Abstract
For many cancers, autologous bone marrow transplantation (BMT) achieves a minimal residual disease state, yet relapse rates remain high. Using a syngeneic murine bone marrow transplant model, we demonstrate that vaccination with irradiated granulocyte-macrophage colony-stimulating factor (GM-CSF)–producing autologous tumor cells is effective in the post-BMT period and actually results in a greater tumor-free survival than vaccination in the nontransplant setting. Employing T cells specific for a model tumor-antigen, we find that transplantation of the tumor-bearing host results in a massive expansion and activation of tumor-specific T cells in the early posttransplant period, but this response rapidly declines in association with tumor progression. Immunization with irradiated GM-CSF tumor cells during the period of immune reconstitution results in the sustained amplification and activation of this response that closely correlates with freedom from relapse. These results demonstrate the feasibility of integrating GM-CSF vaccines in the postautologous BMT setting and suggest mechanisms that may contribute to the observed efficacy of immunization during the critical period of immune reconstitution.
The past several decades have seen considerable advances in the treatment of hematologic malignancies. These are in large part attributable to the significant impact conferred by bone marrow transplantation (BMT). Dose escalation of cytotoxic therapy, together with improvements in supportive care, has resulted in prolonged disease-free survival and reduced transplant-related mortality. Despite this progress, a significant proportion of patients transplanted for the treatment of hematologic malignancies will eventually relapse and die of their disease.
A growing body of evidence suggests that immune-mediated effects may contribute to tumor cell killing in patients with leukemia, lymphoma, and multiple myeloma. In fact, the reduced relapse rates observed in the setting of allogeneic transplantation when compared with autologous BMT is likely the result of immune-mediated “graft-versus-tumor” effects. Unfortunately, because of the lack of tumor specificity of the allo-response, what is gained by a reduction in relapse rates is lost in the morbidity and mortality of graft-versus-host disease (GVHD) and its treatment. Indeed, efforts to reduce the incidence and severity of GVHD, such as more rigorous T-cell depletion of the grafts or more intensive systemic immunosuppression of the recipient, are likely to also reduce the magnitude of the allogeneic graft-versus-tumor effect. Furthermore, the lack of a suitable donor or the advanced age of the patient often precludes the option of allogeneic translantation. In contrast, autologous transplantation has a relatively favorable safety profile, while preserving the benefit of chemoradiation used at doses beyond what can be given without stem cell support. Accordingly, efforts to augment host antitumor immunity in the setting of autologous transplantation may provide a means to diminish relapse rates without a concomittant increase in toxicity.
Priming of systemic, tumor-specific immunity with vaccines against tumor-associated antigens holds significant promise as a therapeutic strategy. Animal models have demonstrated that immune responses can be generated capable of eradicating small preestablished tumor burdens, and early-phase clinical trials currently examining the clinical efficacy of therapeutic cancer vaccines have reported the induction of immune responses that are qualitatively similar to that observed in mouse models.1-5 Despite the enthusiasm for this approach, substantial evidence suggests that the effect of active immunotherapy may be limited in the presence of advanced disease.6-8 Therefore, immunization in the adjuvant setting may have the greatest potential to impact on tumor progression. For many malignancies, especially those of hematologic origin, myeloablative chemotherapy and autologous BMT may offer the best chance of achieving a state of minimal residual disease. Unfortunately, the period of immune reconstitution following BMT has been characterized as a time-decreased responsiveness to antigenic stimulation.9Nevertheless, preclinical models10 and some clinical trials11-14 have demonstrated the capacity of the transplant recipient to respond to several different vaccine formulations, underscoring the potential to manipulate host antitumor immunity early during immune reconstitution. Indeed, the normal homeostatic mechanisms that regulate adaptive immunity are profoundly altered in the early posttransplant period, potentially leading to augmented clonal expansion of antigen-specific lymphocytes upon priming, “skewing” of thereconstituting T-cell repertoire toward recognition of tumor-specific antigens,15 and enhanced response to vaccination through the disruption of host tolerogenic mechanisms.
We have used a mouse B-cell lymphoma model to develop strategies that seek to integrate granulocyte-macrophage colony-stimulating factor (GM-CSF)–based tumor cell vaccines in the postautologous BMT setting. Immunization with irradiated autologous tumor cells engineered to secrete GM-CSF has been shown to induce specific and long-lasting antitumor immunity even against poorly immunogenic tumor models when administered as a therapeutic vaccine in the treatment of small established tumor burdens.16 We find that vaccination with irradiated GM-CSF–producing autologous tumor cells is effective in the post-BMT period and actually results in a greater degree of tumor-free survival than is achieved following vaccination in the nontransplant setting. Mature T cells accompanying the graft participate substantially in this response. In a minimal residual disease model, tumor-specific T cells undergo a massive clonal expansion and activation in the early posttransplant period, which precipitously declines in close temporal association with the development of macroscopic relapse. Vaccination with irradiated GM-CSF–producing tumor cells during immune reconstitution substantially decreased the incidence of tumor relapse and was accompanied by the persistence of an expanded population of activated tumor-specific T cells. These studies suggest that a “graft-versus-tumor effect” also occurs in the autologous BMT setting, but it is not sustained. Repeated immunizations during immune reconstitution may serve to maintain the increased precursor frequency and activation state of tumor-specific T cells that is required to prevent relapse.
Materials and methods
Mice
Six- to eight-week-old BALB/c mice or BALB/c athymic nude mice were obtained from the National Institutes of Health (Frederick, MD). T-cell receptor (TCR) transgenic mice expressing an αβTCR specific for influenza hemagglutinin (HA) peptide (amino acids 110-120) presented by I-Ed were a generous gift of Harald von Boehmer.17 All experiments involving the use of mice were performed in accordance with protocols approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Tumors cells
A20 cells were obtained from the American Type Culture Collection (Rockville, MD). Cells were cultured in vitro in RPMI 1640 media, supplemented with 10% fetal calf serum, penicillin/streptomycin (50 U/mL), L-glutamine (2 mM), and 2-mercaptoethanol (50 mM), and grown in suspension at 37°C, 5% CO2. Electroporation of A20 cells was used for plasmid transfection in the creation of A20HA as previously reported.18
Syngeneic bone marrow transplantation
The femurs and tibiae were obtained from 6- to 8-week-old donor BALB/c mice, and BM was harvested by flushing the bones with RPMI at 4°C. The marrow was treated with Low-Tox-M rabbit complement (Cedarlane Laboratories) for 30 minutes at 37°C in the presence of monoclonal antibody (MAb) J1J (anti-Thy1), MAb C3PO (anti-CD2), MAb RL172 (anti-CD4), and MAb 3-155 (anti-CD8) to obtain a T-cell–depleted BM. Single-cell suspensions were obtained from the spleens following FicollHypaque centrifugation. The graft consisted of 4 × 106 BM cells with or without the addition of 4 × 107 splenocytes (as indicated). Recipients were 6- to 8-week-old BALB/c mice that were irradiated with 850 cGy, followed by intravenous injection of the graft in a volume of 0.2 mL. The transplanted animals were maintained in sterile micro-isolator cages and received sterile food and water. Overall transplant-related mortality was less than 5%.
Tumor purging of donor bone marrow and spleen
In the indicated experiments, donor BM and splenocytes were depleted of A20 tumor by incubating with either an anti-IgG2a-biotin (Pharmingen, San Diego, CA) only or IgG2a-biotin, RA3.3A1-biotin (anti-B cell surface glycoprotein, B220), and 14.4.4-biotin (anti-I-Ed) for 30 minutes at 4°C in 2% fetal calf serum in phosphate-buffered saline. The cells were then incubated with streptavidin-conjugated Dynabeads (Dynal, Oslo, Norway) at a ratio of 4 beads/cell for 1 hour at 4°C and depleted by magnetic separation.
Adoptive transfer
Single-cell suspensions were made from peripheral lymph nodes and spleen that were harvested from TCR transgenic donors. The percentage of CD4+, TCR clonotype-positive lymphocytes was determined by flow cytometry as described below. Nontransplanted mice received 2.5 × 106 CD4+ anti-HA TCR T cells. In the BMT animals, the clonotypic T cells composed 1% of the splenic component of the graft.
Vaccine preparation and administration
A20/GM-CSF and A20HA/GM-CSF were created by retroviral transduction using the retroviral construct MFG-mGM-CSF as previously described.16 The cells were washed 3 times in sterile Hank's balanced salt solution, irradiated with 5000 cGy, and injected subcutaneously in the right flank (1 × 106cells/0.1 mL).
Tumor survival experiments
All live tumor challenge with A20 wild-type or A20HA cells occurred via tail vein injection. In this model of systemic lymphoma, tumor traffics to spleen, mesenteric lymph nodes, liver, and can be found in blood and marrow at late stages. Progressive tumor is detected by the presence of increased abdominal girth and palpable splenomegaly, which is confirmed at autopsy by direct visualization. Ten mice were included per group, including unvaccinated controls, and each survival experiment was repeated at least once. Statistical analysis was performed using Kaplan-Meier survival and the log-rank (Mantel-Cox) test. Starview 4.5 software (San Francisco, CA) was used for the analysis.
Flow cytometric analysis
T cells were stained with fluorescein isothiocyanate–conjugated goat antimouse CD4 (Caltag, Burlingame, CA) and biotinylated rat anticlonotypic TCR MAb 6.5, followed by phycoerythrin-conjugated streptavidin (Caltag). A total of 50 000 gated events were collected on a FACScan (Becton Dickinson, San Jose, CA). Data represent the mean + SEM of the percentage of cells expressing the clonotypic TCR. Background staining of splenocytes from naı̈ve BALB/c mice was less than 0.10%.
γ-interferon release
T cells enriched by nylon wool purification (5 × 104 cells/well) were mixed with fresh splenocytes (8 × 104 cells/well) from a naı̈ve BALB/c mouse to which 12.5 μg/mL of synthetic HA peptide (amino acids 110-120; SFERFEIFPKE) was or was not added. The supernatants were collected 48 hours later, and γ-interferon (IFN) production was measured by enzyme-linked immunosorbent assay (ELISA; R & D Systems, Minneapolis, MN). Values for T cells cultured in media alone were less than 10% of the values for HA peptide–stimulated T cells.
Results
Kinetics of immune reconstitution and responsiveness to posttransplant immunization with irradiated GM-CSF–producing tumor cells
The immediate posttransplant period is accompanied by significant immunosuppression likely resulting from the profound reduction in the number of normal immune effector cells. We sought to determine the point in time after BMT that a therapeutic vaccination could elicit an effective antitumor response. To directly compare the response to vaccination in the transplant versus nontransplant setting, we first established a model in which tumor was not present until after the recipient was irradiated, so that any differences observed between transplanted and nontransplanted mice would reflect the capacity of the immune system to respond to an equivalent tumor challenge. Transplants were staggered at weekly intervals (10 mice per group), followed by the intravenous challenge of all mice with 1 × 105BALB/c-derived lymphoma cells (A20 wild type). Five days after tumor challenge, mice were vaccinated subcutaneously with 1 × 106 irradiated autologous tumor cells transduced to express GM-CSF (A20/GM-CSF). Normal BALB/c mice not having undergone a BMT were also challenged with this same tumor dose and vaccinated in an identical fashion (Figure1A). Similar to our previously reported experience,18 vaccination of the nontransplanted cohort 5 days after tumor challenge conferred a 40% survival advantage over the nonvaccinated group (P < .04). In the BMT setting, early tumor challenge and vaccination (weeks 1 and 2) did not result in significant tumor rejection. However, a substantial antitumor effect of vaccination was observed at the 3-week point (70% long-term tumor-free survival). Indeed, the survival of mice challenged and immunized 3 to 6 weeks after BMT (40 mice in total) actually exceeded the survival of the nontransplant group given the identical tumor challenge and vaccination scheme (P < .03). A parallel kinetic analysis of lymphoid recovery revealed that the absolute number of CD4+ T cells (from pooled peripheral lymph nodes) 3 weeks post-BMT was less than 20% of that present in untransplanted (“normal”) BALB/c mice, and CD8+ T cells were 33% of normal. By 6 weeks post-BMT, CD4+ T cells were 66% of normal and CD8+ T cells were 50% of normal. These results therefore demonstrate that a substantial response to this form of immunization can be generated prior to full immune reconstitution.
Posttransplant vaccination generates a more effective antitumor immune response than vaccination in the nontransplant setting.
(A) BALB/c mice underwent a syngeneic BMT from non–tumor-bearing donors consisting of 4 × 106 T-cell–depleted BM cells plus 4 × 107 splenocytes as described in “Materials and methods.” Nontransplanted mice were included for comparison. At the indicated times post-BMT, mice were challenged with A20 tumor (1 × 105 intravenously) and vaccinated 5 days later by a subcutaneous injection of irradiated A20/GM-CSF (1 × 106). Mice were followed twice a week for the presence of tumor, which was confirmed at autopsy. (B) Mice underwent syngeneic BMT with grafts consisting of 4 × 106 T-cell–depleted marrow alone or 4 × 106 T-cell–depleted marrow plus 4 × 107 splenocytes as indicated. Three weeks after transplantation, they were challenged with a 10-fold greater dose of A20 tumor (1 × 106), followed 5 days later by subcutaneous vaccination with irradiated A20/GM-CSF (1 × 106). Nontransplanted mice received the same tumor challenge and vaccination.
Posttransplant vaccination generates a more effective antitumor immune response than vaccination in the nontransplant setting.
(A) BALB/c mice underwent a syngeneic BMT from non–tumor-bearing donors consisting of 4 × 106 T-cell–depleted BM cells plus 4 × 107 splenocytes as described in “Materials and methods.” Nontransplanted mice were included for comparison. At the indicated times post-BMT, mice were challenged with A20 tumor (1 × 105 intravenously) and vaccinated 5 days later by a subcutaneous injection of irradiated A20/GM-CSF (1 × 106). Mice were followed twice a week for the presence of tumor, which was confirmed at autopsy. (B) Mice underwent syngeneic BMT with grafts consisting of 4 × 106 T-cell–depleted marrow alone or 4 × 106 T-cell–depleted marrow plus 4 × 107 splenocytes as indicated. Three weeks after transplantation, they were challenged with a 10-fold greater dose of A20 tumor (1 × 106), followed 5 days later by subcutaneous vaccination with irradiated A20/GM-CSF (1 × 106). Nontransplanted mice received the same tumor challenge and vaccination.
This experiment was repeated using a 10-fold greater tumor challenge, focusing on the earliest time post-BMT that measurable vaccine-induced tumor rejection was observed (ie, tumor challenge at 3 weeks, vaccination 5 days later). The response to vaccination was compared in (a) untransplanted mice, (b) mice transplanted with T-cell–depleted marrow cells alone, or (c) mice transplanted with T-cell–depleted marrow plus splenocytes (Figure 1B). With this larger tumor burden, therapeutic vaccination was completely ineffective in the nontransplant setting (open vs closed triangles). Interestingly, even in the absence of vaccination, transplantation with marrow plus splenocytes (open squares) resulted in a delay in tumor growth (by about 20 days) as compared with transplantation with marrow alone (open circles), although all unvaccinated mice ultimately relapsed. In contrast, vaccination of mice post-BMT resulted in some long-term tumor-free survivors, with the greatest antitumor effect observed in vaccinated mice transplanted with marrow plus splenocytes (closed squares) (P < .001 when compared with mice vaccinated without BMT). Taken together, these results suggest that the mature lymphocytes accompanying the graft can mediate a “syngeneic graft-versus-tumor effect,” as has been previously reported,19 and this effect can be augmented by posttransplant immunization.
The enhanced antitumor effect in BMT is T-cell dependent
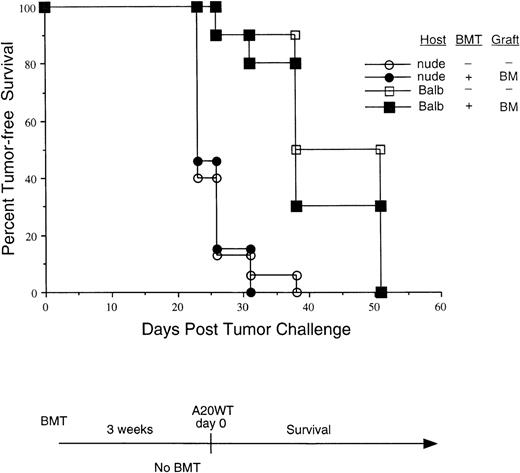
While multiple cellular effector mechanisms participate in the response to vaccination with irradiated GM-CSF–producing tumor cells,16,20,21 a functional T-cell reservoir is absolutely required for the successful induction of tumor rejection.22The early posttransplant period is characterized by profound alterations in the nascent immune repertoire, including that of B cells, NK cells, as well as T cells.9 To examine the contribution of T cells to tumor rejection in the BMT setting, transplants were carried out in either euthymic BALB/c mice or syngeneic athymic nude mice. In these experiments, the grafts consisted of T-cell–depleted BM alone, without the addition of mature T cells. Three weeks following BMT, these mice as well as unirradiated (nontransplanted) mice were challenged with A20WT tumor (1 × 106 intravenously) and followed for the kinetics of tumor progression (Figure2). Tumor progression was most rapid in nude mice, irrespective of their transplant status. Given that NK cell function has been shown to be normal or even exaggerated in nude mice, these results suggest that enhanced NK activity during the posttransplant period cannot completely account for the improved tumor rejection previously observed. Tumor progression in euthymic mice transplanted with T-cell–depleted marrow was delayed relative to nude mice (with or without T-cell–depleted BMT, P < .001), suggesting that recent thymic emigrants may mediate some degree of antitumor effect in this experiment (where tumor challenge occurred 21 days post-BMT). Nevertheless, in contrast to transplantation with marrow plus splenocytes (Figure 1), the kinetics of tumor growth in euthymic mice transplanted with T-cell–depleted marrow alone was virtually identical to that observed in untransplanted BALB/c mice. These results provide evidence for T-cell–mediated antitumor immunity during the early phases of immune reconstitution, although tumor ultimately progresses in the absence of immunization.
The enhanced antitumor response is T-cell dependent.
BALB/c mice or BALB/c athymic nude mice were transplanted as indicated. Grafts consisted of T-cell–depleted BM only. Three weeks following the BMT, these groups, as well as mice that did not undergo a transplant, were all challenged with live A20 tumor (1 × 106intravenously) and observed twice a week for the presence of tumor.
The enhanced antitumor response is T-cell dependent.
BALB/c mice or BALB/c athymic nude mice were transplanted as indicated. Grafts consisted of T-cell–depleted BM only. Three weeks following the BMT, these groups, as well as mice that did not undergo a transplant, were all challenged with live A20 tumor (1 × 106intravenously) and observed twice a week for the presence of tumor.
Enhanced response of tumor-antigen–specific T cells to vaccination during immune reconstitution
The above findings point to the repopulating T-cell compartment as a critical component of the antitumor response during the early posttransplant period. Given the favorable effects of vaccination with irradiated GM-CSF–transduced tumor cells during immune reconstitution, we wished to quantify the response of a defined tumor-specific T-cell population to vaccination in the transplant versus nontransplant settings. We used a well-characterized system employing the adoptive transfer of TCR transgenic CD4+ T cells specific for an MHC class II (I-Ed) restricted epitope of influenza HA. BALB/c mice underwent a syngeneic transplant consisting of T-cell–depleted BM plus splenocytes containing 1% HA-specific clonotypic T cells. Also present were nontransplanted (unirradiated) animals into which clonotypic T cells were adoptively transferred, as well as animals that underwent BMT in the absence of transgenic T cells. We examined the response of HA-specific T cells to immunization with a GM-CSF–producing tumor vaccine transfected to coexpress the model antigen (A20HA/GM-CSF). As a specificity control, a cohort of mice was vaccinated with A20/GM-CSF, which does not express HA. All animals were vaccinated 1 day following transplant (or adoptive transfer) and analyzed 14 days later. As we have previously observed in the nontransplant setting, priming with an irradiated GM-CSF–producing tumor vaccine expressing this model antigen fails to elicit a demonstrable clonal expansion of HA-specific T cells (Figure3). In contrast, immunization with the identical vaccine in the posttransplant period resulted in a significant clonal expansion of antigen-specific T cells that accompanied the graft. Although T-cell repopulation of the peripheral compartment ultimately “dilutes” this percentage of clonotype-positive T cells, an expanded population of memory T cells was detectable 6 weeks after immunization (data not shown). These findings indicate that, even in the immediate posttransplant period, the host is capable of mounting a response to vaccination with irradiated GM-CSF–producing tumor cells as reflected in the antigen-specific clonal expansion of T cells that accompany the graft. Furthermore, radiation-induced changes in the recipient early after BMT permit a greater “burst” of T-cell expansion in response to immunization than occurs in immunized, nonirradiated mice.
Enhanced clonal expansion of tumor-specific T cells in response to vaccination post-BMT.
BALB/c mice underwent a syngeneic BMT in which the splenic component of the graft contained a final concentration of 1% HA-specific TCR transgenic T cells (double positive for CD4 and clonotype-positive TCR by FACS). For comparsion, nontransplanted (unirradiated) mice received a comparable number of transgenic T cells. One day following the transplant (or T cell transfer), the indicated groups were vaccinated subcutaneously with 1 × 106 irradiated GM-CSF–producing tumor cells that either expressed the model antigen (A20HA/GM-CSF) or did not (A20/GM-CSF). Mice were killed 14 days later, and purified splenic T cells were analyzed by 2-color flow cytometry staining for CD4 versus anti-HA TCR clonotype. Values represent the mean + SE of percentage of T cells expressing the clonotypic TCR for 3 mice per group.
Enhanced clonal expansion of tumor-specific T cells in response to vaccination post-BMT.
BALB/c mice underwent a syngeneic BMT in which the splenic component of the graft contained a final concentration of 1% HA-specific TCR transgenic T cells (double positive for CD4 and clonotype-positive TCR by FACS). For comparsion, nontransplanted (unirradiated) mice received a comparable number of transgenic T cells. One day following the transplant (or T cell transfer), the indicated groups were vaccinated subcutaneously with 1 × 106 irradiated GM-CSF–producing tumor cells that either expressed the model antigen (A20HA/GM-CSF) or did not (A20/GM-CSF). Mice were killed 14 days later, and purified splenic T cells were analyzed by 2-color flow cytometry staining for CD4 versus anti-HA TCR clonotype. Values represent the mean + SE of percentage of T cells expressing the clonotypic TCR for 3 mice per group.
Grafts from tumor-bearing donors can mediate a graft-versus-tumor effect
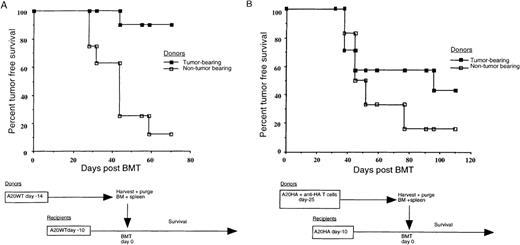
The above results demonstrate that mature T cells accompanying the graft can participate in an endogenous graft-versus-tumor effect and are responsive to posttransplant vaccination. However, these experiments using tumor-free syngeneic donors may not accurately reflect alterations in T-cell function that could exist in grafts obtained from a tumor-bearing host. To more closely model the autologous transplant setting, we examined the effect of established tumor in the syngeneic donors. BALB/c mice to be used as donors were given 1 × 106 A20 cells 14 days prior to graft harvest or were left tumor-free. On the day of transplantation, donor marrow and splenocytes were harvested and “purged” of lymphoma cells by magnetic separation. Transplant recipients were challenged with 1 × 106 A20 cells 10 days prior to BMT, which was performed using grafts obtained from the tumor-bearing or non–tumor-bearing donors. This tumor burden, established prior to transplantation, utimately results in tumor progression in most mice reconstituted with grafts from naı̈ve donors (Figure 4A). Surprisingly, however, grafts obtained from donor mice harboring A20 cells for 14 days prior to harvest actually mediated a substantial antitumor effect upon reconstitution of tumor-bearing recipients (P < .001). Furthermore, this unexpected result occurred in the face of incomplete elimination of tumor from the donor grafts, as demonstrated by the outgrowth of “contaminating” A20 cells upon in vitro culture of an aliquot of the graft after purging. Despite this, transplantation of most tumor-bearing recipients (as well as 5 of 5 non–tumor-bearing recipients—data not shown) did not result in tumor growth in vivo, suggesting that failure to completely eliminate tumor from the graft does not preclude long-term tumor-free survival of the recipient. These results suggest that some fraction of the donor lymphocytes from tumor-bearing mice are primed in response to tumor-antigen and remain responsive upon transplantation. While it is possible that the tumor-bearing donors in this experiment were not fully tolerant to A20 antigens, the tumor burden present when they were harvested (1 × 106 A20 cells given 14 days earlier) significantly exceeds what can be cured by vaccination alone in the nontransplant setting (Figure 1B).
Grafts from tumor-bearing donors can mediate a graft-versus-tumor effect.
(A) Tumor-bearing BALB/c donor mice were challenged 14 days prior to graft harvest, and all recipients were challenged 10 days prior to transplant with 1 × 106 A20 wild-type tumor cells given intravenously. BM and spleens were harvested from the donors, and tumor was depleted as described in “Materials and methods.” Recipient mice were irradiated, reconstituted with the purged grafts, and followed twice weekly for the progression of tumor. (B) Donor BALB/c mice received 2.5 × 106anti-HA–specific T cells with or without a tumor challenge (1 × 106 A20HA cells intravenously) 25 days before graft harvest. BM and spleens from both groups were harvested and tumor depleted as described. Recipient mice were challenged with 1 × 106 A20HA cells 10 days before being irradiated, transplanted with the purged grafts from non–tumor-bearing or non–tumor-bearing donors, and followed for tumor progression.
Grafts from tumor-bearing donors can mediate a graft-versus-tumor effect.
(A) Tumor-bearing BALB/c donor mice were challenged 14 days prior to graft harvest, and all recipients were challenged 10 days prior to transplant with 1 × 106 A20 wild-type tumor cells given intravenously. BM and spleens were harvested from the donors, and tumor was depleted as described in “Materials and methods.” Recipient mice were irradiated, reconstituted with the purged grafts, and followed twice weekly for the progression of tumor. (B) Donor BALB/c mice received 2.5 × 106anti-HA–specific T cells with or without a tumor challenge (1 × 106 A20HA cells intravenously) 25 days before graft harvest. BM and spleens from both groups were harvested and tumor depleted as described. Recipient mice were challenged with 1 × 106 A20HA cells 10 days before being irradiated, transplanted with the purged grafts from non–tumor-bearing or non–tumor-bearing donors, and followed for tumor progression.
Given the above, we wished to examine the effect of a more extensive tumor burden on the donor and to document tumor-antigen–specific T-cell unresponsiveness in the graft. The experiment was therefore repeated using a model tumor-antigen system. BALB/c mice to be used as donors were injected intravenously with 2.5 × 106anti-HA TCR transgenic T cells with or without 1 × 106 A20 cells stably transfected to express HA (A20HA). Twenty-five days later, grafts were harvested from these donors. At this time, donor mice that had been challenged with A20HA were found to have an extensive tumor burden, with 3- to 5-mm lymphoma nodules studding the surface of the liver and spleen and confluent tumor masses effacing the mesenteric lymph nodes. HA-specific T-cell responsiveness was assessed in tumor-bearing versus non–tumor-bearing mice in a subgroup of donors that were randomly removed from the donor cohort. Specifically, 6 days prior to graft harvest, 3 tumor-challenged and 3 non–tumor-challenged mice were injected with a recombinant vaccinia virus encoding HA, and the response to immunization was monitored by measuring clonal expansion of HA-specific T cells and proliferation and γ-IFN release in response to HA peptide in vitro. As we have previously documented in this system,23 24 each of these parameters of T-cell response to vaccination was markedly impaired in mice harboring A20HA as compared with tumor-free donors (data not shown). Nevertheless, despite the extensive tumor burden of the donors and evidence for impaired tumor-antigen–specific T-cell function, purged grafts from tumor-bearing donors again conferred an antitumor effect when used to transplant recipient mice harboring A20HA for 10 days prior to BMT (Figure 4B).
Endogenous activation of tumor-specific T cells during immune reconstitution
Given the evidence for a T-cell–mediated graft-versus-tumor effect, we sought to examine the fate of tumor-antigen–specific T cells in the tumor-bearing transplant recipient during immune reconstitution. We employed a model in which tumor was established prior to transplantation to examine the consequences of radiation-induced tumor cell killing (and antigen release) as well as the effect of residual tumor present throughout the period of immune reconstitution. BALB/c mice were challenged with 1 × 106 A20HA cells 10 days prior to transplant or were left tumor-free. Following irradiation, they received grafts containing HA-specific TCR transgenic T cells. For comparison, nontransplanted (unirradiated) BALB/c mice with or without the same tumor challenge received an equivalent number of mature TCR transgenic T cells. Three weeks after BMT (or T cell transfer), mice were killed, and the percentage of HA-specific, clonotype-positive T cells was analyzed by fluorescence-activated cell sorter (FACS) (Figure 5). As we have previously reported, in the absence of BMT there was a modest increase in the percentage (from 0.45% to 0.78%) of clonotype-positive T cells upon adoptive transfer into tumor-bearing mice (Figure 5A). Despite this, these cells have been shown to have a markedly diminished capacity to proliferate and produce interleukin-2 and γ-IFN in response to the nominal peptide antigen in vitro.23 In contrast to the nontransplanted tumor-bearing mice, there was a dramatic clonal expansion of HA-specific CD4+ T cells in transplanted mice harboring A20HA (from 0.57% to 13.78%). The magnitude of this expansion far exceeded what we have previously observed with any antigen-specific vaccine strategy in the nontransplant setting and is substantially greater than the response of non–tumor-bearing transplanted mice to vaccination with irradiated A20HA/GM-CSF cells alone (Figure 3). Notably, at the time of this analysis (day 21 post-BMT), there was no macroscopic evidence of lymphoma present in the transplanted mice.
Tumor-specific T cells undergo endogenous activation during immune reconstitution of the tumor-bearing transplant recipient.
BALB/c mice were challenged intravenously with 1 × 106 A20HA cells or remained tumor-free. Ten days later, half the mice were irradiated and transplanted with T-cell–depleted BALB/c marrow mixed with marrow from HA-specific TCR transgenic mice (10:1 ratio), plus splenocytes containing 1% CD4+ TCR clonotype-positive transgenic T cells. The other half received a similar number of HA-specific TCR transgenic T cells without irradiation. The animals were killed 21 days later. Nylon wool–purified splenic T cells were analyzed by 2-color flow cytometry staining for CD4 versus anti-HA TCR clonotype. Three mice were included per group. Shown are representative FACS plots from individual mice in each group.
Tumor-specific T cells undergo endogenous activation during immune reconstitution of the tumor-bearing transplant recipient.
BALB/c mice were challenged intravenously with 1 × 106 A20HA cells or remained tumor-free. Ten days later, half the mice were irradiated and transplanted with T-cell–depleted BALB/c marrow mixed with marrow from HA-specific TCR transgenic mice (10:1 ratio), plus splenocytes containing 1% CD4+ TCR clonotype-positive transgenic T cells. The other half received a similar number of HA-specific TCR transgenic T cells without irradiation. The animals were killed 21 days later. Nylon wool–purified splenic T cells were analyzed by 2-color flow cytometry staining for CD4 versus anti-HA TCR clonotype. Three mice were included per group. Shown are representative FACS plots from individual mice in each group.
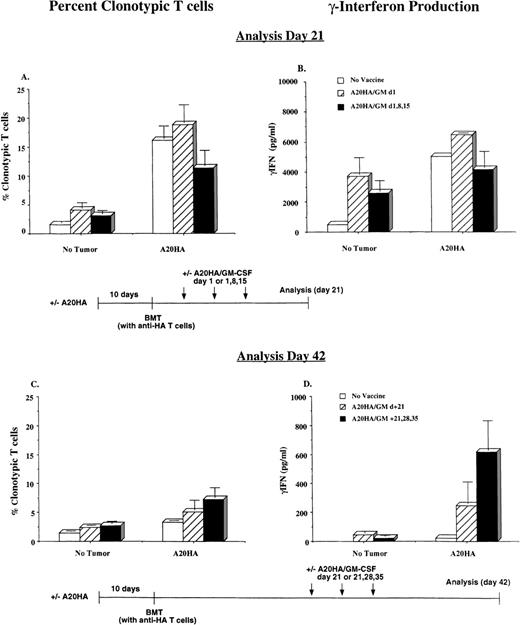
Immunization with irradiated GM-CSF–producing tumor cells during immune reconstitution sustains the activation of tumor-specific T cells
The clonal expansion of tumor-specific T cells illustrated in Figure5 occurred in a setting where parallel survival experiments demonstrated that tumor would ultimately progress in all unvaccinated mice. We therefore wished to examine the fate of these cells at a later time point as well as to determine the impact of posttransplant immunization on the state of tumor-specific T-cell activation. The experimental design was as described for Figure 5, with the addition of treatment groups that were immunized with irradiated A20HA/GM-CSF early (day +1 alone vs days +1, +8, +15, analysis day +21) or late (day +21 alone vs days +21, +28, +35, analysis on day +42). As before, 3 weeks after transplanting A20HA-bearing recipients there was a vigorous exansion of HA-specific CD4+ T cells (Figure 6A). The magnitude of this expansion was far greater than the effect of immunizing non–tumor-bearing transplant recipients with irradiated A20HA/GM-CSF. In fact, vaccination of tumor-bearing transplant recipients at the early time points did not result in a measurable increase in HA-specific T cell expansion beyond what occurred with transplantation alone. This clonal expansion was accompanied by substantial γ-IFN production in response to HA peptide in vitro, indicative of differentiation into T-helper 1 (Th-1) effector cells (Figure 6B). As before, 21 days after BMT no evidence of macroscopic lymphoma was evident at dissection.
Vaccination during immune reconstitution effectively sustains tumor-specific T-cell activation in tumor-bearing recipients.
BALB/c were challenged intravenously with 1 × 106A20HA cells or remained tumor-free. Ten days later, all mice were irradiated and transplanted with a graft as described in Figure 5. Mice were vaccinated with 1 × 106 irradiated A20HA/GM-CSF cells subcutaneously in the left flank on the days indicated and were killed for analysis 21 days (A and B) or 42 days (C and D) post-BMT. Three mice were included per group for each time point. (A and C) Percentage of HA-specific CD4+ TCR clonotype-positive T cells as analyzed by 2-color flow cytometry. (B and D) γ-IFN production in response to a 48-hour incubation with HA peptide, as measured by ELISA. Values are the mean + SE. Values for T cells cultured in the absence of HA peptide were below the limit of detection for the ELISA kit.
Vaccination during immune reconstitution effectively sustains tumor-specific T-cell activation in tumor-bearing recipients.
BALB/c were challenged intravenously with 1 × 106A20HA cells or remained tumor-free. Ten days later, all mice were irradiated and transplanted with a graft as described in Figure 5. Mice were vaccinated with 1 × 106 irradiated A20HA/GM-CSF cells subcutaneously in the left flank on the days indicated and were killed for analysis 21 days (A and B) or 42 days (C and D) post-BMT. Three mice were included per group for each time point. (A and C) Percentage of HA-specific CD4+ TCR clonotype-positive T cells as analyzed by 2-color flow cytometry. (B and D) γ-IFN production in response to a 48-hour incubation with HA peptide, as measured by ELISA. Values are the mean + SE. Values for T cells cultured in the absence of HA peptide were below the limit of detection for the ELISA kit.
Analysis at day 42, however, revealed numerous 3- to 5-mm lymphoma nodules in the spleen and mesentary of all unimmunized transplant recipients that had been given A20HA 10 days prior to BMT. The percentage of HA-specific CD4+ T cells in this cohort declined significantly from the levels present 3 weeks earlier (Figure6C vs 6A; 3.5% down from 15%). Even more striking was the precipitous reduction in antigen-specific γ-IFN release by these cells on day 42 compared with the levels observed on day 21 (Figure 6D vs 6B; 214 vs 5017 pg/mL, respectively).
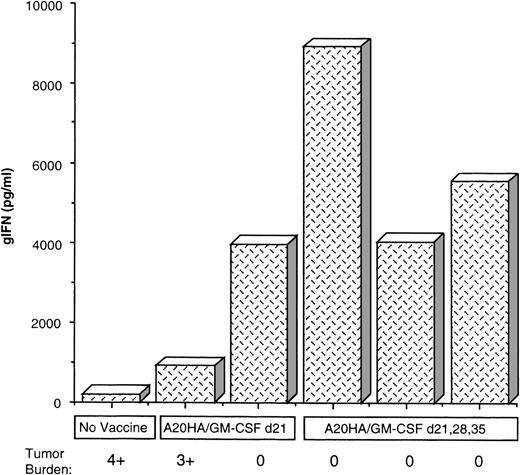
In contrast to the findings in unimmunized mice, vaccination starting on day 21 partially sustained the degree of clonal expansion of clonotype-positive T cells relative to unvaccinated mice at this time point, although this was still below the frequency detected on day 21 in response to tumor alone (Figure 6A vs 6C). Nevertheless, these T cells remained activated in the groups vaccinated post-BMT, as indicated by HA-specific γ-IFN release. In fact, for mice immunized on days 21, 28, and 35, the level of γ-IFN production measured on day 42 was comparable to the peak response observed 3 weeks earlier (Figure6D vs 6B). Furthermore, the preservation of antigen-specific T-cell function in response to vaccination with irradiated A20HA/GM-CSF cells closely correlated with tumor rejection. Indeed, within each group, analysis of individual mice demonstrated an inverse correlation between the presence of detectable tumor and γ-IFN production (Figure7).
Th-1 responsiveness of tumor-specific T cells directly correlates with sustained tumor remission post-BMT.
γ-IFN production in individual samples as related to tumor burden. The samples were obtained from the experiment described in Figure 6. Shown are the values for γ-IFN production in response to HA peptide from T cells obtained from individual mice in each group 42 days post-BMT. Treatment group and relative tumor burden observed at autopsy are as indicated. 0 = no tumor, 1+ = 1 to 4 nodules, 2+ = 5 to 10 nodules less than 1 cm, 3+ = more than 10 nodules or any nodule more than 1 cm, 4+ = confluent tumor + ascites.
Th-1 responsiveness of tumor-specific T cells directly correlates with sustained tumor remission post-BMT.
γ-IFN production in individual samples as related to tumor burden. The samples were obtained from the experiment described in Figure 6. Shown are the values for γ-IFN production in response to HA peptide from T cells obtained from individual mice in each group 42 days post-BMT. Treatment group and relative tumor burden observed at autopsy are as indicated. 0 = no tumor, 1+ = 1 to 4 nodules, 2+ = 5 to 10 nodules less than 1 cm, 3+ = more than 10 nodules or any nodule more than 1 cm, 4+ = confluent tumor + ascites.
Discussion
The impact of the graft-versus-leukemia (GVL) effect in allogeneic stem cell transplantation25-28 and the positive results obtained with donor leukocyte infusions29-31 have led to an increased recognition of the role played by the immune response in the treatment of hematologic malignancies. Indeed, the capacity for immune-mediated tumor cell killing of chemo-resistant cell lines32,33 underscores the potential for this non–cross-resistant treatment modality as an adjunct to dose-intensive chemoradiation. Whereas there is much evidence to support the existence of a T-cell–mediated graft-versus-tumor effect in the allogeneic transplant setting, this effect has not been thought to contribute significantly to tumor-free survival in autotransplants, which have been largely viewed as a means to bypass the dose-limiting toxicities of chemotherapy and radiation through “stem cell rescue.” Nevertheless, previous studies in mouse models have demonstrated that the addition of syngeneic donor T cells to marrow grafts can prolong tumor-free survival in a dose-dependent manner.19Despite this effect, however, tumor relapse remains the major source of failure for autologous BMT, and the immunologic events that accompany tumor progression have been largely unexplored in this setting.
The studies presented here indicate that transplantation of the tumor-bearing recipient results in a significant degree of tumor-specific T-cell activation in the early posttransplant period (Figures 5 and 6) that may favorably impact on tumor-free survival (Figures 1 and 2). Nevertheless, this response rapidly declines in association with tumor progression. Immunization during immune reconstitution can sustain this response (Figure 6), resulting in diminished rates of relapse from the minimal residual disease state achieved by the preparative regimen.
Given these results, it is useful to consider why the graft-versus-tumor effect has been less clinically apparent in the autologous transplant setting than with allogeneic BMT. Significantly, the spectrum of antigens capable of serving as tumor rejection antigens in the allo-GVL reaction is not limited to tumor-specific antigens but also includes minor antigen differences between donor and host. Unfortunately, while T-cell recognition of minor antigens whose expression is restricted to recipient hematopoietic cells may promote an antileukemic effect as well as engraftment,34allorecognition of more widely expressed minor antigens contributes to the morbidity of GVHD. In contrast, in the autologous setting, the graft-versus-tumor effect likely requires T-cell recognition of less abundant tumor-associated antigens, underscoring the utility of sustaining or augmenting this effect with posttransplant immunization using vaccines that enhance tumor-specific immunity. While a similar strategy may be possible following allogeneic BMT, several barriers would have to be overcome, including the need for T-cell depletion of the graft, the immunosuppression of GVH prophylaxis, and the immune dysfunction and immunopathology of GVHD itself.
Barriers also exist in the setting of autologous transplantation that may impede the ability to fully exploit the autologous graft-versus-tumor effect, including altered function and decreased numbers of T cells obtained from the patient during graft procurement, as well as tumor contamination of the graft itself. Considerable attention has been given to alterations in T-cell function in the tumor-bearing host, both in the form of antigen-specific T-cell tolerance23 as well as the more global dysfunction that has been associated with large tumor burdens.7,8,35,36Interestingly, however, we were still able to demonstrate a graft-versus-tumor effect when tumor-bearing mice were transplanted with grafts obtained from tumor-bearing syngeneic donors, more closely modeling “autologous” BMT (Figure 4). In fact, grafts from tumor-bearing donors imparted an enhanced antitumor effect upon transfer into irradiated recipients, reminiscient of earlier studies of concomitant immunity.37 Surprisingly, this effect was seen even with a substantial donor tumor burden. While the basis for this is currently unclear, it is possible that the physical removal of T cells from the tumor environment,38,39 together with the antigen-driven clonal expansion of these cells40 in the immediate posttransplant period (as seen in Figures 5 and 6) may serve to restore their functional responsiveness.
While many studies have examined the generation of humoral responses to defined antigens following posttransplant vaccination, few reports characterize T cell immunity in this setting. Kwak et al reported that vaccination in the early post-BMT period with B-cell lymphoma idiotype protein conjugated to KLH resulted in detectable antibody responses to both the idiotype and KLH.14 Cellular responses were also detected to KLH, although such responses to idiotype protein were only seen in patients immunized 10 months post-ABMT. Reichardt et al subsequently demonstrated effective antigen-specific proliferative and cytolytic T-cell responses to idiotype vaccines in the autologous BMT setting for multiple myeloma when vaccinating patients as early as 3 months posttransplant.41 Consistent with these finding, our results show measurable responses to vaccination with irradiated GM-CSF–producing tumor cells significantly before full phenotypic immune reconstitution. In fact, tumor rejection was noticeably greater following posttransplant immunization than what was observed in vaccinated, nontransplanted mice given the same tumor challenge (Figure1). It is clear from these data that recovery of normal numbers of host lymphocytes is not a prerequisite for the generation of effective responses to tumor cell GM-CSF–based vaccines.
Interestingly, a fully intact immune system may in fact exert a negative effect on the magnitude of the vaccine response, as suggested by differences in tumor-free survival (Figure 1) as well as in the clonal expansion of antigen-specific T cells following immunization of transplanted versus immunologically intact mice (Figure 3). These results are consistent with earlier observations demonstrating inhibition of T-cell expansion upon adoptive transfer into T-cell–reconstituted compared with T-cell–deficient mice.42 While the mechanisms responsible for maintaining homeostasis of the peripheral T-cell pool are incompletely understood, decreased competition for survival or proliferative signals following transfer into irradiated recipients may favor the expansion of T cells specific for antigens encountered during immune reconstitution. Indeed, Mackall et al demonstrated that in a setting where thymic output is minimized, T-cell repopulation following BMT is highly dependent upon expansion of peripheral T cells.43Furthermore, the developing T-cell repertoire is “skewed” toward the recognition of antigens present during immune reconstitution.15,44 Skewing of the developing repertoire has been clearly demonstrated in response to viral infections,45 minor histocompatibility antigens,46,47 and tumor-antigens.48
Tumor-specific T cells underwent a striking clonal expansion following irradiation of mice harboring established tumor (A20HA) at the time of BMT (Figure 6; 14% vs 0.57% clonotypic T cells present in non–tumor-bearing transplanted mice). We have previously reported that the adoptive transfer of tumor-specific CD4+ T cells into nonirradiated non–tumor-bearing mice results in a much more modest expansion of clonotype-positive T cells (Figure 5), which is accompanied by the loss of the naı̈ve phenotype.23,24,49 Nevertheless, in this setting these cells have a markedly diminished functional response to HA peptide in vitro and cannot be primed in vivo. In contrast, the response observed during the early stages of immune reconstitution is accompanied by a substantial degree of peptide-specific γ-IFN release, one hallmark of fully developed effector function that has been shown to be critical for tumor rejection.22
Two factors that may contribute to this activation response are the antigen dose and the context in which it is encountered. It is possible that a major attribute of the transplant is irradiation-induced tumor cell death in vivo, generating antigens that are immunogenic in nature, as has been suggested by studies of irradiation-induced apoptotic bodies.50-52 The liberation of these antigens in vivo in the context of the altered T-cell homeostasis that exists during immune reconstitution may be the initiating factor driving the graft-versus-tumor effect.
Despite the initial burst of tumor-specific T-cell activation in the early posttransplant period, this response was not sustained in unvaccinated mice that went on to relapse. The failure to fully eliminate antigen-bearing cells during the initial stages of immune reconstitution has been shown to result in 2 distinct pathways of T-cell tolerance, ie, anergy and exhaustion.53 This, in turn, appears to be influenced by the relative quantity of antigen encountered. Accordingly, the amount of tumor going into the transplant and the extent of tumor reduction achieved by the preparative regimen are likely to have a significant impact on both the immunologic and clinical outcomes. Because this outcome appears to be heavily influenced by events that occur during the early stages of immune reconstitution, attempts at sustaining the host response through posttransplant immunization should occur prior to the establishment of tumor-specific T-cell tolerance, ie, long before “full recovery” of the host immune system.
Taken together, the period of immune reconstitution that accompanies autologous BMT appears to provide numerous opportunities to enhance the antitumor immune response, hopefully resulting in improvement in relapse-free survival in the clinical setting.
Supported by PHS grants CA15396-23 and CA78658-01.
I.B. is a Fellow of the Leukemia Society of America. E.M.S. is a Fellow of the Lymphoma Research Foundation of America. H.I.L. is a Scholar of the Leukemia Society of America.
Reprints:Hyam Levitsky, Johns Hopkins Oncology Center, 452 Bunting Blaustine Cancer Research Building, 1650 Orleans St, Baltimore, MD 21231; e-mail: hy@jhmi.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal