Ag (U/dL) . | FVIII:C (U/dL) . | vWF:Ag (U/dL) . | vWF:RCo (U/dL) . | Mutations . |
|---|---|---|---|---|---|---|
| 1 | > 20 | 4 | 1.5 | 23 | 20 | Cys25Tyr/(c)93Ter |
| 2 | 8 | 30 | 26 | 65 | 64 | Cys25Tyr/Arg91Gln |
| 3 | 4 | 44 | 30 | 100 | 90 | Cys95Phe/Arg91Gln |
| Normal | < 10 | 50-150 | 50-150 | 50-175 | 50-150 |
| Patients . | Bleeding Time (min) . | FVIII:Ag (U/dL) . | FVIII:C (U/dL) . | vWF:Ag (U/dL) . | vWF:RCo (U/dL) . | Mutations . |
|---|---|---|---|---|---|---|
| 1 | > 20 | 4 | 1.5 | 23 | 20 | Cys25Tyr/(c)93Ter |
| 2 | 8 | 30 | 26 | 65 | 64 | Cys25Tyr/Arg91Gln |
| 3 | 4 | 44 | 30 | 100 | 90 | Cys95Phe/Arg91Gln |
| Normal | < 10 | 50-150 | 50-150 | 50-175 | 50-150 |
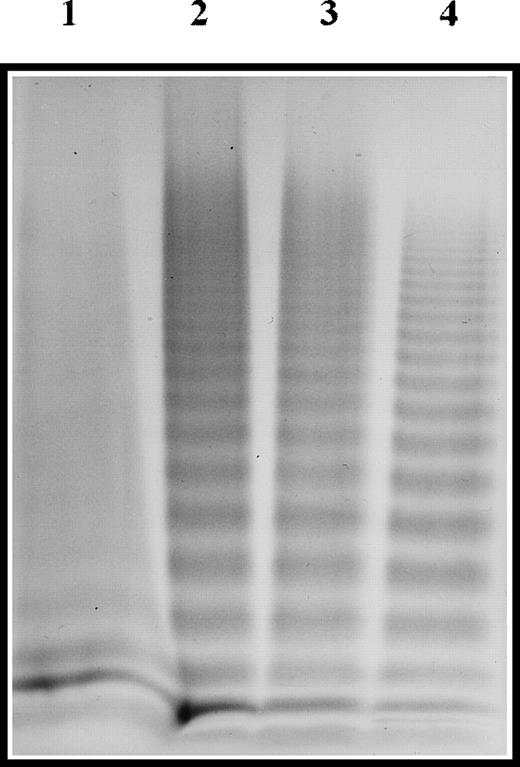
Further analyses, including FVIII binding assay and multimeric analysis of vWF, were performed on the patient's plasma vWF. Compared with a pool of normal plasma, the FVIII binding capacity of patient 1 was nil, whereas it was approximately 6% of normal for patients 2 and 3 (data not shown). In patient 1, vWF displayed an unusual multimeric pattern with a loss of HMW (10 mer or more) and a smeared aspect of the profile. The vWF multimeric profiles of patients 2 and 3 were normal (Figure 1).
Multimeric analysis of plasma vWF.
Plasma samples were electrophoresed in SDS-1.5% agarose gel, and vWF was visualized with alkaline-phosphatase–conjugated anti-vWF polyclonal antibodies. Lane 1, patient 1; lane 2, patient 2; lane 3, patient 3; lane 4, pool of normal plasma.
Multimeric analysis of plasma vWF.
Plasma samples were electrophoresed in SDS-1.5% agarose gel, and vWF was visualized with alkaline-phosphatase–conjugated anti-vWF polyclonal antibodies. Lane 1, patient 1; lane 2, patient 2; lane 3, patient 3; lane 4, pool of normal plasma.
Identification of mutations
To identify the abnormality in vWF gene responsible for the FVIII binding defect, PCR-amplified exons 18 to 23 were sequenced. In exon 18, a G→A transition at nt 2363 was detected in patients 1 and 2, predicting the replacement of Cys 25 of the mature vWF subunit by Tyr. Sequence analysis of exon 20 revealed 2 single-base substitutions. A G2561→A transition, which modifies the encoded aa residue Arg91 to Gln, was found in patients 2 and 3. Moreover, another substitution, a G→T transversion at nt 2573, was identified in patient 3. This transversion causes the aa substitution of Phe for Cys 95. According to the sequence data, all these mutations are present at a heterozygous state in the 3 patients.
In patient 1, a second mutation was suspected, and the vWF gene was analyzed by multiplex single-stranded conformation polymorphism. The aberrant electrophoretic pattern observed for exon 4 indicated a mutation. Direct sequencing of exon 4 allowed detection of a thymidine insertion within a 6-thymidine repeat at the nt position 271 to 276. This insertion changes a GAC to a TGA codon and causes the substitution of a stop codon for aspartic acid residue at position 93 of vWF propeptide (D93 ter). The patient and her mother and sister were found heterozygous for this mutation.
No family members of patient 3 were available to demonstrate that the 2 mutations reside on separate alleles. PCR-amplified exon 20 was digested by MspI, as described in “Materials and methods.” The sequencing pattern of the undigested fragment revealed only the G2561→A transition. This result proved that the Arg91Gln and Cys95Phe mutations were localized on separate alleles.
Expression of recombinant vWF
To determine the effect of the missense mutations on vWF structure and function, site-directed mutagenesis was used to introduce G2363A, G2573T, and G2561A nt substitutions individually into the full-length vWF expression vector. After 5 separate transient expression experiments in COS-7 cells of WT and mutant plasmids, the secretion amounts of rvWF in conditioned media were quantified. As shown in Figure 2, WT and Gln91rvWF were secreted efficiently, achieving a concentration of 10.6 ± 0.4 U/dL and 10.7 ± 1.6 U/dL (mean ± SD), respectively. In contrast, the secretion level of Tyr25rvWF was significantly reduced to 4.7 ± 0.2 U/dL, whereas hybrid Tyr25/Gln91 and Tyr25/WT rvWF, obtained by cotransfection of a 1:1 ratio of corresponding plasmids, were secreted to 5.9 ± 0.4 and 6.6 ± 0.06 U/dL, respectively. The Cys95Phe mutation induced a poor secretion level of rvWF, reaching a concentration of only 2.0 ± 0.3 U/dL in conditioned media. Secretion levels of hybrid Phe95/Gln91 and Phe95/WT rvWF were both decreased to approximately 5.4 U/dL.
Amount of rvWF secreted by transiently transfected COS-7 cells.
The concentration of WT and mutant rvWF in conditioned media was determined by ELISA. Absorbance at 492 nm was converted to units of vWF:Ag using a standard curve generated from dilutions of a calibrated pool of normal plasma. The results represent the mean ± SD of 5 independent transfections.
Amount of rvWF secreted by transiently transfected COS-7 cells.
The concentration of WT and mutant rvWF in conditioned media was determined by ELISA. Absorbance at 492 nm was converted to units of vWF:Ag using a standard curve generated from dilutions of a calibrated pool of normal plasma. The results represent the mean ± SD of 5 independent transfections.
Effect of mutations on FVIII binding capacity of rvWF
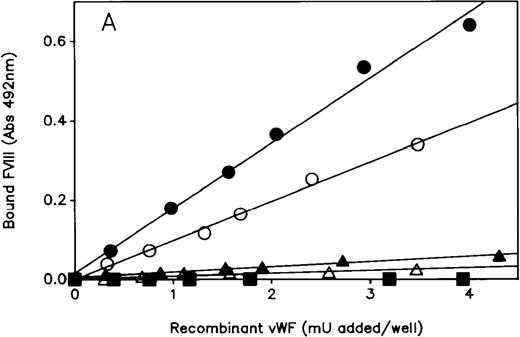
The ability of the mutated rvWF to bind FVIII was measured using the solid-phase binding assay described in “Materials and methods.” Mutated Tyr25rvWF failed to bind FVIII, whereas the slope value for the regression line obtained with Gln91rvWF was reduced to 10% compared with WT rvWF (Figure3A). The hybrid Tyr25/Gln91 rvWF gave an FVIII binding regression line intermediate between that of Tyr25 and Gln91rvWF. As expected, the FVIII binding capacity of hybrid Tyr25/WT rvWF was decreased to 50% of WT, in agreement with the recessive feature of type 2N mutations.
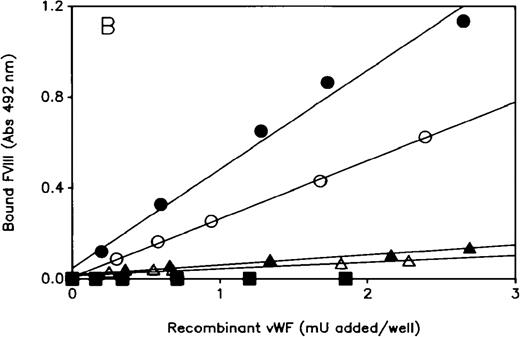
FVIII binding assay of rvWF.
The ability of WT and mutant rvWF to bind rFVIII was determined by ELISA using a biotinylated anti-FVIII mAb to detect bound FVIII, as described in “Materials and Methods.” Each plot is representative of at least 3 similar experiments performed with the conditioned media of 3 independent transfections. Each point is the average of duplicate measurements. (A) Mutant and hybrid Tyr25rvWF: (•) WT; (▪) Tyr25; (▴) Gln91; (▵) hybrid Tyr25/Gln91; (○) hybrid Tyr25/WT. (B) Mutant and hybrid Phe95rvWF: (•) WT; (▪) Phe95; (▴) Gln91; (▵) hybrid Phe95/Gln91; (○) hybrid Phe95/WT.
FVIII binding assay of rvWF.
The ability of WT and mutant rvWF to bind rFVIII was determined by ELISA using a biotinylated anti-FVIII mAb to detect bound FVIII, as described in “Materials and Methods.” Each plot is representative of at least 3 similar experiments performed with the conditioned media of 3 independent transfections. Each point is the average of duplicate measurements. (A) Mutant and hybrid Tyr25rvWF: (•) WT; (▪) Tyr25; (▴) Gln91; (▵) hybrid Tyr25/Gln91; (○) hybrid Tyr25/WT. (B) Mutant and hybrid Phe95rvWF: (•) WT; (▪) Phe95; (▴) Gln91; (▵) hybrid Phe95/Gln91; (○) hybrid Phe95/WT.
Results of FVIII binding capacity of Phe95rvWF, hybrid Phe95/Gln91, and Phe95/WT rvWF were identical to those of Tyr25rvWF and corresponding hybrids, respectively (Figure 3B).
Multimeric pattern of rvWF
For both WT and Gln91rvWF, multimers varied in size from protomer to HMW multimers (15 mer or more) (Figure 4A, lanes 1 and 3). The Tyr25rvWF gave an abnormal multimeric profile characterized on one hand by a slight decrease in multimers by 5 mer or more and on the other hand by a smeared feature of HMW multimers (Figure 4A, lane 2). However, the pattern of hybrid Tyr25/Gln91 and Tyr25/WT rvWF appeared normal (Figure 4A, lanes 4 and 5).
Multimeric analysis of rvWF.
Conditioned media samples containing WT or mutant rvWF were electrophoresed in SDS-1.5% agarose gels, and multimers were visualized with alkaline-phosphatase–conjugated anti-vWF polyclonal antibodies. (A) Lane 1, WT; lane 2, Tyr25; lane 3, Gln91; lane 4, Tyr25/Gln91; lane 5, Tyr25/WT. (B) lane 1, WT; lane 2, Phe95; lane 3, Phe95/Gln91; lane 4, Phe95/WT.
Multimeric analysis of rvWF.
Conditioned media samples containing WT or mutant rvWF were electrophoresed in SDS-1.5% agarose gels, and multimers were visualized with alkaline-phosphatase–conjugated anti-vWF polyclonal antibodies. (A) Lane 1, WT; lane 2, Tyr25; lane 3, Gln91; lane 4, Tyr25/Gln91; lane 5, Tyr25/WT. (B) lane 1, WT; lane 2, Phe95; lane 3, Phe95/Gln91; lane 4, Phe95/WT.
Mutation Cys95Phe induced a deep impairment of rvWF multimerization evidenced by a dramatic loss of HMW multimers with a scant amount of intermediate multimeric forms and, consequently, by a relative large increase in protomer (Figure 4B, lane 2). The multimeric structure of both hybrid Phe95/Gln91 and Phe95/WT rvWF was only partially restored because no HMW multimers were detected (Figure 4B, lanes 3 and 4).
Because the multimerization of vWF dimers depends on the intersubunit disulfide bonds, conditioned media were collected before agarose gel electrophoresis in 5 mmol/L NEM to block free sulfhydryl groups and to prevent disulfide interchange in rvWF molecules. As shown in Table3, the multimeric structure of WTrvWF was identical regardless of whether it was treated by NEM. In contrast, NEM treatment of Tyr25 and Phe95rvWF improved the multimeric pattern because the percentage of protomer was decreased and, in parallel, the multimers 5 mer or larger were increased.
Multimeric analysis of rvWF before and after NEM treatment
| . | Multimers (%) . | ||
|---|---|---|---|
| Protomer . | ≥ 5 mer . | ≥ 10 mer . | |
| WTrvWF | |||
| −NEM | 7.4 | 73.5 | 49.6 |
| +NEM | 7.7 | 71.0 | 46.1 |
| Tyr25rvWF | |||
| −NEM | 20.4 | 50.0 | ND |
| +NEM | 11.5 | 59.2 | ND |
| Phe95rvWF | |||
| −NEM | 64.0 | 18.4 | ND |
| +NEM | 26.7 | 42.8 | ND |
| . | Multimers (%) . | ||
|---|---|---|---|
| Protomer . | ≥ 5 mer . | ≥ 10 mer . | |
| WTrvWF | |||
| −NEM | 7.4 | 73.5 | 49.6 |
| +NEM | 7.7 | 71.0 | 46.1 |
| Tyr25rvWF | |||
| −NEM | 20.4 | 50.0 | ND |
| +NEM | 11.5 | 59.2 | ND |
| Phe95rvWF | |||
| −NEM | 64.0 | 18.4 | ND |
| +NEM | 26.7 | 42.8 | ND |
ND, not detectable.
Detection of free cysteine residues
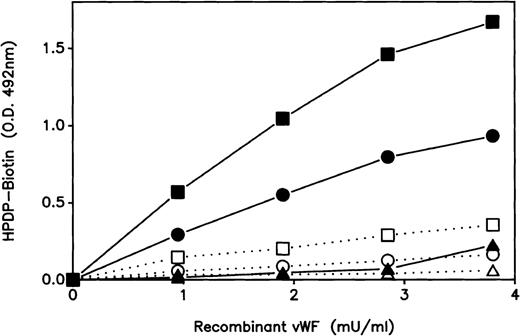
To compare the presence and accessibility of free cysteine residues in the 2 mutant proteins, rvWF was biotinylated using EZ-link biotin HPDP that reacts specifically with thiol groups, as described in “Materials and methods.” As depicted in Figure5, though the extent of Tyr25 and Phe95 rvWF biotinylation before guanidine HCl treatment remained faint, it was significantly increased in comparison with WTrvWF. Moreover, Phe95rvWF was 2-fold more biotinylated than Tyr25rvWF. After guanidine HCl treatment, the biotinylation of WTrvWF was hardly increased. In contrast, the incorporation of biotin–HPDP was dramatically increased in Tyr25 and Phe95rvWF pretreated with guanidine HCl. In addition, the extent of biotin incorporation into Phe95rvWF was still higher than into Tyr25rvWF.
Reactivity of rvWF with HPDP-biotin.
Biotinylation of rvWF by HPDP-biotin was performed as described in “Materials and methods.” Serial dilutions of biotinylated rvWF were added in duplicate into microtiter wells coated with anti-vWF polyclonal antibodies. On half the wells, the quantity of bound rvWF was detected by peroxidase-conjugated anti-vWF polyclonal antibodies; on the other half, the amount of biotin linked to vWF was detected by streptavidin–peroxidase. Dotted lines and empty symbols represent the native rvWF, whereas the full lines and full symbols represent the rvWF in 4 mol/L guanidine HCl. ▵, ▴, WT; ○, •, Tyr25; □, ▪, Phe95.
Reactivity of rvWF with HPDP-biotin.
Biotinylation of rvWF by HPDP-biotin was performed as described in “Materials and methods.” Serial dilutions of biotinylated rvWF were added in duplicate into microtiter wells coated with anti-vWF polyclonal antibodies. On half the wells, the quantity of bound rvWF was detected by peroxidase-conjugated anti-vWF polyclonal antibodies; on the other half, the amount of biotin linked to vWF was detected by streptavidin–peroxidase. Dotted lines and empty symbols represent the native rvWF, whereas the full lines and full symbols represent the rvWF in 4 mol/L guanidine HCl. ▵, ▴, WT; ○, •, Tyr25; □, ▪, Phe95.
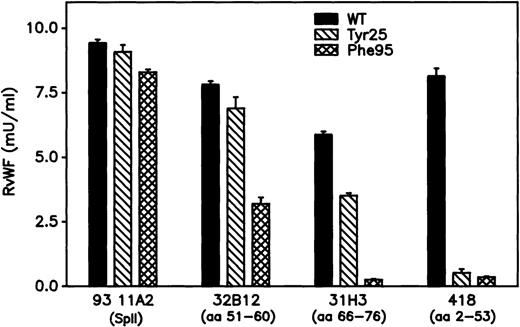
Recognition of rvWF by mAbs
To investigate potential conformational changes induced by mutations in the D′ domain of the mature rvWF subunit, antigen-captured ELISA was performed with various anti-vWF mAbs. mAb 21A11, directed to the C-terminal moiety of vWF, bound similarly both WT and mutant rvWF. mAb 32B12, which recognizes reduced vWF, bound similarly WT and Tyr25 rvWF but showed a 50% binding capacity for Phe95rvWF. mAb 31H3, which recognizes only nonreduced vWF, failed to bind Phe95rvWF and displayed a reduced capacity for binding Tyr25rvWF. Finally, mAb 418, which is also sensitive to the reduction of vWF, failed to bind either Tyr25 or Phe95 rvWF (Figure 6).
Recognition of rvWF by mAbs, of which the previously identified epitope is mentioned in parentheses.
Conditioned media samples diluted to approximately 0.01 U/mL vWF:Ag were incubated for 1 hour at 37°C into a microtiter plate coated with each mAb. After washing, the bound rvWF was detected with peroxidase-conjugated anti-vWF polyclonal antibodies. Absorbance at 492 nm was converted to milliunits of vWF:Ag using standard curves generated for each capture mAb using dilutions of a calibrated pool of normal plasmas. Each sample of rvWF was tested in triplicate, and data are illustrated as mean ± SD.
Recognition of rvWF by mAbs, of which the previously identified epitope is mentioned in parentheses.
Conditioned media samples diluted to approximately 0.01 U/mL vWF:Ag were incubated for 1 hour at 37°C into a microtiter plate coated with each mAb. After washing, the bound rvWF was detected with peroxidase-conjugated anti-vWF polyclonal antibodies. Absorbance at 492 nm was converted to milliunits of vWF:Ag using standard curves generated for each capture mAb using dilutions of a calibrated pool of normal plasmas. Each sample of rvWF was tested in triplicate, and data are illustrated as mean ± SD.
Discussion
In this report, we describe 2 new missense mutations within the N-terminal part of the mature vWF subunit that were identified in 3 patients and were characterized by a discrepancy between FVIII and vWF levels, consistent with a markedly decreased capacity of vWF to bind FVIII. A Tyr substitution for Cys at position 25 of the mature vWF subunit was detected, at heterozygous state, in the vWF gene of the related patients 1 and 2. Patient 1 appeared to be a compound heterozygote for 2 recessive mutations: 1 quantitative (D93ter) type 3 mutation inherited from her mother and 1 qualitative (Cys25Tyr) type 2N mutation inherited from her father. In patient 2, the first cousin of patient 1, the second allele was found to contain the Arg91Gln type 2N mutation.28 This frequent mutation was also found in patient 3 in association with a Phe95 for Cys substitution originating from the other allele. The phenotype of patients 2 and 3, who are compound heterozygotes with 2 type 2N mutations, is typical of type 2N vWD—ie, vWF was normal except for a markedly decreased affinity for FVIII.6 In contrast, the phenotype of patient 1, who has compound type 3/2N vWD, was characterized by—in addition to a loss of FVIII binding affinity to vWF—a prolonged bleeding time associated with a very low level of vWF and multimeric impairment.
To assess the impact of these 2 mutations affecting cysteine residues on vWF function and structure, WT and mutant vWF molecules were transiently expressed in COS-7 cells. The first effect of these 2 mutations on rvWF was the quantitative defect in expression levels. Indeed, vWF:Ag rates of Tyr25 and Phe95rvWF were reduced by 60% and 80%, respectively, compared with WT. Thus, the low level of plasma vWF:Ag found in patient 1 was probably caused by the presence of the null allele caused by the D93ter mutation and the secretion defect induced by the Cys25Tyr mutation. It is noteworthy that another mutation, Cys25Arg, was found at the homozygous state in a Turkish patient who was classified as type 1 vWD on the basis of a low vWF:Ag level.29 These data are in favor of the requirement of a Cys residue at position 25 for the normal secretion of vWF. The Tyr25 and Phe95 rvWF also showed complete abolition of their capacity to bind FVIII. The hybrid rvWF, resulting from cotransfection of mutant and WT vWF cDNA, showed an intermediate FVIII binding capacity in agreement with the recessive inheritance pattern of type 2N vWD.6 The hybrid Tyr25/Gln91 and Phe95/Gln91 rvWF, prepared to mimic the plasma vWF of patients 2 and 3, respectively, displayed a slight affinity for FVIII by reproducing the results obtained with the patients' plasma vWF. Furthermore, the multimeric structure of both mutant rvWF was different from that of WTrvWF. The pattern of Tyr25rvWF displayed a slight decrease in high multimeric forms and a peculiar smeared aspect of the profile, whereas the corresponding hybrid rvWF showed a normal multimeric pattern. The Phe95rvWF multimeric pattern was more disturbed with a large decrease in high and intermediate molecular weight multimers and was not restored by cotransfection of pSV95Phe with either pSVvWF or pSV91Gln expression vectors. Thus, mutant and hybrid Tyr25rvWF reproduced the multimeric patterns obtained with the plasma vWF of patients 1 and 2. On the other hand, the multimeric profile exhibited by the hybrid Phe95/Gln91rvWF was still disturbed, in contrast with patient 3 plasma vWF. To explain this discrepancy, we hypothesize that the vWF allele containing the Cys95Phe mutation may not be expressed in patient 3. Actually, her phenotype, which is typical of type 2N vWD, is consistent with a possible null/Arg91Gln (type 3/2N) genotype.
To define the mechanism by which these mutations caused a FVIII binding defect and an abnormal multimerization of vWF, we considered the conformation of the N-terminal part of the mature vWF subunit. The D′ domain (aa 6-102) is a highly structured region containing 24 paired cysteine residues. Cys25 has been found to form a disulfide bridge with Cys36 (E. Sadler, personal communication, 1993), whereas the pairing of Cys95 has not been determined yet. Our mAb binding studies showed that both the aa Cys25 and Cys95 are critical residues for the conformation of the epitope of mAbs 418 (aa 2-53) and 31H3 (aa 66-76), which are inhibitors of the FVIII/vWF interaction and recognize only nonreduced vWF. Furthermore, the epitope of mAb 32B12, a potent inhibitor of the FVIII/vWF interaction not sensitive to the reduction of vWF, was affected by Cys95Phe but not by Cys25Tyr mutation. The epitope of mAb 32B12 has been previously mapped to aa 51-60 of mature vWF, within the Cys 47–Cys 58 loop. This loop contains Arg53, which plays an essential role in FVIII binding,30 and Arg57, which is a critical residue in the epitope of mAb 32B12 (Sylvie Jorieux, personal observation, 1995). The fact that mAb 32B12 displayed a large decrease in affinity for Phe95rvWF may indicate that the Cys95Phe mutation induces a conformational change, different from that obtained by opening the Cys47-58 loop, reducing the accessibility of Arg57 for this mAb and that of Arg53 for FVIII. As expected, with regard to the nature of substituted aa residues, our data support the conclusion that the defective FVIII binding observed for the Tyr25 and Phe95 rvWF is caused by a conformational change in the D′ domain.
Multimerization is a complex process that requires, in addition to the vWF propeptide, D′ and D3 domains of the mature vWF subunit for multimer assembly.31 The D3 domain contains sulfhydryl groups that are involved in intramolecular and intermolecular disulfide bonds,32 but the possible role of D′ in multimerization is thus far unknown. Although the 2 new mutations reported here affect cysteine residues not directly involved in the multimer assembly, when conditioned media were collected in NEM before multimer analysis, the multimeric distribution of mutant rvWF, especially the Phe95rvWF one, was improved. In regard to the difference of the extent of multimeric defect between the Tyr25 and Phe95rvWF, we assumed a difference in the degree of disulfide bond impairment induced by each mutation. In fact, the detection of free sulfhydryl groups by a biotinylated reagent provided evidence that the Phe95 substitution induces more of them than the Tyr25 mutation. Furthermore, for both mutations free sulfhydryl groups are more accessible after treatment by a chaotropic agent. Considering these data, we hypothesize that the Tyr25 and Phe95 mutations may induce a destabilization of disulfide bonds up to the D3 domain. The conformational change induced by these 2 mutations in the D′ domain likely hinders the correct noncovalent interaction between the vWF propeptide and vWF dimers that allows the propeptide to promote interchain disulfide bonding in the D3 domain.
In summary, all these data indicate that the Cys25Tyr and Cys95Phe mutations, like the Asp116Asn mutation,33 are able to induce a peculiar 2N vWD phenotype characterized by a defective FVIII binding associated with an abnormal multimerization of vWF. Moreover, they demonstrate that the native conformation of the D′ domain is required not only for FVIII binding but also for normal multimerization of vWF.
Acknowledgments
We thank Baxter Healthcare Corporation for the generous gift of recombinant FVIII. We also thank V. Barylo, S. Belmont, and D. Hoguet for their excellent technical assistance, and V. Tancré for typing the manuscript.
Reprints:S. Jorieux, Département Recherche et Développement, Laboratoire Français du Fractionnement et des Biotechnologies, 59, rue de Trévise, BP 2006, 59,011 Lille cédex, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal