Abstract
Recently we reported that the down-regulation of CD99 (Mic2) is a primary requirement for the generation of Hodgkin's and Reed-Sternberg (H-RS) cells seen in Hodgkin's disease. In this study, we provide evidence that the down-regulation of CD99 is induced by high expression of Epstein-Barr virus (EBV) latent membrane protein 1 (LMP-1), which is highly expressed in H-RS cells of EBV-associated Hodgkin's disease. To investigate the effect of LMP-1 on the expression of CD99 in vitro, we established a stable cell line by transfecting an SV40-early promoter driven-LMP-1 expression construct into a neoplastic lymphoblastoid B cell line, IM9, in which the level of endogenous LMP-1 expression is almost negligible. In this cell line, the overexpression of LMP-1 led to the down-regulation of CD99 and the acquisition of morphological and functional characteristics of H-RS cells indistinguishable from those in lymph nodes of Hodgkin's disease patients and in CD99-deficient B cells. In addition, induced LMP-1 expression in an EBV-negative B cell clone, BJAB, directly caused the down-regulation of surface CD99 expression. Northern and Western analysis data, showing that overexpression of LMP-1 negatively influenced the expression of CD99, were supported by experiments in which a CD99 promoter-driven luciferase promoter reporter construct transfected into 293T cells was down-regulated when LMP-1 was coexpressed. Therefore, our data strongly suggest that the EBV LMP-1 protein plays a pivotal role in the down-regulation of CD99 via transcriptional regulation, which leads to the generation of the H-RS cells. (Blood. 2000;95:294-300)
Hodgkin's disease is a malignant disorder morphologically characterized by low occurrence of mononuclear Hodgkin (H) and multinucleated Reed-Sternberg (RS) cells encompassed by abundant non-neoplastic infiltrates.1,2 Advances in the comprehension of H-RS cell origin have been made by several studies that include immunophenotyping, genotyping, and cytokine production of H-RS cells in Hodgkin's disease specimens or cell lines from Hodgkin's disease tissues.3,4 Indeed, uncontrolled production of various cytokines and high levels of transcription factors are common features of H-RS cells in Hodgkin's disease biopsy specimens and Hodgkin's disease–derived cell lines.5,6While the use of biopsy material and isolated H-RS cells has the potential to answer many questions, the cellular origin of H-RS cells is still a matter of debate. Recently we reported that H-RS cells from lymph nodes of Hodgkin's disease patients are consistently devoid of CD99 expression at their surfaces and that loss of CD99 in neoplastic B cells leads to the generation of cells with H-RS phenotype.7
Epstein-Barr virus (EBV), a ubiquitous human herpesvirus, is implicated in several human malignancies such as endemic Burkitt's lymphoma, nasopharyngeal carcinoma, and posttransplantation lymphoproliferative disease. The virus is a potent transforming agent for normal human B cells, and infection of resting B cells in vitro regularly leads to the establishment of EBV-immortalized lymphoblastoid cell lines in which about 9 viral proteins are expressed. Several of these so-called latent viral proteins (including the nuclear antigens EBNA LP, EBNA1, EBNA2, EBNA3A, and EBNA3C and the latent membrane protein [LMP]–1) cooperate to effect EBV-mediated transformation of normal B lymphocytes.8 Of these latent genes, LMP-1 not only is essential for the in vitro transformation of B cells by EBV,9 but also has pleiotropic effects on cellular phenotype in vitro and in animal models,10-14 consistent with its playing an important role in the initiation or maintenance of EBV-induced tumors. With respect to Hodgkin's disease, it has been reported that EBV was detected in the lesions of about 50% of Hodgkin's disease cases studied,15,16 and that LMP-1 was highly expressed in H-RS cells in EBV-associated Hodgkin's disease.17-19 In addition, overexpression of LMP-1 favors the formation of multinucleated H-RS cells in L-428, a Hodgkin's disease–derived cell line.20
Because LMP-1 is highly expressed in H-RS cells from EBV-associated Hodgkin's disease patients and down-regulation of CD99 contributes to the production of H-RS–like cells, we investigated the possibility that LMP-1 might be involved in the regulation of CD99 expression. We show here that overexpression of LMP-1 leads to the down-regulation of CD99, which eventually causes the generation of H-RS–like cells.
Materials and methods
Tissues and cells
Lymph nodes were obtained from patients with Hodgkin's disease. IM9 (immunoglobulin-secreting lymphoblast) and 293T (human embryonic kidney cell line) cells were obtained from American Type Culture Collection (ATCC, Rockville, MD). Stable-transfected Burkitt's lymphoma B-cell line (BJAB) clones with tetracycline-regulatable expression of LMP-1 were described previously.21EBV-transformed cell lines were prepared in this laboratory as previously described.22 The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (IM9 and 293T) or RPMI 1640 (BJAB) (GIBCO BRL) supplemented with 10% fetal bovine serum (FBS), antibiotics, and 2 mM glutamine in a humidified incubator atmosphere of 5% CO2. BJAB-LMP-1 cells were maintained in 1 μg/mL tetracycline, and LMP-1 expression was induced by reculturing them in tetracycline-free medium for 2 days.
Gene constructs and stable transfection
LMP-1 expression constructs pSG5-LMP-1 and pcDNA3-LMP-1 were gifts of Dr. E. Kieff (Harvard University, Boston, MA) and Dr. C.V. Paya (Mayo Clinic, Rochester, NY), respectively. Some of the sequence of the 5′ flanking region of human CD99 has been published previously,23 but additional upstream 5′ flanking sequences were obtained in this study by the use of genomic library screening, subcloning, and DNA-sequencing methods described by Sambrook et al.24 An EMBL phage containing 6 kb was cloned by screening a human female peripheral blood leukocyte genomic library (Clontech Laboratories Inc, Palo Alto, CA), and an NcoI-EcoRI fragment containing 2430 nucleotides of the 5′-flanking region of CD99 was sequenced (unpublished data). The region from −1650 to +130 relative to the transcriptional initiation site was amplified by polymerase chain reaction by the use of primers 5′-CCCATGGTCACTCATATGTGGCTCAG-3′ (sense strand) and 5′-GGGGTACCGAAGGCGGCAGGACAGATAC-3′ (antisense strand) and subsequently ligated into Basic/pGL-2 (promoterless and enhancerless luciferase reporter vector, Promega) for transient expression assay in 293T cells. The construction of stable-transfected IM9 cells with pSG5 or pSG5-LMP-1 (Vec-TF or LMP-1-TF, respectively) was established as described elsewhere.7 Establishment and subcloning of stable cell lines were accomplished by culturing primary transfectants in the presence of 500 μg/mL of G-418 (GIBCO BRL) for 1 month. Establishment of tetracycline-regulated LMP-1-BJAB clones was accomplished by first transfecting BJAB cells with transactivator plasmid pJEF3, and selecting with 500 μg/mL Hygromycin-B (Boehringer), then transfecting with the LMP-1-responder plasmid, pJEF6, and selecting with 2 mg/mL G418.21
Immunofluorescence staining and flow cytometric analysis
Samples of 106 cells were first incubated with relevant monoclonal antibodies (Abs) (10 μg/mL) in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 0.1% sodium azide for 30 minutes at 4°C. These cells were then washed with PBS and stained with fluorescein isothiocyanate (FITC)–conjugated goat anti-mouse immunoglobulin (Ig)G antibody. Flow cytometric analysis was performed on a FACScan (Becton Dickinson, San Jose, CA). The antibodies were either obtained from hybridoma cultures or purchased: major histocompatibility complex (MHC) class I (W6/32, ATCC), CD15 (Becton Dickinson), and calnexin (Transduction Laboratories, Lexington, KY). The monoclonal antibody to CD99 (DN16) was developed in this laboratory.7 The secondary antibody used was an FITC-conjugated goat anti-mouse IgG Ab (Dako, Glostrup, Denmark).
Confocal microscopic analysis
For immunofluorescence labeling, 5 × 104 cells were cytospun onto poly-L-lysine–coated slides, permeabilized, and fixed in cold acetone/methanol (50%/50% vol/vol) for 10 minutes, and then blocked in PBS containing 10% FBS. The slides were stained and examined by confocal microscopy (BioRad 1024; Bio-Rad Laboratories, Hercules, CA).
Cell cycle analysis
An asynchronous population of IM9 transfectants in the log-phase of cell growth was examined for DNA content by flow cytometric analysis. The cells were fixed in 70% ethanol in PBS on ice, pelleted with RNase A (0.1 ng/mL) for 30 minutes at 37°C, and then stained with propidium iodide (40 μg/mL). The cell-cycle profiles (10,000 cells per sample) were analyzed on a FACScan (Becton Dickinson).
Western blotting
Cells were solubilized with 1% NP-40 in 50 mM Tris-HCl, pH7.4, 50 mM ethylenediaminetetraacetic acid, and 1 mM phenyl methyl sulfonyl fluoride. After the insoluble pellets were removed, the lysates were separated by 12.5% sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis, electroblotted onto the nitrocellulose filters for probing with CS.1-4 (LMP-1), DN16 (anti-CD99), and anti-calnexin monoclonal antibodies. Specifically bound antibodies were detected with the use of peroxidase-conjugated goat anti-mouse IgG (Zymed, San Francisco, CA), and visualized with the enhanced chemiluminescence detection system (Amersham, Arlington Heights, IL).
Northern blot analysis
Total RNA was prepared from cells with TRIzol reagent (Life Technologies, Grand Island, NY), separated by electrophoresis on a 1.0% agarose/formaldehyde gel, and then transferred onto nylon membrane filters (Hybond-N+; Amersham International, Amersham, UK). The filters were hybridized at 42°C overnight with [α-32P-dCTP]-labeled cDNA fragments, then washed under stringent conditions (65°C for 30 minutes in washing buffer composed of 0.2 × standard saline citrate and 0.1% SDS), and detected by autoradiography. A full-length CD99 cDNA was used as a probe. The filters were stripped and rehybridized with a cDNA probe for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control.
Transient transfection and luciferase assay
To test the effect of LMP-1 expression on the CD99 promoter activity, 293T cells (1 × 106 cells per 60 mm plate) were transfected with 1 μg of the CD99 (−1650/+130)–driven luciferase construct, 3 μg of an LMP-1 construct (pSG5LMP-1 or pcDNA3-LMP-1), and 1 μg of the internal control plasmid by the calcium phosphate precipitation method. After overnight incubation, cells were washed and cultured in complete culture media for 36 hours. Luciferase activity was measured for a 15-second time course with the use of a luminometer (Turner Designs, TD-20/20; Promega, Madison, WI) and luciferase assay system (Promega) with one-tenth of the total volume of cell extract. The human polypeptide binding protein (BiP)-based chloramphenicol acetyl transferase (CAT) reporter vector (containing the BiP promoter, kindly provided by Dr. Chao, Chang Gung Medical College, Taoyuan, Taiwan25) was used as an internal control for transfection efficiency. CAT assays were performed as previously described.26 Protein concentrations of cell extracts were measured with the use of the bicinchoninic acid protein assay reagent kit (Pierce, Rockford, IL). The luciferase activity of each sample was subsequently adjusted according to CAT activity and protein concentration per sample.
Results
Generation of H-RS–like cells by LMP-1 overexpression in IM-9, a lymphoblastoid B cell line
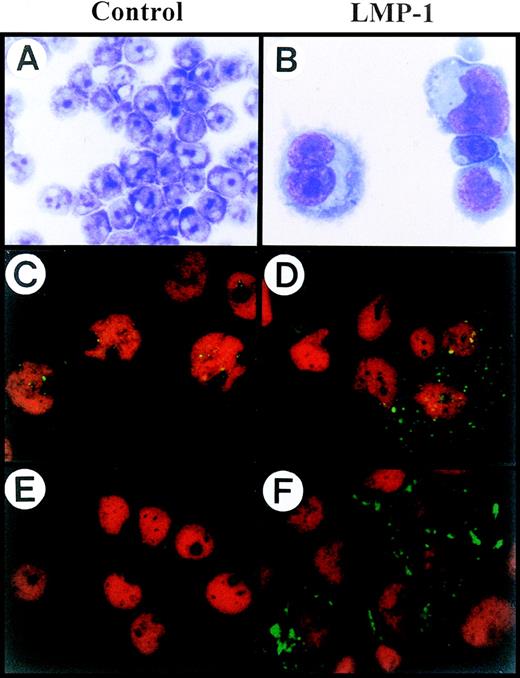
The strong association of high expression of LMP-1 with multinuclear cell formation and our previous finding that down-regulation of CD99 induces the generation of H-RS–like cells prompted us to investigate the possible relationship between LMP-1 and CD99 molecules in H-RS cells. To investigate whether the overexpression of LMP-1 correlates with the modulation of surface expression of CD99 and produces cells with an H-RS phenotype, we first established a stable IM9 cell line transfected with SV40 immediate early promoter-driven LMP-1 gene (defined as LMP-1-TF). Interestingly, a significant number of LMP-1 TF cells showed the typical H-RS cell morphology, such as abundant cytoplasm and multilobed or multinucleated nuclei with amphophilic owl-eyed nucleoli compared with the vector-transfected IM9 cells (defined as Vec-TF) (Figure 1A, 1B). A high level of LMP-1 expression in LMP-1-TF IM9 cells compared with Vec-TF was confirmed by confocal microscopic analysis (Figure 1C,1D) and immunoblot analysis (Figure 3B). This was also the case in the overexpression by LMP-1 of EBV-transformed B cells (data not shown).
H-RS phenotype generated by LMP-1 overexpression in IM9 cells.
(A, B) Both Vec-TF (A) and LMP-1-TF (B) IM9 cells were morphologically examined with Wright’s and Giemsa staining. LMP-1 overexpressing IM9 cells showed typical H-RS morphology. (C, D) Vec-TF (C) IM9 cells and LMP-1-TF (D) IM9 cells were stained with anti–LMP-1 monoclonal antibody. LMP-1 expression was identified by incubation with FITC-conjugated goat anti-mouse antibody, and then was analyzed by confocal microscopy. Vec-TF cells showed minimal expression of LMP-1. (E, F) Vec-TF (E) IM9 cells and LMP-1-TF (F) IM9 cells were stained with FITC-conjugated anti-CD15 monoclonal antibody. While no CD15 expression was detectable in Vec-TF cells, the majority of enlarged LMP-1-TF cells showed relatively high expression of CD15 in Golgi and cytoplasmic regions. PI staining was included for nuclear staining in all samples except A and B. All slides were processed in parallel and photographed under identical magnification (original magnification ×630).
H-RS phenotype generated by LMP-1 overexpression in IM9 cells.
(A, B) Both Vec-TF (A) and LMP-1-TF (B) IM9 cells were morphologically examined with Wright’s and Giemsa staining. LMP-1 overexpressing IM9 cells showed typical H-RS morphology. (C, D) Vec-TF (C) IM9 cells and LMP-1-TF (D) IM9 cells were stained with anti–LMP-1 monoclonal antibody. LMP-1 expression was identified by incubation with FITC-conjugated goat anti-mouse antibody, and then was analyzed by confocal microscopy. Vec-TF cells showed minimal expression of LMP-1. (E, F) Vec-TF (E) IM9 cells and LMP-1-TF (F) IM9 cells were stained with FITC-conjugated anti-CD15 monoclonal antibody. While no CD15 expression was detectable in Vec-TF cells, the majority of enlarged LMP-1-TF cells showed relatively high expression of CD15 in Golgi and cytoplasmic regions. PI staining was included for nuclear staining in all samples except A and B. All slides were processed in parallel and photographed under identical magnification (original magnification ×630).
Characterization of H-RS–like cells generated by LMP-1 overexpression
It has been reported that a significant proportion of H-RS cells display the CD15+CD30+ phenotype, which is the immunophenotypic criterion for the diagnosis of Hodgkin's disease.27-31 Since H-RS–like cells were generated by LMP-1 overexpression in B cell lines in the present study, we investigated whether these H-RS–like cells share common immunological characteristics shown in H-RS cells from Hodgkin's disease and CD99-deficient H-RS–like cells previously reported.7Enlarged LMP-1-TF IM9 cells expressed a low level of CD15 on their surface as confirmed by flow cytometric analysis (data not shown). However, confocal microscopic examination clearly demonstrated the unique pattern of CD15 localization in the majority of enlarged LMP-1-TF cells but not in Vec-TF cells (Figure 1E, 1F). Most of the H-RS–like cells showed intense expression of CD15 in Golgi/ER and cytoplasmic regions, whereas expression was either faint or almost completely absent on the plasma membrane (Figure 1E,1F). These findings further support the idea that LMP-1 overexpression is accompanied by the immunophenotypic changes seen in H-RS cells. Neither LMP-1-TF nor Vec-TF cells showed any change in their expression levels of other surface molecules such as CD19, CD21, CD23, CD25, CD40, MHC class I/II, LFA-3, and CD80 (data not shown). The observation that MHC class I is expressed at similar levels in LMP-1-TF and Vec-TF cells is in agreement with several recent reports that H-RS cells in EBV-positive Hodgkin's disease express a considerable amount of MHC class I, whereas EBV-negative H-RS cells lack MHC class I expression.32,33
We next investigated the proliferation profiles of LMP-1-TF cells. LMP-1-TF IM9 cells showed slower kinetics of cell proliferation than did Vec-TF cells (Figure 2A), which was confirmed by 3H]-thymidine uptake assay (data not shown). Similar results were obtained in LMP-1-TF EBV-transformed B cells (data not shown).
Characterization of LMP-1 overexpressing IM9 cells.
(A) Slow growth rate of LMP-1 overexpressing cells. The same number (5 × 104/mL) of cells were plated in 10% FBS-DMEM media at day 0. The total cell numbers were counted at the indicated days. LMP-1 overexpressing cells show slower kinetics of cell proliferation than do vector-transfected cells. (B) Cell cycle analysis. Flow cytometric analysis was performed on asynchronously proliferating vector-transfected or LMP-1–transfected IM-9 cells.
Characterization of LMP-1 overexpressing IM9 cells.
(A) Slow growth rate of LMP-1 overexpressing cells. The same number (5 × 104/mL) of cells were plated in 10% FBS-DMEM media at day 0. The total cell numbers were counted at the indicated days. LMP-1 overexpressing cells show slower kinetics of cell proliferation than do vector-transfected cells. (B) Cell cycle analysis. Flow cytometric analysis was performed on asynchronously proliferating vector-transfected or LMP-1–transfected IM-9 cells.
In order to elucidate the phase-distribution of cell cycle and possible arrest during a specific phase of cell cycle, the DNA contents of Vec-TF and LMP-1-TF IM9 cells were evaluated. LMP-1-TF cells revealed a substantial accumulation of 4N cells (5.21 → 24.7%) and contained a number of aneuploidy cells that were larger than 4N (0.23 → 7.11%) (Figure 2B). This finding indicates that the LMP-1-TF cells either are blocked at the mitotic (M) phase or are defective in cytokinesis.
Overexpression of LMP-1 induces the down-regulation of CD99
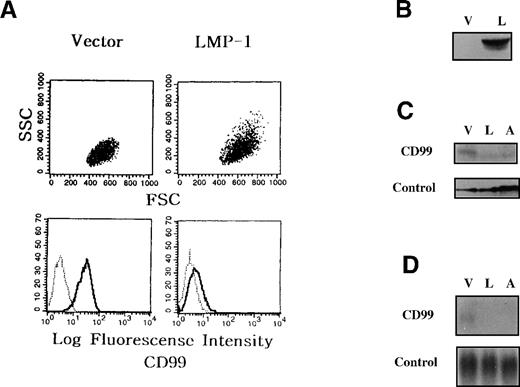
This H-RS phenotype shown in the LMP-1-TF cells was accompanied by a substantial reduction of CD99 expression as revealed by flow cytometric analyses (Figure 3A). The down-regulation of CD99 molecules was also confirmed by immunoblot (Figure 3C) and Northern blot (Figure 3D), suggesting that CD99 down-regulation induced by LMP-1 overexpression is attributable mainly to a decrease in the synthesis of CD99 at the transcriptional level. These findings are consistent with the postulate that LMP-1 overexpression leads to the generation of cells with H-RS morphology through down-regulation of CD99. Since IM9 cells are originally infected with EBV, however, the possibility cannot be excluded that EBV proteins other than LMP-1 in LMP-1-TF cells reduce CD99 expression.
Down-regulation of CD99 in LMP-1 overexpressing IM9 cells.
IM9 B cells were transfected with either vector (Vec-TF), LMP-1 (LMP-1-TF), or antisense-CD99 (AS-TF) expression construct, and G418-resistant stable clones were obtained. (A) Flow cytometric analyses of LMP-1-TF cells. The cells were first stained with control monoclonal antibody or CD99 monoclonal antibody, and then with FITC-conjugated goat anti-mouse IgG antibody. (B) Vec-TF (V) and LMP-1-TF (L) IM9 cells were examined for LMP-1 expression by immunoblot. (C and D) Immunoblot and Northern blot analyses of LMP-1-TF IM9 cells. Expression of CD99 molecules was examined in Vec-TF (V), LMP-1-TF (L), and AS-TF (A) IM9 cells by immunoblot (C) and Northern blot (D) analysis. Calnexin (C) and GAPDH (D) were tested as internal controls. LMP-1-TF cells displayed down-regulation of CD99 expression at the transcriptional level.
Down-regulation of CD99 in LMP-1 overexpressing IM9 cells.
IM9 B cells were transfected with either vector (Vec-TF), LMP-1 (LMP-1-TF), or antisense-CD99 (AS-TF) expression construct, and G418-resistant stable clones were obtained. (A) Flow cytometric analyses of LMP-1-TF cells. The cells were first stained with control monoclonal antibody or CD99 monoclonal antibody, and then with FITC-conjugated goat anti-mouse IgG antibody. (B) Vec-TF (V) and LMP-1-TF (L) IM9 cells were examined for LMP-1 expression by immunoblot. (C and D) Immunoblot and Northern blot analyses of LMP-1-TF IM9 cells. Expression of CD99 molecules was examined in Vec-TF (V), LMP-1-TF (L), and AS-TF (A) IM9 cells by immunoblot (C) and Northern blot (D) analysis. Calnexin (C) and GAPDH (D) were tested as internal controls. LMP-1-TF cells displayed down-regulation of CD99 expression at the transcriptional level.
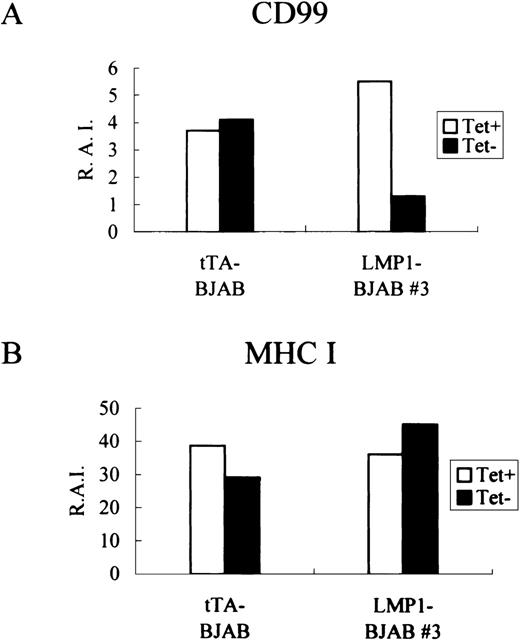
To confirm whether the overexpression of LMP-1 has a direct correlationship with the modulation of expression of CD99, we reinvestigated the relationship using stable-transfected EBV-negative BJAB cell clones with tetracycline-regulatable expression of LMP-1. Induction of LMP-1 expression in these LMP-1–BJAB cells following tetracycline removal was confirmed by immunohistochemical analysis (data not shown). The induced LMP-1 expression coincided with the appearance of H-RS–like cells (data not shown) and, in accordance with previous results,21 with the accumulation of cells at the G2/M phase of the cell cycle. Whereas induced expression of LMP-1 was observed in most of the cells in the absence of tetracycline, the multinucleated giant cells with H-RS morphology were more intensively stained for LMP-1. Consistent with CD99 down-regulation in LMP-1-TF IM9 cell clones, induction of LMP-1 in 1 LMP-1-BJAB clone (LMP-1 BJAB #3) resulted in a threefold to fourfold reduction in the levels of surface CD99, with no change of MHC class I expression (Figure4).
Effects of induced LMP-1 expression on CD99 molecules.
tTA-BJAB and LMP-1-BJAB cells were grown in the presence (+) or absence (−) of tetracycline for 2 days before being harvested for indirect immnunofluorescence staining for CD99 (A) and MHC class I (B) with the use of the DN16 and W6/32 monoclonal antibodies, respectively. Stained cells were analyzed by flow cytometry, and the relative antigen intensity was calculated.
Effects of induced LMP-1 expression on CD99 molecules.
tTA-BJAB and LMP-1-BJAB cells were grown in the presence (+) or absence (−) of tetracycline for 2 days before being harvested for indirect immnunofluorescence staining for CD99 (A) and MHC class I (B) with the use of the DN16 and W6/32 monoclonal antibodies, respectively. Stained cells were analyzed by flow cytometry, and the relative antigen intensity was calculated.
LMP-1 down-regulates CD99 promoter activity when transiently co-transfected into 293T cells
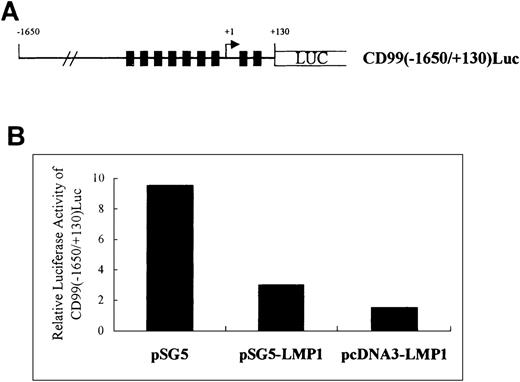
To confirm that LMP-1 modulates the CD99 expression at the transcriptional level, we performed transient transfection experiments with a CD99 promoter-driven luciferase reporter construct, pCD99(−1650/+130)Luc, which contains 1780 nucleotides of CD99 5′ flanking region including all putative Sp1 binding sites (see “Materials and Methods” and Figure5A). The ubiquitously expressed transcription factor Sp1 binds GC-rich DNA elements and has been found to play a major role in the positive regulation of many TATA-less promoters.34,35 According to the sequencing data from others21 and our laboratory (unpublished data), the 5′-flanking region of CD99 contains no recognizable TATA element but a large number of binding sites for Sp1. In the present experiment, we utilized 293T cells, which express the endogenous CD99 gene with high promoter activity, to maximize the effect of co-transfected effector LMP-1 on pCD99(−1650/+130)Luc. Upon co-transfection with SV40 early or CMV IE promoter-driven LMP-1, the CD99 promoter activity was markedly down-regulated. The effect of CMV IE promoter-driven LMP-1 was more dramatic than that of SV40 early promoter-driven, possibly owing to the higher expression of LMP-1. This finding strongly supports the previous Northern analysis data indicating the down-regulation of CD99 expression by LMP-1 at the transcriptional level.
Transiently expressed LMP-1 down-regulates CD99 promoter-driven luciferase activity in 293T.
(A) Schematic presentation of the CD99 promoter-driven luciferase construct pCD99(−1650/+130)luc. Closed bars denote putative Sp1-binding sequences located at −287, −255, −164, −107, −61, −18, +11, and +61 relative to the transcription start site indicated as an arrow head. (B) Effect of LMP-1 expression on CD99 promoter activity. The mean values for 2 experiments are shown. The histogram shows the relative lucifersase activity normalized by the internal control plasmid, pBiP670CAT.
Transiently expressed LMP-1 down-regulates CD99 promoter-driven luciferase activity in 293T.
(A) Schematic presentation of the CD99 promoter-driven luciferase construct pCD99(−1650/+130)luc. Closed bars denote putative Sp1-binding sequences located at −287, −255, −164, −107, −61, −18, +11, and +61 relative to the transcription start site indicated as an arrow head. (B) Effect of LMP-1 expression on CD99 promoter activity. The mean values for 2 experiments are shown. The histogram shows the relative lucifersase activity normalized by the internal control plasmid, pBiP670CAT.
In our experiment, we used a BiP promoter-driven CAT construct as an internal control plasmid to avoid using promoters that can be modulated by coexpressed LMP-1 or CD99, such as a β-actin promoter. Transiently transfected LMP-1 or antisense-CD99 displayed little effect on the expression of BiP. This result was confirmed by comparison of the levels of BiP expression among the 3 stable-transfected IM-9 cell lines, Vec-TF, LMP-1-TF, and anti–CD99-TF in Western blot analysis (data not shown). Although it has been reported that the expression level of BiP is transiently modulated in cells in response to certain stresses,36 37 its expression is known to be fairly steady as a chaperone molecule under normal conditions.
Down-expression of CD99 in H-RS cells from EBV-associated Hodgkin's disease patients
We previously showed lack of CD99 expression in H-RS cells from Hodgkin's disease patients by immunohistochemical analysis.7 However, since these data were obtained in all but 3 cases with the use of paraffin-embedded tissues, we reexamined the level of CD99 expression by staining frozen Hodgkin's disease tissues. In 8 cases of EBV-negative Hodgkin's disease studied, H-RS cells were completely negative for CD99 expression by immunohistochemical and immunofluorescence analyses. In 5 cases examined, H-RS cells from EBV-positive Hodgkin's disease were all negative for the cell surface expression of CD99, except for 1 case with faint cytoplasmic expression in a few H-RS cells (Table1).
Discussion
We previously reported7 that down-expression of CD99 induces the generation of cells with the H-RS phenotype. Since more than 50% of Hodgkin's disease cases are known to be associated with the expression of EBV antigens and H-RS cells are frequently LMP-1-positive,15-19 we examined whether there was any correlation between the expression of CD99 and LMP-1 molecules. Upon stably transfecting an LMP-1 expressing construct into IM9 cells, the overexpressed LMP-1 led to the marked down-regulation of CD99 molecules, subsequently generating H-RS–like cells.
Based on our observations, it is evident that LMP-1 causes a reduced expression of CD99 molecules mainly at the transcriptional level. Furthermore, the fact that the down-regulation of CD99 molecules was induced by regulated overexpression of LMP-1 in EBV-negative BJAB cells, argues against the possibility that CD99 down-regulation is an indirect spontaneous process of an as yet unidentified cellular adaptation to LMP-1 overexpression in EBV-positive IM9 cells. Although these results indicate that LMP-1 signaling events may suppress CD99 promoter activity via regulation of some molecules responsible for the transcription of CD99, the mechanism of the down-regulation of CD99 molecules by LMP-1 is not known at present. LMP-1 mediates its pleiotropic activities through at least 3 independent signaling pathways. LMP-1 triggers cellular NF-κB activity through the TRAF signaling pathway,38-40 AP-1 activity through the SEK/JNK/c-JUN pathway,41,42 and the JAK/STAT pathway through binding and activation of JAK3.43 The involvement of these signaling pathways in the regulation of CD99 by LMP-1 is currently under investigation.
LMP-1 overexpression in IM9 cells showed a negative effect on the rate of cell growth and an accumulation of cells at the G2/M phase, consistent with the previous report on the effect of LMP-1 expression in other cell lines.21 LMP-1-TF cells also exhibited H-RS morphology as shown in CD99-deficient B cells, suggesting that M-phase blockade by CD99 down-regulation is necessary for the production of H-RS cells in EBV-positive Hodgkin's disease. In support of this notion, it has been reported that multinuclear H-RS cells are derived from mononuclear Hodgkin's cells through disturbance of cytokinesis.20 Our data suggest that the formation of multinuclear cells by LMP-1 expression in L-428 and 293T is also attributed to LMP-1-mediated CD99 down-regulation.20
MHC class I down-regulation in H-RS cells was originally demonstrated by Poppema and Visser.32 However, several recent reports described high-level expression of MHC class I molecules on the H-RS cells of EBV-associated Hodgkin's disease, while expression of MHC class I molecules on EBV-negative H-RS cells was either extremely low or completely absent.33 44 In accordance with these reports, we confirmed that up-regulation of LMP-1 led to no change of MHC class I expression on the cell surfaces as compared with control cells, whereas CD99-deficient EBV-negative H-RS cells showed the down-regulation of MHC class I molecules.7
Our findings address a primary role of the down-regulation of CD99 by LMP-1 in the generation of the H-RS phenotype in EBV-associated Hodgkin's disease. In the pathogenesis of EBV-associated Hodgkin's disease, LMP-1 overexpression may be triggered by unknown factors induced in the microenvironmental milieu or genetic alterations in EBV-infected H-RS–precursor B cells, leading to the generation of H-RS cells by a subsequent decrease in the expression of CD99 at the transcriptional level. Previously, we demonstrated that the reduced expression of CD99 is a critical event in the development of Hodgkin's disease.7 In the case of EBV-associated Hodgkin's disease, the viral gene product LMP-1 is likely to play an important role in CD99 down-regulation. However, it is not clear by which mechanism CD99 expression is down-regulated in EBV-negative cases. Although some events, such as mutations in genes regulating CD99 expression or changes in cytokine milieu, are plausible, it remains to be determined what cellular factors are involved in CD99 down-regulation in the case of Hodgkin's disease not associated with EBV.
Acknowledgments
We are grateful to Dr E. Kieff (Harvard University, Boston, MA), Dr C.V. Paya (Mayo Clinic, Rochester, NY), and Dr Chao (Chang Gung Medical College, Taoyuan, Taiwan) for providing us with LMP-1 gene and BiP promoter constructs, and to Sean Bong Lee (MGH Cancer Center, Boston, MA) for helpful comments on the manuscript.
S.H.K. and Y.K.S. contributed equally to this work.
Supported by the ‘99 DiNonA Inc R&D Project, Seoul, Korea. MR was supported by the Leukemia Research Fund, London, UK.
Reprints:Seong Hoe Park, Department of Pathology, Seoul National University College of Medicine, 28 Yongon-dong Chongno-gu, Seoul 110-799, Korea.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal