Abstract
Decreased expression of functional IIbβ3 complexes on the platelet surface produces Glanzmann thrombasthenia. We have identified mutations of IIbP145 in 3 ethnically distinct families affected by Glanzmann thrombasthenia. Affected Mennonite and Dutch patients were homozygous and doubly heterozygous, respectively, for a P145A substitution, whereas a Chinese patient was doubly heterozygous for a P145L substitution. The mutations affect expression levels of surface IIbβ3 receptors on their platelets, which was confirmed by co-transfection of IIbP145A and β3 cDNA constructs in COS-1 cells. Each mutation also impaired the ability of IIbβ3 on affected platelets to interact with ligands. Moreover, when IIbP145A and β3 were stably coexpressed in Chinese hamster ovary cells, IIbβ3 was readily detected on the cell surface, but the cells were unable to adhere to immobilized fibrinogen or to bind soluble fluorescein isothiocyanate–fibrinogen after IIbβ3 activation by the activating monoclonal antibody PT25-2. Nonetheless, incubating affected platelets with the peptide LSARLAF, which binds to IIb, induced PF4 secretion, indicating that the mutant IIbβ3 retained the ability to mediate outside-in signaling. These studies indicate that mutations involving IIbP145 impair surface expression of IIbβ3 and that the IIbP145A mutation abrogates ligand binding to the activated integrin. A comparative analysis of other IIb mutations with a similar phenotype suggests that these mutations may cluster into a single region on the surface of the IIb and may define a domain influencing ligand binding. (Blood. 2000;95:180188)
The platelet-specific integrin αIIbβ3 (GPIIb/IIIa, CD41/CD61) binds fibrinogen and other ligands following platelet activation.1,2 Because ligand binding to αIIbβ3 is required for platelet aggregation, inherited decreases in the amount of functional αIIbβ3 on the platelet surface cause a bleeding disorder, Glanzmann thrombasthenia.3,4 To date, 59 molecular defects have been identified in 48 kindreds4-10; 19 of these mutations are compound heterozygous and 29 are homozygous. The identified mutations cover the range of known molecular defects, including gene rearrangements or deletions, messenger RNA splicing abnormalities, frameshifts, nonsense mutations, and missense mutations. All of these mutations have quantitative and/or qualitative effects on the αIIbβ3.
Studying the functional consequences of a variety of naturally occurring and chemically induced β3 mutations has made it possible to designate 2 regions in β3 that are probably involved in ligand binding. A naturally occurring αIIbL183Pmutation has recently been found to impair both αIIbβ3 expression and its ligand-binding activity, suggesting that L183 is in proximity to a ligand-binding site in αIIb.6 Consistent with this possibility, 2 series of chemically induced mutations in αIIb involving amino acids from G184 through G193 and at D224 prevented the interaction of αIIbβ3 with fibrinogen.11 12
In this paper, we report studies of 3 unrelated families with Glanzmann thrombasthenia due to mutations of αIIbP145. Affected members of Mennonite and Dutch families were homozygous and compound heterozygous, respectively, for a P145A mutation, whereas a Chinese patient was compound heterozygous for a P145L substitution. When αIIb containing the P145A substitution was co-expressed heterologously with β3 in COS and Chinese hamster ovary (CHO) cells, decreased numbers of αIIbβ3 heterodimers were present on the cell surface compared with cells expressing wild-type αIIbβ3. Moreover, the mutant heterodimers that were expressed were unable to interact with fibrinogen. Thus, these studies indicate that the presence of proline at position 145 in αIIb is required both for the efficient expression of αIIbβ3 and for its ability to interact with ligands. When viewed in the context of the β-propeller model of the amino terminus of integrin α-subunits13 and the other point mutations in αIIb known to perturb ligand binding to αIIbβ3, our results define a domain influencing ligand binding on the surface of αIIb.
Materials and methods
Case reports
Mennonite family.
We studied 2 affected sibs (LW and GW). LW is a 22-year-old woman who was first noted to have recurrent epistaxis and purpura at the age of 2. Until the age of 7, she required platelet transfusions for epistaxis, but she has not required transfusion in the subsequent 15 years. Currently, she notes scattered petechiae and purpura, but epistaxis is infrequent. Her platelet count is normal, but her platelets fail to aggregate in response to thrombin, adenosine diphosphate (ADP), epinephrine, or collagen, although they agglutinate normally in the presence of ristocetin. GW is a 24-year-old man who was noted to have excessive bruising at age 4 and was also found to have platelet function studies consistent with a diagnosis of Glanzmann thrombasthenia. He has never required platelet transfusions for bleeding. A detailed family tree documents no consanguinity in the family for at least 5 generations. The studies described below focus on GW, although LW had an identical αIIb mutation.
Dutch family.
A male patient from the Netherlands (JF) presented at birth with epistaxis and subsequently suffered from excessive bruising, gastric hemorrhage, and hematuria. From ages 2 to 16, the patient was hospitalized on multiple occasions for persistent epistaxis requiring platelet and red blood cell transfusions. He also required multiple red cell and platelet transfusions following dental extractions and after the removal of kidney stones. Laboratory studies revealed that his platelets failed to aggregate in response to ADP, epinephrine, collagen, or thrombin. Although his platelets initially aggregated in response to ristocetin, this was followed by partial disaggregation. The patient's bleeding time was > 15 minutes, and minimal clot retraction was observed. Platelet fibrinogen levels were markedly decreased (∼9% of normal).
Chinese family.
The patient (Chinese-14) is a male from the Hunan province of the People's Republic of China who was noted to have epistaxis, gingival hemorrhage, and purpura at 3 years of age. A laboratory evaluation revealed no platelet aggregation in response to ADP, epinephrine, or collagen. The initial slope of ristocetin-induced platelet aggregation was normal, but the extent of aggregation, as judged by the maximal change in light transmittance, was minimally decreased. Bleeding manifestations, primarily epistaxis, have been severe, requiring multiple blood transfusions. Platelet fibrinogen levels were markedly deficient.
Flow cytometry
Expression of αIIbβ3 on the platelet surface was measured by flow cytometry with the use of a panel of anti-platelet monoclonal antibodies and a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ) as previously described.14 Monoclonal antibodies used were A2A9, a monoclonal antibody that interacts with an epitope expressed on the extracellular domain of the intact αIIbβ3 heterodimer15,16; B1B5, a monoclonal antibody that recognizes an epitope located on αIIb16; SSA6, a monoclonal antibody that recognizes an epitope located on β316; PAC-1, a monoclonal antibody that recognizes an epitope expressed exclusively by the activated conformation of αIIbβ317; and AP-1, a monoclonal antibody specific for platelet GPIb.18 Monoclonal antibody binding was detected with the use of fluorescein-conjugated anti-murine IgG (Boehringer-Mannheim, Indianapolis, IN). Measurements of PAC-1 binding were performed after stimulating platelets with 0.2 μM phorbol myristate acetate for 5 minutes at 25°C.
Immunoblotting.
Platelets of JF and Chinese-14 (5 × 108platelets/mL) were dissolved in an equal volume of sodium dodecyl sulfate (SDS) and electrophoresed in 0.1% SDS, 7.5% polyacrylamide gels. The resolved platelet proteins were then transferred to polyvinylidene difluoride membranes and immunoblotted.19Control and patient samples were electrophoresed under reducing conditions for αIIb analysis and under nonreducing conditions for β3 analysis. The membranes were incubated with the anti-αIIb heavy-chain–specific murine monoclonal antibody PMI-116,20or with the anti-β3–specific murine monoclonal antibody 7H2.21
Identification of the thrombasthenic mutation
Genomic DNA was isolated from blood as previously described.22 Screening for mutations was performed with the use of single-stranded conformation polymorphism analysis of polymerase chain reaction (PCR)–amplified DNA of each αIIb and β3 exon and of the 500 base pairs (bp) of DNA immediately upstream of each transcriptional start site23-25 as previously described.26
DNA fragments that migrated abnormally in the single-stranded conformation polymorphism analysis gel were directly sequenced with the use of the fmol DNA Cycle Sequencing Kit (Promega, Madison, WI) as described.14 26 DNA fragments were also subcloned with the use of a commercial TA cloning kit (Invitrogen, San Diego, CA) and sequenced with the use of Sp6 and T7 primers and a commercial Sequenase sequencing kit (USB, Cleveland, OH).
For JF and Chinese-14, platelet expression of αvβ3 was quantified with the use of radiolabeled monoclonal antibodies,27 and the results of this assay suggested that the mutation in these patients involved the gene for αIIb. The 30 exons of the αIIb gene23 were amplified with the use of PCR and directly sequenced. One of the 2 PCR primers in each of the 25 pairs of primers used for the amplifications was biotinylated. The resulting strand of DNA with the 5′-biotin group in the PCR-amplified fragment was purified by attachment to streptavidin-coated magnetic beads and alkali-denaturation according to the manufacturer's instructions (Dynal, Lake Success, NY). The attached DNA was directly sequenced with the use of nested primers and a commercial Sequenase sequencing kit (USB).
Heterologous expression of IIbβ3
To determine the effect of mutation of αIIbP145 on αIIbβ3 expression, αIIb containing a mutation at this position was expressed in COS-1 cells as previously described.26Briefly, the codon for P145 in wild-type αIIb cDNA was mutated with the use of an overlap PCR technique.28 PCR amplification was performed with the use of VENT polymerase (Promega) to decrease the frequency of PCR-induced mutations. The resulting mutated PCR products were inserted into wild-type αIIb cDNA in PUC19 (Gibco/BRL, Gaithersburg, MD). Following sequencing to ensure the fidelity of the PCR reaction, the DNA was shuttled into the expression vector pMT2ADA.29
The pMT2ADA αIIb-expression vector was introduced in COS-1 cells, either alone or with a similar vector for β3, with the use of Lipofectin Reagent (Gibco/BRL).14,26 Forty-eight hours after transfection, the cells were metabolically labeled with35S-methionine (NEN Life Sciences Products, Boston, MA) at 200 μCi/mL or surface-labeled with 125I (NEN Life Sciences Products) and extracted with a 0.02 mol/L Tris-HCl buffer, pH 7.8, containing 1% Triton X-100 (Sigma, St. Louis, MO).14αIIb and β3 were then immunoprecipitated from the cell extracts with the use of either B1B5 or SSA6. The radiolabeled, immunoprecipitated proteins were electrophoresed on 0.1% SDS–7.5% polyacrylamide slab gels, dried, and analyzed by autoradiography as previously described.14
To determine the effect of mutation of αIIb residue 145 on αIIbβ3 function, αIIbβ3 was stably expressed in CHO cells. cDNAs for wild-type αIIb and αIIbP145A were shuttled into pcDNA 3.1Neo+ (Invitrogen), and a cDNA for β3 was shuttled into pcDNA 3.1Zeo+ (Invitrogen). CHO cells, cultured in Ham's F12 media (Hyclone Laboratories Inc, Logan, UT) supplemented with 10% fetal bovine serum (FBS) (Hyclone) were co-transfected with the vectors for αIIb and β3 with the use of FUGENE transfection according to the manufacturer's instructions (Boehringer-Mannheim). Transfected cells were transferred 2 days later to selection media containing G418 (500 μg/mL) (Gibco/BRL) and Zeocin (300 μg/mL) (Invitrogen). After 3 weeks of growth in selection media, 1 × 106 cells were examined for αIIbβ3 expression by flow cytometric analysis with the use of the β3-specific monoclonal antibody SSA6.
The ability of αIIbβ3 expressed by CHO cells to interact with fibrinogen was tested by measuring cell adhesion to immobilized fibrinogen30 and the binding of soluble fluorescein isothiocyanate (FITC)–fibrinogen to αIIbβ3.31 To measure cell adhesion, 1.5 × 105 CHO cells were labeled metabolically overnight with 35S-methionine (Dupont, Wilmington, DE) at 200 μCi/mL. The labeled cells were then suspended in 100 μL of 50 mM Tris-HCl buffer, pH 7.4, containing 150 mM NaCl, 0.5 mM CaCl2, 0.1% glucose, and 1% FBS, and incubated with 10 μg/mL of the αIIbβ3-activating monoclonal antibody PT25-2.32 The cells were added to wells of microtiter plates precoated with human fibrinogen (Sigma) at a concentration of 5 μg/mL. Following a 30-minute incubation at 37°C without agitation, the plates were vigorously washed 4 times with the suspension buffer; the adherent cells were dissolved with the use of 2% SDS; and the SDS solution was analyzed for 35S in a liquid scintillation counter.
To measure the binding of soluble fibrinogen to αIIbβ3 on CHO cells, purified human fibrinogen (Sigma) was labeled with FITC with the use of a Calbiochem FITC-labeling Kit (Calbiochem, San Diego, CA). Fibrinogen labeled with FITC in this manner remained monomeric as assessed by gel filtration chromatography, supported platelet aggregation as well as unlabeled fibrinogen, and was 95% clottable with thrombin.33CHO cells (1.5 × 105) were then suspended in 100 μL of suspension buffer (10 mM sodium phosphate buffer, pH 7.4, containing 137 mM NaCl, 1 mM CaCl2, and 1% bovine serum albumin). The cells were then incubated with 200 μg/mL FITC-fibrinogen in the presence or absence of 10 μg/mL of PT25-2 monoclonal antibody for 30 minutes at 37°C. After being washed once with suspension buffer, the cells were resuspended in a fixation solution consisting of 10 mM sodium phosphate buffer, pH 7.4, containing 137 mM NaCl and 0.37% formalin. After being rewashed once with the suspension buffer, the cells were analyzed by flow cytometry as described previously.31
Platelet factor 4 secretion stimulated by the peptide LSARLAF
LSARLAF (LSA), the control peptide FRALASL (FRA), and the thrombin receptor activating peptide SFLLRN (TRAP) were synthesized and characterized as previously described.33,34 To measure peptide-stimulated platelet factor (PF) 4 secretion, platelets were stirred for 3 minutes in the presence of various concentrations of peptide. Following sedimentation of the platelets in a microfuge, secreted PF4 was measured in the supernatant with the use of an anti-PF4 antibody enzyme-linked immunosorbent assay (Asserachrome kit) as previously described.34
Results
Quantitation of IIbβ3 in affected platelets
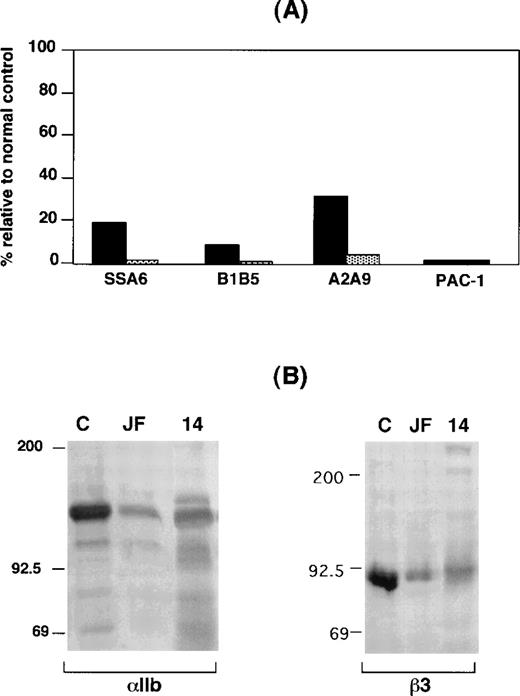
Expression of αIIbβ3 on the surface of GW's platelets was analyzed by flow cytometry, and radiolabeled monoclonal antibody binding35 was used for patients JF and Chinese-14. As shown in Figure 1A, staining GW's platelets with monoclonal antibodies specific for αIIb, β3, and αIIbβ3 revealed that they respectively bound ∼10%, ∼20%, and ∼30% as much monoclonal antibody as control platelets, substantially more than was seen with platelets from FLD, a patient with Type 1 thrombasthenia due to a mutation in αIIb that prevents surface αIIbβ3 expression.16 Binding of the GPIb-specific monoclonal antibody AP1 to the platelets of both GW and FLD was within the normal range (data not shown). Despite the presence of αIIbβ3 on their surface, however, GW's platelets were unable to bind the activation-dependent monoclonal antibody PAC-1 following platelet stimulation by phorbol myristate acetate. Identical data were obtained when αIIbβ3 expression on the surface of LW's platelets was analyzed (data not shown). Thus, these results indicate that there are both quantitative and qualititative αIIbβ3 abnormalities in GW's and LW's platelets.
IIbβ3 expression in platelets from patients GW, JF, and Chinese-14.
(A) Flow cytometric analysis of platelets from patient GW (solid bars) and a patient with a known deficiency of surface αIIbβ3 receptors (FLD)14 (shaded bars). Data are expressed relative to a concurrently studied normal control (100%), whose platelets are known to express normal amounts of αIIbβ3.14 26 Measurements of the binding of the β3-specific monoclonal antibody SSA6, the αIIb-specific monoclonal antibody B1B5, the αIIbβ3-specific monoclonal antibody A2A9, and the GPIb-specific monoclonal antibody AP1 were performed with the use of unstimulated platelets. PAC1 binding was measured after stimulating platelets with the phorbol myristate acetate. (B) Immunoblots of separated proteins from the platelets of patients JF and Chinese-14 and a normal control (labeled C) were performed with the use of the anti-αIIb heavy-chain–specific monoclonal antibody PMI-1 and the anti-β3–specific monoclonal antibody 7H2. Identical amounts of platelet protein from each subject were immunoblotted.
IIbβ3 expression in platelets from patients GW, JF, and Chinese-14.
(A) Flow cytometric analysis of platelets from patient GW (solid bars) and a patient with a known deficiency of surface αIIbβ3 receptors (FLD)14 (shaded bars). Data are expressed relative to a concurrently studied normal control (100%), whose platelets are known to express normal amounts of αIIbβ3.14 26 Measurements of the binding of the β3-specific monoclonal antibody SSA6, the αIIb-specific monoclonal antibody B1B5, the αIIbβ3-specific monoclonal antibody A2A9, and the GPIb-specific monoclonal antibody AP1 were performed with the use of unstimulated platelets. PAC1 binding was measured after stimulating platelets with the phorbol myristate acetate. (B) Immunoblots of separated proteins from the platelets of patients JF and Chinese-14 and a normal control (labeled C) were performed with the use of the anti-αIIb heavy-chain–specific monoclonal antibody PMI-1 and the anti-β3–specific monoclonal antibody 7H2. Identical amounts of platelet protein from each subject were immunoblotted.
Radiolabeled monoclonal antibody–binding data for patient JF revealed that binding of the αIIbβ3-specific monoclonal antibody 10E5 and the αIIbβ3+αvβ3–specific monoclonal antibody 7E3 were 5% and <1%, respectively, of the control values. This suggests that the αIIbβ3 expressed on surface of JF's platelets was not recognized by the conformation-dependent monoclonal antibody 7E3. On the other hand, the platelets of Chinese-14 did not bind detectable levels of either antibody. To estimate the total amounts of αIIb and β3 in JF's and Chinese-14's platelets, immunoblots were performed with the use of the αIIb heavy-chain–specific monoclonal antibody PMI-119,36 and the β3-specific monoclonal antibody 7H2.37 As shown in Figure 1B, αIIb and β3 were readily detectable in detergent extracts of platelets of both patients, but the amounts were substantially decreased compared with control platelets. The immunoblots of αIIb were performed under reducing conditions. Thus, it is notable that most of the immunodetectable αIIb in JF's and Chinese-14's platelets corresponded to the αIIb heavy chain. This indicates that a substantial proportion of the pro-αIIb in the megakaryocytes of both patients was able to reach the Golgi complex where pro-αIIb is cleaved into heavy and light chains.
Identification of mutations responsible for Glanzmann thrombasthenia in the Mennonite, Dutch, and Chinese families
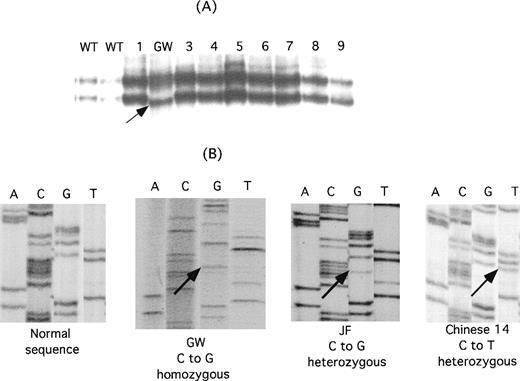
To identify the molecular basis for the thrombasthenia in the Mennonite family, genomic DNA from GW was screened with the use of single-stranded conformation polymorphism analysis and oligonucleotide primers designed to amplify DNA from each exon of the αIIb and β3 genes and from the 500 bp of DNA immediately upstream of each gene's transcriptional start site.23-25 As shown in Figure2A, single-stranded conformation polymorphism analysis of exon 4 of GW's αIIb gene revealed a new, faster migrating band. Direct sequence analysis of the PCR products from the patient and a normal control revealed that the patient's DNA was homozygous for a C→G nucleotide substitution in the codon for amino acid 145, resulting in the replacement of proline in the wild-type sequence with alanine (Figure 2B). LW was also homozygous for this mutation, and their parents were heterozygous (data not shown).
Identification of mutations in IIb responsible for the thrombasthenia phenotype of patients GW, JF, and Chinese-14.
(A) Single-stranded conformation polymorphism analysis of αIIb exon 4 in 2 normal controls (WT), 8 unrelated thrombasthenic patients (lanes 1 and 3-9), and patient GW. An aberrantly migrating band in the sample from GW is indicated by the arrow. (B) Direct genomic sequence analysis of the region of interest of the αIIb gene from a normal individual, GW, JF, and Chinese-14. Differences from the normal sequence are indicated by the arrows. GW is homozygous for a mutation in the codon for P145, whereas both a normal and a mutant base are present in the JF and Chinese-14 sequences, indicating that they are heterozygous for this mutation.
Identification of mutations in IIb responsible for the thrombasthenia phenotype of patients GW, JF, and Chinese-14.
(A) Single-stranded conformation polymorphism analysis of αIIb exon 4 in 2 normal controls (WT), 8 unrelated thrombasthenic patients (lanes 1 and 3-9), and patient GW. An aberrantly migrating band in the sample from GW is indicated by the arrow. (B) Direct genomic sequence analysis of the region of interest of the αIIb gene from a normal individual, GW, JF, and Chinese-14. Differences from the normal sequence are indicated by the arrows. GW is homozygous for a mutation in the codon for P145, whereas both a normal and a mutant base are present in the JF and Chinese-14 sequences, indicating that they are heterozygous for this mutation.
In the Dutch and Chinese patients, radiolabeled antibody binding studies27 38 using the αv-specific monoclonal antibody LM142 and the αvβ3-specific monoclonal antibody LM609 revealed normal to increased amounts of αvβ3, suggesting that the mutational defect was in the gene encoding αIIb, rather than β3 (data not shown). Direct PCR amplification and sequence analysis of the αIIb exons in patient JF revealed that he was heterozygous for a C→G nucleotide substitution that results in a P145A substitution (Figure 2B). The other mutation has not been identified. The patient Chinese-14 was found to be heterozygous for a C→T nucleotide substitution at the second position of the same codon, resulting in a Pro145Leu substitution (Figure 2B); the other mutation was identified as a deletion of the G nucleotide in the AG splice acceptor site of exon 16 and is designated IVS15(-1)Gdel (deletion of the first nucleotide, G, at the 3′ end of intervening sequence, intron, 15).
Effect of mutation of IIbP145 on IIbβ3 expression and function
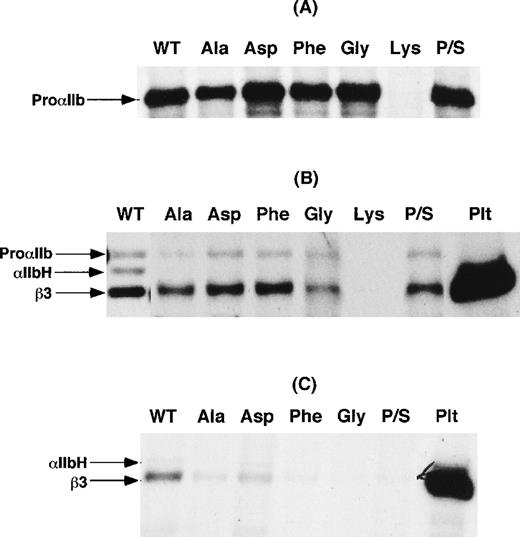
To examine the effect of the mutation of αIIbP145 on αIIbβ3 expression, cDNA constructs expressing P145A, P145G, P145D, P145K, and P145F were generated. In addition, another construct was generated in which the codons for serine at amino acid residue 144 and proline at residue 145 were inverted (P/S swap mutation) to retain the structural consequences of a proline residue in this region of αIIb. Wild-type αIIb and the various αIIb mutants were then coexpressed with β3 in COS-1 cells, and αIIbβ3 expression was examined in cells metabolically labeled with 35S-methionine or surface-labeled with 125I. As shown in Figure3A, except for lysine, none of the substitutions at position 145 impaired αIIb synthesis. In 4 separate experiments, we were never able to detect a synthesis product with the P145K mutation. Moreover, as shown in Figure 3B, none of the mutations, except for lysine, affected the assembly of αIIbβ3 heterodimers. On the other hand, none of the immunoprecipitates of αIIbβ3 from cells expressing the αIIbP145 mutations and the P/S swap mutation contained the αIIb heavy chain. These data suggest that the presence of proline at position 145 is required for efficient export of αIIbβ3 complexes from the endoplasmic reticulum to the Golgi complex, where αIIb cleavage into heavy and light chains occurs.39 Consistent with this interpretation, little αIIbβ3 was detectable on the surface of these cells (Figure 3C).
Transient expression of IIbP145 mutations in COS-1 cells.
(A) Wild-type αIIb (WT) and the indicated αIIbP145mutants were expressed in COS-1. An equal number of cells for each transfection were then labeled with 35S-methionine, and αIIb was immunoprecipitated with the use of the αIIb-specific monoclonal antibody B1B5. P/S refers to a proline and serine swap at amino acid residues 144 and 145. (B) COS-1 cells co-transfected with either wild-type αIIb or the indicated αIIbP145 mutants and β3. After the cells were labeled with 35S-methionine, αIIbβ3 was immunoprecipitated with the β3-specific monoclonal antibody SSA6. The identity of β3 was confirmed by immunoprecipitation of control platelets (Plt) surface-labeled with125I. (C) Immunoprecipitation of αIIbβ3 with the use of SSA6 from cells, cotransfected with either wild-type αIIb or the indicated αIIbP145 mutants and β3, which were surface-labeled with 125I. The data shown are representative of 3 separate experiments.
Transient expression of IIbP145 mutations in COS-1 cells.
(A) Wild-type αIIb (WT) and the indicated αIIbP145mutants were expressed in COS-1. An equal number of cells for each transfection were then labeled with 35S-methionine, and αIIb was immunoprecipitated with the use of the αIIb-specific monoclonal antibody B1B5. P/S refers to a proline and serine swap at amino acid residues 144 and 145. (B) COS-1 cells co-transfected with either wild-type αIIb or the indicated αIIbP145 mutants and β3. After the cells were labeled with 35S-methionine, αIIbβ3 was immunoprecipitated with the β3-specific monoclonal antibody SSA6. The identity of β3 was confirmed by immunoprecipitation of control platelets (Plt) surface-labeled with125I. (C) Immunoprecipitation of αIIbβ3 with the use of SSA6 from cells, cotransfected with either wild-type αIIb or the indicated αIIbP145 mutants and β3, which were surface-labeled with 125I. The data shown are representative of 3 separate experiments.
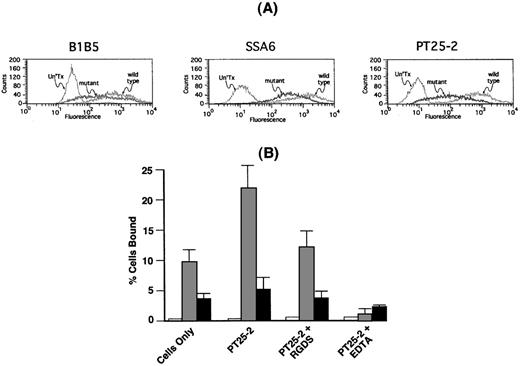
To determine whether the αIIbP145Aβ3 that was present on the cell surface was able to interact with fibrinogen, we stably expressed αIIbP145Aβ3 and wild-type αIIbβ3 in CHO cells. The cells were then sorted by flow cytometry with the use of the anti-β3 monoclonal antibody SSA6 to obtain populations of cells expressing comparable levels of each integrin on their surface. Because SSA6 can bind to αvβ3, as well as αIIbβ3, we confirmed that comparable levels of mutant and wild-type αIIbβ3 were expressed on the surface of the sorted cells by also staining the cells with the αIIb-specific monoclonal antibody B1B516 and the β3-specific monoclonal antibody PT25-2.32 Figure4A demonstrates that comparable amounts of each of 3 monoclonal antibodies bound to cells expressing mutant and wild-type αIIbβ3.
Adhesion of CHO cells expressing IIbP145Aβ3 to immobilized fibrinogen.
(A) CHO cells were co-transfected with either wild-type αIIb or αIIbP145A and β3. Untransfected cells (Un'Tx), cells expressing wild-type αIIbβ3 (wild type), and cells expressing αIIP145Aβ3 (mutant) were sorted by flow cytometry with the use of the anti-β3 monoclonal antibody SSA6. Comparable expression of αIIbβ3 on the transfected cells was confirmed with the use of the anti-αIIb monoclonal antibody B1B5 and the anti-β3 monoclonal antibody PT25-2. (B) Adhesion of untransfected CHO cells (open bars) and CHO cells expressing comparable levels of either wild-type αIIbβ3 (shaded bars) or αIIbP145Aβ3 (solid bars) to immobilized fibrinogen was measured in the absence or presence of the αIIbβ3-activating β3-specific monoclonal antibody PT25-2. Reduction of adhesion to baseline levels by 1 mM EDTA indicates that adhesion to fibrinogen was integrin-specific. The data show the mean ± SD that was done in 3 separate runs.
Adhesion of CHO cells expressing IIbP145Aβ3 to immobilized fibrinogen.
(A) CHO cells were co-transfected with either wild-type αIIb or αIIbP145A and β3. Untransfected cells (Un'Tx), cells expressing wild-type αIIbβ3 (wild type), and cells expressing αIIP145Aβ3 (mutant) were sorted by flow cytometry with the use of the anti-β3 monoclonal antibody SSA6. Comparable expression of αIIbβ3 on the transfected cells was confirmed with the use of the anti-αIIb monoclonal antibody B1B5 and the anti-β3 monoclonal antibody PT25-2. (B) Adhesion of untransfected CHO cells (open bars) and CHO cells expressing comparable levels of either wild-type αIIbβ3 (shaded bars) or αIIbP145Aβ3 (solid bars) to immobilized fibrinogen was measured in the absence or presence of the αIIbβ3-activating β3-specific monoclonal antibody PT25-2. Reduction of adhesion to baseline levels by 1 mM EDTA indicates that adhesion to fibrinogen was integrin-specific. The data show the mean ± SD that was done in 3 separate runs.
As shown in Figure 4B, cells expressing wild-type αIIbβ3 readily adhered to immobilized fibrinogen, and adherence was ≈2-fold greater following exposure of the cells to the αIIbβ3-activating monoclonal antibody PT25-2. The presence of 1 mM RGDS returned PT25-2–stimulated adhesion to nearly baseline levels, whereas the presence of 1 mM ethylenediaminetetraacetic acid (EDTA) reduced the level of adhesion to that of nontransfected cells. By contrast, there was ≈2.5-fold less spontaneous adhesion of cells expressing αIIbP145Aβ3 to immobilized fibrinogen, and there was little increase in adhesion following exposure of the cells to PT25-2. As in cells expressing wild-type αIIbβ3, adhesion was restored to baseline levels by 1 mM RGDS and nearly to the level of untransfected cells by 1 mM EDTA.
To examine whether mutation of αIIbP145 also affects the ability of αIIbβ3 to bind soluble fibrinogen, CHO cells expressing αIIbP145Aβ3 and wild-type αIIbβ3 were incubated with soluble FITC-labeled fibrinogen in the absence or presence of the αIIbβ3-activating monoclonal antibody PT25-2. FITC-fibrinogen binding was then assessed by flow cytometry. In the absence of PT25-2, neither cell line bound FITC-fibrinogen (data not shown). However, as shown in Figure 5, whereas there was substantial PT25-2–stimulated fibrinogen binding to cells expressing wild-type αIIbβ3, there was none to cells expressing αIIbP145Aβ3. FITC-fibrinogen binding to cells expressing wild-type αIIbβ3 was undetectable in the presence of 1 mM RGDS or 1 mM EDTA, indicating that the binding was specific for αIIbβ3, a conclusion consistent with the inability of untransfected cells to bind fibrinogen. Thus, these experiments indicate that not only does mutation of αIIbP145A attenuate the ability of αIIbβ3 to interact with immobilized fibrinogen, but it abolishes the ability of αIIbβ3 to bind soluble fibrinogen.
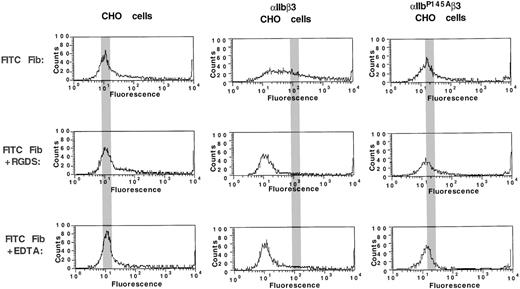
FITC-fibrinogen binding to CHO cells expressing IIbP145Aβ3.
Untransfected CHO cells and CHO cells expressing comparable levels of either wild-type αIIbβ3 or mutant αIIbP145Aβ3 were incubated with 200 μg/mL FITC-fibrinogen in the absence or presence of the αIIbβ3-activating β3-specific monoclonal antibody PT25-2. The amount of FITC-fibrinogen bound was then determined by flow cytometry. Reduction of fibrinogen binding to baseline levels by 1 mM RGDS and 1 mM EDTA indicates that the binding was αIIbβ3-specific. The gray bar indicates the location of the mean fluorescence intensity in the histograms of cells incubated with PT25-2.
FITC-fibrinogen binding to CHO cells expressing IIbP145Aβ3.
Untransfected CHO cells and CHO cells expressing comparable levels of either wild-type αIIbβ3 or mutant αIIbP145Aβ3 were incubated with 200 μg/mL FITC-fibrinogen in the absence or presence of the αIIbβ3-activating β3-specific monoclonal antibody PT25-2. The amount of FITC-fibrinogen bound was then determined by flow cytometry. Reduction of fibrinogen binding to baseline levels by 1 mM RGDS and 1 mM EDTA indicates that the binding was αIIbβ3-specific. The gray bar indicates the location of the mean fluorescence intensity in the histograms of cells incubated with PT25-2.
Effect of mutation of IIbP145 on IIbβ3-mediated outside-in signaling
Ligand binding to αIIbβ3 initiates intraplatelet signaling (outside-in signaling), which can be mimicked by exposing platelets to the peptide LSARLAF (LSA).33,34 40 To determine whether the αIIbP145A mutation also perturbs the ability of αIIbβ3 to mediate outside-in signaling, we exposed GW's platelets, normal platelets, and FLD's platelets to LSA, as well as to the scrambled control peptide FRALASL (FRA) and to TRAP, and measured platelet PF4 secretion. As shown in Table1, TRAP-stimulated PF4 secretion from GW's and FLD's platelets were ∼60% that of normal platelets. In comparison with TRAP, LSA stimulated 44%, 21%, and 4% as much PF4 from control, GW, and FLD platelets, respectively, whereas the amount of PF4 released from platelets exposed to FRA was no different from the amount released from platelets incubated in the absence of peptide. When the secretion data were normalized for PF4 secretion in response to TRAP, the LSA-induced increments in secretion from control and GW platelets were nearly equal, suggesting that outside-in signaling mediated by ligand binding to αIIbP145Aβ3 is essentially intact.
PF4 releasate by platelet activation
| Platelets . | Treatment . | |||
|---|---|---|---|---|
| TRAP . | LSA . | FRA . | No Peptide . | |
| Normal | 100%* | 44.0 ± 0.09% | 10.8 ± 0.8% | 11.4 ± 0.9% |
| GW | 67.5 ± 3.8% | 21.1 ± 1.6% | 12.0 ± 0.8% | 7.3 ± 2.6% |
| FLD | 54.7 ± 2.4% | 3.7 ± 0.7% | 3.1 ± 0.6% | 1.7 ± 0.3% |
| Platelets . | Treatment . | |||
|---|---|---|---|---|
| TRAP . | LSA . | FRA . | No Peptide . | |
| Normal | 100%* | 44.0 ± 0.09% | 10.8 ± 0.8% | 11.4 ± 0.9% |
| GW | 67.5 ± 3.8% | 21.1 ± 1.6% | 12.0 ± 0.8% | 7.3 ± 2.6% |
| FLD | 54.7 ± 2.4% | 3.7 ± 0.7% | 3.1 ± 0.6% | 1.7 ± 0.3% |
Discussion
We have identified mutations involving P145of αIIb that have resulted in Glanzmann thrombasthenia in 3 separate kindreds. Affected members of a Mennonite family were homozygous for an αIIbP145A mutation, and the affected member of a Dutch family was compound heterozygous for the same mutation. In addition, a Chinese patient was compound heterozygous for an independent mutation of the P145 codon, which has resulted in an αIIbP145L substitution. It is noteworthy that identical mutations were found in the Mennonite and Dutch families because the Mennonites immigrated to North America from the Netherlands, southern Germany, and Switzerland in the second half of the 18th century. Moreover, a family tree provided by the Mennonite family whose affected members were homozygous indicated no consanguinity for at least 5 generations, suggesting the possibility that the αIIbP145A mutation is resident at a low frequency in the Dutch/Mennonite population. Additional examples of resident mutations common to the Dutch and Mennonite populations have been previously described for other genes.41
Mutation of P145 is similar to the previously described point mutations and small deletions in αIIb that decreased αIIbβ3 expression on the platelet surface by impairing the intracellular transit of the complex.14,26,42,43 Thus, when a series of αIIbP145 mutants, including P145A, were transiently coexpressed in COS-1 cells with β3, there was no apparent effect on αIIb synthesis or on the assembly of αIIbβ3 heterodimers. Nonetheless, little αIIbβ3 was transported to the cell surface, and αIIb heavy chain was not detected in immunoprecipitates from metabolically labeled cells. Because αIIb is cleaved into heavy and light chains in the trans-Golgi network,39,44,45 the inability to detect αIIb heavy chain indicates that most of the αIIbβ3 assembled in these cells failed to pass through this compartment. Previous studies of retained αIIbβ3 in the platelets of other patients with Glanzmann thrombasthenia have also found that the αIIb in the mutant complexes fails to become resistant to the enzyme Endo H.14 These data imply that the block in αIIbβ3 transport is proximal to the mid-Golgi stacks, most likely at the level of the endoplasmic reticulum. Interestingly, the block in αIIbβ3 transit was greatest in cells of human (platelets) and primate (COS) origin, whereas it was possible to select for CHO cells in which wild-type αIIbβ3 and αIIbP145Aβ3 were expressed at more comparable levels. This suggests that the quality-control function, at least with regard to abnormally folded human αIIbβ3, is more rigorous for the former cells than for the latter.
Because αIIbP145Aβ3 was present at reduced levels on the platelet surface of the affected Mennonite kindred, one might expect the platelets of these patients to bind comparable amounts of ligand. However, there was negligible binding of the αIIbβ3 ligand mimetic monoclonal antibody PAC1 to phorbol myristate acetate–stimulated GW and LW platelets. In addition, when αIIbP145Aβ3 receptors were stably expressed in CHO cells, the cells were unable to adhere to immobilized fibrinogen or bind soluble fibrinogen. Taken together, these observations suggest that besides influencing overall αIIbβ3 folding, P145is either part of, or regulates the conformation of, its ligand-binding domain.
The portion of αIIb that interacts with ligands has been localized to the amino-terminal third of the molecule,46,47 but the specific residues that define its ligand-binding domain are uncertain. Previous studies have suggested that amino acids 294 through 314 in the vicinity of the putative second calcium-binding loop interact with the carboxyl terminus of the fibrinogen γ chain,46 although recent studies of mutations involving amino acids 183, 184, 189, 190, 191, 193, and 224 also suggest that these amino acids interact with αIIbβ3 ligands.6,11,12A mutation involving amino acid 183 (L183P) is noteworthy because it occurred in a thrombasthenic patient whose platelets expressed ≈12% of the normal amount of αIIbβ3 on their surface.6 Moreover, when the mutant was coexpressed with β3 in CHO cells, the level of αIIbβ3 expression was ≈60% of normal, but the cells were unable to bind PAC1 or adhere to immobilized fibrinogen.
Although mutation of P145 impaired ligand binding to αIIbβ3, the mutant integrin αIIbP145A retained the ability to generate the outside-in signals required for PF4 secretion when GW platelets were exposed to the LSA peptide. LSA was designed to bind to αIIb residues 315 through 321 and following binding to αIIb on platelets, it mimics the effects of strong platelet agonists by inducing platelet aggregation and secretion.34 40 Thus, these data suggest that although αIIbP145Aβ3 is unable to interact with fibrinogen, presumably owing to disruption of its ligand-binding domain, the domain that binds LSA, presumably the fibrinogen γ chain cross-linking site in αIIb, is intact and able to undergo the conformational change responsible for αIIbβ3-mediated outside-in signaling.
Amino acid P145 is located immediately proximal to an invariant α-subunit cysteine residue (αIIbC146) and to a small loop formed by a disulfide bond between cysteine residues 146 and 169 that is present in all integrin α-subunits that do not contain an inserted domain (“I-domain”). (For review, see reference 48.) A homologous proline is also present in rat αIIb and in α2, α4, α5, αv, and α9, implying that a proline at this position is important for the structural stability and/or function of these subunits. Proline contains a pyrrolidine ring that limits the number of its accessible conformations. Thus, it is possible that an absence of flexibility is required to establish the correct conformation of this region of αIIb, perhaps by directing the formation of the disulfide bond between C146 and C.169
Molecular modeling of the amino-terminal, ligand-binding region of integrin α-subunits predicts that they are folded into a 7-bladed β-propeller configuration,13 although there are as yet no definitive data to support the model.49-51 The β-propeller model is shown in Figure 6, with the locations of P145 and other αIIb point mutations that disrupt ligand binding to αIIbβ3 superimposed. It is noteworthy that although these mutations span 80 amino acid residues in the linear αIIb sequence, they are juxtaposed in the folded model on the upper surface of a single quadrant of the propeller, suggesting that this region of αIIb constitutes a portion of the ligand-binding site on αIIbβ3. Moreover, because β3 residues 1-243 appear to be sufficient to form a heterodimer with αIIb and contain at least a portion of its ligand-binding site,52 it is conceivable that the region of αIIb encompassing P145 is the region that binds to β3. We and others have also described a number of mutations in the amino-terminus of αIIb that produce Type I thrombasthenia owing to the intracellular retention of misfolded αIIbβ3 heterodimers.14,26,42,43However, when the location of these mutations are projected on the β-propeller model, they are clustered on its under surface, a region proposed as being involved in ion binding, and away from the putative surface associated with ligand binding.13
Location of the P145 in the β-propeller model of the amino-terminus of an IIb -chain subunit.
This figure was adapted from that of Springer13 and shows views of the αIIb amino-terminus looking down from the top (A) and laterally (B). In the model, P145, indicated as the blue circle numbered 1, is located at the transition between the W2 loop and the W2 blade. A disulfide bond between C146 and C169 is shown in red. Seven other mutations that affect ligand binding to αIIb are shown as purple (#2-7) and pink (#8) circles. The naturally occurring mutation αIIbL183P (#2) produces a phenotype similar to αIIbP145A.6The αIIbL183P and 5 of the other mutations (G184A [#3], Y189A [#4], Y190A [#5], F191A [#6], and G193A [#7]) are present in the second surface loop of W311 and the seventh mutation (D224V [#8]) is found on the next loop at the interface between W3 and W4.12 As best appreciated from the lateral view shown in (B), these mutations lie on the upper and outer side of the β-propeller. The location of mutations that completely prevent αIIbβ3 expression on the platelet surface14,26,44 45 are indicated as green squares in the left side of the figure, and can be seen to lie at the bottom and/or opposite side of the propeller.
Location of the P145 in the β-propeller model of the amino-terminus of an IIb -chain subunit.
This figure was adapted from that of Springer13 and shows views of the αIIb amino-terminus looking down from the top (A) and laterally (B). In the model, P145, indicated as the blue circle numbered 1, is located at the transition between the W2 loop and the W2 blade. A disulfide bond between C146 and C169 is shown in red. Seven other mutations that affect ligand binding to αIIb are shown as purple (#2-7) and pink (#8) circles. The naturally occurring mutation αIIbL183P (#2) produces a phenotype similar to αIIbP145A.6The αIIbL183P and 5 of the other mutations (G184A [#3], Y189A [#4], Y190A [#5], F191A [#6], and G193A [#7]) are present in the second surface loop of W311 and the seventh mutation (D224V [#8]) is found on the next loop at the interface between W3 and W4.12 As best appreciated from the lateral view shown in (B), these mutations lie on the upper and outer side of the β-propeller. The location of mutations that completely prevent αIIbβ3 expression on the platelet surface14,26,44 45 are indicated as green squares in the left side of the figure, and can be seen to lie at the bottom and/or opposite side of the propeller.
In summary, we have identified mutations of αIIbP145 in 3 separate Mennonite, Dutch, and Chinese families that reduce αIIbβ3 expression on the platelet surface. The α IIbP145Amutation was also shown to substantially impair the ability of αIIbβ3 to interact with ligands. These studies not only indicate that the presence of proline at position 145 is required for proper αIIb folding; they also suggest that the integrity of the region of αIIb encompassing P145 is required for fibrinogen binding to αIIbβ3.
Acknowledgments
We thank Dr Y. Ikeda at Keio University, Tokyo, for providing the LIBS monoclonal antibody PT25-2. We thank Lesley Scudder and Jihong Li for their expert technical assistance.
Supported in part by a grant from the National Institutes of Health HL40387 (JSB, MP), HL19278 (BSC), and HL56369 (TKG), a grant from the Schulman Foundation (MP) and Plummer Family (MP), grant 3152 from The Council for Tobacco Research-USA, Inc (MP), and grant 9750841A (DLF) from the American Heart Association Heritage Affiliate Inc.
Reprints:Mortimer Poncz, The Children's Hospital of Philadelphia, 34th Street and Civic Center Boulevard, Philadelphia, PA 19104.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 6. Location of the P145 in the β-propeller model of the amino-terminus of an IIb -chain subunit. / This figure was adapted from that of Springer13 and shows views of the αIIb amino-terminus looking down from the top (A) and laterally (B). In the model, P145, indicated as the blue circle numbered 1, is located at the transition between the W2 loop and the W2 blade. A disulfide bond between C146 and C169 is shown in red. Seven other mutations that affect ligand binding to αIIb are shown as purple (#2-7) and pink (#8) circles. The naturally occurring mutation αIIbL183P (#2) produces a phenotype similar to αIIbP145A.6The αIIbL183P and 5 of the other mutations (G184A [#3], Y189A [#4], Y190A [#5], F191A [#6], and G193A [#7]) are present in the second surface loop of W311 and the seventh mutation (D224V [#8]) is found on the next loop at the interface between W3 and W4.12 As best appreciated from the lateral view shown in (B), these mutations lie on the upper and outer side of the β-propeller. The location of mutations that completely prevent αIIbβ3 expression on the platelet surface14264445 are indicated as green squares in the left side of the figure, and can be seen to lie at the bottom and/or opposite side of the propeller.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/1/10.1182_blood.v95.1.180/5/m_bloo0011606z.jpeg?Expires=1769107240&Signature=DIF8De2l2zbDq-qxWzyhdga0rNLIzhMp4ivonN0nMm9hhj-S0UQaA5oX~YJS00m5qI3x2qIp0Zi0R7J5jzDYa-D4-lTJYf~TIQ3b8Wk5WL2uaSP9MAwFf-PhVG-FmIpBvJwztBeGELSIKsGmyYNB8uwtucU8LpwPsuF3XaFavTD2mLKoU9cHuhwtAFnJJI~6nFmfIhZEtD-CvGFsFPo~UpUrNLPShrnnAlDEqCUfnSgVLw2Pc6HiBMeyQqNQJt6hn~yrX~QxqKPzoFAYBEKI-NaV~e6GNilY9HbTHChq7mebb8NWUwX4H5Ep8fZ4jVM-n9m-lGpllyBC7hvP8SwyQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal