Abstract
We examined the incidence, clinical course, and outcome of patients with newly diagnosed acute promyelocytic leukemia (APL) who developed the retinoic acid syndrome (RAS) treated on the Intergroup Protocol 0129, which prospectively evaluated the role of alltrans retinoic acid (ATRA) alone during induction and as maintenance therapy. Forty-four of 167 (26%) patients receiving ATRA for induction developed the syndrome at a median of 11 days of ATRA (range, 2-47). The median white blood cell (WBC) count was 1450/μL at diagnosis and was 31 000/μL (range, 6800-72 000/μL) at the time the syndrome developed. ATRA was discontinued in 36 of the 44 patients (82%) and continued in 8 patients (18%), with subsequent resolution of the syndrome in 7 of the 8. ATRA was resumed in 19 of the 36 patients (53%) in whom ATRA was stopped and not in 17 (47%). The syndrome recurred in 3 of those 19 patients, with 1 death attributable to resumption of the drug. Ten of these 36 patients received chemotherapy without further ATRA, and 8 achieved complete remission (CR). Among 7 patients in whom ATRA was not restarted and were not treated with chemotherapy, 5 achieved CR and 2 died. Two deaths were definitely attributable to the syndrome. No patient receiving ATRA as maintenance developed the syndrome. (Blood. 2000;95:90-95)
Randomized clinical trials have shown that the vitamin A derivative all-trans retinoic acid (ATRA) significantly improves the outcome of patients with acute promyelocytic leukemia (APL).1,2 Although ATRA is generally well tolerated, some patients develop the retinoic acid syndrome (RAS), manifested by unexplained fever, weight gain, respiratory distress, interstitial pulmonary infiltrates, pleural and pericardial effusions, episodic hypotension, and acute renal failure.3 This syndrome is the most serious toxicity of ATRA and is often, but not always, associated with the development of hyperleukocytosis.4-6 It has been suggested that patients who have a white blood cell count (WBC) that exceeds 5000/μL on day 1, 6000/μL on day 5, 10 000/μL on day 10, or 15 000/μL on day 15 are at high risk for the development of RAS.1
The pathogenesis of the syndrome is not completely understood. However, several possible mediators have been identified, including cathepsin G, a serine protease that enhances capillary permeability7; cell adhesion molecules on APL cells such as CD15s(Lex) and integrins CD11a and CD11b, which interact with the endothelial cell receptor ICAM (intercellular adhesion molecule)-1; and hematopoietic growth factors such as interleukin (IL)-1β, tumor necrosis factor (TNF)α and IL-6, which promote leukocyte activation.8-10 Increased expression of IL-1 in leukemic promyelocytes may induce endothelial cell expression of ICAM-1 and vascular cell adhesion molecule (VCAM)-1, which may allow for leukemic cell binding to endothelium.11 Finally, exposure of the promyelocytic leukemia cell line NB4 to ATRA promotes formation of leukoaggregates via LFA-1/ICAM-2 interaction, providing another potential mechanism contributing to the syndrome.12
The best approach to predict, prevent, or treat the syndrome has not been established. To further characterize this complication, we examined the incidence, clinical manifestations, lung pathology, clinical course, and outcome of patients with newly diagnosed APL who developed RAS treated on the National Cancer Institute Intergroup Protocol 0129, which prospectively evaluated the role of ATRA alone during induction and as maintenance therapy.2
Materials and methods
Treatment with ATRA
Intergroup Protocol 0129 was a prospective trial conducted between April 1992 and February 1995 in which patients with newly diagnosed APL established by morphology were randomized to either ATRA 45 mg/m2/d divided into 2 daily doses orally or daunorubicin 45mg/m2 by intravenous (IV) bolus on days 1 to 3 and cytosine arabinoside 100 mg/m2 by continuous IV infusion on days 1 to 7 for induction.2 Patients who achieved a complete remission (CR) received 2 cycles of consolidation chemotherapy. They were then randomized to either maintenance therapy with daily ATRA 45 mg/m2/d for 1 year or observation. Patients < 3 years of age were randomized to receive ATRA as just described or daunorubicin 1.5 mg/kg/d by IV bolus on days 1 to 3 plus cytosine arabinoside 3.3 mg/kg/d by continuous IV infusion on days 1 to 7. All patients on the ATRA induction arm were required to have a WBC ≤10 000/μL either at presentation, or after hydroxyurea, prior to initiating ATRA. Hydroxyurea was given any time the WBC increased to ≥ 30 000/μL. Patients failing to respond to ATRA for induction after a maximum of 90 days, or failing before 90 days of ATRA because of toxicity or progressive disease, were allowed to cross over to the chemotherapy arm. Patients randomized to induction chemotherapy who did not achieve CR after 2 cycles did not cross over to the ATRA arm but were removed from the study and treated at the physician's discretion.
Patients
A total of 172 patients were evaluable for analysis on the ATRA arm. Details of the patient characteristics and outcome have been previously reported.2 Five of these patients never received ATRA (2 patients crossed over before any treatment, 2 patients were randomized to ATRA but received chemotherapy, and 1 patient died of intracerebral hemorrhage after the first dose) and are excluded from this analysis. Forty-four of the remaining 167 (26%; 95% CI, 20%-34%) patients developed definite RAS during induction therapy with ATRA.
Diagnosis of the retinoic acid syndrome
The diagnosis of RAS was made clinically by the presence of otherwise unexplained fever, weight gain, respiratory distress, interstitial pulmonary infiltrates, and pleural or pericardial effusions during treatment with ATRA. No single sign or symptom itself was considered diagnostic of the syndrome.3 Cases were identified by reviewing records of patients reported to have a grade 2 or higher pulmonary or cardiac toxicity as well as by reviewing cases identified by the institution as having the syndrome from adverse drug reporting. Seven other patients had signs or symptoms consistent with the syndrome, but all had concurrent medical problems, such as bacteremia, sepsis, or congestive heart failure, making accurate diagnosis of RAS impossible. Therefore, these 7 patients were indeterminate3 and are not included in the cohort of patients categorized as definitely having the syndrome.
Forty-eight patients treated with chemotherapy who developed grade 2 or worse pulmonary or cardiac toxicity were analyzed for the presence of a cardiorespiratory distress syndrome similar to that associated with ATRA, which has been reported rarely.13 One patient developed Streptococcal mitus bacteremia with adult respiratory distress syndrome, 1 had an arrhythmia, 8 sustained substantial declines in the left ventricular ejection fraction, 4 had fungal pneumonia, 1 had sternal heaviness, and the remainder had either pneumonia, pulmonary infiltrates of undetermined etiology, fluid overload, or pleural effusion. One patient had pulmonary infiltrates 2 days prior to study entry with a WBC 34 600/μL and an oxygen saturation of 85%. On day 1 of chemotherapy, the patient underwent leukapheresis for a WBC of 42 500/μL and the pulmonary symptoms resolved, reflecting disease-related pulmonary symptoms.
Treatment of the retinoic acid syndrome
At the earliest sign or symptom of the syndrome, ATRA was to be discontinued and dexamethasone initiated at 10 mg IV twice daily. If the syndrome resolved, ATRA was to be reinstituted at 75% of the initial dose and then escalated to the full dose after 3 to 5 days if the syndrome did not recur. All but 3 patients were given 10 mg per day of dexamethasone. Two patients were given 20 mg per day, and 1 was given 1 mg per day. Among the patients receiving 10 mg per day, the mean duration of dexamethasone was 9 days (range, 1-49 days). One of the 2 patients given 20 mg per day of dexamethasone received 1 day of the drug; the other received 7 days of the drug; and the single patient treated with 1 mg per day received 17 days of the drug.
Statistical methods
Univariate analyses of the association between ever-developing RAS and dichotomous predictors were conducted with Fisher's exact test. Evaluations of the association of continuous predictors and of multicovariate models were performed with logistic regression (SAS V6.12). P values <.05 were considered to be significant.
Results
Clinical characteristics of patients with the retinoic acid syndrome
Table 1 presents the initial characteristics of the patients with and without RAS. Briefly, the median age was 42 years among patients who developed RAS and 35 years among those who did not. The WBC at diagnosis was slightly lower among those who developed RAS than those who did not, with medians of 1450/μL and 2000/μL, respectively. One patient (2%) among those who developed RAS was reported to have the microgranular variant M3v of APL in the original data set.2 However, more detailed review for this analysis indicated that another patient had M3v. Twenty-one (17%) of the cases who did not develop RAS had M3v. Among those who were evaluated, slightly more than 50% in each group had the long form of the PML/RARα fusion protein. Three (7%) patients received hydroxyurea prior to ATRA among those who developed RAS, compared to 19 (15%) among those who did not. No patients receiving ATRA for maintenance developed the syndrome.
Initial characteristics and clinical outcome
| Characteristic . | RAS* (n = 44) . | Non-RAS (n = 123) . |
|---|---|---|
| Age, y | ||
| Median (Range) | 42 (4-81) | 35 (1-76) |
| >55 | 11 (25%) | 21 (17%) |
| Gender | ||
| Male | 23 (52%) | 57 (46%) |
| Performance status | ||
| 0 | 17 (39%) | 54 (43%) |
| 1 | 17 (39%) | 51 (41%) |
| >1 | 10 (22%) | 18 (16%) |
| White blood cell count at diagnosis (per μL) | ||
| Median (Range) | 1450 (400-35 900) | 2000 (300-64 800) |
| >10 000 | 3 (7%) | 20 (17%) |
| M3v | 2 (4%) | 21 (17%) |
| PML-RARα | ||
| BCR1 (L) | 16 (59%) | 43 (53%) |
| BCR2 (V) | 0 | 7 (9%) |
| BCR3 (S) | 10 (37%) | 27 (33%) |
| True negative | 1 (4%) | 4 (5%) |
| Hydroxyurea at entry | 3 (4%) | 19 (15%) |
| Outcome | ||
| Complete response | 28 (64%) | 93 (76%) |
| 3-y disease-free survival | 74% | 66% |
| Characteristic . | RAS* (n = 44) . | Non-RAS (n = 123) . |
|---|---|---|
| Age, y | ||
| Median (Range) | 42 (4-81) | 35 (1-76) |
| >55 | 11 (25%) | 21 (17%) |
| Gender | ||
| Male | 23 (52%) | 57 (46%) |
| Performance status | ||
| 0 | 17 (39%) | 54 (43%) |
| 1 | 17 (39%) | 51 (41%) |
| >1 | 10 (22%) | 18 (16%) |
| White blood cell count at diagnosis (per μL) | ||
| Median (Range) | 1450 (400-35 900) | 2000 (300-64 800) |
| >10 000 | 3 (7%) | 20 (17%) |
| M3v | 2 (4%) | 21 (17%) |
| PML-RARα | ||
| BCR1 (L) | 16 (59%) | 43 (53%) |
| BCR2 (V) | 0 | 7 (9%) |
| BCR3 (S) | 10 (37%) | 27 (33%) |
| True negative | 1 (4%) | 4 (5%) |
| Hydroxyurea at entry | 3 (4%) | 19 (15%) |
| Outcome | ||
| Complete response | 28 (64%) | 93 (76%) |
| 3-y disease-free survival | 74% | 66% |
RAS indicates the retinoic acid syndrome; BCR, breakpoint cluster region; L, long form; V, variable form; S, short form.
There was no significant difference in the CR rate between those who developed RAS (64%) and those who did not (76%), and there was no difference in disease-free survival between the 2 groups among those who did achieve a CR (74% and 66% at 3 years, respectively).
Timing of the retinoic acid syndrome
RAS developed after a median of 11 days of ATRA (range, 2-47 days).
White blood cell count at the time of the retinoic acid syndrome
The maximum WBC count among the 44 patients who developed the syndrome ranged from 6800/μL to 72 000/μL (median, 31 000/μL).
Major manifestations of the retinoic acid syndrome
The major manifestations (≥10% incidence) of RAS included respiratory distress, fever, pulmonary edema, pulmonary infiltrates, pleural or pericardial effusions, hypotension, bone pain, headache, congestive heart failure, and acute renal failure. (Table2). Mechanical ventilation was required in 26% of patients with the syndrome.
Major manifestations of the retinoic acid syndrome
| Manifestation . | % of Patients . |
|---|---|
| Respiratory distress | 84 |
| Fever | 81 |
| Pulmonary edema | 54 |
| Pulmonary infiltrates | 52 |
| Pleural/pericardial effusion | 36 |
| Hypotension | 18 |
| Bone pain | 14 |
| Headache | 14 |
| Congestive heart failure | 11 |
| Acute renal failure | 11 |
| Manifestation . | % of Patients . |
|---|---|
| Respiratory distress | 84 |
| Fever | 81 |
| Pulmonary edema | 54 |
| Pulmonary infiltrates | 52 |
| Pleural/pericardial effusion | 36 |
| Hypotension | 18 |
| Bone pain | 14 |
| Headache | 14 |
| Congestive heart failure | 11 |
| Acute renal failure | 11 |
Outcome of the retinoic acid syndrome
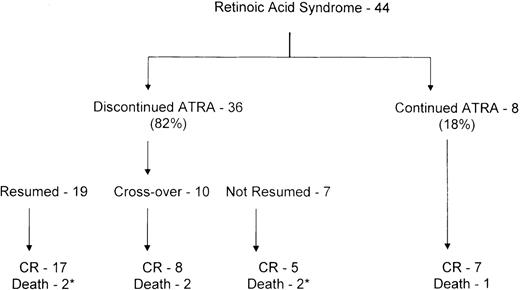
All but 2 of the 44 patients were treated with dexamethasone. ATRA was discontinued when the syndrome developed in 36 of the 44 patients (82%) and was continued in 8 patients (18%) (Figure1). The syndrome resolved in all 8 patients in whom ATRA was continued, but 1 patient died of intracerebral hemorrhage attributable to the underlying disease. Among the 36 patients in whom ATRA was stopped, it was resumed in 19 (53%), 11 (58%) under coverage of steroids. Ten of these 36 patients crossed over to chemotherapy without restarting ATRA, and 8 achieved CR. The syndrome recurred in 3 of 19 patients after reinstitution of ATRA (2 under coverage of steroids), with 1 death attributable to recurrence of the syndrome, 1 death due to cardiorespiratory arrest and sepsis, and 17 CRs. Among the 7 patients in whom ATRA was not resumed and who were not crossed over to chemotherapy, 5 achieved CR after having received 14, 16, 19, 24, and 26 days of ATRA, and 2 patients died, 1 of intracerebral hemorrhage due to progressive disease and 1 of multiorgan failure attributable to the syndrome.
Outcome of 44 patients with retinoic acid syndrome.
*Retinoic acid syndrome, 1 each.
Outcome of 44 patients with retinoic acid syndrome.
*Retinoic acid syndrome, 1 each.
Deaths due to the retinoic acid syndrome
There were 2 deaths definitely attributed to RAS. A 52-year-old man presented with a WBC count of 3100/μL and developed patchy bilateral pulmonary infiltrates, fever, and pleural effusions on day 4 of ATRA, with a peak WBC count of 56 100/μL on day 6. ATRA was discontinued on day 4, and dexamethasone was administered. ATRA was resumed on day 12 at 75% dose after the syndrome resolved, and the WBC had decreased to 11 700/μL while he was still receiving tapering doses of dexamethasone (<10 mg every 12 hours), but ATRA was discontinued again on day 18 because the syndrome recurred. The WBC had increased in 24 hours from 8800/μL to 21 300/μL when ATRA was discontinued the second time and to 55 800/μL on the day of death. He sustained a myocardial infarction and died on day 20. The second patient was a 4-year-old girl who presented with a WBC count of 6000/μL and developed a peak WBC count of 58 100/μL on day 10, when hydroxyurea was begun. RAS developed on day 29. Despite 17 days of dexamethasone (initially started on day 13 for increased intracranial pressure due to pseudotumor cerebri and tapered beginning on day 27), she died on day 30 with pulmonary infiltrates and hypotension.14
Histologic findings in cases with fatal retinoic acid syndrome
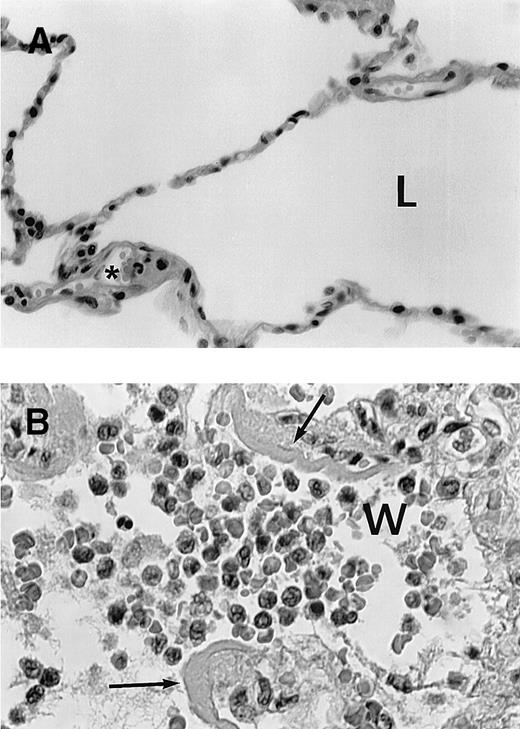
We obtained formalin-fixed postmortem lung tissue for histologic evaluation from the 2 patients who died. The histologic findings in the lungs of these 2 patients were consistent with the concept that ATRA therapy led to differentiation, endothelial cell damage, and leukocyte infiltration into the lung (Figure 2). ATRA promotes in vitro differentiation of APL cells over a period of days and, indeed, circulating myeloid precursors at various stages of differentiation were present in the microvasculature of both cases. An intra-alveolar myeloid infiltrate was prominent in 1 case and mild in the other, consistent with the hypothesis that ATRA exposure alters adhesive properties of differentiating APL cells that may lead to interaction with the endothelium and extravasation from the blood.11 12 In the case with a prominent intra-alveolar infiltrate, additional findings indicative of endothelial cell damage were present and included intra-alveolar edema, interalveolar hemorrhage, and fibrinous exudates.
Histologic findings in cases with fatal retinoic acid syndrome.
(A) Histologic appearance of normal lung tissue. L shows lung alveolae in normal lung; * indicates normal neutrophil in microvasculature. (B) Lung tissue showing infiltration of alveolae with leukocytes from a patient who succumbed to the retinoic acid syndrome. W shows myeloid cells in the airspace (most leukocytes to left of “w”); the lobated nuclei of myeloid cells is prominently seen. Arrows indicate fibrinous exudate due to vascular leak of serum fibrin.
Histologic findings in cases with fatal retinoic acid syndrome.
(A) Histologic appearance of normal lung tissue. L shows lung alveolae in normal lung; * indicates normal neutrophil in microvasculature. (B) Lung tissue showing infiltration of alveolae with leukocytes from a patient who succumbed to the retinoic acid syndrome. W shows myeloid cells in the airspace (most leukocytes to left of “w”); the lobated nuclei of myeloid cells is prominently seen. Arrows indicate fibrinous exudate due to vascular leak of serum fibrin.
Prognostic factors for the development of RAS
It has been suggested that patients with a WBC that exceeds 5000/μL on day 1, 6000/μL on day 5, 10 000/μL on day 10, or 15 000/μL on day 15 are at increased risk for RAS.2Table 3 presents the number of patients who met these criteria and whether they ever developed RAS.
Number of patients whose white blood cell count met criteria predicting the development of the retinoic acid syndrome (RAS)
| . | None . | Any . | Total . | No. of Patients Meeting Criteria3-150 (%) . | |||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | ||||
| No RAS | 44 | 79 | 123 | 30 (24%) | 28 (23%) | 16 (13%) | 5 (4%) |
| RAS | 13 | 31 | 44 | 18 (41%) | 10 (23%) | 1 (2%) | 2 (5%) |
| Total | 57 | 110 | 167 | 48 (29%) | 38 (23%) | 17 (10%) | 7 (4%) |
| . | None . | Any . | Total . | No. of Patients Meeting Criteria3-150 (%) . | |||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | ||||
| No RAS | 44 | 79 | 123 | 30 (24%) | 28 (23%) | 16 (13%) | 5 (4%) |
| RAS | 13 | 31 | 44 | 18 (41%) | 10 (23%) | 1 (2%) | 2 (5%) |
| Total | 57 | 110 | 167 | 48 (29%) | 38 (23%) | 17 (10%) | 7 (4%) |
One criterion equals one of the following; 2 criteria equal any two of the following; 3 criteria, 3 of the following; 4 criteria, 4 of the following: white blood cell (WBC) count higher than 5000/μL on day 1, WBC count higher than 6000/μL on day 6, WBC count higher than 10 000/μL on day 10, WBC count equal to or higher than 15 000/μL on day 15.
A total of 110 of 167 patients (66%) met at least 1 of the criteria, only 31 of whom ever developed RAS. Thus, the sensitivity (probability of meeting the criteria given that a patient ever developed RAS) is 31/44, or 70% (95% CI, 55%-83%), and the specificity (probability of not meeting the criteria given that a patient never developed RAS) is only 44/123, or 36% (95% CI, 27%-45%). The overall false-positive rate is 64%, and a number of patients met 2 or more criteria without ever developing RAS. There is no discrimination between those who developed RAS and those who did not with these criteria in this large population. In addition, a time-varying model that considered the predictive value of WBC at entry and days 5, 10, and 15 for development of RAS in a subsequent 5-day period also showed no discrimination between patients who developed RAS and those who did not.
The on-study characteristics listed in Table 1 were analyzed for their predictive value for the development of RAS. As noted above, only 1 of 44 patients who developed RAS was confirmed to have M3v at the time the original manuscript was prepared. However, the subsequent detailed review for this analysis identified a second patient who had M3v. In multicovariate logistic regression models, only M3v status was significantly associated with protection from developing RAS whether considering 1 of 44 (P = .01) or 2 of 44 (P = .042). Age (P = .27), gender (P = .60), performance status (P = .60), molecular break point (P = .82), and receipt of hydroxyurea prior to ATRA (P = .20) were not associated with the development of RAS in this population. There was no association of WBC at diagnosis with duration of the syndrome.
Discussion
All-trans retinoic acid represents a major advance that has made APL the most curable subtype of acute myeloid leukemia (AML) in adults.1,2 However, a major toxicity of ATRA has been RAS.3 Neither the pathogenesis nor the optimal way to prevent or treat the syndrome has been established.
Among 167 patients with newly diagnosed APL treated with ATRA alone for induction, RAS developed in 26%. An additional 7 patients may have had RAS, but this could not be determined because of concurrent medical problems. None of these indeterminate cases were treated with corticosteroids. Although the syndrome usually resolved rapidly with the early administration of dexamethasone even among patients who continued ATRA, the deaths of 2 patients (5%) were definitely attributable to the syndrome. The incidence of the syndrome has varied in other reports from 5% to 27% and the mortality from 5% to 29%.6,13-19 (Table 4). A cohort of patients similar to that reported here, treated with ATRA alone, had a similar incidence of 27%.6 However, the mortality rate of patients with the syndrome in the present report is substantially lower (5% vs 29%), likely reflecting the earlier recognition and institution of dexamethasone, as experience with ATRA has accumulated.
Comparison of the incidence and outcome of the retinoic acid syndrome (RAS) in other reports
| Study . | No. of Patients . | Induction . | Incidence (%) . | Mortality (%)4-150 of Patients With RAS . | Mortality (%)4-151 of All Treated Patients . |
|---|---|---|---|---|---|
| Vahdat6 | 78 | ATRA | 27 | 29 | 8 |
| Wiley15 | 22 | ATRA + steroids | 9 | 50 | 5 |
| Avvisati17 | 20 | ATRA + chemo | 10 | 0 | 0 |
| Mandelli18 | 240 | ATRA + chemo | 3 | 17 | 0.4 |
| Asou19 | 196 | ATRA ± chemo | 6 | 9 | 0.5 |
| De Botton13 | 413 | ATRA ± chemo | 15 | 8 | 1 |
| Tallman2 | 167 | ATRA | 26 | 5 | 1 |
| Study . | No. of Patients . | Induction . | Incidence (%) . | Mortality (%)4-150 of Patients With RAS . | Mortality (%)4-151 of All Treated Patients . |
|---|---|---|---|---|---|
| Vahdat6 | 78 | ATRA | 27 | 29 | 8 |
| Wiley15 | 22 | ATRA + steroids | 9 | 50 | 5 |
| Avvisati17 | 20 | ATRA + chemo | 10 | 0 | 0 |
| Mandelli18 | 240 | ATRA + chemo | 3 | 17 | 0.4 |
| Asou19 | 196 | ATRA ± chemo | 6 | 9 | 0.5 |
| De Botton13 | 413 | ATRA ± chemo | 15 | 8 | 1 |
| Tallman2 | 167 | ATRA | 26 | 5 | 1 |
Percentage of patients with RAS with death attributable to RAS.
Percentage of all treated patients with death attributable to RAS.
The concurrent administration of chemotherapy with ATRA may decrease the incidence of the syndrome. However, this has not clearly been established. In the first report of the AIDA (all-transretinoic acid plus idarubicin) trial, in which all patients receive idarubicin concurrently with ATRA, the incidence of the syndrome was 10%.17 As the experience with concurrent ATRA plus idarubicin expanded, the incidence of the syndrome decreased.18 Among 240 patients treated with the AIDA regimen in the most recent trials, only 6 (3%) patients developed the syndrome and 1 patient died. An additional 11 patients had a constellations of signs and symptoms that led to an “indeterminate” classification. The incidence of the syndrome in the Japanese Adult Leukemia Study Group (JALSG) trial, in which chemotherapy was introduced early for the prevention of hyperleukocytosis, was 11 of 196 patients (6%) with 1 death, which occurred early in the trial when there was less experience with the syndrome.19 An overall incidence of 15% was reported by the European APL group, with a mortality rate of 8% of patients with the syndrome.13 No difference in the incidence of the syndrome was observed among patients treated with concurrent versus sequential ATRA and chemotherapy. In this trial, some patients received concurrent chemotherapy for a rapidly rising WBC count. These series suggest that although the incidence of RAS may be reduced, the mortality rate due to the syndrome is low and is not different than the mortality in our series when the syndrome is recognized and treated early.
There were no pretreatment variables predictive of the syndrome, including median age, gender, WBC count, or breakpoint location20 except for M3v. Patients with M3v appear to be protected from the syndrome, but the reason(s) is not clear. Furthermore, we did not find a WBC count above 5000/μL on day 1, or above 6000/μL, 10 000/μL, or 15 000/μL on days 5, 10, or 15 of ATRA, respectively, predictive for the development of the syndrome, as was observed by Fenaux and colleagues.21 Similarly, Vahdat and colleagues found that the initial WBC count as well as the rate of rise was not statistically correlated with the development of the syndrome.6
The pathogenesis of the syndrome has not been completely determined. However, the autopsy findings reported here provide insight. The constellation of histologic findings (edema, hemorrhage, fibrinous exudates, and leukocyte infiltration) likely results from microvasculature damage, because similar histologic findings are seen in a variety of diseases, including trauma, sepsis, and the adult respiratory distress syndrome.22-27 The final common pathway is an insult to the endothelium followed by a predictable series of events, including edema, hemorrhage, fibrinous exudates, neutrophilic infiltration, and respiratory failure.
Patients with AML and hyperleukocytosis with pulmonary compromise may have indistinguishable clinical presentations from patients with APL who develop RAS. However, in cases of AML with hyperleukocytosis, pulmonary compromise appears to result primarily from formation of leukoaggregates in the circulation.28 In contrast, Frankel et al reported leukocytic infiltration without leukoaggregate formation in 2 cases of RAS.3 The histologic findings in the 2 fatal cases of RAS reported here were identical to those described by Frankel et al.3 Taken together, these observations indicate that different cellular and molecular mechanisms cause RAS in contrast to leukostasis in AML, although the clinical scenario is similar. Migration of leukocytes into tissue such as lung is important in the pathogenesis of RAS, but leukemic cell adhesion to each other and the formation of leukoaggregates may be important in leukostasis in AML.
Strategies to prevent the syndrome have been explored. Wiley and Firkin tested the prophylactic administration of corticosteroids in 12 patients whose WBC rose above 10 000/μL on ATRA.15 None of these 12 patients developed any pulmonary toxicity despite a peak WBC count of up to 112 000/μL. Although this decreased incidence compared to other trials suggests a benefit, the number of patients studied was small, and no prospective randomized trial has been conducted.16-18 Two groups of investigators have reported the use of a lower dose of ATRA. Castaigne and colleagues showed that the administration of a lower dose of ATRA of 25 mg/m2/d resulted in a similar CR rate and similar toxicity profile, including a similar incidence of RAS as the standard dose.29 However, an even lower dose of 15-20 mg/m2/d appeared to result in less frequent development of the syndrome.30 Whether novel retinoids such as 9-cis retinoic acid and AM-80 will be associated with a decreased incidence of the syndrome is not known.31 32
Several observations regarding the clinical course of patients with the syndrome can be made. First, ATRA need not necessarily be discontinued if RAS develops, providing that dexamethasone is instituted at the earliest sign or symptom. ATRA was continued in 8 patients and, in 7 of the 8 patients, the syndrome resolved and CR was achieved uneventfully. However, the success of that approach may depend on the severity of the syndrome and the rapidity of institution of dexamethasone. Second, ATRA can be successfully reintroduced following resolution of the syndrome without concomitant steroids. The ability to resume ATRA may be attributable to suppression of cytokines by corticosteroids together with a continuous reduction in the cell burden. However, the syndrome can recur despite prophylactic steroids, and it led to the death of 1 patient in this series. Third, there does not appear to be a risk of the syndrome when ATRA is given as maintenance therapy to patients in CR. This issue is important because recent data suggest the value of maintenance ATRA, even in patients who present with WBC counts ≤5000/μL.2 16 Finally, mortality from the syndrome is now very low.
What, then, is the recommended approach to prevent and treat the syndrome? Because recent data suggest that concurrent ATRA plus chemotherapy may prevent relapse, and because the incidence of the syndrome may be lower, this approach is emerging as a routine strategy for all patients.16 Prophylactic corticosteroids cannot be recommended for routine use at the present time. Studies evaluating the role of corticosteroid prophylaxis are underway. If moderate or severe RAS develops, it is prudent to discontinue ATRA. Prompt administration of dexamethasone is critical, not only when the diagnosis is definitively established, but also at the first sign of unexplained dyspnea, fever, weight gain, or pulmonary infiltrate. When the syndrome resolves, ATRA can safely be restarted in most patients; however, continued close observation is warranted. Further studies of growth factor expression and modulation of adhesion molecules may provide further insights into the pathogenesis of the syndrome and lead to its prevention.
Study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, Chair) and supported in part by US Public Health Service grants CA17145, CA23318, CA31983, CA20319, CA03161, CA11083, CA32102, CA14958, CA66636, and CA21115 from the National Cancer Institute, National Institutes of Health, Bethesda, MD, and the US Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Reprints:Martin S. Tallman, Northwestern University Medical School, Division of Hematology/Oncology, Department of Medicine, Robert H. Lurie Comprehensive Cancer Center, 233 East Erie St, #700, Chicago, IL 60611; e-mail: m-tallman@nwu.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal