Abstract
Donor lymphocyte infusion (DLI) was originally administered as a single, relatively large dose of lymphocytes called a bulk dose regimen (BDR). It has since been suggested that the use of an escalating dose regimen (EDR) may be equally effective against leukemia while it induces less graft-versus-host disease (GVHD). We therefore compared the efficacy and incidence of complications in a nonrandomized sequential study of the 2 regimens in 48 consecutive patients who had relapses with cytogenetic or hematologic evidence of chronic myeloid leukemia after allogeneic stem cell transplantation. Twenty-eight patients were treated on a BDR (August 1990 to November 1995) and 20 were treated on an EDR (December 1995 to January 1998). Although the probability of achieving cytogenetic remission within 2 years of starting DLI did not differ significantly between the 2 groups (EDR, 91% [CI, 63%–98%] vs. BDR, 67% [CI,49%–83%],P = .70), the incidence of GVHD was much lower using EDR (10% vs. 44%, P = .011). When we considered only subsets of patients treated by BDR or EDR who had received comparable total lymphoid cell doses, the incidence and severity of acute and chronic GVHD were both significantly lower for recipients treated by EDR than for recipients treated by BDR (P = .005 andP = .031, respectively). These findings suggest that the incidence of GVHD associated with the EDR is low, not because the final cell dose is small, but because lymphocytes are administered over a considerable number of months. (Blood. 2000;95:67-71)
Donor lymphocyte infusion (DLI) is an effective therapeutic option to treat chronic myeloid leukemia (CML) in relapse after allogeneic stem cell transplantation (SCT).1,2Response rates vary between 64% and 86%, but the beneficial effects are often associated with a high incidence of graft-versus-host disease (GVHD) that can affect up to 50% of the treated patients (reviewed by Dazzi and Goldman3).
The conventional approach to DLI has been to infuse single “bulk” doses containing variable numbers of CD3+ T cells, but this is associated with significant incidences of acute and chronic GVHD and occasionally with death.2,4-6 Two approaches have been introduced to reduce the incidence of GVHD. One was based on the selective depletion from the infusion of CD8+ lymphocytes, which are thought to include most of the cells responsible for mediating GVHD.7,8 The other strategy relies on the transfusion of donor lymphocytes in multiple aliquots, starting at low cell numbers and escalating the dosage at variable intervals as required.9 The assumption underlying the use of an escalating dose regimen (EDR) is that the incidence of GVHD increases with the total cell dose administered. Thus identification of the minimal cell dose capable of inducing remission would minimize the risk for GVHD. We have adopted this approach at our institution and report here a comparison of the incidence of response and GVHD in 30 patients treated for CML in relapse by bulk dose regimen (BDR) and in 21 patients treated by EDR.
Patients and methods
Patients
Fifty-eight consecutive patients with Philadelphia (Ph) chromosome-positive CML who had relapses after allogeneic SCT were treated with DLI in a nonrandomized, sequential study at the Hammersmith Hospital in London between August 1991 to January 1998. Informed consent was obtained before patients were enrolled in the study. Five patients were excluded from further analysis because they were changed from one regimen to the other and could not be assessed or because their disease was already in the blastic phase when treatment with DLI was initiated. Five other patients treated with DLI for molecular relapse (without evidence of cytogenetic relapse) were also omitted. Results of treatment in 48 patients were included in this study (Table 1). The respective donor was a genetically human leukocyte antigen (HLA)-identical sibling (SIB) or a serologically HLA-matched volunteer unrelated donor (VUD). If the donor was unrelated, isoelectric focusing and molecular typing for DRB1 were used to confirm HLA identity at class 1 and 2 loci, respectively. If a choice between unrelated donors existed, the cytotoxic T-cell precursor assay was used to aid donor selection. Transplant conditioning and GVHD prophylaxis were performed according to our standard procedures as previously described.10,11 Of the 48 patients, 18 received nonmanipulated marrow cells from their respective donors, and 30 received donor cells treated in vitro with a murine monoclonal antibody of the Campath series (CD52) or received a Campath monoclonal antibody intravenously for the prevention of GVHD.11 12
Patient characteristics according to donor lymphocyte infusion regimen used
| Features . | Bulk (n = 28) . | Escalating (n = 20) . | Overall (n = 48) . |
|---|---|---|---|
| Donor type | |||
| SIB/VUD | 18/10 | 10/10 | 28/20 |
| Patient gender: | |||
| Male/female | 8/20 | 16/4 | 24/24 |
| GVHD prophylaxis at SCT: | |||
| TCD/T-replete | 18/10 | 12/8 | 30/18 |
| GVHD after SCT: | |||
| Grade 2-4 acute | 10 | 7 | 17 |
| Extensive chronic | 10 | 2 | 12 |
| Relapse | |||
| Cytogenetic alone | 7 | 9 | 16 |
| Hematologic-CP | 17 | 6 | 23 |
| Hematologic-AP | 4 | 5 | 9 |
| Interval SCT → relapse | 16.6 | 12.2 | 12.5 |
| Months (range) | 3-103 | 4-51 | 3-103 |
| Interval relapse → DLI | 10.9 | 12.9 | 11.1 |
| Months (range) | 0-70 | 1-53 | 0-70 |
| Features . | Bulk (n = 28) . | Escalating (n = 20) . | Overall (n = 48) . |
|---|---|---|---|
| Donor type | |||
| SIB/VUD | 18/10 | 10/10 | 28/20 |
| Patient gender: | |||
| Male/female | 8/20 | 16/4 | 24/24 |
| GVHD prophylaxis at SCT: | |||
| TCD/T-replete | 18/10 | 12/8 | 30/18 |
| GVHD after SCT: | |||
| Grade 2-4 acute | 10 | 7 | 17 |
| Extensive chronic | 10 | 2 | 12 |
| Relapse | |||
| Cytogenetic alone | 7 | 9 | 16 |
| Hematologic-CP | 17 | 6 | 23 |
| Hematologic-AP | 4 | 5 | 9 |
| Interval SCT → relapse | 16.6 | 12.2 | 12.5 |
| Months (range) | 3-103 | 4-51 | 3-103 |
| Interval relapse → DLI | 10.9 | 12.9 | 11.1 |
| Months (range) | 0-70 | 1-53 | 0-70 |
SIB, sibling donor; VUD, volunteer unrelated donor; SCT, stem cell transplant; GVHD, graft-versus-host disease; TCD, T-cell depleted SCT; hematologic-CP, chronic phase; hematologic-AP, hematologic relapse in advanced phase.
Cytogenetics and quantitation of BCR-ABL mRNA
Patients considered to be in remission after allogeneic SCT were monitored at intervals not exceeding 3 months and usually more frequently. When relapse was diagnosed (see below), the frequency with which patients were monitored was increased. At each clinic visit, full blood counts were taken. Peripheral blood was examined for BCR-ABL transcripts by multiplex, 2-step reverse transcription–polymerase chain reaction (RT-PCR), or both as reported previously.13If BCR-ABL transcript numbers were raised, cytogenetic studies using standard techniques were performed on bone marrow metaphases. At least 30 marrow metaphases were analyzed whenever possible.
Definitions of relapse
Patients who experience relapse after allogeneic SCT probably do so in a sequential manner with relapse recognizable first at the molecular level, then at the cytogenetic level, and finally with hematologic evidence of leukemia. The 48 patients in this series satisfied criteria for cytogenetic or hematologic relapse when treatment with DLI was initiated (Table 1). A patient was considered to be in cytogenetic relapse if 1 or more Ph-positive metaphase was detected without evidence of hematologic relapse. Hematologic relapse was defined as peripheral blood leukocytosis, usually with a predominance of myelocytes and neutrophils in the differential count, accompanied by a hypercellular bone marrow with Ph-chromosome positivity on cytogenetic analysis. The phase of CML was classified in accordance with criteria proposed by the International Bone Marrow Transplant Registry.14
Donor leukocyte infusions
Donor cells were collected on a continuous flow blood cell separator (Cobe Spectra, Gloucester, UK). The dose of CD3+ cells was calculated by cytofluorimeter analysis after staining with a CD3 monoclonal antibody (Becton Dickinson, Oxford, UK). Cells were transfused to the respective patient on the day of collection or were frozen and stored in liquid nitrogen for future use as described below.
Twenty-eight patients received DLI by the BDR from August 1990 through November 1995; subsequently 20 patients started treatment with DLI on an EDR from December 1995 through January 1998 (Table 1). The 2 groups were comparable with regard to donor type, use of T-cell depletion for the original transplant, incidence of acute GVHD after BMT, intervals from SCT to relapse, and intervals from relapse to DLI. More women received BDR, and more men received EDR (Table 1). Chronic GVHD occurred more frequently after the original SCT in the recipients of BDR.
For the patients treated with BDR, the median dose of lymphocytes infused was 1.5 × 108/kg (range, 0.6–5.3). For those treated with EDR, each patient was assessed with RT-PCR, cytogenetic studies, or both 12 weeks after the preceding dose, and an additional dose of lymphocytes was planned if there was no clear evidence of response; in practice, the median interval between doses was 20 weeks (range, 12–33 weeks). The median total lymphocyte dose was 1.9 × 108/kg (range, 0.01–3.3). Dose schedules designed for recipients of SIB transplants were higher than for recipients of VUD transplants because we anticipated that graft-versus-leukemia (GVL) effects at comparable lymphocyte dose levels would be greater using cells from nongenetically HLA-identical donors. Thus for SIB transplant recipients, the target doses of CD3+ cells/kg were sequentially 107→ 5 × 107 → 108, whereas for VUD recipients the target doses were 106 → 107→ 5 × 107 → 108, Mononuclear cells were collected from the donor, and the appropriate dose of CD3+ cells was calculated and transfused immediately to the recipient. The remaining cells were frozen and stored in aliquots for future use. All patients included in this study completed the full protocol, that is, they were treated until they responded or until they were deemed refractory to DLI; responders were followed up for at least 3 months.
The management of patients before and after BDR DLI has been described elsewhere.15 In the EDR group, patients in cytogenetic relapse did not receive any cytotoxic drugs, and none received cyclosporine at the time of DLI. Patients in hematologic relapse with leukocyte counts >30 × 109/L were treated with hydroxyurea as required, but this drug was discontinued on the day before DLI. If the leukocyte count exceeded 100 × 109/L during DLI treatment, patients underwent leukapheresis for cytoreduction. Only 1 patient needed hydroxyurea shortly after DLI; 2 patients received hydroxyurea to reduce the leukocyte count before DLI. Neither interferon-α nor immunosuppressive agents were administered in conjunction with DLI.
Assessment of response
Hematologic remission was achieved if a patient with previous evidence of hematologic relapse attained a normal blood count. The patient was considered to have achieved cytogenetic remission if no Ph-positive metaphases were detected in the marrow.
Acute GVHD was graded according to the Seattle criteria.16Chronic GVHD was defined as none, limited, or extensive.
Statistics
The Fisher exact test, the chi-square test or the chi-square trend test, and the Mann-Whitney U test were used to compare groups as appropriate. Outcome probabilities were calculated by the method of Kaplan and Meier.17 The log-rank test was used to compare survival curves. Proportional hazards regression analysis was specifically used to investigate the effect of chronic GVHD occurring after the SCT on the development of GVHD after DLI. All quotedP values are 2-sided, and confidence intervals refer to 95% boundaries.
Results
Response and side effects
Response.
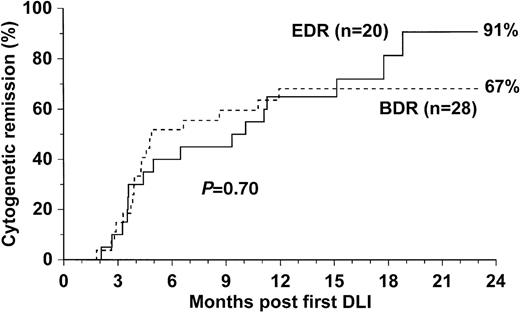
The efficacy of the 2 treatments and the incidence of acute and chronic GVHD are shown in Table 2. The probability of achieving cytogenetic remission was higher for recipients treated by EDR than for those treated by BDR (91% [CI, 63%–98%] vs. 67% [CI, 49%–83%]), but the difference was not statistically significant (P = .70) (Fig. 1). The median time to achieve cytogenetic remission was shorter for recipients of BDR than for recipients of EDR (125 days [range, 55–363 days] vs. 163 days [range, 63–540 days]), but the difference was not significant (P = .32).
Response and incidence of graft-versus-host disease after treatment with bulk or escalating dose donor lymphocyte infusion
| . | Donor Lymphocyte Infusion Dose Regimen . | |
|---|---|---|
| Bulk (n = 28) . | Escalating (n = 20) . | |
| Probability of cytogenetic remission at 2 years (95% CI) | 67% (49%-83%) | 91% (63%-98%) |
| GVHD | ||
| Acute | ||
| Grade 0 | 10* (37%) | 15 (75%) |
| Grade 1 | 5* (19%) | 3 (15%) |
| Grade 2 | 5* (19%) | 2 (10%) |
| Grades 3-4 | 7* (26%) | 0 (0%) |
| Chronic | ||
| Non/limited | 15*,† (59%) | 17‡ (89%) |
| Extensive | 11*,† (41%) | 2‡ (11%) |
| . | Donor Lymphocyte Infusion Dose Regimen . | |
|---|---|---|
| Bulk (n = 28) . | Escalating (n = 20) . | |
| Probability of cytogenetic remission at 2 years (95% CI) | 67% (49%-83%) | 91% (63%-98%) |
| GVHD | ||
| Acute | ||
| Grade 0 | 10* (37%) | 15 (75%) |
| Grade 1 | 5* (19%) | 3 (15%) |
| Grade 2 | 5* (19%) | 2 (10%) |
| Grades 3-4 | 7* (26%) | 0 (0%) |
| Chronic | ||
| Non/limited | 15*,† (59%) | 17‡ (89%) |
| Extensive | 11*,† (41%) | 2‡ (11%) |
CI, confidence interval; GVHD, graft-versus-host disease; DLI, donor lymphocyte infusion.
GVHD data for 1 patient are unknown.
One patient died of grade 4 acute GVHD 46 days after DLI and could not be evaluated for response or chronic GVHD.
The follow-up of 1 patient was too short from DLI (<5 months) to evaluate chronic GVHD.
Probability of cytogenetic remission.
Probability of achieving cytogenetic remission for 48 patients who received escalating-dose or bulk-dose infusion regimens dated from the first (or only) infusion of donor lymphocytes.
Probability of cytogenetic remission.
Probability of achieving cytogenetic remission for 48 patients who received escalating-dose or bulk-dose infusion regimens dated from the first (or only) infusion of donor lymphocytes.
Graft-versus-host disease.
The incidence of acute GVHD (AGVHD) (grades 2–4) was higher in the BDR group than in the EDR group (44% vs. 10%; P = .011). Extensive chronic GVHD (CGVHD) occurred more frequently in the BDR recipients than in the EDR recipients (41% vs. 11%;P = .02) (Table 2). The probabilities of grades 2 to 4 GVHD and extensive CGVHD developing after the last DLI dose are shown in Figure 2.
Probability of acute and chronic graft-versus-host disease.
Probabilities of developing grade 2-4 acute GVHD (A) and extensive chronic GVHD (B) in 37 patients treated by escalating-dose or bulk-dose infusion regimens of donor lymphocytes in the 0.4 to 3.3 × 108/kg range.
Probability of acute and chronic graft-versus-host disease.
Probabilities of developing grade 2-4 acute GVHD (A) and extensive chronic GVHD (B) in 37 patients treated by escalating-dose or bulk-dose infusion regimens of donor lymphocytes in the 0.4 to 3.3 × 108/kg range.
The higher frequency of GVHD in the BDR group may be attributed to the fact that these patients experienced a higher incidence of CGVHD after SCT (Table 1). However, multivariate analysis showed that the schedule of administration was the most significant factor (P = .08), whereas extensive CGVHD after SCT was uninformative (P = .54). The median duration of follow-up after the last DLI was significantly longer for the BDR group (46 months; range, 7–99 months) than for the EDR patients (20 months; range, 5–33 months) (P = .001). However, 11 of the 13 patients who developed extensive CGVHD post DLI did so within 6 months of the last dose, and therefore it is unlikely that the difference in follow-up contributes to a reduction in the observed incidence of CGVHD in the EDR group.
The higher incidence of GVHD in the BDR group might have resulted because patients receiving EDR achieved responses at lower doses than patients receiving BDR and thus did not receive additional unnecessary infusions. Therefore, we compared the incidence and severity of GVHD by subgroups of patients who received comparable total numbers of lymphocytes by BDR and EDR. We identified patients whose total lymphocyte dose lay within a range with its upper limit defined by the highest number of lymphocytes administered by EDR (3.3 × 108/kg) and its lower limited defined by the lowest number administered by BDR (0.4 × 108/kg). Within this range 25 patients were treated by BDR (total lymphocytes infused median 1.5 × 108/kg, range 0.4-3.1) and 12 patients treated by EDR total lymphocytes infused, (median 1.9 × 108/kg; range, 0.7–3.3) (Table3). In these 2 groups the incidences of acute and chronic GVHD were substantially greater in recipients of DLI by BDR than by EDR (P = .005 and P = .031 respectively).
Comparison of incidences of graft-versus-host disease in subgroups of patients treated by bulk dose regimen or escalating-dose regimen
| . | BDR (n = 25) . | EDR (n = 12) . |
|---|---|---|
| Relapse stage | ||
| Cytogenetic | 5 | 4 |
| Hematologic–CP | 16 | 3 |
| Hematologic–AP | 4 | 5 |
| Complete remission | 17 (68%) | 8 (67%) |
| GVHD: | ||
| Acute | ||
| Grade 0 | 8 | 10 |
| Grade 1 | 5 | 1 |
| Grade 2 | 5 | 1 |
| Grades 3-4 | 7 | 0 |
| Chronic | ||
| None/limited | 14 | 11 |
| Extensive | 11 | 1 |
| . | BDR (n = 25) . | EDR (n = 12) . |
|---|---|---|
| Relapse stage | ||
| Cytogenetic | 5 | 4 |
| Hematologic–CP | 16 | 3 |
| Hematologic–AP | 4 | 5 |
| Complete remission | 17 (68%) | 8 (67%) |
| GVHD: | ||
| Acute | ||
| Grade 0 | 8 | 10 |
| Grade 1 | 5 | 1 |
| Grade 2 | 5 | 1 |
| Grades 3-4 | 7 | 0 |
| Chronic | ||
| None/limited | 14 | 11 |
| Extensive | 11 | 1 |
GVHD, graft-versus-host disease; BDR, bulk-dose regimen; EDR, escalating-dose regimen. Lymphocyte doses ranged from 0.4-3.3 × 108/kg.
The results derived from the analysis of patients receiving different dose levels by BDR were consistent with the previous comparison. The incidence of GVHD did not differ between patients transfused with <1.5 × 108 lymphocytes/kg and those transfused with >1.5 × 108 lymphocytes/kg (in both groups, acute GVHD grades 2–4 developed in 45% of patients). Moreover the probability of achieving cytogenetic remission did not statistically differ between the 2 groups (Table 4).
Comparison of incidences of graft-versus-host disease in subgroups of patients treated by bulk dose regimen
| . | <1.5 × 108 (n = 11) . | ≥1.5 × 108 (n = 11) . |
|---|---|---|
| Relapse stage | ||
| Cytogenetic | 2 | 5 |
| Hematologic–CP | 8 | 5 |
| Hematologic–AP | 1 | 1 |
| Complete remission | 6 (54%) | 9 (81%) |
| GVHD: | ||
| Acute | ||
| Grades 0-1 | 6 | 6 |
| Grade 2 | 1 | 3 |
| Grades 3-4 | 4 | 2 |
| Chronic | ||
| None/limited | 6 | 5 |
| Extensive | 4 | 6 |
| . | <1.5 × 108 (n = 11) . | ≥1.5 × 108 (n = 11) . |
|---|---|---|
| Relapse stage | ||
| Cytogenetic | 2 | 5 |
| Hematologic–CP | 8 | 5 |
| Hematologic–AP | 1 | 1 |
| Complete remission | 6 (54%) | 9 (81%) |
| GVHD: | ||
| Acute | ||
| Grades 0-1 | 6 | 6 |
| Grade 2 | 1 | 3 |
| Grades 3-4 | 4 | 2 |
| Chronic | ||
| None/limited | 6 | 5 |
| Extensive | 4 | 6 |
GVHD, graft-versus-host disease.
Lymphocyte doses ranged from <1.5 × 108/kg to >1.5 × 108/kg.
Marrow aplasia.
In the BDR group pancytopenia with marrow aplasia was observed in 5 of 21 patients treated for hematologic relapse, but it was observed in none of the patients treated in cytogenetic relapse. In the EDR group, marrow aplasia was observed in 2 of 11 patients treated for hematologic relapse, but it was observed in none of those treated for cytogenetic relapse. These results are consistent with the fact that residual donor hematopoiesis is severely impaired in advanced disease.18
Factors influencing response
In the EDR group, all 9 patients treated for cytogenetic relapse attained cytogenetic remission, whereas only 7 of 11 in hematologic relapse did so (P = .052). In the BDR group, all 7 patients treated for cytogenetic relapse achieved cytogenetic remission; of the 21 patients treated for hematologic relapse, 11 achieved cytogenetic remission (P = .031).
The donor type did not influence outcomes among patients receiving either bulk-dose DLI or escalating-dose DLI. With BDR complete remission was attained in 12 of 18 recipients of SIB transplants and in 6 of 10 recipients of VUD transplants. With EDR complete remission was attained in 8 of 10 recipients of SIB transplants and in 8 of 10 VUD recipients.
Durability of response
The durability of response was assessed for patients who achieved cytogenetic remission. Seventeen transfused with bulk DLI remained Ph-negative for a median time of 47 months (range, 13–86 months); 1 patient relapsed into chronic phase. Of the 14 patients who achieved cytogenetic remission after treatment on the EDR 1 relapsed into blastic phase. However, the median duration of follow-up for these patients was shorter (median time, 15 months; range, 6–30 months) than for BDR recipients.
Discussion
We have compared the incidences of response and of GVHD in 2 patient groups, 1 treated with DLI administered in the conventional manner (BDR) and the other treated on an escalating-dose regimen (EDR). The incidence of cytogenetic response was higher among patients treated by EDR than among patients treated by BDR, though the difference was not significant. The incidence of GVHD, both acute and chronic, was significantly lower in recipients of DLI by EDR than in recipients treated by BDR (Table 2). Although the durability of the response does not seem to differ between the 2 groups, the shorter duration of follow-up for the patients treated by EDR does not yet allow us to draw any conclusions.
Although the lower incidence of GVHD may be ascribed to the fact that patients in the EDR group received lower total cell doses than patients in the BDR group, the incidence of GVHD was still significantly lower in recipients treated by EDR when we analyzed results by subgroups of patients treated by BDR and EDR who received comparable total cell doses (Table 3). These results imply that it is the administration of lymphoid cells in escalating aliquots for a number of months rather than a lower total cell dose that is responsible for the lower incidence of GVHD in the recipients of EDR. This interpretation is supported by the observation that within the BDR group the incidence of GVHD was similar in recipients of low and high total cell doses (Table4).
Why a protracted administration schedule of donor lymphocytes should be associated with a lower incidence of GVHD is unknown. It is possible that the administration of DLI by EDR means that the total time from the original transplant procedure before an effective antileukemic dose is achieved is longer for recipients of EDR than for recipients of BDR. For example, the delayed administration of donor splenocytes in a murine model of allogeneic bone marrow transplantation greatly reduces the incidence and severity of GVHD.19 It should be noted parenthetically that the incidence of GVHD in our study was lower than that observed in 2 other reports9 20 of the use of escalating-dose DLI; in both studies, the median intervals between successive doses for recipients of DLI by EDR were much shorter than in our study (3 to 10 weeks vs. 20 weeks).
Alternatively, one could speculate that the initial low dose or doses of donor cells that are inadequate to exert a useful GVL effect are “anergized” by contact with recipient tissues and thereby reduce the capacity of the subsequent higher dose or doses to produce an unwanted GVHD. This speculation is supported by the notion of “transferable anergy,” whereby anergic T cells inhibit an allospecific T-cell response.21 If this speculation were valid, a possible clinical strategy would be first to infuse a low dose of donor CD3+ cells to establish a population of donor-derived immunoregulatory cells and subsequently to infuse a high dose to induce an effective but safe GVL effect.
In summary our data show that the administration of donor lymphocytes on a protracted escalating-dose schedule is preferable to a bulk dose regimen and should be considered as the optimal regimen. The observation that the type of regimen has a greater impact on the incidence GVHD than the total cell dose warrants further investigation because it may form the basis for developing safer and more effective protocols for adoptive immunotherapy.
Acknowledgments
We acknowledge the technical contributions of John Davis, Christine MacDonald, and Sarah Chilcott, all of whom prepared donor cells for infusion. We also thank the clinicians and nurses responsible for patient care at Hammersmith Hospital for collecting blood specimens.
Reprints:John M. Goldman, Department of Haematology, Imperial College School of Medicine/Hammersmith Hospital, Du Cane Road, London W12 0NN, UK; e-mail: jgoldman@ic.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal