Abstract
Warm autoimmune hemolytic anemia (WAIHA) is characterized by an accelerated clearance of red blood cells (RBCs) associated with the presence of anti-RBC immunoglobulin (Ig)G autoantibodies. In the present study, we analyzed the self-reactive IgG and IgM antibody repertoires of patients with WAIHA using a technique of quantitative immunoblotting on a panel of whole tissue extracts as sources of self-antigens. Data were compared by means of multiparametric statistical analysis. We demonstrate that self-reactive antibody repertoires of IgG purified from plasma and of IgG purified from RBC eluates do not differ between healthy donors and patients with WAIHA, whereas autoreactive repertoires of IgM from patients exhibit broadly altered patterns of reactivity as compared with those of healthy controls. We further demonstrate that IgG purified from eluates of RBCs of healthy donors induces agglutination of RBCs in an indirect Coombs assay to a similar extent as IgG purified from eluates of RBCs of patients with WAIHA. The capability of IgG to induce agglutination of RBCs is suppressed in unfractionated eluates of healthy donors' cells, whereas it is readily found in unfractionated eluates of patients' RBCs. IgM is an essential factor in controlling the ability of IgG in unfractionated RBC eluates to induce agglutination of RBCs. These observations indicate that anti-RBC IgG autoantibodies of patients with WAIHA share extensive similarity with natural antiRBC autoantibodies of healthy donors and suggest that defective control of IgG autoreactivity by autologous IgM is an underlying mechanism for autoimmune hemolysis in WAIHA. (Blood. 2000;95:328-335)
Warm autoimmune hemolytic anemia (WAIHA) is characterized by an accelerated clearance of red blood cells (RBCs) associated with the presence of polyclonal anti-RBC immunoglobulin (Ig)G autoantibodies that optimally bind to erythrocytes at 37°C.1 Anti-RBC IgG of patients with WAIHA reacts with a variety of blood group–related RBC antigens and other membrane components of autologous and homologous RBCs.2 The most common target antigens are the band-3 anion transporter, glycophorin A, and Rh-related proteins.3
Autoimmunity in WAIHA is related to a breakdown in tolerance mechanisms toward RBC antigens. The basis for such failure is not understood. Current concepts on the understanding of self-/nonself discrimination focus on the central induction of self-tolerance in thymus and bone marrow by deleting, receptor-editing, or anergizing self-reactive T- and B-cell clones, and on the active maintenance of self-tolerance in the periphery.4,5 However, self-reactive T cells,6 self-reactive B cells, which have been positively selected and are maintained on the basis of their autoreactivity,7 and natural self-reactive autoantibodies of the IgG, IgM, and IgA isotypes8 are present in healthy human individuals. Thus, self-reactive T cells with specificity for the Rhesus polypeptide9 and naturally occurring autoantibodies to RBC band 3,10 spectrin,11 and ABO blood group antigens12 are found in healthy individuals. Under normal conditions, autoreactivity of IgG is largely masked in serum by idiotypic interactions between self-reactive IgG and autologous IgM.13,14 The inhibitory activity of autologous IgM toward autoreactive IgG is altered in patients with active Hashimoto's thyroiditis14 and systemic lupus erythematosus (SLE).15 Such a failure in the peripheral control of autoreactivity also has been suggested as an underlying mechanism of hemolytic anemia in NZB mice.16
In the present study, we analyzed the self-reactive IgG and IgM antibody repertoires of patients with WAIHA during acute hemolysis using a technique that allows comparative assessment of antibody reactivities of different individuals toward a wide range of self-antigens by means of quantitative immunoblotting with multiparametric statistical analysis.17,18 This method has been used to characterize the self-reactive antibody repertoires in healthy individuals and in various pathologic conditions.15 18-23 Our observations in patients with WAIHA indicate that anti-RBC IgG autoantibodies of patients share extensive similarity with natural anti-RBC autoantibodies of healthy blood donors and suggest that defective peripheral control of IgG autoreactivity by a broadly altered autologous IgM repertoire is an underlying mechanism for autoimmune hemolysis in patients with WAIHA.
Materials and methods
Patients and controls
Heparin-plasma samples were obtained from 20 patients with WAIHA (10 men and 10 women) aged 33 to 82 years (mean SEM, 65 ± 13 years) and from 20 healthy sex-matched blood donors aged 20 to 50 years (mean, 31 ± 8 years). WAIHA was idiopathic in 4 patients and secondary to malignant disease in 14 patients (B-cell chronic lymphocytic leukemia [B-CLL] in 8 patients, myeloproliferative syndrome in 2 patients, osteomyelofibrosis with secondary acute myelocytic leukemia in 1 patient, heavy-chain disease in 1 patient, angioimmunoblastic lymphoma in 1 patient, and B-cell non-Hodgkin's lymphoma in 1 patient). Whether autoimmune hemolysis was idiopathic or secondary was unknown in 2 patients. Samples were obtained at the time of acute hemolysis, as defined by hemoglobin levels < 12 g/dL, haptoglobin concentrations < 50 mg/dL, lactate dehydrogenase activity > 250 U/L, and serum bilirubin levels > 1.0 mg/dL. None of the patients had received intravenous immunoglobulin (IVIg) before the collection of blood samples. In 2 patients, plasma samples were also obtained during clinical remission, and in 1 patient, a plasma sample was available 1 year before disease onset. Plasma was aliquoted and stored at −80°C until use.
Immunohematologic procedures were performed in accordance with the guidelines of the American Association of Blood Banks (AABB).24 Antisera from Biotest (Biotest Diagnostics, Dreieich, Germany), Gull Laboratories (Bad Homburg, Germany), and DiaMed (Cressier sur Morat, Switzerland) were used for RBC typing. Test cells for antibody screening and identification were obtained from DiaMed. Immunohematologic investigations, including the direct antiglobulin test (DAT) with polyspecific as well as with monospecific antisera, were performed using the DiaMed Micro Typing ID gel system. The DAT was positive in all of the patients. The DAT was positive with anti-IgG in 19 patients, positive with both anti-IgG and anti-C3d in 11 patients, positive with anti-IgG and anti-IgM in 2 patients, and positive with both anti-IgG and anti-IgA in 1 patient. In 1 patient, the DAT was negative with anti-IgG and positive with anti-IgM and anti-C3d; the RBC eluate of the latter patient contained, however, small amounts of IgG in addition to IgM. IgG eluted from patients' RBCs reacted broadly with homologous RBCs without detectable specificity of anti-RBC IgG. In 1 patient, however, we found a stronger reactivity of eluted anti-RBC IgG with Rh(D)-positive RBCs than with Rh(D)-negative RBCs. Titers of warm autoantibodies in plasma ranged between undetectable and > 4.000. Anti-Lea, anti-E, anti-Jka, anti-Cw, or anti-S alloantibodies were present in the plasma of 5 of the patients. Healthy blood donors exhibited a negative DAT. Alloantibodies against RBC antigens were not detectable in their plasma.
Immunoglobulins in plasma and in RBC eluates
The concentrations of plasma IgM and IgG in patients' samples were (mean ± SD) 1.39 ± 2.10 g/L (range, 0.23-9.92 g/L) and 8.74 ± 6.20 g/L (range, 2.67-25.40 g/L), respectively, as determined by nephelometry. The concentrations of plasma IgM and IgG in healthy controls were (mean ± SD) 1.23 ± 0.63 g/L (range, 0.34-2.64 g/L) and 9.21 ± 1.94 g/L (range, 5.45-12.40 g/L), respectively. IgG was purified from plasma by affinity chromatography on protein G–Sepharose (Pharmacia Biotech, Uppsala, Sweden). The concentration of IgG was determined spectrophotometrically at 280 nm. Purity of IgG was assessed by enzyme-linked immunosorbent assay (ELISA) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). IgM was purified from the serum of 3 donors and 3 patients by affinity chromatography using anti-human IgM coupled to CNBr-activated Sepharose (Pharmacia). The concentration of IgM was determined spectrophotometrically. Normal human IgG for therapeutic intravenous use (IVIg) (Sandoglobulin; a gift of the Central Laboratory of the Swiss Red Cross, Bern, Switzerland) was used as a standard for IgG. A reference preparation of normal IgM (IVIgM) was obtained by submitting Pentaglobin (Biotest Pharma, Dreieich, Germany), an IgM-enriched therapeutic preparation of normal human immunoglobulin, to size-exclusion chromatography on Sephacryl HR S-300. F(ab′)2 fragments were prepared from purified IgG and from IVIg by pepsin digestion and affinity chromatography on Protein G–Sepharose (Pharmacia). The purity of F(ab′)2fragments was assessed by SDS-PAGE.
Immunoglobulins were eluted on ice, using citric acid,25from extensively washed patients' and donors' RBCs (5 × 105 RBCs/individual). The washing procedures were performed with phosphate-buffered saline (PBS), pH 7.4, at 37°C, and the final wash solutions were shown to be free of IgG and IgM by ELISA and SDS-PAGE. The eluates were immediately neutralized to a pH of 7.0 and dialyzed against PBS containing 0.01% NaN3. IgG was purified from eluates by affinity chromatography on protein G–Sepharose (Pharmacia). Purified IgG was free of IgM as assessed by ELISA. The concentrations of IgG and IgM in the eluates and in purified IgG fractions from the eluates were determined by ELISA. The capability of eluted IgG to induce agglutination of RBCs in an indirect Coombs assay was assessed using the DiaMed-ID Micro Typing System and the test cell reagents ID-DiaCell I+II (homologous RBCs; DiaMed) in the case of patients and of healthy donors. Briefly, RBCs were incubated with eluted anti-RBC IgG. The binding of anti-RBC IgG antibodies to RBCs was visualized by an agglutination reaction using rabbit anti-human IgG as the agglutinating reagent. In addition to homologous test cells, autologous RBCs were used for agglutination assays in the case of healthy donors. Homologous and autologous RBCs were used suspended in LISS (low ionic strength solution, ID-diluent 2; DiaMed) as well as in PBS at pH 7.4.
Analysis of antibody repertoires by quantitative immunoblotting
To analyze antibody repertoires, we used a quantitative immunoblotting technique that allows for the simultaneous investigation of reactivities of different sources of antibodies with a large number of antigens in normal homologous tissue extracts.17,18Sources of self-antigens were extracts of histologically normal human liver, kidney, and stomach obtained during surgical procedures; extracts of pooled RBCs of 5 healthy individuals (blood group 0 Rh[D+]); pooled RBC membranes (ghosts) of 5 healthy individuals (blood group 0 Rh[D+]); pooled RBC membranes of 3 patients with WAIHA (blood group 0 Rh[D+]); and F(ab′)2 fragments of IVIg. Extracts were prepared from tissues and RBCs in 2% SDS, 1.45 mol/L 2-mercaptoethanol, and 125 mmol/L Tris/HCl, pH 6.8, containing 1.0 mg/mL aprotinin, 1.0 mg/mL pepstatin A, and 1.0 mmol/L ethylenediaminetetra-acetic acid (EDTA) on ice. The extracts were centrifuged, boiled for 5 minutes, and dialyzed against PBS, pH 7.4. RBC membranes were prepared according to Lutz et al26; briefly, leukocyte-free RBC suspensions were submitted to hypotonic lysis in 5 mmol/L phosphate, 1 mmol/L EDTA, pH 7.4, on ice. The RBC membranes were then incubated in the presence of 1 mmol/L phenylmethylsulfonyl fluoride, 1.0 mg/mL aprotinin, and 1.0 mg/mL pepstatin A on ice; washed; aliquoted; and stored at −80°C until use in the presence of 1% SDS and 5 mmol/LN-ethylmaleimide. The amount of solubilized tissue proteins subjected to electrophoresis ranged between 200 and 600 μg/gel, depending on the tissue extract, and was maintained constant for a given tissue in all experiments. Low amounts of carbohydrates were detected in solubilized protein samples that represented approximately 1:10 to 1:20 of protein on a weight basis. Proteins were subjected to preparative SDS-PAGE in 10% polyacrylamide. The proteins were then transferred onto nitrocellulose (Schleicher & Schuell, Dassel, Germany) for 1 hour at 0.8 mA/cm2 using a Semi Dry Electroblotter model A (Ancos, Hφjby, Denmark). Membranes were blocked for 1 hour at room temperature with PBS containing 0.2% Tween 20. The antibodies to be tested were incubated with the membranes after the addition of 1 sample per slot in a Cassette Miniblot system (Immunetics Inc., Cambridge, MA). The membranes were incubated for 4 hours at room temperature, washed, and revealed with μ-chain–specific secondary rabbit anti-human IgM antibody or γ-chain–specific secondary rabbit anti-human IgG antibody coupled to alkaline phosphatase (Dako, Glostrup, Denmark) for 90 minutes at room temperature. Immunoreactivities were revealed using the NBT/BCIP (nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate) substrate (Sigma, St. Louis, MO). Antibodies were tested at concentrations of 20 μg/mL and 200 μg/mL of plasma or purified plasma immunoglobulin in the case of IgM and IgG, respectively. The concentration of IgG was adjusted to 30 μg/mL in experiments aimed at analyzing the reactivity profiles of IgG purified from RBC eluates. Quantitation of immunoreactivities was performed by scanning the membranes (SnapScan 600; Agfa Gevaert, New York, NY). Blotted proteins were then stained with colloidal gold (Protogold; BioCell, Cardiff, Wales) and subjected to a second densitometric analysis to record the protein profile and to quantitate transferred proteins. Data were analyzed using a Power Mac G3 computer (Apple Computer Inc., Cupertino, CA) and IGOR software (Wavemetrics, Lake Oswego, OR). Densitometric profiles of immunoreactivity were compared by referring to their corresponding protein profile after correction of the migration defects by superimposition of protein peaks. A sample of the reference IgM (IVIgM) or IgG (IVIg) preparations was included in each blot, allowing us to rescale the different membranes transferred with a given protein extract and to adjust for the intensity of staining of different membranes. Migration distance (X-axis) and optical density (Y-axis) were expressed as arbitrary units (AU). Migration distances of 200, 600, and 1000 AU corresponded to apparent molecular weights of 200, 65, and 20 kDa, respectively.21
Statistical analysis
Multivariate statistical treatment of the data was performed using IGOR software, including specially written software packages, and StatView software. Densitometric profiles of reactivity of IgM and IgG of patients and healthy donors with antigens in each tissue extract were divided into sections corresponding to individual peaks of immunoreactivity. Respective peak areas under the curve were calculated for each section. To discriminate between groups of individuals, areas corresponding to sections were submitted to principal component analysis (PCA).27 The repertoire of reactivities of each individual in a given sample was represented as a single symbol in a 2-dimensional linear subspace accounting for between 60% and 90% of the data. Discrimination between repertoires of groups of individuals was investigated by submitting the PCA data to linear discriminant analysis (LDA) and by subsequently comparing factors 1 of the LDA by means of a Mann-Whitney U test. Differences were considered statistically significant if P values were < .05 as assessed by the Mann-Whitney U test. The PCA of repertoires of antibody reactivities performed individually for each group of subjects allowed the calculation of respective variances. Variances were compared using the F test.
Results
Self-reactive antibody repertoires of plasma IgG
We analyzed the patterns of reactivity of IgG purified from plasma and of IgG in whole unfractionated plasma of patients with WAIHA and healthy blood donors toward antigens in liver, kidney, stomach, and RBC extracts, and in RBC membranes. The patterns of reactivity of purified IgG were highly homogeneous among patients and among controls in the case of all tissue extracts that we tested (Figure1). IgG of patients recognized the same antigenic bands in the tissue extracts as did IgG of healthy donors, although with a higher intensity of reactivity (Figure2). Enhanced reactivity of patients' IgG toward self-antigens accounted for the fact that multiparametric analysis of the data discriminated between repertoires of patients and controls (.0002 < P < .02, depending on the tissue extract). These results were substantiated by the observation that the intensity of reactivity of IgG obtained from 3 patients at the time of acute disease was higher than that of IgG obtained at the time of remission or before acute hemolysis, although the antibody repertoires did not differ in these samples in terms of the nature of the antigenic bands that were recognized (data not shown). We also found no difference in antibody repertoires of purified IgG of patients and healthy blood donors when analyzed toward pooled membranes prepared from RBCs of 3 patients with WAIHA (data not shown). The specificity of reactivity was confirmed in experiments showing that the same antigenic bands in tissues were recognized by F(ab′)2 fragments of IgG and by uncleaved IgG, and that the binding of F(ab′)2 fragments of IgG to RBC extracts was inhibited by soluble RBC extracts, but not by soluble liver antigens (data not shown).
Western blotting of IgG with extracts of normal human RBCs.
IgG purified from plasma of patients with WAIHA and healthy blood donors was immunoblotted at 200 μg/mL with a soluble extract of pooled RBCs of healthy donors, as described in Materials and Methods. Lanes 1 to 10: IgG of patients with WAIHA; lanes 15 to 24: IgG of healthy blood donors; lanes 12 and 13: normal reference IgG (IVIg); lanes 11 and 14: secondary anti-Fcγ antibody alone.
Western blotting of IgG with extracts of normal human RBCs.
IgG purified from plasma of patients with WAIHA and healthy blood donors was immunoblotted at 200 μg/mL with a soluble extract of pooled RBCs of healthy donors, as described in Materials and Methods. Lanes 1 to 10: IgG of patients with WAIHA; lanes 15 to 24: IgG of healthy blood donors; lanes 12 and 13: normal reference IgG (IVIg); lanes 11 and 14: secondary anti-Fcγ antibody alone.
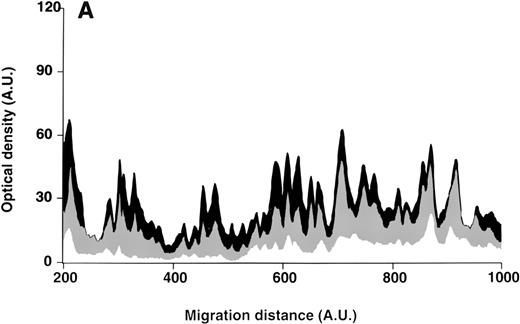
Patterns of reactivity of IgG purified from plasma of patients and healthy blood donors with self-antigens.
IgG purified from plasma of patients with WAIHA and healthy blood donors was interacted at 200 μg/mL with self-antigens, as described in Materials and Methods. The figure depicts the mean densitometric profiles of reactivity of purified IgG (i.e., the arithmetic mean of the recorded intensities constituting the reactivity profile of each individual) of 20 patients with WAIHA (black area) and 20 healthy blood donors (gray area) with antigens in soluble extracts of normal RBCs (A) and normal stomach (B). White areas depict the pattern observed in the presence of secondary anti-Fcγ antibody alone. Optical densities and migration distances are expressed as arbitrary units (AU). Migration distances of 200, 600, and 1000 AU correspond to molecular weights of 200, 65, and 20 kDa, respectively.
Patterns of reactivity of IgG purified from plasma of patients and healthy blood donors with self-antigens.
IgG purified from plasma of patients with WAIHA and healthy blood donors was interacted at 200 μg/mL with self-antigens, as described in Materials and Methods. The figure depicts the mean densitometric profiles of reactivity of purified IgG (i.e., the arithmetic mean of the recorded intensities constituting the reactivity profile of each individual) of 20 patients with WAIHA (black area) and 20 healthy blood donors (gray area) with antigens in soluble extracts of normal RBCs (A) and normal stomach (B). White areas depict the pattern observed in the presence of secondary anti-Fcγ antibody alone. Optical densities and migration distances are expressed as arbitrary units (AU). Migration distances of 200, 600, and 1000 AU correspond to molecular weights of 200, 65, and 20 kDa, respectively.
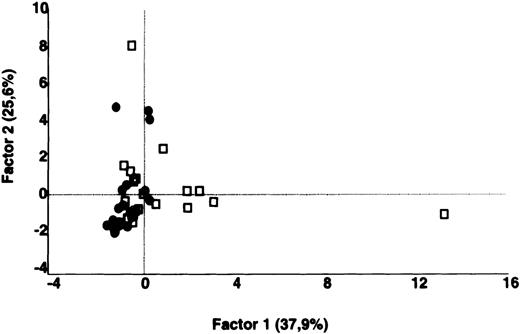
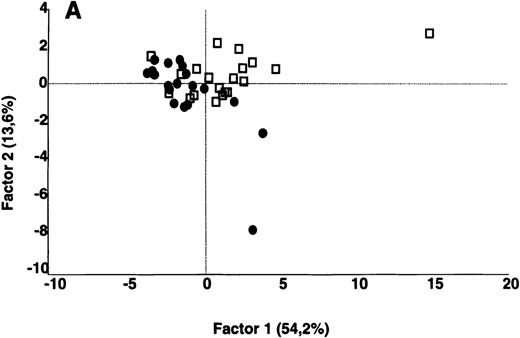
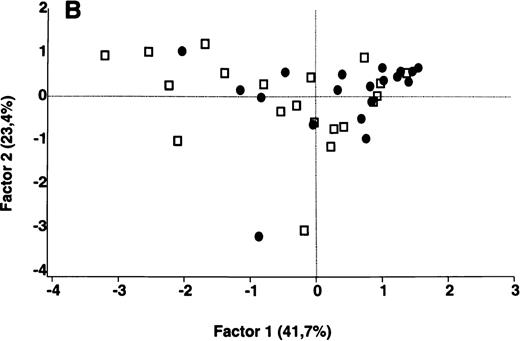
In contrast to the results obtained with purified IgG, the patterns of reactivity of IgG in unfractionated plasma were heterogeneous among patients and among healthy controls. Self-reactive antibody repertoires of IgG in plasma of patients differed from those of healthy donors in terms of both intensity of reactivity and the nature of the antigenic bands that were recognized, and were discriminated by PCA (P = .001) (Figure 3).
Comparative analysis of the patterns of reactivity of IgG in unfractionated plasma of patients and healthy blood donors with self-antigens.
IgG in unfractionated plasma of patients with WAIHA and healthy blood donors was interacted at 200 μg/mL with self-antigens in extracts of kidney, liver, stomach, and RBCs, as described in Materials and Methods. For each individual, the densitometric profile of reactivity with a given tissue extract was divided into sections corresponding to peaks of reactivity. The areas under the curve of each peak were calculated in the case of each tissue extract. The data were subjected to PCA and fitted within a 2-dimensional linear subspace (factor 1/factor 2). The figure shows the result of a cumulative PCA of the repertoires of reactivities of IgG in unfractionated plasma toward the antigens in all of the self-tissues tested. Percentages of variance accounted for by factor 1 and factor 2 are indicated on the abscissa and ordinate, respectively. Each symbol represents the reactivity of IgG of a single individual for the group of patients with WAIHA (●) and healthy blood donors (□). PCA discriminated between repertoires of patients and controls (P = .001 by the Mann-Whitney U test).
Comparative analysis of the patterns of reactivity of IgG in unfractionated plasma of patients and healthy blood donors with self-antigens.
IgG in unfractionated plasma of patients with WAIHA and healthy blood donors was interacted at 200 μg/mL with self-antigens in extracts of kidney, liver, stomach, and RBCs, as described in Materials and Methods. For each individual, the densitometric profile of reactivity with a given tissue extract was divided into sections corresponding to peaks of reactivity. The areas under the curve of each peak were calculated in the case of each tissue extract. The data were subjected to PCA and fitted within a 2-dimensional linear subspace (factor 1/factor 2). The figure shows the result of a cumulative PCA of the repertoires of reactivities of IgG in unfractionated plasma toward the antigens in all of the self-tissues tested. Percentages of variance accounted for by factor 1 and factor 2 are indicated on the abscissa and ordinate, respectively. Each symbol represents the reactivity of IgG of a single individual for the group of patients with WAIHA (●) and healthy blood donors (□). PCA discriminated between repertoires of patients and controls (P = .001 by the Mann-Whitney U test).
Self-reactive antibody repertoires of plasma IgM
The patterns of reactivity of plasma IgM toward antigens in extracts of liver, kidney, stomach, RBCs, and in RBC membranes differed among the patients and differed from the homogeneous patterns of reactivity observed in the case of healthy blood donors (Figures4 and 5). Self-reactive antibody repertoires of IgM of patients and controls were discriminated by PCA (.0001 < P < .0005, depending on the tissue extract; Figure 6). The individual variances of repertoires of self-reactivities of plasma IgM of patients and controls, calculated in the case of each tissue extract and tested by PCA, did not differ significantly between patients and healthy donors as assessed by the F test, indicating that self-reactive repertoires of IgM were homogenous within the 2 groups of individuals (Table 1). The intensity of reactivity of IgM of WAIHA patients toward F(ab′)2fragments of normal IgG was significantly lower than the intensity of reactivity of IgM of healthy controls (P = .0001, data not shown).
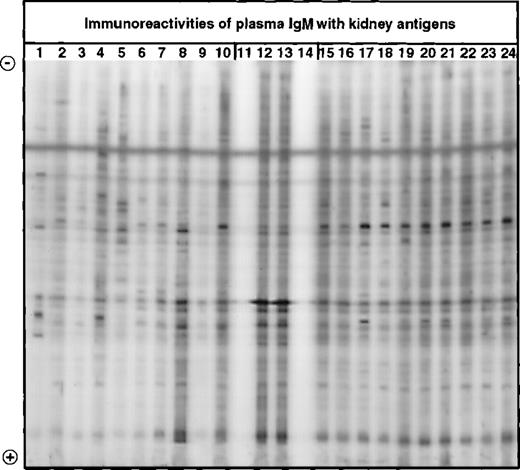
Western blotting of IgM with extracts of normal human kidney.
IgM in the plasma of patients with WAIHA and healthy blood donors was interacted at 20 μg/mL with a soluble extract of kidney antigens, as described in Materials and Methods. Lanes 1 to 10: IgM of patients; lanes 15 to 24: IgM of healthy blood donors; lanes 12 and 13: normal reference IgM (IVIgM); lanes 11 and 14: secondary anti-Fcμ antibody alone.
Western blotting of IgM with extracts of normal human kidney.
IgM in the plasma of patients with WAIHA and healthy blood donors was interacted at 20 μg/mL with a soluble extract of kidney antigens, as described in Materials and Methods. Lanes 1 to 10: IgM of patients; lanes 15 to 24: IgM of healthy blood donors; lanes 12 and 13: normal reference IgM (IVIgM); lanes 11 and 14: secondary anti-Fcμ antibody alone.
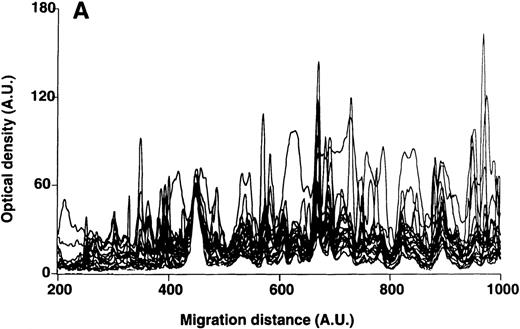
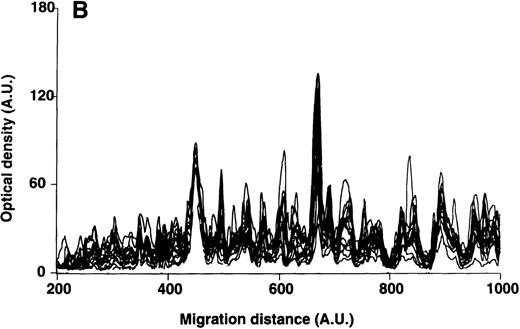
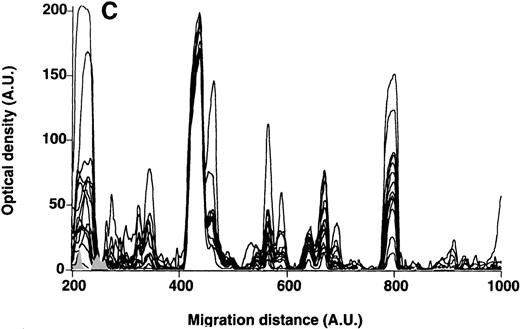
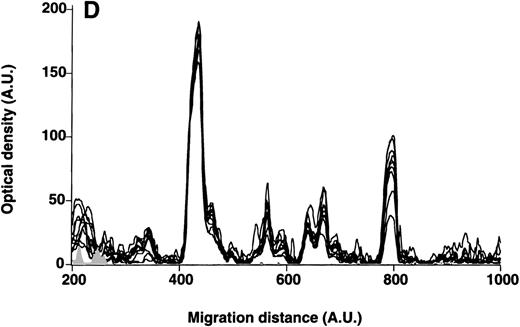
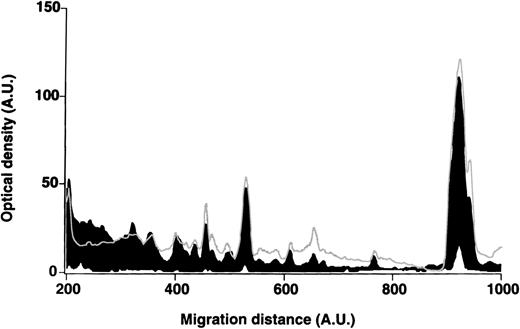
Patterns of reactivity of IgM of patients and healthy blood donors with self-antigens.
The densitometric profiles of reactivity of IgM with antigens in kidney (A,B) and RBC (C,D) extracts are depicted as individual profiles (black curves) in the case of 20 patients with WAIHA (A,C) and as individual profiles (black curves) in the case of 20 healthy donors (B,D). Gray areas show the densitometric pattern observed in the presence of the secondary anti-Fcμ antibody alone. Optical densities and migration distances are expressed as arbitrary units (AU). Migration distances of 200, 600, and 1000 AU correspond to apparent molecular weights of 200, 65, and 20 kDa, respectively.
Patterns of reactivity of IgM of patients and healthy blood donors with self-antigens.
The densitometric profiles of reactivity of IgM with antigens in kidney (A,B) and RBC (C,D) extracts are depicted as individual profiles (black curves) in the case of 20 patients with WAIHA (A,C) and as individual profiles (black curves) in the case of 20 healthy donors (B,D). Gray areas show the densitometric pattern observed in the presence of the secondary anti-Fcμ antibody alone. Optical densities and migration distances are expressed as arbitrary units (AU). Migration distances of 200, 600, and 1000 AU correspond to apparent molecular weights of 200, 65, and 20 kDa, respectively.
Comparative analysis of the patterns of reactivity of IgM of patients and healthy blood donors with self-antigens.
IgM in the plasma of patients with WAIHA and healthy blood donors was interacted at 20 μg/mL with self-antigens, as described in Materials and Methods. For each individual, the densitometric profile of reactivity with a given tissue extract was divided into sections corresponding to peaks of reactivity. Respective peak areas were calculated in the case of each tissue extract. The data were subjected to PCA within a 40- to 56-dimension vector space, depending on the tissue extract, and fitted within a 2-dimensional linear subspace (factor 1/factor 2). PCA discriminated between repertoires of patients and controls in the case of all tissue extracts tested (.0001 < P < .001, depending on the tissue extract, by the Mann-Whitney U test). The figure shows the results of the comparative analyses in the case of kidney antigens (A) and RBC extracts (B). Percentages of variance accounted for by factor 1 and factor 2 are indicated on the abscissa and ordinate, respectively. Each symbol represents the reactivity of IgM of a single individual for the group of patients with WAIHA (●) and healthy blood donors (□).
Comparative analysis of the patterns of reactivity of IgM of patients and healthy blood donors with self-antigens.
IgM in the plasma of patients with WAIHA and healthy blood donors was interacted at 20 μg/mL with self-antigens, as described in Materials and Methods. For each individual, the densitometric profile of reactivity with a given tissue extract was divided into sections corresponding to peaks of reactivity. Respective peak areas were calculated in the case of each tissue extract. The data were subjected to PCA within a 40- to 56-dimension vector space, depending on the tissue extract, and fitted within a 2-dimensional linear subspace (factor 1/factor 2). PCA discriminated between repertoires of patients and controls in the case of all tissue extracts tested (.0001 < P < .001, depending on the tissue extract, by the Mann-Whitney U test). The figure shows the results of the comparative analyses in the case of kidney antigens (A) and RBC extracts (B). Percentages of variance accounted for by factor 1 and factor 2 are indicated on the abscissa and ordinate, respectively. Each symbol represents the reactivity of IgM of a single individual for the group of patients with WAIHA (●) and healthy blood donors (□).
Total variances of the self-reactive antibody repertoires of IgM of patients with WAIHA and healthy blood donors
| Tissue Extract . | Total Variance of Repertoires of Reactivity of IgM . | |
|---|---|---|
| Patients With WAIHA . | Healthy Blood Donors . | |
| Kidney | 323.8 | 367.2 |
| Liver | 265.0 | 246.6 |
| Stomach | 440.8 | 206.5 |
| RBC extracts | 51.4 | 84.6 |
| RBC membranes | 460.0 | 240.0 |
| Tissue Extract . | Total Variance of Repertoires of Reactivity of IgM . | |
|---|---|---|
| Patients With WAIHA . | Healthy Blood Donors . | |
| Kidney | 323.8 | 367.2 |
| Liver | 265.0 | 246.6 |
| Stomach | 440.8 | 206.5 |
| RBC extracts | 51.4 | 84.6 |
| RBC membranes | 460.0 | 240.0 |
Total variances of repertoires of antibody reactivities of IgM in whole plasma with self-antigens in extracts of kidney, liver, stomach, RBCs, and in RBC membranes were calculated separately in a 40- to 60-dimension vector space, depending on the tissue extract. None of the differences between total variances were significant as assessed by the F test.
In contrast to a generally enhanced reactivity of purified IgG of patients toward the antigenic bands in all the tissue extracts during acute disease, we found a higher reactivity of IgM toward certain selected antigenic bands and decreased reactivities toward other antigenic bands during acute disease when compared with the reactivity of IgM in plasma samples obtained at the time of disease remission or before acute hemolysis (data not shown). We verified in the case of 3 patients and 3 healthy donors that, as demonstrated previously,18 reactivities of IgM purified from plasma did not differ from those of IgM tested in unfractionated plasma (data not shown).
Self-reactive antibody repertoires of IgG in eluates of RBCs
Cell-bound immunoglobulins were eluted from RBCs of patients with WAIHA and healthy blood donors. The mean numbers of IgM and IgG molecules per patients' RBCs in the eluates were 37 (< 10-136) and 3300 (14-10 000), respectively. Numbers were 18 (< 10-28) and 173 (< 10-330)/RBCs in the case of healthy donors. We purified IgG from the RBC eluates before testing it for self-reactivity toward antigens in RBC extracts and in RBC membranes. The patterns of reactivity were homogeneous among patients and controls and did not differ between patients and controls (Figure7). The patterns of reactivity with self-antigens of IgG purified from RBC eluates did not differ from those of IgG purified from plasma in the case of patients and healthy donors (data not shown).
Patterns of reactivity of IgG purified from RBC eluates with self-antigens in RBC membranes.
IgG purified from RBC eluates of patients' and blood donors' RBCs was incubated (30 μg/mL) with antigens in RBC membranes, as described in Materials and Methods. The mean densitometric profiles of reactivity of purified IgG (i.e., the arithmetic mean of the recorded intensities constituting the reactivity profile of each individual) of 4 patients with WAIHA (black area) and 4 healthy blood donors (gray curve) with antigens in RBC membranes are depicted. White areas show the densitometric pattern observed in the presence of the secondary antiFcγ antibody alone. Optical densities and migration distances are expressed as arbitrary units.
Patterns of reactivity of IgG purified from RBC eluates with self-antigens in RBC membranes.
IgG purified from RBC eluates of patients' and blood donors' RBCs was incubated (30 μg/mL) with antigens in RBC membranes, as described in Materials and Methods. The mean densitometric profiles of reactivity of purified IgG (i.e., the arithmetic mean of the recorded intensities constituting the reactivity profile of each individual) of 4 patients with WAIHA (black area) and 4 healthy blood donors (gray curve) with antigens in RBC membranes are depicted. White areas show the densitometric pattern observed in the presence of the secondary antiFcγ antibody alone. Optical densities and migration distances are expressed as arbitrary units.
In vitro binding to RBCs of anti-RBC IgG eluted from RBCs
We investigated the ability of anti-RBC IgG in unfractionated RBC eluates and purified from RBC eluates to bind to RBCs by using an indirect Coombs assay. At similar inputs, IgG in unfractionated eluates of patients' RBCs and IgG purified from eluates of patients' RBCs induced agglutination of unselected homologous cells to the same extent. In contrast, IgG in unfractionated eluates of healthy donors' RBCs did not induce agglutination of unselected homologous RBCs, whereas IgG purified from eluates of healthy donors' RBCs did induce agglutination. The extent of agglutination of unselected homologous RBCs induced by IgG purified from eluates of healthy donors' RBCs was similar to that induced by IgG purified from eluates of patients' RBCs (Table 2). In the case of healthy donors, we further observed that IgG purified from RBC eluates induced agglutination of autologous RBCs to a similar extent as of homologous RBCs.
In vitro binding of IgG in unfractionated RBC eluates and of IgG purified from RBC eluates to unselected homologous RBCs
| . | DAT . | IgG in Unfractionated Eluate . | IgG Purified From Eluate . |
|---|---|---|---|
| Patient 1 | ++ | +++ | +++ |
| Patient 2 | ++ | ++ | ++ |
| Patient 3 | +++ | +++ | +++ |
| Patient 4 | +++ | +++ | +++ |
| Patient 5 | ++ | ++ | ++ |
| Blood donor 1 | − | − | +++ |
| Blood donor 2 | − | − | ++ |
| Blood donor 3 | − | − | +++ |
| Blood donor 4 | − | − | +++ |
| Blood donor 5 | − | − | ++ |
| . | DAT . | IgG in Unfractionated Eluate . | IgG Purified From Eluate . |
|---|---|---|---|
| Patient 1 | ++ | +++ | +++ |
| Patient 2 | ++ | ++ | ++ |
| Patient 3 | +++ | +++ | +++ |
| Patient 4 | +++ | +++ | +++ |
| Patient 5 | ++ | ++ | ++ |
| Blood donor 1 | − | − | +++ |
| Blood donor 2 | − | − | ++ |
| Blood donor 3 | − | − | +++ |
| Blood donor 4 | − | − | +++ |
| Blood donor 5 | − | − | ++ |
IgG was eluted from the RBCs of 5 patients with WAIHA and 5 healthy blood donors. The DAT was positive with anti-IgG in the case of all patients, as indicated, and negative with anti-IgG in the case of healthy controls. Binding of IgG in the unfractionated eluate and of IgG purified from the eluate to homologous RBCs was assessed by means of an indirect Coombs assay at 2.3 ng/107 RBCs in the case of patient nos. 1 to 4 and donor nos. 1 to 4, and at 0.3 ng/107 RBCs in the case of patient no. 5 and donor no. 5. Agglutination was graded from − to +++ using a conventional grading system.
The coincubation of IgG purified from RBC eluates of healthy donors with normal plasma at a mol/mol ratio of purified eluted anti-RBC IgG to plasma IgM of 1:350 inhibited the agglutination of homologous RBCs induced by natural anti-RBC IgG. A similar inhibition of agglutination was observed using a 1:1 mixture (wt/wt) of purified plasma IgG and purified plasma IgM of healthy donors instead of whole plasma. Inhibition was not observed when purified IgG or purified IgM of healthy donors was used alone. Agglutination of homologous RBCs induced by natural anti-RBC IgG was not inhibited when a 1:1 mixture of purified plasma IgG of healthy donors and purified plasma IgM of patients with WAIHA was used. Agglutination of homologous RBCs induced by anti-RBC IgG of patients was also inhibited by either healthy donors' plasma or a mixture of purified plasma IgG and purified plasma IgM of healthy donors. Agglutination of homologous RBCs induced by patients' IgG was not inhibited by a mixture of purified IgG of healthy donors and purified IgM of patients or by purified IgG or purified IgM from healthy donors alone. Agglutination of unselected homologous RBCs induced by anti-RBC IgG purified from RBC eluates of healthy donors or of patients was not inhibited by coincubating either with PBS or with serum albumin (Table 3).
Coincubation of anti-RBC IgG purified from RBC eluates with different reagents
| Reagent . | Anti-RBC IgG From RBC Eluate of Patients With WAIHA . | Anti-RBC IgG From RBC Eluate of Healthy Blood Donors . |
|---|---|---|
| PBS | + | + |
| Serum albumin | + | + |
| Plasma (blood donors) | − | − |
| Purified plasma IgG (blood donors) | + | + |
| Purified plasma IgM (blood donors) | + | + |
| Purified plasma IgM (WAIHA patients) | + | + |
| Purified plasma IgG (blood donors) + purified plasma IgM (blood donors) | − | − |
| Purified plasma IgG (blood donors) + purified plasma IgM (WAIHA patients) | + | + |
| Reagent . | Anti-RBC IgG From RBC Eluate of Patients With WAIHA . | Anti-RBC IgG From RBC Eluate of Healthy Blood Donors . |
|---|---|---|
| PBS | + | + |
| Serum albumin | + | + |
| Plasma (blood donors) | − | − |
| Purified plasma IgG (blood donors) | + | + |
| Purified plasma IgM (blood donors) | + | + |
| Purified plasma IgM (WAIHA patients) | + | + |
| Purified plasma IgG (blood donors) + purified plasma IgM (blood donors) | − | − |
| Purified plasma IgG (blood donors) + purified plasma IgM (WAIHA patients) | + | + |
IgG was eluted from RBCs of patients with WAIHA and healthy blood donors and purified by affinity chromatography on protein G. The in vitro binding of purified anti-RBC IgG to homologous RBCs when coincubated with different reagents was assessed by means of an indirect Coombs assay. Purified anti-RBC IgG from eluates of patients and of healthy subjects was tested at a concentration of 1 ng/107 RBCs. It was coincubated with normal plasma at a mol/mol ratio of purified anti-RBC IgG to plasma IgM of 1:350. Purified plasma IgG and purified plasma IgM were used at a concentration of 1 g/L. In those experiments in which IgG and IgM were mixed, a ratio of 1:1 (wt/wt) was used. Serum albumin was used at a concentration of 50 g/L.
Discussion
WAIHA is associated with the presence of IgG autoantibodies directed against surface antigens expressed on RBCs. Bound antibodies mediate the adherence of RBCs to phagocytes, resulting in an accelerated clearance of erythrocytes.1 Investigations in WAIHA so far have focused on the identification of disease-associated autoantigens2,3 or on the mechanisms of Fc/FcR-mediated RBC clearance.28 In contrast, the present study investigates the overall IgG and IgM self-reactive antibody repertoires of patients with WAIHA. The data provide evidence for a normal IgG but broadly distorted IgM autoantibody repertoire and suggest that defective peripheral control of IgG autoreactivity by altered autologous IgM is an underlying mechanism for autoimmune hemolysis in patients with WAIHA.
Complementary idiotypic interactions occur between self-reactive IgG and autologous IgM in normal human serum,14,29-31 providing a mechanism by which IgG autoreactivity is largely “masked” in unfractionated normal serum13,14,19,32 and by which natural autoantibodies may be prevented from expressing pathogenic potential.33-35 There is evidence that such peripheral control of self-reactive IgG by IgM is defective in antineutrophil cytoplasmic autoantibody–positive vasculitis,36 Hashimoto's thyroiditis,14 SLE,15 and in the context of the hemolytic anemia occurring in NZB mice.16 In the present study, we observed that self-reactive antibody repertoires of IgG purified from plasma do not differ between healthy donors and patients with WAIHA when tested on tissue extracts and RBCs from healthy donors. In addition, no difference was observed when tested on RBCs obtained from patients with WAIHA, in which the protein composition may be altered as compared with that of normal RBCs.37 In contrast, the autoreactive antibody repertoires of plasma IgM of patients with WAIHA are distorted and differ significantly from those of healthy controls on multiparametric analysis. These differences were not dependent on heterogeneity between individual patients because the calculated individual variances of repertoires of self-reactivities of IgM did not differ significantly between patients and healthy donors. These observations suggest that altered patterns of self-reactivity of IgG in the unfractionated plasma of patients with WAIHA are related to defective control of IgG autoreactivity by autologous IgM. The hypothesis of defective peripheral control of autoreactive anti-RBC IgG by autologous IgM is consistent with observations in patients with WAIHA secondary to B-CLL, indicating that anti-RBC IgG autoantibodies in these cases are not the product of the malignant clone, which typically expresses IgM, but originate from remnant B lymphocytes.38
Consistent with the results obtained upon testing of purified plasma IgG, the patterns of reactivity of anti-RBC IgG purified from RBC eluates of DAT-positive patients with antigens in homologous RBC extracts and RBC membranes did not differ from those of DAT-negative healthy blood donors. In addition, natural anti-RBC IgG purified from the RBC eluates of healthy blood donors was capable of inducing agglutination of homologous and autologous RBCs in an indirect Coombs assay to a similar extent as IgG purified from eluates of RBCs of patients with WAIHA. Because the LISS buffer used in the standard indirect Coombs assay could favor to some extent a nonspecific binding of IgG to RBCs and enhance the binding of specific low-affinity antibodies,39 we verified that agglutination of RBCs induced by IgG purified from RBC eluates was also observed using PBS instead of LISS in the indirect Coombs assay. The capability of IgG in RBC eluates to induce agglutination of RBCs was suppressed in unfractionated eluates of healthy donors' cells, whereas it was readily found in unfractionated eluates of patients' RBCs. The data suggest that non-IgG molecules, including IgM, inhibit the binding capability of IgG in unfractionated eluates of healthy donors' RBCs and that such inhibitory activity is defective or absent in eluates of RBCs of patients with WAIHA. Indeed, coincubating IgG purified from RBC eluates of healthy donors and of patients with WAIHA with plasma or with a 1:1 mixture of purified plasma IgG and purified plasma IgM of healthy donors inhibited the agglutination induced by natural and by disease-associated anti-RBC IgG, whereas coincubation with plasma IgM of patients instead of plasma IgM of healthy blood donors did not. These results indicate that reconstituting the “regulatory IgM” prevents in vitro anti-RBC IgG autoantibodies in patients with WAIHA from binding to RBCs.
Consistent with data in the literature,40 we observed higher amounts of IgG bound to RBCs in patients as compared with healthy donors. Such an increase in RBC-bound IgG could be due to an increased clonal frequency of RBC-specific B cells or to an increased binding ability of IgG toward RBC antigens. To our knowledge, there is no evidence of an increased frequency of single RBC-specific B-cell clones in WAIHA. In contrast, it has been documented that anti-RBC antibodies in WAIHA due to B-CLL usually show a polyclonal light-chain distribution.38 41 It may be speculated that a dysregulated inhibitory IgM control may influence the binding ability (avidity) of anti-RBC IgG antibodies.
The observation of a lower reactivity of plasma IgM of patients with WAIHA toward F(ab′)2 fragments of normal IgG, as compared with that of IgM of healthy individuals, suggests altered idiotypic interactions between IgM and IgG in patients with WAIHA. Our results are consistent with previous observations showing that RBC eluates of DAT-positive blood donors contain both anti-RBC IgG autoantibodies and IgG anti-idiotypic antibodies directed against the RBC autoantibody42 and suggest that, in addition to IgG anti-idiotypes, IgM anti-idiotypes may participate in the regulation of anti-RBC IgG autoreactivity. At this stage, we provide evidence for a distortion in the functional equilibrium between IgG and IgM in eluates of patients' RBCs as compared with healthy controls. We have no quantitative data on the relative amounts of idiotypes and anti-idiotypes in the eluates, on the precise binding site of the anti-idiotypic IgM on anti-RBC IgG, or on the relative affinities of these antibodies for their respective antigens, as yet. Anti-F(ab′)2 antibodies interact with the variable region of the target antibody in multiple ways and may recognize idiotypic determinants within or outside the paratope43-48or conserved domains of the F(ab′)2.49-52A subpopulation of anti-F(ab′)2 antibodies has been shown to cover a part of the physiologically active Fc domain and nevertheless is able to immobilize the Fab arms, thus exerting immunoregulatory functions.51 52 Thus, IgM anti-(Fab′)2 antibodies recovered in RBC eluates are likely to be heterogeneous with regard to binding sites on the target anti-RBC IgG and, therefore, also with regard to the ability to interfere with the specific binding of anti-RBC IgG antibodies to RBC antigens. It could be expected that such IgG-IgM complexes are copurified during affinity chromatography and thus are found in the fraction of purified IgG, which we observed to be free of IgM. It is possible that complexes undergo dissociation during the washing steps or acid treatment. More direct evidence for the existence of anti-RBC IgG-IgM complexes comes from another set of experiments, in which we adsorbed the anti-RBC IgG autoantibody in patients' plasma to homologous native RBCs before comparing the patterns of reactivity of IgG and IgM in nonabsorbed and absorbed plasma with RBC antigens in the immunoblot assay. We found a highly significant change in the patterns of IgG toward RBC antigen extracts after absorption of the anti-RBC IgG autoantibodies from the plasma. The same change in reactivity was observed in the case of IgM, indeed suggesting that anti-RBC IgG in WAIHA is complexed with IgM (Stahl et al, unpublished observation).
Taken together, the results suggest that defective peripheral control of IgG autoreactivity by autologous IgM is an underlying mechanism for autoimmune hemolysis in patients with WAIHA. The molecular defects in such regulatory IgM are still unclear. Our observations may open the prospect of therapeutic strategies aimed at reconstituting the physiologic regulatory interactions between autologous IgM and self-reactive IgG, rather than nonspecifically suppressing autoantibody production.
Acknowledgments
We thank Constantin Fesel and Matthias Haury, Institut Pasteur, Paris, for advice and discussion; and Jacques Blouin, Marie-Françoise Bloch, and Stéphanie Rose for technical assistance. We also thank many colleagues in the Department of Hematology/Oncology, Heidelberg, and the blood banks of Heidelberg and Hôpital Broussais, Paris, for helping in collecting and processing blood samples.
Supported by the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Centre National de la Recherche Scientifique (CNRS), France; by the Central Laboratory of the Swiss Red Cross, Bern, Switzerland; and by the German Society for Transfusion Medicine and Immunohaematology (DGTI), Germany.
Reprints:Michel D. Kazatchkine, INSERM U430 and Université Pierre et Marie Curie, Hôpital Broussais, 96, rue Didot, 75014 Paris, France; e-mail:michel.kazatchkine@brs.ap-hop-paris.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal