Abstract
High-dose therapy has become a common treatment for myeloma. The objectives of this study were to estimate in a prospective, population-based setting the impact on survival of high-dose therapy in newly diagnosed, symptomatic patients less than 60 years old and to compare the results with those of conventionally treated historic controls. The prospective population comprised 348 patients. Of these, 274 were treated according to a specified intensive-therapy protocol (Nordic Myeloma Study Group [NMSG] #5/94) and constituted the intensive-therapy group. The historic population consisted of 313 patients identified from 5 previous population-based Nordic studies. Of these, 274 fulfilled the eligibility criteria for high-dose therapy stated in NMSG #5/94 and constituted the control group. The expected numbers of patients in the prospective population and the historic population were 450 and 410, respectively, estimated from previously established data on the incidence in this population and the population base for each study. Survival was prolonged in the intensive-therapy group compared with the control group (risk ratio for the control group 1.62; 95% confidence interval 1.22-2.15; P = .001). These groups represented more than 60% of the expected number of patients. When survival for all the registered patients in the 2 populations was compared, representing more than 75% of the expected number of patients, the advantage for the prospective population persisted (risk ratio for the historic population 1.46; 95% confidence interval 1.14-1.86; P = .002). These results indicate that the introduction of high-dose therapy for newly diagnosed myeloma has resulted in prolonged survival for the total patient population aged less than 60 years. (Blood. 2000; 95:7-11)
Intermittent melphalan and prednisone has for many years been the recommended treatment for patients with multiple myeloma. Trials with other drug combinations have not led to any major improvement in clinical outcome. With conventional therapy, only a minority of patients achieve a complete response, and virtually all patients eventually succumb to progressive disease, with a median survival of approximately 3 years.1-3
During the last decade, high-dose therapy with autologous stem cell support has become a common treatment in younger patients with myeloma.4 Although a number of reports have been published, a lack of comparison with conventional chemotherapy and selection bias have hindered a reliable evaluation of the feasibility and value of this treatment.
In 1994, the Nordic Myeloma Study Group (NMSG) started a prospective study in which the primary aim was to evaluate the impact on survival of high-dose therapy in the entire population of patients aged less than 60 years with newly diagnosed, symptomatic myeloma. Survival was compared with that of historic controls, derived from previous Nordic population-based studies on conventional chemotherapy. Secondary aims were to evaluate event-free survival, response rate, toxicity, feasibility, effects on quality of life, and the health economics of a specified intensive-therapy protocol (NMSG #5/94). We present the results of the study, except for the effects on quality of life and cost-effectiveness, which will be addressed in a separate report.
Materials and methods
Patients
Prospective population.
Fourteen participating centers in Denmark, Norway, and Sweden, representing a total population of 15 million inhabitants, were requested to register all newly diagnosed, symptomatic myeloma patients less than 60 years old within their respective regions. The registration started in Norway and Sweden in March 1994 and in Denmark in October 1994, and it was stopped in June 1997. A total of 348 patients were registered in the prospective population. Two hundred seventy-four of these patients, constituting the intensive-therapy group, were treated according to a specified treatment protocol (NMSG #5/94, described later). The reasons for nonentry into the protocol are presented in Table 1.
Reasons for non-inclusion in the intensive-therapy group (prospective population) or the control group (historic population)
| Reason . | Prospective Population . | Historic Population . |
|---|---|---|
| Total number registered, fulfilling diagnostic criteria | 348 | 313 |
| Not receiving intensive therapy for physician-related reasons | 9 | — |
| Treated according to intensive-therapy protocols other than NMSG #5/94 | 27 | — |
| Not eligible for intensive therapy | ||
| Severe coincident heart or lung disease | 6 | 9 |
| Other active malignancy or severe coincident illness | 6 | 1 |
| Psychiatric disease or abuse | 3 | 15 |
| Terminal illness, including refractory uremia | 8 | 9 |
| Patient refusal | 15 | 5 |
| Total number included in the intensive-therapy group or the control group | 274 | 274 |
| Reason . | Prospective Population . | Historic Population . |
|---|---|---|
| Total number registered, fulfilling diagnostic criteria | 348 | 313 |
| Not receiving intensive therapy for physician-related reasons | 9 | — |
| Treated according to intensive-therapy protocols other than NMSG #5/94 | 27 | — |
| Not eligible for intensive therapy | ||
| Severe coincident heart or lung disease | 6 | 9 |
| Other active malignancy or severe coincident illness | 6 | 1 |
| Psychiatric disease or abuse | 3 | 15 |
| Terminal illness, including refractory uremia | 8 | 9 |
| Patient refusal | 15 | 5 |
| Total number included in the intensive-therapy group or the control group | 274 | 274 |
Historic population.
The historic population was identified from 5 previous prospective population-based Nordic studies, of which 3 were incidence studies5-7 and 2 were randomized clinical trials.8,9 Details on the historic population have been presented elsewhere.10 Briefly, 313 patients less than 60 years old were registered in these studies. The records of all the patients were reviewed and updated. Thirty-nine patients were judged retrospectively not to fulfill the eligibility criteria for intensive therapy stated in the NMSG #5/94 protocol for the reasons presented in Table 1. The remaining 274 patients constituted the control group, intended for comparison with the intensive-therapy group.
Expected number of patients.
The crude incidence of multiple myeloma at less than 60 years was calculated to be 0.9 per 100 000 inhabitants annually, based on previous Nordic incidence studies6,7,11 and the official cancer statistics of Sweden.12 The expected number of new cases within the prospective population and in each study representing the historic population was then estimated from this incidence figure, the known population base for each study and the study periods (Table 2).
Expected number of patients in the prospective population and the historic population, proportion of patients registered, and proportion of patients included in the intensive-therapy group or the control group
| Number of Patients . | Prospective Population . | Historic Population . | ||
|---|---|---|---|---|
| Number . | Proportion of Expected . | Number . | Proportion of Expected . | |
| Expected | 450 | — | 410 | — |
| Registered | 348 | 77% | 313 | 76% |
| Included in the intensive-therapy group or the control group | 274 | 61% | 274 | 67% |
| Number of Patients . | Prospective Population . | Historic Population . | ||
|---|---|---|---|---|
| Number . | Proportion of Expected . | Number . | Proportion of Expected . | |
| Expected | 450 | — | 410 | — |
| Registered | 348 | 77% | 313 | 76% |
| Included in the intensive-therapy group or the control group | 274 | 61% | 274 | 67% |
Methods
Diagnostic criteria.
The diagnosis of multiple myeloma was accepted if criteria A+C, A+D, or B+C+D of the following were fulfilled: (A) serum monoclonal component (M-protein) concentration of immunoglobulin (Ig)G > 30 g/L, IgA > 20 g/L, the presence of an M-protein of IgD or IgE regardless of concentration, or Bence-Jones proteinuria > 1 g/24 h; (B) M-protein in serum or urine at a lower concentration than described under A; (C) at least 10% plasma cells in bone marrow aspirate or biopsy-verified plasmacytoma of bone or soft tissue; and (D) osteolytic bone lesions. Only patients with symptomatic disease were registered.
NMSG #5/94 eligibility criteria.
All patients could be treated according to the NMSG #5/94 protocol provided that they were not considered ineligible for the induction therapy because of severe chronic heart or lung disease, other active malignancy or severe coincident illness, psychiatric disease or abuse, terminal illness, or refusal.
NMSG #5/94 treatment protocol.
The treatment was divided into 4 phases: (I) induction therapy with 3 courses of VAD (vincristine 1.6 mg and doxorubicin 36 mg/m2as a continuous intravenous infusion on days 1 to 4; dexamethasone 40 mg/d on days 1 to 4, 9 to 12, and 17 to 20; repeated every fourth week); (II) peripheral blood stem cell harvest of a minimum of 2 × 106 CD34+ cells per kilogram body weight at regeneration after cyclophosphamide 4 g/m2 given as a single dose intravenously and granulocyte colony-stimulating factor (G-CSF; filgrastim) 5 μg/kg daily; (III) high-dose therapy with melphalan 200 mg/m2 given as a single dose intravenously, followed by stem cell infusion 48 hours later, and G-CSF (filgrastim) 5 μg/kg daily from day 4 after grafting until the absolute neutrophil count was more than 1.0 × 109/L for 3 consecutive days; and (IV) maintenance therapy with interferon alfa-2b 3 MU/m2 3 times per week subcutaneously, started 2 months after grafting and maintained until relapse. Patients with progressive disease or with emerging contraindications to phases II to III were taken off the treatment protocol. For patients not achieving at least a partial response (for definition, see later) after phase I, the responsible physician was free to choose between stopping or continuing protocol-regulated treatment. Allogeneic stem cell transplantation was accepted at the responsible physician's discretion if the patient had an HLA identical sibling. For patients leaving protocol-regulated treatment and for relapsing patients, the responsible physician was free to choose therapy.
Definitions.
Complete response was defined as the disappearance of M-protein from serum and urine in agarose gel electrophoresis and < 5% plasma cells in a bone marrow aspirate. Partial response was defined by at least a 50% reduction of the initial serum M-protein concentration and a reduction of Bence-Jones proteinuria to < 0.2 g/24 h. Minor response was defined by a 25% to 50% reduction of the initial serum M-protein concentration and a reduction in Bence-Jones proteinuria by at least 50% but exceeding 0.2 g/24 h. To fulfill the criteria for complete, partial, or minor response, the patients were not allowed to have any other signs of myeloma progression, such as persisting hypercalcemia or progressive renal insufficiency, skeletal disease, or bone marrow insufficiency due to plasma cell infiltration. Progression was defined by a confirmed increase in the serum M-protein concentration by more than 25% from the level at the time of best response, an increase of Bence-Jones proteinuria to more than 1.0 g/24 h, or other unequivocal signs of disease progression, such as hypercalcemia, progressive skeletal disease, or soft-tissue plasmacytomas. Progression, death without progression, and occurrence of a secondary malignancy were all considered as events. Event-free and total survival were calculated from the start of therapy.
Follow-up evaluation.
All patients treated according to the NMSG #5/94 protocol were evaluated before the start of phase II and phase III, and thereafter every sixth week. Patients who did not complete phase I to III treatment were evaluated every sixth week after leaving the protocol. All registered patients were followed until death or September 1998.
Statistical analysis.
The proportions of patients with a given characteristic were compared using Fisher's exact test for variables with frequency scale and Wilcoxon rank-sum test for the remaining variables. Event-free and total survival rates were calculated according to the Kaplan-Meier method, and survival comparisons between groups were made by the log-rank test. The Cox proportional hazards regression model was used to estimate the prognostic importance of different variables. Age, bone marrow plasma cells, blood hemoglobin, serum calcium, serum creatinine, blood platelets, and serum albumin were included as continuous variables. The following variables were dichotomized: sex (male versus female), stage according to Durie and Salmon (I or II versus III), M-protein class (IgG versus other; IgA versus other; light chains only versus other), and osteolytic bone lesions (none versus limited or advanced). In the multivariate analyses, forward stepwise variable selection was used. All analyses were performed on an intention-to-treat basis.
Results
Intensive-therapy group
Baseline characteristics.
Baseline characteristics for the intensive-therapy group are shown in Table 3, together with baseline characteristics for the control group.
Patient characteristics at the time of diagnosis
| Characteristic . | Category . | Intensive-Therapy Group . | Control Group . |
|---|---|---|---|
| Median age (y) | 51 | 54 | |
| Male/female | 172/102 | 165/109 | |
| WHO performance status | 0-1 | 139 (52%) | 65 (45%) |
| 2-4 | 129 (48%) | 80 (55%) | |
| Missing data | 6 | 129 | |
| Stage (Durie and Salmon) | I | 6 (2%) | 16 (6%) |
| II | 76 (28%) | 104 (38%) | |
| III | 192 (70%) | 154 (56%) | |
| M-protein class | IgG | 169 (62%) | 157 (57%) |
| IgA | 66 (24%) | 48 (18%) | |
| IgD | 4 (1%) | 3 (1%) | |
| Light chains only | 35 (13%) | 65 (24%) | |
| Serum creatinine | > 200 μmol/L | 25 (9%) | 36 (13%) |
| Blood hemoglobin | < 100 g/L | 97 (35%) | 79 (29%) |
| Serum calcium | > 2.6 mmol/L | 86 (31%) | 77 (30%) |
| Serum β-2-microglobulin | < 4 mg/L | 136 (58%) | 62 (44%) |
| 4-8 mg/L | 59 (26%) | 52 (36%) | |
| > 8 mg/L | 35 (16%) | 29 (20%) | |
| Missing data | 48 | 131 | |
| Bone marrow plasma cells | > 25% | 140 (51%) | 124 (49%) |
| Advanced osteolytic lesions | 128 (47%) | 108 (40%) |
| Characteristic . | Category . | Intensive-Therapy Group . | Control Group . |
|---|---|---|---|
| Median age (y) | 51 | 54 | |
| Male/female | 172/102 | 165/109 | |
| WHO performance status | 0-1 | 139 (52%) | 65 (45%) |
| 2-4 | 129 (48%) | 80 (55%) | |
| Missing data | 6 | 129 | |
| Stage (Durie and Salmon) | I | 6 (2%) | 16 (6%) |
| II | 76 (28%) | 104 (38%) | |
| III | 192 (70%) | 154 (56%) | |
| M-protein class | IgG | 169 (62%) | 157 (57%) |
| IgA | 66 (24%) | 48 (18%) | |
| IgD | 4 (1%) | 3 (1%) | |
| Light chains only | 35 (13%) | 65 (24%) | |
| Serum creatinine | > 200 μmol/L | 25 (9%) | 36 (13%) |
| Blood hemoglobin | < 100 g/L | 97 (35%) | 79 (29%) |
| Serum calcium | > 2.6 mmol/L | 86 (31%) | 77 (30%) |
| Serum β-2-microglobulin | < 4 mg/L | 136 (58%) | 62 (44%) |
| 4-8 mg/L | 59 (26%) | 52 (36%) | |
| > 8 mg/L | 35 (16%) | 29 (20%) | |
| Missing data | 48 | 131 | |
| Bone marrow plasma cells | > 25% | 140 (51%) | 124 (49%) |
| Advanced osteolytic lesions | 128 (47%) | 108 (40%) |
Significant differences between the groups were found for age, stage according to Durie and Salmon, M-protein class, and serum β-2-microglobulin.
Completion of assigned therapy.
High-dose therapy with autologous stem cell support was performed in 214 patients (78%) at a median time of 5.0 months (range, 3.1-11.4 months) from the start of VAD therapy.
Four patients underwent allogeneic and 1 had syngeneic stem cell transplantation. Fifty-five patients (20%) did not have transplantation because of early death (n = 12; 6 from myeloma, 4 from infections, and 2 from cardiac events), progressive disease (n = 11), no complete or partial response after phase I (n = 12; protocol option), contraindications to high-dose chemotherapy (n = 16), or patient refusal (n = 4). Treatment with interferon alfa-2b was started in 90% of the eligible patients at a median time of 2.9 months (range, 1.2-9.1 months) from the time of high-dose chemotherapy.
Toxicity.
Table 4 summarizes the significant side effects during phases I through IV.
Toxicity grades 2 to 4 according to WHO during treatment phases I, II, III, and IV, respectively, for patients treated according to the intensive-therapy protocol
| Toxicity . | Phase I . | Phase II . | Phase III . | Phase IV . |
|---|---|---|---|---|
| Number of evaluable patients | 274 | 215 | 214 | 211 |
| Infectious | 42 (3) | 52 (1) | 55 (2) | 18 (1) |
| Gastrointestinal | 8 (1) | 11 (0) | 53 (3) | 3 (0) |
| Cardiopulmonary | 4 (1) | 1 (0) | 1 (0) | 1 (1) |
| Neurological/mental | 9 (1) | 3 (0) | 7 (1) | 12 (0) |
| Other4-150 | 14 (0) | 3 (0) | 3 (0) | 14 (1) |
| Toxicity . | Phase I . | Phase II . | Phase III . | Phase IV . |
|---|---|---|---|---|
| Number of evaluable patients | 274 | 215 | 214 | 211 |
| Infectious | 42 (3) | 52 (1) | 55 (2) | 18 (1) |
| Gastrointestinal | 8 (1) | 11 (0) | 53 (3) | 3 (0) |
| Cardiopulmonary | 4 (1) | 1 (0) | 1 (0) | 1 (1) |
| Neurological/mental | 9 (1) | 3 (0) | 7 (1) | 12 (0) |
| Other4-150 | 14 (0) | 3 (0) | 3 (0) | 14 (1) |
The figures indicate the percentages of patients starting each phase who sometime during the phase experienced WHO grade 2 to 4 toxicity (within brackets grade 4 toxicity only).
Other includes metabolic (e.g., diabetes mellitus, hypothyreosis), thromboembolic, musculoskeletal, skin (including damage caused by extravasation of cytostatics), liver, urogenital, and hematologic (excluding expected cytopenia after chemotherapy).
Eleven patients died of causes considered to be at least possibly related to treatment during the study period, resulting in a total toxic death rate of 4.0%. However, only 3 of these patients had achieved at least a minor response at the time of death. Of the 11 toxic deaths, 6 occurred during phase I (4 from infections and 2 from cardiac events), none during phase II, and 3 during phase III (all from infections). Two treatment-related deaths occurred more than 3 months after high-dose therapy (1 from myocardial infarction and 1 from treatment-related acute myelogenous leukemia). The actual toxic death rate was 2.1% during phase I and 1.4% during phase III. No treatment-related deaths occurred in the 5 patients undergoing allogeneic or syngeneic stem cell transplantation.
Response rate.
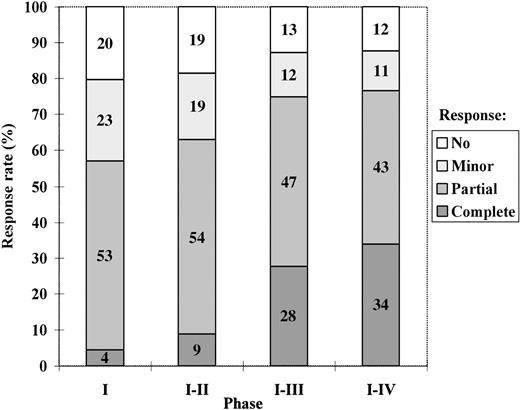
The response rate after each phase, calculated on an intention-to-treat basis, is presented in Figure 1.
The best degree of response achieved with the intensive-therapy protocol after treatment phases I, I to II, I to III, and I to IV, respectively, calculated on an intention-to-treat basis.
The median times from the start of VAD therapy until the evaluation of response after phases I, I to II, and I to III in patients who completed treatment according to the protocol were 3, 5, and 8 months, respectively. For patients not completing treatment according to the protocol, the same times were used for response evaluation.
The best degree of response achieved with the intensive-therapy protocol after treatment phases I, I to II, I to III, and I to IV, respectively, calculated on an intention-to-treat basis.
The median times from the start of VAD therapy until the evaluation of response after phases I, I to II, and I to III in patients who completed treatment according to the protocol were 3, 5, and 8 months, respectively. For patients not completing treatment according to the protocol, the same times were used for response evaluation.
Among those who actually underwent high-dose chemotherapy, 41% achieved a complete response and 48% a partial response. Twenty-three patients achieved their best response more than 6 months after high-dose therapy. Twenty-two of these had ongoing interferon treatment, of whom 20 had received interferon for more than 3 months. Of the 5 patients treated with allogeneic or syngeneic transplantation, 4 achieved a complete response and 1 a partial response.
Event-free survival and salvage therapy.
The median follow-up time was 32 months (range, 15-55 months).
The event-free survival at 3 years was 39% (95% confidence interval 32-46), and the median event-free survival was 27 months. For the patients who actually underwent high-dose chemotherapy, the event-free survival at 3 years was 45% (95% confidence interval 37-54), and the median event-free survival was 32 months. Six patients received a second high-dose course as salvage therapy after relapse. The remaining relapsing patients received conventional chemotherapy, mainly melphalan plus prednisone, VAD, or radiotherapy.
Causes of death.
Seventy-three patients have died, primarily from myeloma progression and related complications.
Four patients died while having at least a minor response at the time of death, due to septicemia with Neisseria meningitidis(not a transplanted patient), cytomegalovirus pneumonia (transplant-related death), acute myelogenous leukemia, and myocardial infarction.
Survival comparison between the intensive-therapy and control groups
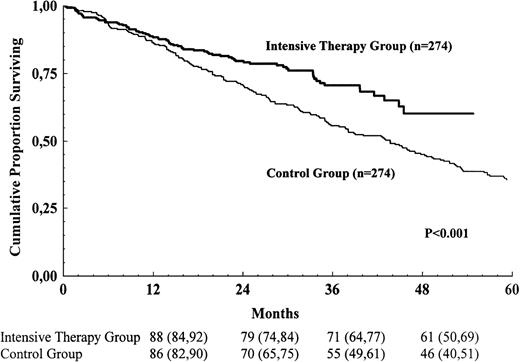
Survival for the intensive-therapy group and the control group is shown in Figure 2. The survival for the intensive-therapy group was prolonged compared with the control group (risk ratio for the control group 1.62; 95% confidence interval 1.22-2.15; P = .001). Median survival was 44 months in the control group and was not reached in the intensive-therapy group. In a multivariate Cox analysis for those variables that were available in at least 80% of the study populations, 4 variables were found to be significantly associated with survival: serum creatinine, bone marrow plasma cells, serum calcium, and blood hemoglobin. The survival advantage for the intensive-therapy group persisted after adjusting for differences between the groups with respect to these variables (risk ratio for the control group 1.56; 95% confidence interval 1.14-2.13;P = .005). Age, sex, stage according to Durie and Salmon, M-protein class, osteolytic bone lesions, serum albumin, and blood platelets were not significantly associated with survival. Data on serum β-2-microglobulin and performance status according to the World Health Organization were available in only about 50% of the control group; these variables were therefore not included in the multivariate analysis.
Survival for the intensive-therapy group and the control group.
The numbers shown below the time points are probabilities of survival in percent, with 95% confidence intervals in brackets.
Survival for the intensive-therapy group and the control group.
The numbers shown below the time points are probabilities of survival in percent, with 95% confidence intervals in brackets.
Survival comparison between the prospective and historic populations
The total number of registered patients in the prospective population was 348, corresponding to 77% of the expected number of new cases. Of these, 274 started treatment according to the NMSG #5/94 protocol, and another 27 were included in other high-dose therapy protocols (i.e., 86% of the registered patients in the prospective population entered protocols in which high-dose therapy was a part of the initial treatment). The total number of registered patients in the historic population was 313, corresponding to 76% of the expected number of new cases.
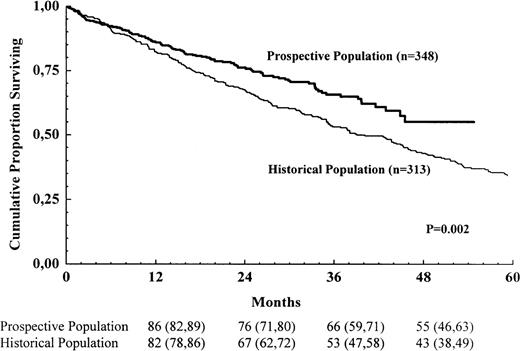
Survival for the 2 populations is shown in Figure3. In this comparison, comprising all known patients and more than 75% of the calculated number of new cases, there was a survival advantage for the prospective population (risk ratio for the historic population 1.46; 95% confidence interval 1.14-1.86; P = .002).
Survival for all registered patients in the prospective population and the historic population.
The numbers shown below the time points are probabilities of survival in percent, with 95% confidence intervals in brackets.
Survival for all registered patients in the prospective population and the historic population.
The numbers shown below the time points are probabilities of survival in percent, with 95% confidence intervals in brackets.
Discussion
Myeloma has been one of the fastest growing indications for high-dose therapy with autologous stem cell transplantation during this decade, and autologous transplantation has been proposed as the optimal treatment for newly diagnosed patients with myeloma younger than 70 years.13 However, the vast majority of the reported results are from single centers14-17 or transplantation registries4,18-20 lacking comparison with conventional chemotherapy. It has also been debated whether autologous transplantation really is superior, considering the historic results with conventional chemotherapy in younger patients.21
Only 1 randomized trial has been published so far.22 In that study, high-dose chemotherapy with autologous bone marrow transplantation was superior to conventional chemotherapy regarding response rate (81% versus 57%; P < .001), event-free survival (5-year probability 28% versus 10%; P = .01), and survival (5-year probability 52% versus 12%; P = .03). In the only other published comparative analysis, using case-matched registry data as controls, Barlogie et al23 found similar advantages for their total therapy protocol in terms of response rate (86% versus 52%; P = .0001), event-free survival (5-year probability 36% versus 19%; P = .0001), and survival (5-year probability 61% versus 39%; P = .01).
Ideally, novel therapies should be evaluated in prospective randomized trials, but history has shown it difficult to perform such trials on issues concerning stem cell transplantation. When the Nordic Myeloma Study Group started this study in 1994, a major concern was that patient accrual for a randomized study might be inferior because of the general preference for high-dose therapy generated by published results. We therefore decided to rely on historic controls for comparison. This carries the risk of selection bias and changes over time in supportive therapy. The first problem was reduced by using population-based studies with a high recruitment of consecutive cases so that the majority of diagnosed patients were included. Both the intensive-therapy group and the control group were highly representative of the entire myeloma population younger than 60 years, representing more than 60% of the expected number of new cases. The risk of selection bias was further reduced by the survival comparison performed on all registered patients, representing more than 75% of the expected number of new cases. Furthermore, in the historic population, which was derived from studies covering more than 20 years, there was no indication that the prognosis has improved over time with conventional therapy.10
We found a significant survival advantage for patients included in the high-dose therapy protocol compared with conventionally treated historic controls, and the survival advantage persisted after multivariate analysis of prognostic factors and adjustment for differences between the groups. Furthermore, when survival was compared for all registered patients, including those who did not enter the intensive-therapy protocol, there was still an advantage for the prospective population. We therefore believe that our results give a realistic estimation of the impact of high-dose therapy on survival for the total myeloma population younger than 60 years.
Although high-dose therapy may be the first treatment in 30 years that significantly improves survival in younger patients with myeloma, some comments are warranted. First, most patients affected with multiple myeloma (i.e., those above 60-65 years) are often not considered to be candidates for this treatment because toxicity might be unacceptably high. In a recent study, outcome for patients aged 65 years or older was not significantly different from that of younger patients in a matched-pair analysis for prognostic factors.24 However, this observation must be confirmed in a randomized or population-based study where selection bias can be minimized. Second, there is no evidence that patients are cured by this therapy. It has not yet been demonstrated that modifications such as more intensive therapy (e.g., double autologous transplantions25) or purging of stem cell harvests from tumor cells can improve the results. Immune-modulating therapy also remains to be evaluated.
The survival advantage for high-dose chemotherapy presented here is of a similar order of magnitude as that reported in 2 other published comparative trials.22 23 Although the designs of the studies are different, the results indicate that high-dose therapy should be a part of the initial treatment up to at least 60 years of age. Results of our studies on the health-related quality of life of these patients and a cost-utility analysis of high-dose therapy are forthcoming and may lend further support to recommendations on high-dose therapy in multiple myeloma.
Acknowledgments
We are indebted to Anders Odén, Kungälv, Sweden, for statistical advice and to the Southern Swedish Regional Tumor Registry at the University Hospital of Lund, especially Ms Monika Andersson and Ms Gertrud Andersson, for secretarial help.
Supported by research grants from the Nordic Cancer Union (grant no. 3588-B94-01); the Georg Danielsson Foundation; and from Amgen, Roche, and Schering-Plough in Denmark, Norway, and Sweden.
Reprints:Stig Lenhoff, Department of Hematology, University Hospital, S-221 85 Lund, Sweden.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.