Abstract
The authors examined the relationship between the time required to enter complete remission (CR) after a first course of chemotherapy for newly diagnosed acute myeloid leukemia (AML), refractory anemia with excess blasts in transformation (RAEB-t), or refractory anemia with excess blasts (RAEB). They also examined subsequent survival time and disease-free survival time after accounting for cytogenetic status, age, and treatment. The data set consisted of 1101 patients with these diagnoses treated at the M. D. Anderson Cancer Center between 1980 and 1996 for whom outcomes were established after first-course therapy. Of the 1101 patients, 740 (67%) were in CR after this time; 508 of these 740 (69%) have died (80% had disease recurrence before death). The authors used the parametric model of Shen and Thall to estimate, in particular, TC (time to CR), TC,D (time from CR to death = residual survival after CR), and TC,R (residual disease-free survival [DFS] after CR) as functions of the covariates noted above and to estimate the dependence of TC,D and TC,R on TC. There was a strong inverse association between TC and both TC,D and TC,R (P < .001 for both) that was independent of cytogenetic status, age, or treatment. The residual survival time of patients who required >50 days to enter CR was closer to the residual survival time of resistant patients than to that of patients known to be in CR within approximately 30 days of the start of treatment. Time to CR is an independent predictor of residual survival and disease-free survival in patients with newly diagnosed AML who achieve CR after 1 course of chemotherapy. (Blood. 2000;95:72-77)
Reports of clinical trials of patients with acute myeloid leukemia (AML) universally note whether a given treatment produces “complete remission” (CR), defined according to standard criteria.1 This focus on CR as a clinical endpoint stems from data published in the 1960s indicating that prolonged survival time in patients with AML is impossible if CR is not achieved.2 The number of courses required to produce CR is also frequently reported, reflecting the knowledge that CR occurring only after more than 1 course of induction therapy is generally shorter than that observed after the first course.3 However, this qualification aside, CR is generally viewed as a binary outcome, ie, it either does or does not occur.
Nonetheless, it appears plausible that events transpiring during a first, successful course of remission induction therapy may influence the subsequent outcome. Based on our observations of the brevity of remission in some patients in whom the criteria for CR—particularly a platelet count >100,000—were met only many weeks after beginning treatment, we have been interested in the relationship between the time required to enter CR after a first course of chemotherapy and subsequent disease-free and overall survival times. In this article we examine these relationships after accounting for treatment and for factors known to influence these times, specifically age and cytogenetic status. In addition to providing an overall analysis, we report separate analyses for patients with de novo AML and for patients with either AML arising after a history of abnormal blood counts or with myelodysplastic syndromes (MDS). To examine whether CR remains a precondition for lengthy survival in the 1980s and 1990s, we also compared survival times from the response dates for patients meeting the criteria for CR after the first course of therapy with survival times from response dates for patients who, considered resistant to this initial course, were taken off-study on this date.
Patients and methods
Between 1980 and 1996, 1498 patients with newly diagnosed AML (acute promyelocytic leukemia excluded), refractory anemia with excess blasts in transition (RAEB-t), or refractory anemia with excess blasts (RAEB) came to the M. D. Anderson Cancer Center for treatment. Our analysis considered 1101 of 1498 (73%) patients in whom response (CR, death, or “resistance”) was established after the first course of induction therapy. Criteria for CR were those we have used previously: a marrow with <5% blasts concomitant with a neutrophil count of >1000/μL and a platelet count of >100 000/μL. Of the 1101 patients, 740 (67%) were in CR after 1 course, 290 (26%) were dead, and the remaining 71 (6%) were removed from their initial treatment protocol because they were considered resistant. Of these 71, 25 (35%) received no additional therapy, and 40 (56%) underwent an alternative treatment protocol (chemotherapy 35, allogeneic transplant 5) that produced CR in 6 patients. In the final 6 of the 71 resistant patients, no information about subsequent therapy is available. Among the 397 patients in whom a response was not established after the first course of induction therapy but who continued to be treated on their initial protocol, 175 (44%) achieved CR, 88 (22%) died, and 132 (33%) were eventually removed from the protocol because of resistance. Thus, 81% of the 915 instances of CR that occurred with the initial treatment protocol were observed after the first course of therapy, as were 77% of the deaths. This observation was made after the first course of therapy, however, in only 35% of the patients considered resistant to the initial protocol.
Figure 1 depicts outcomes in the 1101 patients in whom responses were established after the first course alone. Of the 740 patients in CR after this course, 508 (69%) have subsequently died, as have 65 of the 71 (92%) patients called resistant. Of the 508 patients who died after entering CR, 407 (80%) experienced disease recurrence before death. To assess the effects of the time required to achieve CR on subsequent survival time, the following times are considered: TC is the time from start of treatment to CR date, TC,D is the time from CR date to death (ie, the “residual” survival time after CR), TRis the time from the start of treatment to the date the patient was considered resistant, TR,D is the residual survival time from date of resistance, and TD is the time from start of treatment to death in patients who died before CR occurred or resistance was observed. We wanted to examine the distributions of each of the 5 times TC, TC,D, TR, TR,D, and TD as functions of several baseline variables (covariates) and, in the cases of the residual survival times TC,D and TR,D, also as a function of, respectively, TC and TR. The main question was whether, after accounting for patient covariates, residual survival time after CR, TC,D, was influenced by the time required to achieve CR, TC. The covariates that we included in the analysis were patient age, cytogenetic status, and treatment. Based on previous results,4,5 age was considered as a numerical (ie, continuous) variable, whereas 3 cytogenetic groups were distinguished: 1) normal karyotype (considered the baseline or reference group), 2) inv(16) or t(8;21) and 3) all other karyotypes, including patients with insufficient metaphases for cytogenetic analysis, with these categories as previously defined.4,5The median age of the 1101 patients was 57 years; 43% had normal karyotypes, 11% had inv(16) or t(8;21) and 46% had other abnormal karyotypes or insufficient metaphases. Numerous treatment regimens were investigated during the 1980 to 1996 period, and results with many have been reported.6-9 Eleven regimens each accrued >25 patients. The most commonly used regimens were idarubicin + high-dose ara-C (IA, 127 patients), IA + G-CSF (126 patients), and fludarabine + ara-C + G-CSF (FLAG, 111 patients). For this analysis we grouped the treatment regimens as follows: 1) anthracycline (or amsacrine) ± conventional dose ara-C (each dose <0.5 g/m2), 2) “high-dose” ara-C (each dose ≥0.5g/m2) ± anthracycline (or amsacrine) but without fludarabine, and 3) high-dose ara-C + fludarabine ± idarubicin. Patients in these 3 treatment groups numbered, respectively, 186, 549, and 366.
Therapy outcome.
Outcome of therapy in the 1101 patients whose outcomes (complete remission [CR], death, or resistance) were established after the first course of chemotherapy. TC = time from start of treatment to CR; TC,D = time from CR date to death; TR = time from start of treatment to “resistance”; TR,D = time from resistance date to death; and TD = time to death in patients dying before considered to be in CR or to be resistant.
Therapy outcome.
Outcome of therapy in the 1101 patients whose outcomes (complete remission [CR], death, or resistance) were established after the first course of chemotherapy. TC = time from start of treatment to CR; TC,D = time from CR date to death; TR = time from start of treatment to “resistance”; TR,D = time from resistance date to death; and TD = time to death in patients dying before considered to be in CR or to be resistant.
To estimate each of TC, TC,D, TR,TR,D, and TD as a function of the covariates noted above, and of the dependence of the residual survival times TC,D on TC and TR,D on TR, we used the general parametric model described by Shen and Thall10 for multiple nonfatal competing events (here, CR and resistance) and death. In this formulation, death censors all nonfatal events that have not occurred, the time to a nonfatal event and subsequent residual survival time may be either positively or negatively associated, and the overall survival distribution is a mixture of 3 different distributions corresponding to death after CR, death after resistance, and death without an antecedent nonfatal event. The distribution of each event time is specified marginally by a parametric model that accommodates covariates and the usual administrative censoring. One may wonder whether it would not be simpler to perform the usual Cox regressions for each of TC, TC,D, TR, TR,D, and TD while using the time to each nonfatal event (CR or resistance) as a covariate possibly influencing subsequent residual survival time after these events. This approach, however, requires the unrealistic assumption that the underlying death rates for patients who do or do not experience nonfatal events are the same and that, moreover, achieving CR or being declared resistant does not alter the subsequent risk for death. These assumptions are not made under the model of Shen and Thall, which distinguishes between survival time for patients who achieve CR, TC + TC,D, patients who are declared resistant, TR + TR,D, and patients who die without a nonfatal event, TD. The Cox model, as usually applied, makes no such distinction. Figure2, which compares the estimated survival curve of the 1101 patients as derived from the parametric model to the corresponding Kaplan-Meier curve, illustrates that the parametric model provides an excellent fit to the data.
Estimated survival time.
Estimated survival time curve of the 1101 patients as derived from the parametric model compared to the Kaplan-Meier survival curve for the same patients.
Estimated survival time.
Estimated survival time curve of the 1101 patients as derived from the parametric model compared to the Kaplan-Meier survival curve for the same patients.
The Shen-Thall model was also used to estimate—as functions of TC, age, cytogenetics, and treatment classified as described above—the time from CR to disease recurrence or to death in CR, whichever came first, and the time from CR to disease recurrence. We denote the first of these times, corresponding to disease-free survival among patients achieving CR, as TC,R, and the second time, corresponding to remission duration, as TC,REL. Among the 740 patients who achieved CR, recurrences occurred in 472 (64%) patients and death during CR occurred in 101 (14%) patients. One should bear in mind that analyses of remission duration are confounded because there is no assurance that death in CR and recurrence are independent events. Without such assurance, the practice of censoring patients who die in CR, when conducting analyses of remission duration, is invalid.4 In contrast, no such problems accompany analyses of disease-free survival time among patients in CR.
We also conducted separate analyses of patients with de novo AML (n = 601) and of patients (n = 500) either with AML that arose after an antecedent hematologic disorder (AHD) or with MDS (RAEB or RAEB-t). An AHD is defined as a documented abnormality in the blood count present for at least 1 month before presentation. Of the de novo group 447 (74%) were in CR after the first course of therapy, 133 (22%) died, and 21 (3%) were resistant. Death occurred in 65% of the responders and in 90% of the resistant patients. Among the 500 patient “secondary” group, the CR rate after the first course was 59%, whereas 31% died and 10% were considered resistant. Seventy-five percent of the responders and 92% of the resistant patients died.
Results
Overall results
Table 1 summarizes the estimated effects of the covariates on TC (time to CR) and TC,D(residual survival after CR) and also provides the estimated association parameter describing the relationship between them. For simplicity, additional parameter estimates specifying each event time distribution (3 parameters for each event time) are omitted, because these add no relevant information to the issues under discussion here. Table 1 demonstrates that the only covariate that had a statistically significant (P < .05) effect on TC was age (increasing age associated with longer time to CR). In particular, neither the presence of an inv(16) or a t(8;21) nor the presence of other abnormalities had such an effect on TC relative to what was observed in patients with normal karyotype. There was a trend however (P = .095) for these other abnormalities to be associated with a longer TC. Treatment (regimens containing high-dose cytarabine [HDAC] without fludarabine, or fludarabine + HDAC-containing regimens compared with regimens not containing HDAC) had statistically insignificant effects on TC. In contrast, both cytogenetics and age had the expected highly significant effects on time from CR to death (longer with inv(16) or t(8;21) shorter with other abnormalities or with increasing age). Treatment again had no effect. Of greatest interest was the association parameter describing the relationship between TC and TC,D. The value for this parameter was negative (−0.529, Table 1), indicating that as TCincreased TC,D decreased. The inverse relationship between these 2 times was highly significant (P < .001), and this was the case regardless of whether the model was fit to include treatment, age, and cytogenetics.
Predictors of time to CR (TC) and residual survival time after CR (TC,D) under the multivariate model
| Covariate . | Effect on TC . | P . | Effect on TC,D . | P . |
|---|---|---|---|---|
| Increasing age | TC longer | .002 | TC,D shorter | <.0001 |
| Inv(16) or t(8;21) | TC shorter | .379 | TC,D longer | .004 |
| Other abnormal cytogenetics | TC longer | .095 | TC,D shorter | .008 |
| HDAC | TC shorter | .136 | TC,D longer | .772 |
| Fludarabine + HDAC | TC shorter | .849 | TC,D longer | .749 |
| Covariate . | Effect on TC . | P . | Effect on TC,D . | P . |
|---|---|---|---|---|
| Increasing age | TC longer | .002 | TC,D shorter | <.0001 |
| Inv(16) or t(8;21) | TC shorter | .379 | TC,D longer | .004 |
| Other abnormal cytogenetics | TC longer | .095 | TC,D shorter | .008 |
| HDAC | TC shorter | .136 | TC,D longer | .772 |
| Fludarabine + HDAC | TC shorter | .849 | TC,D longer | .749 |
Association parameter between TC and TC,D= −0.529 (P < .001).
The findings were entirely analogous when TC,R (residual disease-free survival) was examined in place of TC,D(residual survival), consistent with the observation that 80% of the patients who died after achieving CR had a recurrence of disease before death, as noted above. Specifically, the covariate-adjusted association parameter between TC and TC,R was −0.619 with a standard deviation of .112, indicating that the relationship between time to CR and subsequent disease-free survival time was inverse and highly significant (P < .001). As expected, the multivariate model indicated that inv(16) or t(8;21) was associated with a longer TC,R (P = .005), whereas other cytogenetic abnormalities and increasing age were associated with shorter values for this parameter (P = .002 for both age and cytogenetics). Like TC,D, TC,R was unaffected by treatment. Similarly, the covariate-adjusted association parameter between TC and TREL, the latter denoting remission duration, was negative (−0.580) and highly significant (P < .001), indicating that longer times to achieve CR were associated with briefer remissions.
Time to death without achieving CR or being declared resistant (TD) was shorter with increasing age and cytogenetic abnormalities other than inv(16) or t(8;21) (P < .0001 for both), and it was longer with either of the latter 2 abnormalities (P = .016) and for patients given fludarabine + HDAC regimens (P = .022). The presence of prognostically unfavorable cytogenetic abnormalities was strongly associated (P = .009) with a shorter time from start of treatment to declaration of resistance (TR), but no other covariate had a statistically significant effect on this interval. There was the expected inverse relationship (P = .038) between increasing age and residual survival time after a patient was declared resistant (TR,D); other covariates had no effect. In contrast to the inverse relationship between time to CR and residual survival time, time to resistance and subsequent survival time were positively associated (association parameter between TR and TR,D = 0.296), but the relationship was statistically insignificant (P = .52). One should bear in mind, however, that only 71 patients were declared resistant and the undoubted subjectivity associated with the decision to consider patients resistant.
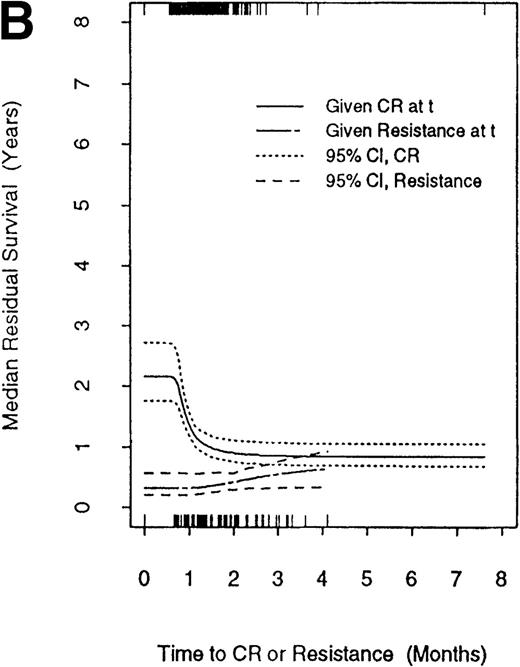
Figure 3 compares median residual survival times in patients who entered CR at a given time, denoted as t, with median residual survival times in patients who were declared resistant at t. The figure includes the 95% confidence interval for each estimated residual median survival time. The times of CR are given along the top of the graph, and the times of declaration of resistance are given along the bottom. Two points should be noted. First, patients who achieve CR at time t live longer than patients who are considered resistant at t regardless of the value oft. Second, reflecting the negative association between TC and TC,D, there is a sharp decline in residual survival as time to enter CR increases. In particular, the residual survival of patients who required more than ∼50 days to enter CR is closer to the residual survival time of resistant patients than to that of patients known to be in CR within approximately 30 days of the start of treatment. It is essential to note that the inverse relationship between TC and TC,D was observed in patients with inv(16) or t(8;21) (Figure4A) and in patients with normal or prognostically poor karyotypes (Figure 4B). However, though median residual survival time decreased with TC in each of these 2 patient subgroups, this effect was dominated by the effect of cytogenetics. Specifically, even if remission induction for an inv(16) or t(8,21) patient took more than 2 months, that patient's estimated median residual survival time remained longer than that of a patient with normal or poor cytogenetics who achieved CR more quickly.
Median residual survival.
Median residual survival time after complete remission (CR) or resistance as functions of the times to these nonfatal events. CR times are indicated along the top of the graph, and declaration of resistance times are indicated along the bottom. Of the 740 patients achieving CR, 19 required ≤20 days, 406 required 21–30 days, 213 required 31–40 days, 65 required 41–50 days, and 37 required >50 days.
Median residual survival.
Median residual survival time after complete remission (CR) or resistance as functions of the times to these nonfatal events. CR times are indicated along the top of the graph, and declaration of resistance times are indicated along the bottom. Of the 740 patients achieving CR, 19 required ≤20 days, 406 required 21–30 days, 213 required 31–40 days, 65 required 41–50 days, and 37 required >50 days.
Mean residual survival as function of time.
(A) Median residual survival time after complete remission (CR) as a function of time to CR in patients with inv(16) or t(8;21). (B) Median residual survival time after CR or resistance as functions of the times to these events in patients with karyotypes other than inv(16) or t(8;21).
Mean residual survival as function of time.
(A) Median residual survival time after complete remission (CR) as a function of time to CR in patients with inv(16) or t(8;21). (B) Median residual survival time after CR or resistance as functions of the times to these events in patients with karyotypes other than inv(16) or t(8;21).
Subset analyses
It was possible that the inverse relationship between time to achieve CR and residual survival time and residual disease-free survival time reflected the presence, in our data set, of a substantial number (500) of patients with AML that developed after AHD or with MDS. Such patients comprised 45% of all 1101 patients. We addressed this possibility by conducting separate analyses for the de novo and secondary groups (Table 2). In both cohorts the association parameter between TC and TC,Dwas negative, indicating an inverse relationship between these 2 times. This relationship reached statistical significance only in the case of the de novo cohort (P = .0004). Table 2 also indicates that the effect of inv(16) or t(8;21) on TC,D was seen only in the de novo cohort, whereas the effect of other cytogenetic abnormalities on this parameter was largely a result of its effect on patients with AML after AHD or with MDS. The effect of age on TC,D was apparent in both cohorts.
Predictors of time to CR (TC) and residual survival time after CR (TC,D) under the multivariate model
| Covariate . | Effect on TC . | P . | Effect on TC,D . | P . |
|---|---|---|---|---|
| De Novo Acute Myeloid | ||||
| Leukemia* | ||||
| Increasing age | TClonger | .011 | TC,D shorter | .002 |
| Inv(16) or t(8;21) | TC shorter | .614 | TC,D longer | <.001 |
| Other abnormal Cytogenetics | TC longer | .607 | TC,D shorter | .465 |
| HDAC | TC shorter | .289 | TC,D shorter | .172 |
| Fludarabine + HDAC | TC shorter | .194 | TC,D shorter | .999 |
| Acute myeloid leukemia arising after AHD; RAEB, RAEB-t† | ||||
| Increasing age | TC longer | .317 | TC,D shorter | .046 |
| Inv(16) or t(8;21) | TC shorter | .332 | TC,D shorter | .950 |
| Other abnormal Cytogenetics | TC longer | .209 | TC,D shorter | <.001 |
| HDAC | TC shorter | .387 | TC,D longer | .022 |
| Fludarabine + HDAC | TC shorter | .621 | TC,D longer | .062 |
| Covariate . | Effect on TC . | P . | Effect on TC,D . | P . |
|---|---|---|---|---|
| De Novo Acute Myeloid | ||||
| Leukemia* | ||||
| Increasing age | TClonger | .011 | TC,D shorter | .002 |
| Inv(16) or t(8;21) | TC shorter | .614 | TC,D longer | <.001 |
| Other abnormal Cytogenetics | TC longer | .607 | TC,D shorter | .465 |
| HDAC | TC shorter | .289 | TC,D shorter | .172 |
| Fludarabine + HDAC | TC shorter | .194 | TC,D shorter | .999 |
| Acute myeloid leukemia arising after AHD; RAEB, RAEB-t† | ||||
| Increasing age | TC longer | .317 | TC,D shorter | .046 |
| Inv(16) or t(8;21) | TC shorter | .332 | TC,D shorter | .950 |
| Other abnormal Cytogenetics | TC longer | .209 | TC,D shorter | <.001 |
| HDAC | TC shorter | .387 | TC,D longer | .022 |
| Fludarabine + HDAC | TC shorter | .621 | TC,D longer | .062 |
Association parameter between TC and TC,D= −0.543 (P = .0004).
Association parameter between TC and TC,D = −0.355 (P = .133).
The finding that under the relevant multivariate models TC, but not treatment, was a predictor of both TC,Rand TC,D strongly suggested that the inverse relationship between TC and the latter times would be observed in each of the 3 treatment groups defined in the Patients and Methods section (no HDAC, HDAC, fludarabine + HDAC). To confirm this suggestion, we computed the association parameters between TC and TC,D in each of these 3 treatment groups, numbering 186 (with 120 CR), 549 (with 395 CR), and 366 (with 225 CR) patients, respectively. In each group, the value for this parameter was negative (−0.248 no HDAC, −0.529 HDAC, −0.922 fludarabine + HDAC), as it had been in the whole data set. The corresponding probability values for the hypothesis that TC and TC,D were unrelated were P = .376,P = .002, and P < .001 for no HDAC, HDAC, and fludarabine + HDAC respectively, recalling that the sample size was smallest in the no HDAC cohort. Thus, the strong negative association between TC and TC,D was present in patients administered HDAC, with or without fludarabine, but the association was not statistically significant in the patients not administered HDAC.
Discussion
Most patients with newly diagnosed AML who enter CR do so after the first course of chemotherapy.11 12 Such patients are considered to constitute a single group regardless of the length of time needed to achieve CR. Our data, exemplified by Figure 3, illustrate that this practice is not necessarily justifiable, assuming that CR is considered a significant outcome at least in part because of its relationship with subsequent survival and disease-free survival times. In particular, the data suggest that time to CR (TC) provides important prognostic information about subsequent survival (TC,D) and subsequent disease-free survival (TC,R) and that this information cannot be supplanted by knowledge of the patient's age, cytogenetic status, or treatment. Indeed, if CR is only achieved after, for example, >50 days, the time to reach CR has more prognostic significance than the fact that CR was actually achieved. Thus, CR attained only after these lengths of time is cosmetic. Our findings are similar for patients with de novo AML and patients with AML arising after AHD or for patients with RAEB/RAEB-t, and are in fact, the inverse relationship between TC and subsequent survival time was more striking in the de novo group (P = .0004). One possible explanation is that the MDS group, to which we added those patients who probably had undocumented MDS based on AHD before AML was diagnosed, consistently had very short remissions. Given this relative homogeneity in outcomes after CR, correlations between the outcome and other parameters would be more difficult to demonstrate than in the de novo group in which outcomes after CR are not as homogeneous. Our findings are also, at least qualitatively, the same for patients who received or did not receive HDAC. The failure to demonstrate a statistically significant association between TC and TC,D in the no HDAC group may reflect the relatively small number of patients achieving CR after the first course of therapy in this group. Specifically, 86% of patients achieving CR in the HDAC groups did so after the first course, whereas in the no HDAC group 61% achieved CR (P < .001).
There are several potential flaws in our analysis. First, blood counts were not performed daily in many patients. At least once, 21 days had elapsed after the start of treatment. Nor were bone marrows necessarily performed on the first date that blood counts met the criteria for CR. Hence, the date on which CR was observed was not necessarily the same as the date on which CR occurred. We are nonetheless unaware of any bias such that the frequency with which blood counts and bone marrows were examined was related to patients' perceived prognoses. Second, there are no objective criteria for declaring a patient resistant to therapy, and so we cannot retrospectively establish why a given patient was considered resistant at a given time after 1 course of therapy. Nor can we ascertain why some patients were considered resistant after 1 course (and therefore included in this analysis) and why some were considered resistant only later (and so not included in the analysis). Indeed, though the 1101 patients whose records we analyzed included 81% of all CRs eventually observed with protocols for newly diagnosed AML, only 35% of the resistant patients (only 71 patients) were included. Hence, it is possible that the resistant patients whom we analyzed were not those that would have been analyzed at another hospital. We obviously could have minimized this difficulty by including all patients resistant after 2 courses of induction therapy, thereby including > 90% of the “resistant” population. Then, however, the comparison (eg, as in Fig. 3) would have to be with patients who were in CR also after 2 courses, if only to find patients who entered CR relatively late so as to compare with patients who were declared resistant relatively late, ie, after 2 courses. In turn, however, this would introduce a new variable, number of courses to CR, which would be strongly confounded with TC and, hence, invalidate the analytical model.
Some of our other findings are also noteworthy. In particular, we are unaware of other studies examining covariates independently related to the time needed to reach CR achieved after 1 course (TC), as opposed to analyses exploring covariates related to achieving CR per se or how many courses are required for this purpose. The observation that TC was unrelated to treatment regimen may reflect a balance between the greater suppression of normal hematopoiesis produced by HDAC-containing regimens and the more rapid anti-AML effect produced by these regimens as opposed to regimens using lower doses of ara-C. Although the general experience is that use of HDAC results in a greater proportion of CR achieved after 1 course, this may reflect a greater reluctance to administer a second course of HDAC than a second course of lower-dose ara-C. Focusing on those CRs achieved after the first course, as we do, eliminates this type of bias. The implication of the relationship between TC and age is discussed below.
There are 2 potential explanations for the effect of TC on TC,D, and TC,R. The first is that time to CR is not only related to intensity of the induction therapy but to the presence of residual AML. Under this hypothesis, recovery of normal hematopoiesis after induction therapy is slow because of residual AML. Thus time to CR is an important clinical marker of “minimal residual disease.” The second explanation, equally plausible, is that a longer time to CR reflects a reduced number, or poorer quality, of residual normal stem cells. Such quantitative or qualitative defects in normal stem cells lead to more rapid disease recurrence or to earlier recognition of it. The association between longer TC and increasing age (Table 1) is consistent with this hypothesis because it is generally accepted that a reduction in stem cell number, function, or both accompanies aging. The clinical implications of these explanations are seemingly different. If TC and TC,D or TC,R are related because TCreflects residual disease, an intensification of induction therapy becomes a therapeutic option in patients with long TC. In contrast, if the relationship between these parameters is a commentary on the status of normal stem cells, this option is less attractive because intensification would be expected to continue to reduce stem cell number or function. One possible approach to this problem would be to recommend allogeneic stem cell transplantation for patients with long TC. Transplantation would in theory provide more effective anti-AML therapy and a new source of stem cells. More generally, a change in therapy, rather than a change in the dose of that therapy given during induction, appears indicated in patients with long TC.
In conclusion, time to CR is an independent predictor of residual survival and disease-free survival times for patients with newly diagnosed AML, RAEB-t, or RAEB who achieve CR after 1 course of chemotherapy. Patients achieving CR only after a lengthy time, eg >50 days, should be considered to have cosmetic CR and should undergo new therapy. Given our data, we believe that time to CR should be reported in detail in chemotherapy studies in these diseases and that CR should no longer be considered an all-or-none phenomenon.
Acknowledgments
The authors thank Sherry Pierce for help with database management, Tania Petts for expert secretarial assistance, and the physicians in the Leukemia Department for providing patient care.
Reprints:Elihu H. Estey, Department of Leukemia, Box 61, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030; e-mail: eestey@odin.mdacc.tmc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. Therapy outcome. / Outcome of therapy in the 1101 patients whose outcomes (complete remission [CR], death, or resistance) were established after the first course of chemotherapy. TC = time from start of treatment to CR; TC,D = time from CR date to death; TR = time from start of treatment to “resistance”; TR,D = time from resistance date to death; and TD = time to death in patients dying before considered to be in CR or to be resistant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/1/10.1182_blood.v95.1.72/5/m_bloo00126001x.jpeg?Expires=1767728482&Signature=GzTDcD~OUn8TQy2o~lv8THVxlRlRoNAqD9EQ7mchBzkB1UxQhJpehGsxgJKLf8GTGptdBNnQte6m2rcb405o~voLKyzw72A~FFAnSGfawVEh7yhbmX4UsYF9VdbDwkb9ScoYGLVqxc0PUJDB3V~nMYKWMkDCZjUpYCOvrrHTTI~gbfShSSyvaiCG2C-AWxJBO7MGx1TuBvPnMXzVqUjvsh76Ki8swyzfQfWcwozXvpDvi-ZONkwz7bEIXFyf6I74tSkoweNcmXnXoCog7Ru0qCsueqdJL~hfotf7h1~Jmp1RcVV1EBEetk21OxPCzFoIhXLBFVYCCKvBfWfNEW7yqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal