Abstract
Preliminary reports have suggested that survivors of childhood cancer and aplastic anemia who are infected with the hepatitis C virus (HCV) have a low risk for progression to significant liver disease. Among our surviving patients who were transfused between 1961 and March 1992, 77 (6.6% of surviving patients tested thus far) have evidence of HCV infection, whereas 4 surviving patients who were transfused after March 1992 are HCV-infected. One patient chronically infected with HCV died of liver failure, and 2 patients died of hepatocellular carcinoma. To characterize the risk for these and other complications, 65 patients are enrolled in a longitudinal study of HCV infection, of whom 58 (89.2%) had circulating HCV RNA at the time of protocol enrollment, with genotypes 1A and 1B most commonly isolated. Most enrolled patients have few or no symptoms, carry out normal activities, and have normal liver function. To date, 35 patients have undergone liver biopsy for abnormal liver function since the diagnosis of primary malignancy; central pathology review shows 28 (80%) have chronic active hepatitis, 25 (71%) have fibrosis, and 3 (9%) have cirrhosis. These preliminary data suggest that though most survivors of childhood cancer who are infected with HCV are clinically well, some are at risk for clinically significant liver disease. Identification of other HCV-infected patients and prospective monitoring of this cohort are ongoing to determine the risk for, and to identify factors associated with the progression of, liver disease.
For at least 80% of patients infected with the hepatitis C virus (HCV), the infection becomes chronic,1with the attendant risk for liver-related morbidity and mortality. Studies indicate that cirrhosis develops in more than 20% of chronically infected adults an average of 20 years after the diagnosis of acute infection2 and that hepatocellular carcinoma (HCC) develops in 1% to 5% of patients an average of 10 years after the onset of cirrhosis.3 Moreover, in the United States, patients with HCV infection are now the most frequent recipients of liver transplantation, which is often the only available intervention for patients with advanced liver disease. These facts emphasize the need for prospective, longitudinal studies of well-defined cohorts to determine the outcomes of patients with HCV infection and to elucidate risk factors predisposing patients to clinically significant liver disease.
Survivors of childhood cancer are a growing and vulnerable population. Patients who were treated for childhood cancer before HCV donor screening was implemented constitute a large population at risk for transfusion-acquired HCV infection. This cohort is unique in comparison with other groups with HCV infection in that these patients acquired the infection when they were young and were likely receiving immunosuppressive or hepatotoxic therapy. Reports with relatively brief follow-up suggest that the risk for progression to clinically significant liver disease is low for survivors of childhood cancer.4-10 To better estimate this risk, however, large cohorts of survivors of childhood cancer must be identified for further study, with consistent long-term monitoring of clinical, laboratory, radiographic, and histologic findings. In addition, the importance of other variables, such as age at the time of initial infection, immunosuppressive regimens, and hepatotoxic therapies, on the ultimate outcomes of these patients must be defined.
In 1995, St. Jude Children's Research Hospital (SJCRH) opened a longitudinal research protocol with the primary objective of defining the risk for progression to significant liver disease for patients infected with HCV. A second aim of the study was to elucidate the effect of variables such as age, hepatotoxic cancer therapy, and immunosuppression on the outcome of HCV infection in these patients. Simultaneously, systematic HCV testing began for all patients who received blood products before the initiation of second-generation HCV testing of donors in March 1992. Although our cohort has not been fully assembled, the preliminary data presented in this report suggest that most survivors of childhood cancer infected with HCV are clinically well, though a few are at risk for significant liver disease, including cirrhosis and HCC. Additional information will be forthcoming once more patients have been identified and recruited to participate in our longitudinal study.
Materials and methods
Identification and testing of patients who received blood products
SJCRH records were audited to determine the number of patients who received blood products before the initiation of HCV donor screening by second-generation enzyme immunoassay (EIA-2; Abbott Diagnostic 2.0; Abbott Laboratories, Chicago, IL) in March 1992. Using the SJCRH Health Information Systems Database, we obtained basic demographic information for these patients, including date of birth, gender, ethnic background, primary cancer diagnosis, date of primary cancer diagnosis, and current status (alive and actively followed up at SJCRH, alive and discharged from SJCRH, or deceased).
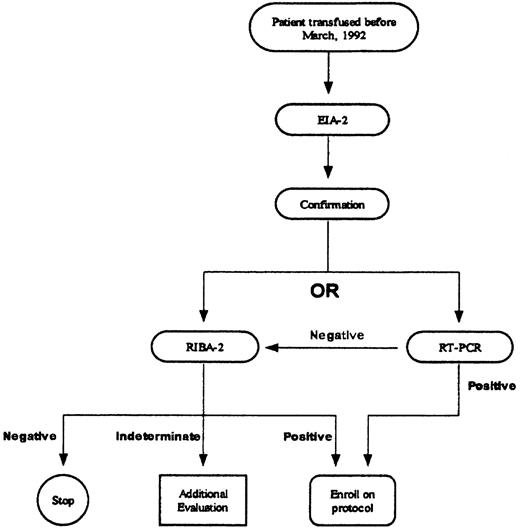
The institutional HCV testing protocol follows recommendations by the Centers for Disease Control and Prevention (Figure1).11 Positive EIA-2 results were confirmed by second-generation recombinant immunoblot assay (RIBA-2; Chiron RIBA HCV 2.0 Strip Immunoblot Assay; Chiron, Emeryville, CA) or quantitative polymerase chain reaction (PCR; Roche Amplicor Monitor HCV; Roche Diagnostic Systems, Somerville, NJ) (sensitivity, 200 copies/mL). If the EIA-2 result was positive and the RIBA-2 result negative, no additional testing was performed. If the RIBA-2 result was indeterminate, PCR testing was performed; if the PCR result was negative, no further testing was conducted.
Laboratory records were reviewed to determine how many patients who were transfused while undergoing treatment for childhood cancer or aplastic anemia had been screened for HCV. Patients were considered to have been infected with HCV if antibodies to HCV (anti-HCV) were detected by both EIA-2 and RIBA-2 or if PCR testing detected HCV RNA, regardless of EIA-2 or RIBA-2 results,
Protocol enrollment and assessment
Patients who had evidence of HCV infection were asked to participate in the longitudinal epidemiologic study of HCV infection and HCV-related outcomes. The protocol was approved by the Institutional Review Board, and informed consent was obtained before patients were enrolled.
After notification of HCV status, the following clinical parameters were recorded at the time of enrollment, and approximately annually thereafter, unless otherwise noted1: a brief, 35-item questionnaire assessing constitutional symptoms that were potentially exacerbated by HCV infection (using a 5-point scale, with 0 equivalent to “none” and 4 equivalent to “extremely,” patients rated the level of their discomfort resulting from symptoms that had occurred over the preceding week; patients were then asked to assess the impact of these symptoms on daily activities—play, leisure, and recreational activities—and work—job, school, housework, child care, studies, and so on)2; assessment of liver function by serum assays of alanine aminotransferase (ALT)3; PCR assessment of viral load and serum HCV genotype (Line Probe Assay INNO-LIPA HCV II; National Genetics Institute, Culver City, CA; performed only at the time of enrollment)4; screening for hepatoma and HCC by liver ultrasonography and determination of alpha-fetoprotein level5; at the time of enrollment only, screening for hepatitis B virus (HBV) core antibodies, HBV surface antibodies, and human immunodeficiency virus (HIV) antibodies and for the presence of HBV surface antigen.
Liver biopsy assessment
Slides were requested from patients who underwent biopsy before protocol enrollment. Liver biopsies were encouraged for patients without recent histologic assessment of liver disease, and they were required for all patients under consideration for antiviral therapy. One pathologist specializing in liver disease evaluated all liver biopsy specimens using a standard format.12 Periportal or periseptal interface hepatitis (piecemeal necrosis) was graded as absent, mild (focal, few portal areas), mild to moderate (focal, most portal areas), moderate (continuous near less than 50% of tracts or septa areas), or severe (continuous near more than 50% of tracts or septa). Fibrosis and cirrhosis were staged on a scale of 0 to 6, as follows: 0, no fibrosis; 1, fibrous expansion of some portal areas, with or without short fibrous septa; 2, fibrous expansion of most portal areas, with or without short fibrous septa; 3, fibrous expansion of portal areas with marked portal-to-portal (P-P) bridging; 4, fibrous expansion of portal areas with marked P-P bridging and portal-to-central (P-C) bridging; 5, marked bridging (P-P, P-C, or both) with occasional nodules (incomplete cirrhosis); and 6, probable or definite cirrhosis. Liver decompensation was determined by characteristic clinical features, and hepatocellular carcinoma was diagnosed by characteristic histologic findings.13
Initiation of antiviral therapy
For patients treated with antiviral agents before protocol enrollment, appropriate medical records were requested for documentation. Criteria for initiation of antiviral therapy after study entry generally followed the recommendations of the National Institutes of Health Consensus Statement on the Management of Hepatitis C,14 including the presence of abnormal ALT levels for at least 6 months and evidence from liver biopsy of portal or bridging fibrosis, with at least moderate inflammation and necrosis. A history of severe or ongoing depressive illness was considered a relative contraindication to therapy because of the potential exacerbation of symptoms by antiviral therapy.
Deceased patients
The medical records of the deceased patients with HCV infection were reviewed to assess the relationship between the cause of death and HCV infection. Because many of these patients received terminal care at SJCRH, extensive documentation was available and often included autopsy results. If the death occurred elsewhere, death certificates were requested and the reported cause of death was verified through telephone conversations with the local physician, the family, or both. When available, reports of postmortem examinations were reviewed. Causes of death were categorized as: 1, progressive primary malignancy; 2, complication of primary treatment (not HCV-related); 3, HCV-related complication; 4, accident or suicide; 5, other causes not related to cancer or treatment; or 6, unknown.
Results
Seroprevalence of HCV infection among survivors
Between 1961 and March 1992, 2311 patients received blood products at SJCRH while undergoing treatment for childhood cancer or aplastic anemia, and they currently survive (Table1). So far, 1521 (65.8%) of these patients have been screened for HCV, and 77 (6.6% of surviving patients tested) have evidence of HCV infection, with the highest proportion (10.7%) found in the patients who were diagnosed with primary malignancy or aplastic anemia before surrogate screening of blood donors. As expected, the number of infected patients declined after the introduction of surrogate marker (3.9%) and EIA-1 screening (0.5%) of blood donors, respectively. Finally, 4 additional patients who were transfused after March 1992 were infected with HCV and survive.
Results of HCV testing of surviving patients who received blood products
| Date of Cancer Diagnosis . | Number of Transfused Survivors . | Number Tested for HCV . | HCV-Infected* (% tested) . |
|---|---|---|---|
| Before 1986† | 1502 | 550 | 59 (10.7) |
| 1986 to 5/3/90‡ | 525 | 436 | 17 (3.9) |
| 5/4/90 to 3/12/921-153 | 284 | 189 | 1 (0.5) |
| Totals, 1961 to 3/12/921-155 | 2311 | 1175 | 77 (6.6) |
| Date of Cancer Diagnosis . | Number of Transfused Survivors . | Number Tested for HCV . | HCV-Infected* (% tested) . |
|---|---|---|---|
| Before 1986† | 1502 | 550 | 59 (10.7) |
| 1986 to 5/3/90‡ | 525 | 436 | 17 (3.9) |
| 5/4/90 to 3/12/921-153 | 284 | 189 | 1 (0.5) |
| Totals, 1961 to 3/12/921-155 | 2311 | 1175 | 77 (6.6) |
Positive EIA-2 and RIBA-2, or positive HCV RNA by PCR.
Before HCV screening of blood products.
Screening of blood products with serum ALT levels.
Screening of blood products with EIA-1.
Four additional patients, diagnosed and transfused after 3/12/92, are also infected with HCV.
HCV infection was confirmed by positive RIBA-2 and PCR results for 52 patients and by positive PCR alone for 17 patients with indeterminate (9 patients) or unavailable (8 patients) RIBA-2 results. Eight patients (9.9%) had a positive RIBA-2 result but did not have circulating HCV RNA, indicating possible resolution of HCV infection or a viral load of less than 200 copies/mL. For 3 patients with positive RIBA-2 results, PCR testing is unavailable. One patient with persistent ALT values during cancer therapy was diagnosed with HCV infection by PCR; the patient completed cancer therapy 6 months ago and has no detectable antibodies to HCV.
The median age of these seropositive patients is 21.2 years (range, 6.2 to 39.9 years). There are 41 (50.6%) females and 40 (49.4%) males; 65 (80.2%) are white, 11 (13.6%) are African American, 2 (2.5%) are Hispanic, and 3 (3.7%) are from other ethnic backgrounds. Primary diagnoses, established when the median age of the patients was 5.1 years (range, 1 month to 20.6 years), were leukemia (62 patients), lymphoma (2 patients), solid tumor (16 patients), and aplastic anemia (1 patient). All patients received at least 1 blood product transfusion, with a median of 10 (range, 1 to 101) transfusions and a median of 21 donor exposures (range, 1 to 483).
Deceased patients
Between 1961 and March 1992, 1456 patients received blood products at SJCRH while undergoing treatment for childhood cancer or aplastic anemia and have subsequently died. Only 346 (23.8%) of these patients have been tested for HCV, and 12 (3.5% of patients tested) had confirmed HCV infection (10 with positive PCR results; 2 with positive EIA-2 and RIBA-2 results yet unavailable PCR results). Causes of death included progressive primary disease (8 patients), primary therapy complications unrelated to HCV (2 patients), and accident (1 patient). One patient with chronic HCV infection died of liver failure at age 29, just 9 years after diagnosis of, and completion of therapy for, acute promyelocytic leukemia.
Two patients with anti-HCV by EIA-2 alone were diagnosed with HCC at 29 and 30 years of age (25 and 27 years after the primary malignancy diagnosis). These patients subsequently died from their secondary hepatic malignancy. Unfortunately, stored serum has not been found to confirm HCV infection by RIBA-2 or PCR.
Characteristics of patients enrolled in longitudinal protocol
Thus far, 65 patients with HCV infection have been enrolled in the longitudinal study. Among these 65 patients, treatment for primary malignancy involved chemotherapy alone (16 patients) chemotherapy and irradiation (29 patients), chemotherapy and surgical resection of tumor (11 patients), chemotherapy with irradiation and surgical resection of tumor (2 patients), chemotherapy and irradiation followed by allogeneic bone marrow transplantation (4 patients), chemotherapy followed by autologous bone marrow transplantation (2 patients), and other immunosuppressive therapy (1 patient).
Evaluation of HCV-related constitutional symptoms
For each symptom listed, the most common response regarding the level of discomfort associated with the symptom was “none”; selected results are listed in Table2. Further evidence of overall well-being was provided by asking patients to provide a single response that best described the extent to which these symptoms had interfered with daily activities and going to work or school during the previous week. Based on 60 responses, most (48%) patients stated that they had felt well and had been able to maintain normal activities, whereas 30% acknowledged some symptoms that were not severe enough to interfere with activities. Only 12% stated that constitutional symptoms had made performing daily activities difficult; 5% were sometimes unable to complete daily activities, and 5% had to take a day or more off from work or school. Preliminary analysis of the patients having the most difficulty with daily activities suggested no correlation to serum ALT or PCR results or histologic changes on liver biopsy. However, 2 of the 3 patients with cirrhosis reported problems completing daily tasks.
Assessment of symptoms interfering with daily activities
| Symptom . | Frequency of Response (% patients) . | ||||
|---|---|---|---|---|---|
| None . | A little bit . | Moderately . | Quite a bit . | Extremely . | |
| Fatigue | 34 | 33 | 14 | 9 | 9 |
| Abdominal or belly pain | 69 | 11 | 8 | 6 | 6 |
| Muscle aches or soreness | 58 | 22 | 9 | 5 | 6 |
| Dark urine | 66 | 25 | 8 | 1 | |
| Jaundice/yellowish eyes or skin | 91 | 6 | 3 | ||
| Itching | 77 | 13 | 3 | 5 | 3 |
| Feeling depressed, sad, or blue | 53 | 20 | 16 | 5 | 6 |
| Worrying too much about things | 34 | 23 | 21 | 10 | 11 |
| Symptom . | Frequency of Response (% patients) . | ||||
|---|---|---|---|---|---|
| None . | A little bit . | Moderately . | Quite a bit . | Extremely . | |
| Fatigue | 34 | 33 | 14 | 9 | 9 |
| Abdominal or belly pain | 69 | 11 | 8 | 6 | 6 |
| Muscle aches or soreness | 58 | 22 | 9 | 5 | 6 |
| Dark urine | 66 | 25 | 8 | 1 | |
| Jaundice/yellowish eyes or skin | 91 | 6 | 3 | ||
| Itching | 77 | 13 | 3 | 5 | 3 |
| Feeling depressed, sad, or blue | 53 | 20 | 16 | 5 | 6 |
| Worrying too much about things | 34 | 23 | 21 | 10 | 11 |
Evaluation of serum ALT and viral characteristics
Of the 65 enrolled patients, 58 had detectable HCV RNA at the time of study entry. At the most recent evaluation, or between protocol enrollment and the initiation of antiviral therapy, the median serum HCV RNA concentration was 381 229 copies/mL (range, 3476 to 2 433 354 copies). Seventeen patients had elevated serum ALT levels (median, 1.51 times the upper limit of normal; range, 1.02 to 2.90 times the upper limit of normal), all of whom had detectable HCV RNA. Serum genotype testing was performed on all patients with a positive PCR result, and genotypes 1a and 1b were most commonly detected (Table3). For 51 of the 58 patients only 1 isolate was identified, but 6 patients were infected with 2 or 3 different HCV subtypes; subtyping was unsuccessful for 1 patient. Of the 8 patients who were PCR-negative on study entry and had no history of receiving antiviral therapy, 5 had been retested and remained negative on 2 or 3 occasions that were separated by a median of 12 months (range, 12 to 17 months). Only 1 of the 65 patients was coinfected with HBV, and none were coinfected with HIV.
Serum genotypes detected among 58 study patients with detectable serum HCV RNA
| Genotype . | Number (%) of genotypes detected . |
|---|---|
| 1 (subtype not specified) | 3 (5.2) |
| 1A | 25 (43.1) |
| 1B | 16 (27.6) |
| 2A | 1 (1.7) |
| 2B | 5 (8.6) |
| 3A | 1 (1.7) |
| 1 (subtype not specified) and 1A | 1 (1.7) |
| 1A and 1B | 3 (5.2) |
| 2A and 2B and 2C | 1 (1.7) |
| 3A and 4A | 1 (1.7) |
| Unsuccessful | 1 (1.7) |
| Genotype . | Number (%) of genotypes detected . |
|---|---|
| 1 (subtype not specified) | 3 (5.2) |
| 1A | 25 (43.1) |
| 1B | 16 (27.6) |
| 2A | 1 (1.7) |
| 2B | 5 (8.6) |
| 3A | 1 (1.7) |
| 1 (subtype not specified) and 1A | 1 (1.7) |
| 1A and 1B | 3 (5.2) |
| 2A and 2B and 2C | 1 (1.7) |
| 3A and 4A | 1 (1.7) |
| Unsuccessful | 1 (1.7) |
Evaluation of liver histology
Thirty-five patients underwent liver biopsy (29 before and 6 after protocol enrollment) for evaluation of abnormal liver function or before the implementation of antiviral treatment. Biopsies were performed a median of 6 years (range, 2 months to 27 years) after the diagnosis of primary malignancy. These histologic specimens were reviewed and scored by our study pathologist (P.J.D.). Chronic active hepatitis was observed in 28 (80%) biopsies and was scored as mild in 17, mild to moderate in 8, and moderate in 3. Fibrosis was noted in 25 (71%) biopsies, whereas 3 (9%) biopsies indicated probable or definite cirrhosis. The 3 biopsies showing cirrhosis were performed 9.6, 20.2, and 27.1 years after diagnosis of the primary malignancy.
Response to antiviral therapy
Eleven patients received antiviral therapy with α-interferon (IFN) alone (10 before and 1 after study enrollment). Unfortunately, only 1 of the 11 patients treated with α-IFN alone had sustained clearance of HCV by PCR evaluation when tested at least 12 months after the discontinuation of therapy (median duration of therapy, 5 months; range, 4 to 12 months).
Four patients were treated with α-IFN and ribavirin after protocol enrollment. Three of these patients completed 12 months of therapy with undetectable HCV RNA, though all stopped therapy less than 6 months ago. The additional patient, currently treated with α-IFN and ribavirin, was negative for HCV RNA after 12 weeks of treatment.
Discussion
Our preliminary results indicate that a significant number of patients who received blood products during treatment for childhood cancer are infected with HCV. Furthermore, because most have normal liver function and few or no symptoms, routine screening of at-risk patients is necessary to identify those infected with HCV. Finally, though most patients with chronic infection have features suggestive of mild liver disease, a subset of patients will eventually progress to clinically significant liver disease. Long-term studies of our surviving cohort should lead to a better estimation of this risk and enable better understanding of the variables contributing to this progression.
HCV infects hosts primarily by parenteral contact with infected blood or bodily secretions, with the highest risk associated with repeated, direct parenteral inoculations of virus, such as blood product transfusions administered before blood donors were routinely tested for HCV. However, the risk for acquiring HCV infection from blood products has decreased significantly since the initiation of routine blood donor screening. Among 2410 patients studied by Donahue et al,153.8% of patients transfused for cardiac surgery before blood donor screening for HCV were HCV− seropositive. After the implementation of surrogate marker screening in 1986 and first-generation anti-HCV screening, this risk declined to 1.5% and 0.6%, respectively. This study also noted a strong correlation between the volume of blood infused and the risk for HCV infection, which likely explains the higher prevalence of HCV infection among our patients tested before anti-HCV testing was available. Geographic variations in HCV seroprevalence may account for the lower risk for HCV infection among our patients tested so far, when compared with risks ranging from 17.8% to 57% in other childhood cancer survivor cohorts.4-6 10 However, because we have not yet tested all patients, the final rate of HCV seropositivity among our patients may be considerably different from that presented in this preliminary report.
The ability to predict HCV outcomes for chronically infected patients is limited because of the inconsistent correlation of histologic findings from liver biopsies with clinical factors and routine laboratory values. For adults, the clinical symptoms of chronic HCV infections are generally nonspecific. Malaise and fatigue are most commonly reported, followed by nausea, abdominal pain, myalgia, and arthritis.12 However, neither the frequency nor the severity of these symptoms seems to correlate with the magnitude of liver disease indicated by subsequent laboratory and histologic evaluations.2 Some investigators have shown a significant correlation between ALT level and liver histology in patients with more advanced HCV-related liver disease,16,17 whereas others have failed to identify a consistent relationship.2,18,19Conversely, the correlation between viremia level, as measured by PCR, and liver histology appears to be strong, though some patients who repeatedly test negative by PCR have detectable HCV in the liver.20 Given the limitations of noninvasive tests, liver biopsy is the most accurate method of evaluating the extent of HCV-related liver disease. Biopsy can also detect and exclude other lesions, measure necroinflammatory activity (grade), follow the progression of liver disease (stage), and evaluate the effectiveness of therapy.12 Unfortunately, the fact that liver biopsies are not consistently performed has limited our ability to interpret studies, including our own, designed to define the natural history of HCV infection. Previously, we were reluctant to request that our patients undergo liver biopsy primarily because a safe and widely effective therapeutic intervention was unavailable. However, with the more recent success of combination antiviral therapy for HCV, our patients are undergoing liver biopsy with increasing frequency.
Variables must be identified that can better predict which patients with HCV are at the highest risk for advanced liver disease. Age at the time of infection appears to be 1 such variable, as suggested by investigators who have observed a higher risk for cirrhosis in patients infected with HCV at an older age.21,22 A recent study of 2235 HCV-infected patients determined that infection in those older than 40 years was significantly associated with more rapid development of liver fibrosis.22 Conversely, fibrosis developed more slowly in patients 20 years of age or younger, whereas the 19 patients who were younger than 10 years had a very low rate of fibrosis. Finally, Vogt et al23 studied 458 patients who had been transfused for cardiac surgery at a mean of 2.8 years of age. There were 67 (14.6%) patients who were seropositive for HCV, though only 55% of these patients had PCR evidence of chronic HCV infection. Of the 17 chronically infected patients who underwent liver biopsy, only 3 had histologic signs of liver disease. The paucity of additional studies evaluating long-term outcomes of patients chronically infected with HCV during childhood limits further conclusions about the relationship between age at time of infection and HCV-related liver outcomes.
The risk for significant liver disease after transfusion-acquired HCV infection during treatment for childhood cancer is unknown. However, the natural history of HCV infection in this cohort may be significantly different than it is in other pediatric cohorts because cancer therapy may significantly alter the initial immune response to HCV. Specifically, whereas specific antibodies against structural and nonstructural HCV proteins develop in most adults with acute HCV infection,24,25 it appears that they do not develop in survivors of pediatric cancer, at least during the initial infection.4,10 However, our data suggest that our patients may have cleared the HCV infection in a frequency similar to that for published adult cohorts,1 providing additional evidence that the production of HCV-specific antibodies has little impact on viral clearance during acute infection.26 Finally, treatment with hepatotoxic antineoplastic therapy, such as methotrexate and 6-mercaptopurine, may increase the risk for progressive liver disease. The hepatotoxic effect of these agents may be similar to that of alcohol, a significant risk factor for progression to clinically significant liver disease for adults infected with HCV.23 27-29
In the largest such study to date, significant liver disease had not developed in any of the 114 HCV-seropositive survivors of childhood leukemia at a mean of 17 years (range, 13 to 27 years) after the completion of cancer therapy.10 The predominance of HCV genotypes 1a and 1b in this study was similar to our own findings and is of uncertain prognostic significance.30-32 The significance of mixed genotypes is unknown. Another Italian study assessed 81 patients with positive PCR results after treatment for childhood leukemia, lymphoma, or solid tumors.5 Again, none of the 81 patients had liver failure a median of 14 years after completion of the primary therapy. In contrast to our study population, 35% of their patients studied were coinfected with HBV; coinfection with other hepatatrophic viruses, such as hepatitis A,33hepatitis B,34 and hepatitis G,35-39 may significantly influence the risk for progression of HCV-related liver disease. Conversely, a study of pediatric and adult survivors of leukemia suggested that these patients were at greater risk for liver disease than were control subjects.40
Previously, treatment with α-IFN alone was the only therapy available for patients with chronic HCV infection. Unfortunately, only approximately 10% of patients respond to this treatment, and side effects are often problematic.41-45 More recently, combination therapy with α-IFN and ribavirin has increased the response rate to nearly 40%.45 Although few patients in our cohort have been treated, the clearance of viremia by combination antiviral therapy is encouraging, but additional follow-up is needed to determine the duration of the response.
In summary, these preliminary findings suggest that survivors of childhood cancer who acquire chronic HCV infection from blood product transfusions are at risk for clinically significant liver disease. Our cohort, when fully assembled, will be 1 of the largest of its kind available for the study of HCV infection in long-term survivors of childhood cancer. Studies of this cohort should increase our understanding of the impact of variables such as age, immunosuppressive therapy, and hepatotoxic chemotherapy and irradiation on the outcome of HCV infection. In addition, our ability to correlate histologic findings and clinical, laboratory, and diagnostic imaging variables to define the risk for progression to clinically significant liver disease should be enhanced.
Acknowledgments
The authors thank Gerald Sharp for organizing the longitudinal protocol, Pamela Branch and Sheila Wilson for data management, Ted Pearson and Margaret Griffith for laboratory testing, and Flo Witte for manuscript editing.
Supported by the National Cancer Institute Cancer Center (grant nos. ROI CA85891-01 and P30 CA21765) and by the American-Lebanese-Syrian Associated Charities.
Reprints:Donald K. Strickland, Department of Hematology-Oncology, St. Jude Children's Research Hospital, 332 North Lauderdale, Memphis TN 38105-2794; e-maildonald.strickland@stjude.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal