Abstract
To assess the relative contribution of genetic factors in the variation of F cells (FC) and other hematologic variables, we conducted a classical twin study in unselected twins. The sample included 264 identical (monozygotic [MZ]) twin pairs and 511 nonidentical (dizygotic [DZ]) same-sex twin pairs (aged 20 to 80 years) from the St. Thomas' UK Adult Twin Register. The FC values were distributed continuously and positively skewed, with values ranging from 0.6% to 22%. FC values were higher in women than in men and decreased with age, with the variables accounting for 2% of the total FC variance. The intraclass correlations of FC values were higher in MZ (rMZ = 0.89) than in DZ (rDZ = 0.49) twins. The XmnI-Gγ polymorphism in the β-globin gene cluster had a significant effect on FC levels, accounting for approximately 13% of the total FC variance. Variance components analysis showed that the FC values were accounted for predominantly by additive genetic and nonshared environmental influences, with an estimate of heritability of 0.89. Hemoglobin levels and red blood cell, white blood cell, and platelet numbers were also substantially heritable, with heritability estimates of 0.37, 0.42, 0.62, and 0.57, respectively.
Previously, studies of sib pairs with sickle cell disease and isolated family studies showed that high levels of Hb F and FC tend to be inherited. Here, our classical twin study demonstrated that the variance of FC levels in healthy adults is largely genetically determined. (Blood. 2000;95:342-346)
The switch from fetal to adult hemoglobin (Hb) synthesis that occurs just before birth is not complete in that it does not lead to a total extinction of fetal hemoglobin (Hb F) in adult life.1 The small amounts of Hb F are not homogeneously distributed but are restricted to a subset of erythrocytes termed F cells (FC),2 and generally there is good correlation between the proportions of Hb F and FC.3 Hb F and FC values in healthy adults vary considerably, with a continuous distribution that is substantially positively skewed.3-7 The high values of Hb F and FC at the upper limits of the population range are transmitted in the condition referred to as heterocellular hereditary persistence of fetal hemoglobin (HPFH), or Swiss HPFH, which should be regarded as a multifactorial quantitative trait, quite distinct from the pancellular HPFHs that are caused by point mutations in the promoters of the γ-globin genes or deletions of the β-globin gene complex.1 Although family studies have shown that high levels of Hb F and FC tend to be inherited, the number of genetic factors involved and the mode of inheritance remain uncertain.
Several factors have been shown to influence Hb F and FC levels in healthy adults, including age,5 sex,3,5 and genetic variants linked7-9 and unlinked to the β-globin locus on chromosome 11p.8,10 Two trans-acting quantitative trait loci (QTLs) for FC variance have been mapped, one on chromosome 6q in an extensive kindred with heterocellular HPFH and β thalassemia11 and the other on Xp in families with sickle cell disease.12 Recently, studies in a large English family indicated the presence of at least one other trans-acting QTL associated with Hb F and FC variance.13 It is clear from the isolated family studies that there are several such QTLs for Hb F and FC, but their frequency in the general population and their contribution to the FC variance are not apparent.
To address this issue, we undertook a classical twin study. The aim of this study was to explore the role of genetic influences on the variance of FC values in healthy adults, in parallel with other hematologic variables, in a sample population of unselected twin pairs. Percentages of FC rather than Hb F were used as the variable for study because measurement of FC by immunofluorescence using an anti-γ-globin chain antibody is more sensitive and reproducible than currently available techniques for measuring the low-range Hb F levels in individuals with heterocellular HPFH.
Subjects and methods
Subjects
Seven hundred seventy-five pairs of twins aged 20-80 years were recruited from the St Thomas' UK Adult Twin Register,14which is a large cohort of volunteer twins unselected for any particular disease or trait. The study population consisted of 264 (27 male and 237 female) monozygotic (MZ) twin pairs and 511 (40 male and 471 female) dizygotic (DZ) same-sex twin pairs. All subjects were of European descent, and both members of a pair attended the clinic on the same day. Informed consent was obtained in all cases before the collection of blood samples. Zygosity was determined by a standard questionnaire15 and confirmed by multiplex DNA analysis of highly polymorphic short tandem repeats (microsatellites). Genotyping and phenotyping were carried out by evaluators blinded to the zygosity of the subjects from whom the samples were obtained.
Hematologic studies.
Blood samples were collected in EDTA as anticoagulant. Hb level, red blood cell (RBC) counts, mean red blood cell volume (MCV), white blood cell (WBC) counts, and platelet counts were determined with an automated blood cell analyzer (Bayer H3 RTX, Newbury, UK). From these measurements, the instrument then derived the values for hematocrit (packed-cell volume [PCV]), mean red blood cell hemoglobin (mean corpuscular hemoglobin [MCH]), and mean red blood cell hemoglobin (mean corpuscular hemoglobin concentration [MCHC]).
FC assays were performed in peripheral blood by using a monoclonal mouse anti-γ-globin chain antibody and fluorescence-activated cell sorting (104 cells counted per assay) in all cases.16 In a proportion of the samples, FC measurements were counterchecked by using the same anti-γ-globin chain antibody and microscopy (2 × 103 RBCs were counted).17 Hb F levels were also estimated in all samples with use of an automated high-performance liquid chromatography system (Bio-Rad Variant, β Thalassemia Short Program, Hercules, CA ).
DNA analysis.
DNA was extracted from peripheral blood leukocytes by using standard procedures. The polymerase chain reaction (PCR) was used to specifically amplify the 5′ region of the Gγ-globin gene and the T-C polymorphism at position −158 of theGγ-globin gene, determined by XmnI restriction analysis of the PCR product.18 Similarly, PCR was used to specifically amplify the Aγ-globin promoter region, and the sequence variant due to a 4-bp deletion at positions −221 to −224 relative to the messenger RNA cap site was detected byFnu4HI restriction analysis of the PCR product.18
Statistical analysis
Because MZ twins share all their genes, any intrapair variation is due to environmental factors (both shared and unique). However, for DZ twins, who share on average half of their genes, any intrapair variation is due to both environmental and genetic factors. Thus, a comparison between the similarity of values in MZ and DZ twins allows estimation of the extent to which genetic factors determine variation in a quantitative trait.
One member of each twin pair was chosen at random for testing the effects of age, sex, and the XmnI-Gγ andAγ 4-bp genotypes. A t test was used to test for differences between men and women in the means of the variables. The equality of variances between the sexes was tested with an F statistic. The effects of age and the XmnI-Gγ andAγ 4-bp genotypes on the variables were tested with Pearson correlation coefficients and linear regression. All the above statistical analyses were done with Statistical Analysis Software.19 The distributions of the variables were tested for deviations from normal by using the Shapiro-Wilk statistic; values for MCV, MCH, and MCHC were squared and the natural logarithm of the FC levels was used to improve the fit to the normal distribution.
A classical twin variance components analysis20 () was carried out by using structural equation modeling with the Mx package.21 Each model (ACE, ADE, AE, and CE in ) was tested for how well it fit the twin data by a χ2goodness-of-fit statistic, with a smaller χ2 indicating a better fit. The number of degrees of freedom for each model was the difference between the number of parameters estimated by the model and the number of statistics in the model. In cases in which more than one model could not be rejected by the goodness-of-fit test, χ2 likelihood ratio tests were done to determine which model fit the data better; generally, a full model (ACE or ADE) was compared with a simpler model (AE or CE). The χ2likelihood ratio test takes the difference between the χ2 goodness-of-fit statistics of the simpler model and the full model, with the number of degrees of freedom equal to the difference in the number of parameters between the 2 models. The full model is rejected in favor of the simpler model if the χ2yields a significance level > 0.05. The simplest model that cannot be rejected by the goodness-of-fit or the likelihood ratio test is the most parsimonious model, and maximum likelihood estimates of the parameters are calculated.
Results
A total of 775 twin pairs aged 20-80 years were recruited. Table1 shows the values for FC and the other hematologic variables measured by the cell analyzer. There were no significant differences between the MZ and DZ twins within each sex in the mean levels or variances of the variables.
Hematologic variables before statistical transformation, according to sex and zygosity
| Variable . | Men . | Women . | ||
|---|---|---|---|---|
| MZ (n = 54) . | DZ (n = 80) . | MZ (n = 474) . | DZ (n = 942) . | |
| Hb (g/L) | 154 (99), 129-178 | 151 (116), 112-175 | 134 (102), 98-164 | 134 (103), 95-165 |
| PCV (ratio) | .47 (.03), .38-.54 | .46 (.04), 35-.54 | .41 (.03), 30-.49 | .41 (.03), .27-.53 |
| RBC (×1012/L) | 5.15 (.29), 4.41-5.88 | 5.01 (.40), 4.04-6.00 | 4.49 (.35), 3.20-5.56 | 4.52 (.37), 3.04-6.70 |
| MCV (fl) | 90.4 (4.01), 83.8-99.4 | 91.9 (5.22), 77.7-108.0 | 91.0 (4.40), 73.4-109.0 | 91.6 (5.27), 72.5-108.0 |
| MCH (pg/RBC) | 29.9 (1.11), 27.0-32.8 | 30.1 (1.64), 24.9-33.8 | 29.9 (1.51), 24.7-35.4 | 29.9 (1.73), 24.2-35.3 |
| MCHC (g/L RBC) | 331 (104), 312-362 | 328 (134), 290-361 | 328 (129), 272-376 | 325 (140), 268-362 |
| WBCs (×1012/L) | 6.01 (2.28), 3.13-14.03 | 5.91 (1.87), 2.86-13.7 | 6.22 (1.81), 2.44-13.6 | 6.23 (1.99), 1.04-15.8 |
| Platelets (×1012/L) | 224.0 (55.6), 133.0-398.0 | 203.0 (49.5), 93.0-356.0 | 235.0 (54.8), 106.0-436.0 | 239.0 (65.5), 84.0-496.0 |
| FCs (% of RBC) | 3.33 (3.06), .65-15.7 | 3.19 (2.58), 53-14.4 | 4.19 (3.04), .60-19.2 | 4.07 (2.78), .57-21.8 |
| Variable . | Men . | Women . | ||
|---|---|---|---|---|
| MZ (n = 54) . | DZ (n = 80) . | MZ (n = 474) . | DZ (n = 942) . | |
| Hb (g/L) | 154 (99), 129-178 | 151 (116), 112-175 | 134 (102), 98-164 | 134 (103), 95-165 |
| PCV (ratio) | .47 (.03), .38-.54 | .46 (.04), 35-.54 | .41 (.03), 30-.49 | .41 (.03), .27-.53 |
| RBC (×1012/L) | 5.15 (.29), 4.41-5.88 | 5.01 (.40), 4.04-6.00 | 4.49 (.35), 3.20-5.56 | 4.52 (.37), 3.04-6.70 |
| MCV (fl) | 90.4 (4.01), 83.8-99.4 | 91.9 (5.22), 77.7-108.0 | 91.0 (4.40), 73.4-109.0 | 91.6 (5.27), 72.5-108.0 |
| MCH (pg/RBC) | 29.9 (1.11), 27.0-32.8 | 30.1 (1.64), 24.9-33.8 | 29.9 (1.51), 24.7-35.4 | 29.9 (1.73), 24.2-35.3 |
| MCHC (g/L RBC) | 331 (104), 312-362 | 328 (134), 290-361 | 328 (129), 272-376 | 325 (140), 268-362 |
| WBCs (×1012/L) | 6.01 (2.28), 3.13-14.03 | 5.91 (1.87), 2.86-13.7 | 6.22 (1.81), 2.44-13.6 | 6.23 (1.99), 1.04-15.8 |
| Platelets (×1012/L) | 224.0 (55.6), 133.0-398.0 | 203.0 (49.5), 93.0-356.0 | 235.0 (54.8), 106.0-436.0 | 239.0 (65.5), 84.0-496.0 |
| FCs (% of RBC) | 3.33 (3.06), .65-15.7 | 3.19 (2.58), 53-14.4 | 4.19 (3.04), .60-19.2 | 4.07 (2.78), .57-21.8 |
Values are mean (SE), range. MZ indicates monozygotic; DZ, dizygotic; Hb, hemoglobin; PCV, packed-cell volume; RBC, red blood cells; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cells; and FCs, F cells.
Hematologic variables and the effects of the covariates
Table 2 shows the t statistics and the Pearson correlation coefficients (r) between the hematologic variables and age and sex that were significant at theP < 0.05 level, as well as the percentage of variance attributed to the covariates calculated as the total coefficient of determination (R2). A positive t statistic indicates a higher mean in men, and a positive r indicates an increase with age. FC levels were, on average, higher in women than in men (t = −2.57, P = .01) and had a negative correlation with age (r = −.12, P < .001), with the variables accounting for only 2% of the total FC variance. Men had significantly higher Hb, PCV, and RBC levels than women, with age and sex accounting for 24%, 18%, and 18%, respectively, of the total variance in the variables. Hb, MCH, and MCHC levels increased with age; however, age accounted for only 1% of the variance in each instance. Women had a significantly higher mean value for platelets; however, the effect accounted for only 1% of the variance in the variable.
Effects of age and sex on hematologic traits
| Trait . | Sex (t statistic, P value) . | Age (r, P value) . | Total R2 . |
|---|---|---|---|
| FC | −2.57, .01 | −.12, <.001 | .02 |
| Hb | 14.31, <.001 | .10, .007 | .24 |
| PCV | 12.25, <.001 | NS | .18 |
| RBC | 12.41, <.001 | NS | .18 |
| MCV | NS | NS | — |
| MCH | NS | .08, .05 | .01 |
| MCHC | NS | .08, .05 | .01 |
| WBC | NS | NS | — |
| Platelets | −2.96, .003 | NS | .01 |
| Trait . | Sex (t statistic, P value) . | Age (r, P value) . | Total R2 . |
|---|---|---|---|
| FC | −2.57, .01 | −.12, <.001 | .02 |
| Hb | 14.31, <.001 | .10, .007 | .24 |
| PCV | 12.25, <.001 | NS | .18 |
| RBC | 12.41, <.001 | NS | .18 |
| MCV | NS | NS | — |
| MCH | NS | .08, .05 | .01 |
| MCHC | NS | .08, .05 | .01 |
| WBC | NS | NS | — |
| Platelets | −2.96, .003 | NS | .01 |
NS indicates not significant. t statistics are shown if significant (P < 0.5) for sex differences. Pearson correlation coefficients (r) are shown if significant (P < 0.5) for age effects with coefficients of determination (R2). A positive t statistic indicates a higher mean in men; a positive correlation coefficient indicates an increase with age.
The effects of the XmnI-Gγ and Aγ 4-bp genotypes on the variables were estimated by linear regression analysis. FC level was the only variable that had a significant relation with the β-globin complex sites. TheXmnI-Gγ polymorphism had a much larger effect than the Aγ 4-bp site (R2 = 0.13 compared with R2 = 0.03), indicating that it was more strongly associated with the high-FC trait.
Variance components analysis
The number of twin pairs used in the variance components analysis of each standardized variable and the Pearson correlation coefficientscalculated for MZ and DZ twin pairs are shown in Table3. Although there was a difference between men and women in the means for 5 of the variables, an F test for the equality of the variances showed that there was not a significant difference between men and women in the variance for any of the variables (results not shown). Therefore, the male and female twins were treated as a single group for each zygosity class. The intraclass correlation for MZ twins greatly exceeded that for DZ twins for all the measured variables (FC, Hb, RBCs, MCV, WBCs, and platelets), with the range of intraclass correlations for MZ twins being 0.61 to 0.89 and that for DZ twins being 0.36 to 0.53.
Pearson correlation coefficients between monozygotic (MZ) and dizygotic (DZ) twin pairs for standardized hematologic traits, calculated with variance components analysis
| Trait . | MZ (No. of Twin Pairs) . | DZ (No. of Twin Pairs) . | Intraclass Correlation, SE . | |
|---|---|---|---|---|
| rMZ . | rDZ . | |||
| FC | 257 | 497 | .89 | .49 |
| Hb | 235 | 480 | .66 | .48 |
| PCV | 234 | 477 | .70 | .56 |
| RBC | 234 | 477 | .72 | .53 |
| MCV | 235 | 481 | .65 | .56 |
| MCH | 231 | 466 | .65 | .46 |
| MCHC | 234 | 476 | .80 | .78 |
| WBC | 234 | 480 | .61 | .36 |
| Platelets | 234 | 478 | .76 | .46 |
| Trait . | MZ (No. of Twin Pairs) . | DZ (No. of Twin Pairs) . | Intraclass Correlation, SE . | |
|---|---|---|---|---|
| rMZ . | rDZ . | |||
| FC | 257 | 497 | .89 | .49 |
| Hb | 235 | 480 | .66 | .48 |
| PCV | 234 | 477 | .70 | .56 |
| RBC | 234 | 477 | .72 | .53 |
| MCV | 235 | 481 | .65 | .56 |
| MCH | 231 | 466 | .65 | .46 |
| MCHC | 234 | 476 | .80 | .78 |
| WBC | 234 | 480 | .61 | .36 |
| Platelets | 234 | 478 | .76 | .46 |
The numbers in each assay vary slightly because outliers were removed from the analysis.
Results of the variance components analysis are shown in Table4. χ2 goodness-of-fit statistics and the corresponding significance levels are given for each model tested. The model with a dominance genetic variance component and the model of no family resemblance, E, were rejected for all variables. The most parsimonious model (ie, the simplest model that cannot be rejected) for each variable is shown. All the variables except MCHC had best-fitting models with an additive variance component (ie, AE or ACE). The maximum likelihood estimates (h2, c2, and e2) for each variable under the best-fitting model are also shown in Table 4. Heritability (h2) estimates between 0.20 and 0.42 were found for Hb, PCV, RBCs, MCV, and MCH. These variables had estimates of the common and specific environmental effects in a similar range, and all fit the full ACE model best. There was no significant additive genetic effect for MCHC (χ2 likelihood ratio = 1.288-0.258 = 1.03, 1 df); the family resemblance was explained entirely by common and specific environment (CE model). The highest heritability estimates were found for FC values, WBC counts, and platelet counts (0.89, 0.62, and 0.57, respectively). A common environmental effect could be rejected for FC (χ2 likelihood ratio = 6.409-4.825 = 1.584, 1df) and WBCs (χ2 likelihood ratio = 2.303-0.885 = 1.48, 1 df) so that, for FC and WBCs, the best models included additive genetic and specific environmental effects only (ie, the AE model). It should be noted that the power to exclude the C or D components of variance is weak in this structural equation modeling method.22 The variance of platelet numbers fit the ACE model best. Of all the hematologic variables, FC had the highest heritability estimate (0.89).
Results of the variance components analysis
| Trait . | χ2Goodness-of-fit of Model, χ2/r . | Parameters of Best-Fitting Model4-151 . | |||||
|---|---|---|---|---|---|---|---|
| ACE df = 3 . | ADE df = 3 . | AE df = 4 . | CE df = 4 . | h2 . | c2 . | e2 . | |
| FC | 4.825 | 6.409 | 6.4094-150 | 166.9 | .89 | [0] | .11 |
| (.185) | (.093) | (.171) | (<.001) | ||||
| Hb | .0984-150 | 14.72 | 14.72 | 15.94 | .37 | .30 | .33 |
| (.992) | (.002) | (.005) | (.003) | ||||
| PCV | .2654-150 | 30.17 | 30.17 | 13.16 | .30 | .40 | .30 |
| (.966) | (<.001) | (<.001) | (.011) | ||||
| RBC | .7074-150 | 17.70 | 17.70 | 24.75 | .42 | .30 | .28 |
| (.872) | (.001) | (.001) | (<.001) | ||||
| MCV | 1.3174-150 | 40.25 | 40.25 | 5.556 | .20 | .46 | .34 |
| (.725) | (<.001) | (<.001) | (.161) | ||||
| MCH | .9964-150 | 11.13 | 11.13 | 16.19 | .39 | .26 | .35 |
| (.802) | (.011) | (.025) | (.003) | ||||
| MCHC | .258 | 175.6 | 175.6 | 1.2884-150 | [0] | .79 | .21 |
| (.968) | (<.001) | (<.001) | (.863) | ||||
| WBC | .885 | 2.303 | 2.3034-150 | 21.97 | .62 | [0] | .38 |
| (.829) | (.512) | (.608) | (<.001) | ||||
| Platelets | 1.3724-150 | 6.560 | 6.560 | 44.02 | .57 | .17 | .26 |
| (.712) | (.087) | (.161) | (<.001) | ||||
| Trait . | χ2Goodness-of-fit of Model, χ2/r . | Parameters of Best-Fitting Model4-151 . | |||||
|---|---|---|---|---|---|---|---|
| ACE df = 3 . | ADE df = 3 . | AE df = 4 . | CE df = 4 . | h2 . | c2 . | e2 . | |
| FC | 4.825 | 6.409 | 6.4094-150 | 166.9 | .89 | [0] | .11 |
| (.185) | (.093) | (.171) | (<.001) | ||||
| Hb | .0984-150 | 14.72 | 14.72 | 15.94 | .37 | .30 | .33 |
| (.992) | (.002) | (.005) | (.003) | ||||
| PCV | .2654-150 | 30.17 | 30.17 | 13.16 | .30 | .40 | .30 |
| (.966) | (<.001) | (<.001) | (.011) | ||||
| RBC | .7074-150 | 17.70 | 17.70 | 24.75 | .42 | .30 | .28 |
| (.872) | (.001) | (.001) | (<.001) | ||||
| MCV | 1.3174-150 | 40.25 | 40.25 | 5.556 | .20 | .46 | .34 |
| (.725) | (<.001) | (<.001) | (.161) | ||||
| MCH | .9964-150 | 11.13 | 11.13 | 16.19 | .39 | .26 | .35 |
| (.802) | (.011) | (.025) | (.003) | ||||
| MCHC | .258 | 175.6 | 175.6 | 1.2884-150 | [0] | .79 | .21 |
| (.968) | (<.001) | (<.001) | (.863) | ||||
| WBC | .885 | 2.303 | 2.3034-150 | 21.97 | .62 | [0] | .38 |
| (.829) | (.512) | (.608) | (<.001) | ||||
| Platelets | 1.3724-150 | 6.560 | 6.560 | 44.02 | .57 | .17 | .26 |
| (.712) | (.087) | (.161) | (<.001) | ||||
Values in parentheses are the corresponding P value for each model tested on each variable.
ACE, ADE, AE, and CE are models described in the .
Most parsimonious model.
Maximum likelihood estimates of the parameters.
Discussion
It has long been suspected that the values of Hb F and FC in healthy adults are genetically influenced. The evidence, however, was circumstantial and based on population and family studies showing that individuals with Hb F and FC levels at the upper limits of the population range, who are considered to have heterocellular HPFH, tend to have at least one parent with similarly increased Hb F (or FC levels).4 23-26 Hence, there has been a tendency to regard heterocellular HPFHs as discrete variables controlled by single genes. We would like to make the case for considering Hb F or FC levels a quantitative trait, with heterocellular HPFH representing the upper tail of the trait distribution. The number of QTLs contributing to the trait remains to be determined.
To explore the role and extent of genetic factors in controlling FC levels, we analyzed FC variance in a sample of unselected MZ and DZ twin pairs of European descent. Our findings provide overwhelming evidence for a strong genetic component in the control of Hb F and FC in healthy adults. FC levels were consistently more similar in identical twins (rMZ = 0.89) than in nonidentical twins (rDZ = 0.49), and 89% of the FC variance could be attributed to genetic factors.
Many genetic variants have been associated with elevated FC levels in healthy adults, including several in the β-globin gene complex–XmnI-Gγ site,7,27 the 4-bp deletion at positions −224 to −221 of theAγ-globin gene,28,29 sequence variations in the DNase 1 hypersensitive site 2 of the β-locus control region,30,31 and several variants unlinked to the β complex, such as the QTLs on Xp12 and 6q.11 In this study, the effects of the XmnI-Gγ site andAγ 4-bp deletion were estimated for all the hematologic variables. FC level was the only variable that had a significant relation with the β-globin complex sites. Our data confirm previous reports of the effects of age and sex on FC: levels were higher in women and decreased with age, and age and sex combined accounted for 2% of the total FC variance. Regression analysis indicated that only 13% of the total FC variation could be attributed to theXmnI-Gγ site, thereby implicating the presence of one or more other QTLs controlling FC levels in adults. These data should be relevant to the variation in Hb F levels observed in patients with β thalassemia and sickle cell disease who have different racial or genetic backgrounds. Inheritance of certain QTLs controlling FC production could also explain the different Hb F response in situations of acute erythroid expansion, such as in bone marrow regeneration after bone marrow transplantation,32 in healthy individuals after acute blood loss,33 and in patients with severe iron deficiency anemia after treatment with iron.34
We also found evidence of a genetic effect on several hematologic variables other than FC levels. Additive genetic effects accounted for 37%, 42%, 62%, and 57%, respectively, of the phenotypic variance in Hb levels, RBC counts, WBC counts, and platelet counts. These results support the data from a previous heritability study that showed that WBC and platelet numbers were accounted for by genetic and nonshared environmental influences only.35 Unlike the previous study, however, our study found that the phenotypic variance related to the RBC mass (Hb levels, RBC count, and MCV) can be attributed in equal proportions to additive genetic effects (A), shared environment (C), and nonshared environment (E).
A general conclusion that can be drawn from this study is that variation in the proportions of FC, WBC numbers, and platelet numbers and, to some extent, RBC numbers, is largely genetically determined. The aim now must be to determine the combination of common genes that pleiotropically influence the variance of these traits. Previous sib-pair analyses of patients with sickle cell anemia demonstrated the presence of genetic factors controlling the production of FC, both linked and unlinked to the β-globin cluster.36,37 A more recent study of Jamaican sickle cell sib pairs estimated that the locus on Xp, the β-globin cluster, the α-globin genes, and age accounted for about 50% of the Hb F variation observed in these subjects, with the Xp locus being the major contributor (35% to 41% of the variation). Nonetheless, about 50% of the variance in Hb F levels in the subjects remained unexplained.38 We propose using data from the DZ twins in a genome scan to map for the principal QTLs influencing these traits and to test the trans-acting factors that have been reported on chromosomes 6q2311 and Xp22.2-22.3.12 DZ twins have several advantages over ordinary sib pairs: the confounding effect of age is removed, common environment is likely to be more similar, and nonpaternity is less of a problem. In this study, the confounding effect of sex was removed because only same-sex DZ twins were recruited and, with respect to FC, the presence of hemoglobinopathies was not a confounder.
QTLs that are candidates for influencing the hematologic variables we studied include a combination of the numerous hemopoietic growth factors and lineage-specific cytokines, such as thrombopoietin, thrombopoietin receptor, erythropoietin, various colony-stimulating factors (CSF) (granulocyte-macrophage CSF and granulocyte CSF), interleukins (IL-3 and IL-6), and negative regulators such as transforming growth factor β1.39 40 The results of analyses of these influences will have immense practical implications in medicine. For example, an understanding of the role and extent of the genetic factors responsible for increased Hb F and FC levels would not only provide further insights into the normal developmental control of Hb F production in general, but could also pave the way to innovative approaches for therapeutic manipulations of Hb F in the treatment of sickle cell disease and β thalassemia.
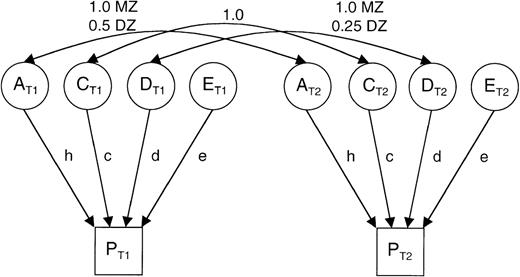
Classic path model for analyzing a sample of monozygotic (MZ) and dizygotic (DZ) twins.
The single-headed arrows (h, c, d, e) reflect the additive genetic (A), dominance genetic (D), common environmental (C), and specific environmental (E) effects on the phenotypes of twins (PT1and PT2). The double-headed arrows across the top show the expected relations among the additive genetic, dominance genetic, and common environmental effects for MZ and DZ twins.
Classic path model for analyzing a sample of monozygotic (MZ) and dizygotic (DZ) twins.
The single-headed arrows (h, c, d, e) reflect the additive genetic (A), dominance genetic (D), common environmental (C), and specific environmental (E) effects on the phenotypes of twins (PT1and PT2). The double-headed arrows across the top show the expected relations among the additive genetic, dominance genetic, and common environmental effects for MZ and DZ twins.
Acknowledgments
We thank Milly Graver and Liz Rose for preparation of the manuscript and Professor Sir David Weatherall for his continuing encouragement and support. We thank Professor Peter Beverley for allowing us to use the anti-γ-globin antibody.
Classical variance component twin analysis using the Mx package
Under the models tested, the phenotypic variance can be partitioned into (A) the additive genetic effects or heritability, (D) dominance genetic effects, (C) the common or shared environment, and (E) the specific environment with the expected relations indicated in the Figure. The following 5 models were tested: (1) family resemblance due to additive genetic and common environmental effects (the ACE model); (2) family resemblance due to additive and dominance genetic effects (the ADE model); (3) family resemblance due to additive genetic effects alone (the AE model); (4) family resemblance due to common environmental effects alone (the CE model); and (5) no family resemblance (the E model).
The parameters D and C are confounded in that the dominance effects cause twins to be less similar and common environmental effects cause twins to be more similar; therefore, a model with both these parameters is not possible. χ2 goodness-of-fit statistics were calculated for each model, and the most parsimonious model was determined by a χ2 likelihood ratio test with degrees of freedom equal to the difference in the number of parameters estimated by the 2 models. The maximum likelihood estimates of the parameters under the most parsimonious model are reported.
Supported by the Medical Research Council, United Kingdom. St Thomas' UK Adult Twin Registry is supported in part by grants from the Arthritis ResearchCampaign, the Wellcome Trust, the MRC, the Chronic Disease Research Foundation, and Gemini Holdings PLC.
Reprints:S. L. Thein, MRC Molecular Hematology Unit, Institute of Molecular Medicine, John Radcliffe Hospital, Headington, Oxford, OX3 9DS, UK; e-mail: swee.thein@imm.ox.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal