Abstract
We have shown that stromal O-sulfated heparan sulfate glycosaminoglycans (O-S-GAGs) regulate primitive human hematopoietic progenitor cell (HPC) growth and differentiation by colocalizing heparin-binding cytokines and matrix proteins with HPC in stem cell “niches” in the marrow microenvironment. We now show that long-term culture-initiating cells (LTC-IC) are maintained for 5 weeks in the absence of stroma when O-S-GAGs are added to IL-3 and either MIP-1 or PF4 (LTC-IC maintenance without GAGs, 32 ± 2%; with GAGs, 95 ± 7%; P < .001). When cultured with 5 additional cytokines, O-S-GAGs, IL-3, and MIP-1, LTC-IC expanded 2- to 4-fold at 2 weeks, and 92 ± 8% LTC-IC were maintained at 5 weeks. Similar results were seen when PF4 replaced MIP-1. Although O-S-GAG omission did not affect 2-week expansion, only 20% LTC-IC were maintained for 5 weeks. When O-S-heparin was replaced by completely desulfated-, N-sulfated (O-desulfated), or unmodified heparins, LTC-IC maintenance at week 5 was not better than with cytokines alone. Unmodified- and O-S-heparin, but not desulfated- or N-sulfated heparin, bound to MIP-1, IL-3, PF4, VEGF, thrombospondin, and fibronectin. However, the affinity of heparin for thrombospondin and PF4, and the association and dissociation rates of heparin for PF4, were higher than those of O-S-heparin. We conclude that (i) although cytokines may suffice to induce early expansion, adult human LTC-IC maintenance for longer than 1 month requires O-S-GAGs, and (ii) HPC support may depend not only on the ability of GAGs to bind proteins, but also on optimal affinity and kinetics of interactions that affect presentation of proteins in a biologically active manner to progenitors. (Blood. 2000;95:147-155)
Stem cells may exist in “stem cell niches” in the marrow microenvironment in vivo, where stromal cells, specific extracellular matrix (ECM) components, and cytokines bound to stromal cell surfaces or to ECM macromolecules provide the appropriate balance of signals that preserves the stem cell pool while permitting controlled proliferation and differentiation.1-4 A number of cytokines, chemokines, and matrix components considered to be essential for the localization, conservation, and proliferation of primitive hematopoietic progenitors cells (HPC) are heparin-binding proteins, including platelet factor 4 (PF4), basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), interleukin-3 (IL-3), vascular endothelial growth factor (VEGF), macrophage inflammatory protein-1α (MIP-1α), thrombospondin (TSP), and fibronectin (FN).5-20 Specific sulfation patterns of heparan sulfate (HS) are required for binding and modulation of the activity of cytokines that have activity on hematopoietic progenitors.6,8,21,22 Additionally, the ability of HS to modulate cytokine activity on target cells may depend on the affinity and kinetics of association (“on” rate) between cytokines and HS.23 O-sulfation of glycosaminoglycans (GAGs) is required for binding of several growth factors, including PF4 and platelet-derived growth factor.24,25 We have recently shown that highly O-sulfated stromal cell-derived HS GAGs help to mediate the formation of such “stem cell niches” by colocalizing specific heparin-binding proteins with HPC, thereby orchestrating the controlled growth and differentiation of stem cells.26
Of the numerous cytokines that modulate human HPC survival and proliferation in vitro, the heparin-binding cytokine IL-3 and chemokines MIP-1α or PF4 are particularly critical. We have recently demonstrated that the human long-term culture-initiating cell (LTC-IC)-maintaining capability of stromal supernatant27,28is improved by the addition of nanogram concentrations of IL-3 + MIP-1α.29 In these stromal supernatant cultures, MIP-1α can be replaced by PF4,30 a GAG-binding chemokine that has effects similar to MIP-1α on hematopoietic progenitors.31Moreover, IL-3 and MIP-1α are required for the ex vivo survival of human HPC with myeloid and lymphoid differentiation potential.29,32,33 HS proteoglycans may be essential for the localization of both MIP-1α and IL-3 within the marrow stromal microenvironment9,26,34-36 and may interact with IL-3 to augment adhesion and colocalization of HPC within the microenvironment.37
Thrombospondin is a matrix protein present in serum, which is synthesized by both hematopoietic and stromal cells, which directly mediates the adhesion of HPC.38 TSP in soluble or immobilized form also modulates cytokine activity, synergizing with stem cell factor (SCF) to stimulate HPC adhesion and proliferation but inhibiting IL-3–mediated HPC proliferation.5 TSP has a high affinity for binding to heparin and interacts with stromal HS proteoglycans to increase HPC adhesion.37 HS GAGs also influence the growth-modulatory activity of TSP, because low molecular weight heparin neutralizes TSP-mediated inhibition of multilineage colony-forming units-granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM) and CFU-megakaryocyte (CFU-MK) growth that is induced by cytokines including IL-3, SCF, granulocyte-macrophage colony-stimulating factor (GM-CSF), bFGF, and thrombopoietin.39
We hypothesized that in the absence of stroma, specifically sulfated HS GAGs are required for IL-3 + chemokine (MIP-1α or PF4)-mediated LTC-IC maintenance and that this biological activity is dependent on optimal binding characteristics of the GAGs for early-acting chemokines (eg, PF4) and matrix components (eg, TSP). In the present studies, we have examined the ability of purified, differentially sulfated heparin GAGs to support IL-3 + chemokine-mediated LTC-IC maintenance and the binding characteristics of these GAGs for cytokines and matrix components essential for HPC localization and survival.
Methods
Cell separation
Bone marrow was aspirated in preservative-free heparin from the posterior iliac crest of healthy young volunteers after informed consent. Bone marrow mononuclear cells were separated by Ficoll-Hypaque centrifugation (s.g., 1.077), and CD34+ enriched cells were obtained using Ceprate® LC CD34 selection columns (CellPro Inc, Bothell, WA). CD34+/HLA-DR−cells were obtained by flow cytometry, using a FACStar Plus® flow cytometry system equipped with a Consort 32 computer as previously described.26 34
Stromal feeders and conditioned media
Human primary bone marrow stromal feeders were established from human bone marrow mononuclear cells as previously described, irradiated at 1250 rad when confluent, and maintained in long-term bone marrow culture (LTBMC) medium.26 34 Supernatant from irradiated stromal cultures was harvested 2 to 3 days after a half-medium change, centrifuged to remove cell debris, and frozen at −70°C until use.
Cytokines
Recombinant human cytokines used in long-term cultures included 500 pg/mL granulocyte colony-stimulating factor (G-CSF) (Neupogen®; Amgen, Thousand Oaks, CA), 50 pg/mL GM-CSF (Immunex Corp, Seattle, WA), 200 pg/mL SCF (a kind gift from Amgen), 50 pg/mL leukemia inhibitory factor (LIF; R & D Systems Inc, Minneapolis, MN), 200 pg/mL MIP-1α (R & D Systems) and 2 ng/mL IL-6 (a kind gift from Dr G. Wong, Genetics Institute).
As indicated, medium in some long-term cultures was supplemented with G-CSF, GM-CSF, SCF, LIF, and IL-6 in the same concentrations as described above, along with 5 ng/mL IL-3 (a kind gift from Dr G. Wong) and either 10 ng/mL MIP-1α or 200 ng/mL PF4 (purified from human platelets).
Glycosaminoglycans
HS, chondroitin sulfate, and heparin were obtained from Sigma (St. Louis, MO). The differentially sulfated heparins used in this study, derived from the same parent unmodified heparin molecule by N-desulfation and N-reacetylation (O-sulfated heparin), N- and O-desulfation and N-reacetylation (completely desulfated heparin), or N- and O-desulfation and N-resulfation, were obtained from Seikagaku America (Falmouth, MA). Disaccharide compositional analysis of the unmodified heparin and differentially sulfated heparins (Seikagaku America, personal communication) is shown in the Table. The completely desulfated heparin was largely composed of desulfated disaccharides. The O-sulfated heparin was largely composed of O-disulfated disaccharides {-UA(2-OSO3)-GlcNAc(6-OSO3)-} and 6-O-sulfated disaccharides {-UA-GlcNAc(6-OSO3)-} (where the UA was mainly iduronic acid) and contained only trace amounts (<1%) of the trisulfated disaccharides {-IdoA (2-OSO3)-GlcNS (6-OSO3)-}, whereas unmodified heparin was mainly composed of trisulfated disaccharides, with a smaller proportion of O-disulfated units. The N-sulfated heparin was depleted of O-sulfated units (not shown). For several growth factors, the regions of transition between highly sulfated GlcNS-containing blocks and unsulfated GlcNAc-containing blocks are believed to be important for binding and biological activity of HS. Because of the specificity of the sulfotransferase enzymes, the terminal GlcNAc (adjacent to GlcNS) in such blocks may be 6-O-sulfated in such regions.40 41 Disaccharides at such transition points thus are 6-sulfated, with or without 2-O sulfation of the hexuronic acid: Most disaccharides in O-sulfated heparin are identical to such units and more closely resemble sulfation patterns of HS rather than heparin.
Disaccharide composition of unmodified and desulfated heparins
| Sulfated Disaccharide . | Positions of Sulfation on Disaccharides . | Percent of Total Disaccharides . | |
|---|---|---|---|
| O-sulfated Heparin . | Unmodified Heparin . | ||
| UA-GlcNAc- | None | 6.8 | 3.8 |
| -UA-GlcNSO3- | N | 1.1 | 2.2 |
| -UA-GlcNAc(6-OSO3)- | 6 | 10.8 | 3.1 |
| -UA(2-OSO3)-GlcNAc- | 2 | 12.4 | 1.9 |
| -UA(2-OSO3)-GlcNSO3- | 2, N | 0.8 | 9.1 |
| -UA(2-OSO3)-GlcNAc(6-OSO3)- | 2, 6 | 67.3 | 8.1 |
| -UA(2-OSO3)-GlcNSO3(6-OSO3)-† | 2, N, 6 | 0.8 | 71.7 |
| Sulfated Disaccharide . | Positions of Sulfation on Disaccharides . | Percent of Total Disaccharides . | |
|---|---|---|---|
| O-sulfated Heparin . | Unmodified Heparin . | ||
| UA-GlcNAc- | None | 6.8 | 3.8 |
| -UA-GlcNSO3- | N | 1.1 | 2.2 |
| -UA-GlcNAc(6-OSO3)- | 6 | 10.8 | 3.1 |
| -UA(2-OSO3)-GlcNAc- | 2 | 12.4 | 1.9 |
| -UA(2-OSO3)-GlcNSO3- | 2, N | 0.8 | 9.1 |
| -UA(2-OSO3)-GlcNAc(6-OSO3)- | 2, 6 | 67.3 | 8.1 |
| -UA(2-OSO3)-GlcNSO3(6-OSO3)-† | 2, N, 6 | 0.8 | 71.7 |
Derived from data from Seikagaku America, Inc (personal communication).
Highly (“default”) sulfated disaccharide; UA indicates hexuronic acid (iduronic acid or glucuronic acid); GlcN, glucosamine.
The GAGs were added to long-term cultures at a final concentration of 5 μg/mL; we have previously shown this concentration to be in the optimal range for LTC-IC maintenance.26
Preparation of synthetic proteoglycan-like molecules
GAGs and either ovalbumin or human albumin were dissolved (2 mg each) in 1 mL distilled water, and 20 mg of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) in 50 μL water was added (all chemicals from Sigma). The mixture was shaken at 4°C for 12 hours to couple GAGs to ovalbumin or albumin via the carboxyl groups by amide bonds.42 Low molecular weight by-products were removed by dialysis in 12 000-14 000 molecular weight cut-off (MWCO) dialysis tubing against phosphate-buffered saline (PBS). For use in cultures, the protein-coupled GAGs (final concentration 5 μg/mL) were dissolved in fresh LTBMC medium and sterile-filtered.
Long-term cultures
LTBMC medium.
The medium consisted of Iscove's modified Dulbecco's medium with 12.5% fetal calf serum, 12.5% horse serum, 2 mM L-glutamine, 1000 U/mL penicillin, 100 U/mL streptomycin, and 10−6mol/L hydrocortisone.43
Conditioned medium cultures.
A total of 10 × 103 to 14 × 103DR− cells were suspended in a transwell insert with a microporous 0.4-μm membrane placed in stroma-free wells of 6- or 24-well plates. Media in the lower well were replaced 5 times weekly with 3 mL (for 6-well plates) or 0.8 mL (for 24-well plates) fresh unsupplemented LTBMC medium, LTBMC medium supplemented with cytokines alone or in combination with cell line–derived or artificially synthesized PGs or GAG chains, or conditioned medium from M2-10B4 or human stromal feeders. After 2 or 5 weeks of culture, progeny of DR− cells were recovered from the transwells by vigorous washing and counted on a hemocytometer. Cells were plated in methylcellulose cultures to enumerate colony forming cells (CFC) and in limiting dilution analysis to determine the absolute number of LTC-IC present, as described.28 44 All cultures were maintained at 37°C in humidified 5% CO2 atmosphere.
Short-term methylcellulose cultures.
Day 0 DR− cells and the progeny of DR− cells recovered at 2 or 5 weeks from long-term cultures were plated in methylcellulose-containing medium supplemented with 30% fetal calf serum (FCS), 3 IU/mL erythropoietin, and 5 ng/mL IL-3 and CFC enumerated at day 14. All cultures were maintained at 37°C in humidified 5% CO2 atmosphere.
Enumeration of the absolute number of LTC-IC by limited dilution analysis.
Day 0 DR− cells (22 replicates per concentration) or progeny of DR− cells recovered from long-term cultures were plated at limiting dilutions onto irradiated M2-10B4 feeders in 96-well plates, as described.26,28,34 Cultures were fed by weekly replacement of 75 μL fresh LTBMC medium. After 5 weeks, all the medium was removed and the wells overlaid with methylcellulose-containing medium supplemented with 30% FCS, 3 IU/mL erythropoietin, and 5 ng/mL IL-3. All cultures were maintained at 37°C in humidified 5% CO2 atmosphere. Wells were scored for the presence or absence of secondary CFC at day 14. The absolute number of LTC-IC was calculated as the reciprocal of the concentration of test cells that yields 37% negative wells, using Poisson statistics and the weighted mean method.28 44
Iodination of differentially sulfated heparins
Heparins were coupled with tyramine14,15 and were iodinated using carrier-free Na2125I (Amersham, Arlington Heights, IL) and the Iodo-gen iodination reagent (Pierce, Rockford, IL).14 Free 125I was removed from the labeled HS by separation on a Sephadex G-25 column, followed by exhaustive dialysis. The specific activity was 5 to 70 μCi/μg for the iodinated GAGs.
Affinity coelectrophoresis (ACE)
Binding of iodinated heparins to cytokines including IL-3 (R & D Systems), MIP-1α, bFGF (R & D Systems), and VEGF (isoforms VEGF165 and VEGF121; kind gifts from Dr S. Ramakrishnan, University of Minnesota) and to ECM molecules including TSP (a kind gift from Dr Robert Hebbel, University of Minnesota) and FN (Sigma) was examined using ACE. ACE was performed as described.26 45 Briefly, a 1% agarose gel (low melting point Seaplaque; FMC, Rockland, MD) in 50 mM sodium MOPSO (Sigma), pH 7.0; 125 mM NaCl; 0.5% CHAPS buffer was cast with a strip well (for HS) and a perpendicular 8-lane comb (for proteins). Protein samples (TSP, bFGF or IL-3) prepared at twice the desired concentration in MOPSO/NaCl/CHAPS electrophoresis buffer were mixed with an equal volume of 2% agarose and allowed to gel in appropriate wells created by the 8-lane comb. The heparin preparation in MOPSO/NaCl/CHAPS electrophoresis buffer containing bromophenol blue and sucrose was added to the strip well. Electrophoresis was performed in a Hoefer electrophoresis apparatus in MOPSO/NaCl running buffer prepared without CHAPS at 330 mA, 45 to 60 V for 2.5 to 2.75 hours at 20°C to 25°C. Gels were air-dried and autoradiographed at −80°C. For VEGF, FN, and MIP-1α, the gels were cast in 50 mM MOPSO, pH 7.0; 50 mM sodium acetate (NaAc). Protein samples and the heparins were also prepared in the same buffer. Electrophoresis was performed as above, for 2 to 2.5 hours.
Determination of half-maximal binding (EC50).
125I-labeled heparin or O-sulfated heparin was electrophoresed through varying concentrations of TSP (1-100 nM) in the affinity coelectrophoresis system. The retardation coefficient45 was calculated as R = (M0 − M)/M0, where M0 was the mobility of free GAGs (taken as mobility in presence of ovalbumin), and M was the mobility of GAGs through the TSP-containing lanes. Mobility was measured as the distance from the loading well at the top of the gel to the upper end (most retarded) of the GAG smear. Because retardation is a function of the binding affinity of the GAG to TSP, a plot of retardation (binding) versus concentration of TSP was used to calculate half-maximal binding concentration, as a measure of the affinity of the 2 GAGs for TSP.
Surface plasmon resonance.
The binding of GAGs to PF4 was examined on a BIAcore®biosensor equipment (Pharmacia, Uppsala, Sweden) using surface plasmon resonance, which is highly sensitive and monitors real-time binding of an analyte (GAG) to a ligand (PF4) immobilized on a sensor chip.46,47 This is measured in resonance units (RU), which correlates with the amount of analyte bound (1 RU = 1 pg/mm2). Binding of GAGs in solution to immobilized PF4 was examined as described.26 The KD was calculated using BIAevaluation 2.1 software (Pharmacia Biosensor, Uppsala, Sweden) from the average of ka (association rate, “run-on” phase) and kd (dissociation rate, “wash-out” phase) kinetics: KD = av. kd/ka (ks).
Statistics.
Results of data are reported as the mean ± SEM. Levels of significance were determined by the 2-sided Student's t test.
Results
HS in combination with IL-3 and MIP-1 supports long-term maintenance of LTC-IC
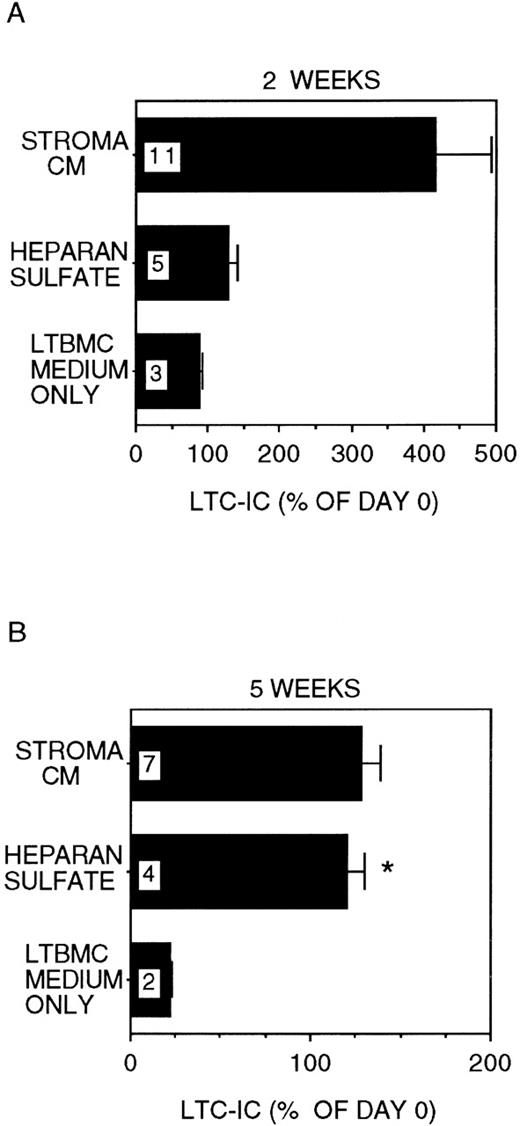
In stroma-free cultures supplemented with IL-3 + MIP-1α, only 22 ± 1% of LTC-IC were recovered after 5 weeks (P < .001 vs day 0 LTC-IC frequency; Figure1), even though 89 ± 3% of day-0 LTC-IC were maintained for 2 weeks. In cultures where 5 μg/mL HS was added together with IL-3 + MIP-1α, 121 ± 10% of input numbers of LTC-IC were maintained for up to 5 weeks (P = .002 vs cultures with IL-3 + MIP-1α alone). A lower concentration of HS (1 μg/mL) was less effective (LTC-IC maintenance 73 ± 5% at 5 weeks; P = .001 vs 5 μg/mL HS). High concentrations of GAGs (20 μg/mL HS) inhibited cell proliferation and LTC-IC maintenance (data not shown). Further, maintenance of LTC-IC at week 5 in HS + IL-3 + MIP-1α cultures was similar to that seen in cultures supplemented with stroma-conditioned medium + IL-3 + MIP-1α (134 ± 12%). LTC-IC expanded 4 ± 0.8-fold at 2 weeks in stroma-conditioned medium-supplemented cultures, whereas LTC-IC expansion in HS + IL-3 + MIP-1α cultures was only 1.3 ± 0.1-fold at week 2. These results suggest that, in the presence of HS, long-term in vitro maintenance of LTC-IC can be obtained by addition of a single growth-promoting cytokine (IL-3) and a single growth-inhibitory chemokine (MIP-1α). LTC-IC expansion, in contrast, may require the presence of additional cytokines and/or factors present in stroma-conditioned medium.
LTC-IC maintenance in the presence of HS with IL-3 and MIP-1.
A total of 10 000 to 20 000 DR− cells were plated in 0.4-μm transwell inserts in 6-well tissue culture clusters. Medium in the lower chambers of the wells was replaced daily by either stroma-conditioned medium (Stroma CM) or long-term bone marrow culture (LTBMC) medium supplemented with or without 5 μg/mL HS. A total of 5 ng/mL IL-3 and 10 ng/mL MIP-1α were added to all cultures, including Stroma CM. Cultures were harvested after 2 weeks (A) or 5 weeks (B) and cells replated at limiting dilutions for estimation of LTC-IC frequency, as described in Methods. Numbers within the bars indicate the number of experiments. Comparison between LTBMC medium only and other conditions: *P < .005.
LTC-IC maintenance in the presence of HS with IL-3 and MIP-1.
A total of 10 000 to 20 000 DR− cells were plated in 0.4-μm transwell inserts in 6-well tissue culture clusters. Medium in the lower chambers of the wells was replaced daily by either stroma-conditioned medium (Stroma CM) or long-term bone marrow culture (LTBMC) medium supplemented with or without 5 μg/mL HS. A total of 5 ng/mL IL-3 and 10 ng/mL MIP-1α were added to all cultures, including Stroma CM. Cultures were harvested after 2 weeks (A) or 5 weeks (B) and cells replated at limiting dilutions for estimation of LTC-IC frequency, as described in Methods. Numbers within the bars indicate the number of experiments. Comparison between LTBMC medium only and other conditions: *P < .005.
Cytokines alone induce short-term expansion of LTC-IC, but O-sulfated GAGs are required for long-term LTC-IC maintenance
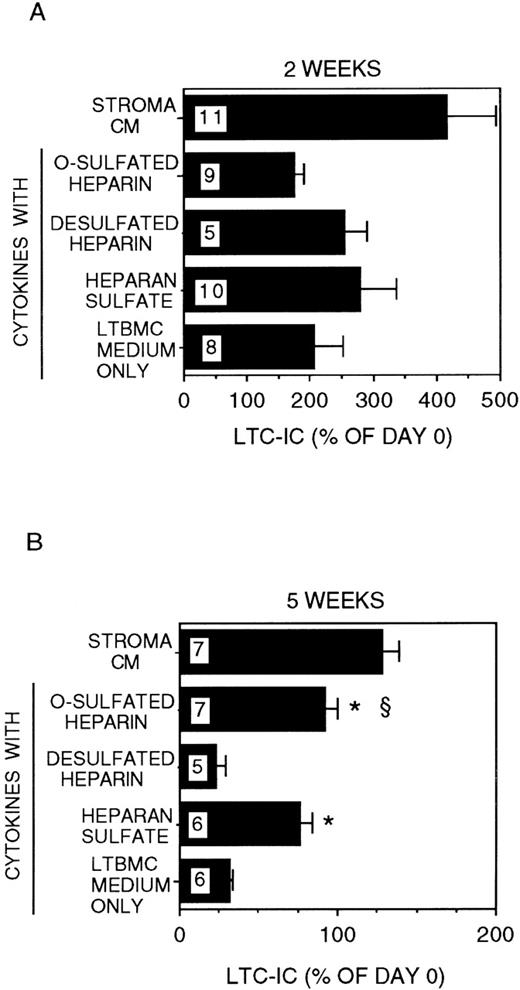
We have demonstrated that stroma-conditioned medium can be replaced by a combination of low-dose cytokines (CK) + HS.34 We therefore cultured DR− cells for 2 to 5 weeks in stroma-free cultures supplemented with IL-3 (5 ng/mL) and MIP-1α (10 ng/mL) with or without a combination of CK in the concentrations detected in stroma-conditioned medium and with or without bovine kidney HS. The combination of IL-3 + MIP-1α + CK but no HS induced a 2 ± 0.5-fold expansion of LTC-IC at 2 weeks (Figure2). However, the majority of LTC-IC was lost at 5 weeks (LTC-IC recovery 32 ± 2%). The addition of HS to the combination of IL-3 + MIP-1α + CK resulted in a 2.8 ± 0.6-fold expansion of LTC-IC at 2 weeks. In contrast to IL-3 + MIP-1α + CK, cultures supplemented with IL-3 + MIP-1α + CK + HS resulted in long-term maintenance of a significantly greater proportion of LTC-IC (76 ± 7%) at 5 weeks (P < .001 vs IL-3 + MIP-1α + CK cultures).
Modulation of LTC-IC maintenance by differentially sulfated GAGs in the presence of multiple growth-promoting cytokines with IL-3 and MIP-1.
A total of 10 000 to 20 000 DR− cells were plated in 0.4-μm transwell inserts in 6-well tissue culture clusters. Medium in the lower chambers of the wells was replaced daily by either stroma-conditioned medium (Stroma CM) or by LTBMC medium supplemented with or without a combination of cytokines (500 pg/mL G-CSF, 50 pg/mL GM-CSF, 200 pg/mL SCF, 50 pg/mL LIF, and 2 ng/nl IL-6) and with or without 5 μg/mL each of GAGs. A total of 5 ng/mL IL-3 and 10 ng/mL MIP-1α were added to all cultures, including Stroma CM. Cultures were harvested after 2 weeks (A) or 5 weeks (B). The equivalent of 500 to 1000 DR− cells plated at day 0 were replated after harvesting in methylcellulose cultures for estimation of CFC generation, and the remaining cells were replated at limiting dilutions for estimation of LTC-IC frequency, as described in Methods. Numbers within the bars indicate the number of experiments. Comparison between cytokines only and other conditions: *P < .002; comparison between desulfated heparin and other conditions: §P < .001.
Modulation of LTC-IC maintenance by differentially sulfated GAGs in the presence of multiple growth-promoting cytokines with IL-3 and MIP-1.
A total of 10 000 to 20 000 DR− cells were plated in 0.4-μm transwell inserts in 6-well tissue culture clusters. Medium in the lower chambers of the wells was replaced daily by either stroma-conditioned medium (Stroma CM) or by LTBMC medium supplemented with or without a combination of cytokines (500 pg/mL G-CSF, 50 pg/mL GM-CSF, 200 pg/mL SCF, 50 pg/mL LIF, and 2 ng/nl IL-6) and with or without 5 μg/mL each of GAGs. A total of 5 ng/mL IL-3 and 10 ng/mL MIP-1α were added to all cultures, including Stroma CM. Cultures were harvested after 2 weeks (A) or 5 weeks (B). The equivalent of 500 to 1000 DR− cells plated at day 0 were replated after harvesting in methylcellulose cultures for estimation of CFC generation, and the remaining cells were replated at limiting dilutions for estimation of LTC-IC frequency, as described in Methods. Numbers within the bars indicate the number of experiments. Comparison between cytokines only and other conditions: *P < .002; comparison between desulfated heparin and other conditions: §P < .001.
We have recently shown that chemically differentially sulfated heparins have different LTC-IC supportive capabilities, such that LTC-IC maintenance is supported by O-sulfated heparin in combination with low concentrations of CK but not by desulfated-, N-sulfated- or unmodified-heparin.26 We therefore examined the effect of completely desulfated and O-sulfated heparins on IL-3 + MIP-1α–mediated LTC-IC maintenance (Figure 2). Addition of O-sulfated heparin to IL-3 + MIP-1α + CK resulted in 1.7 ± 0.2-fold LTC-IC expansion at week 2, which was lower than with HS. However, 95 ± 7% of LTC-IC were maintained at week 5. When desulfated heparin was combined with IL-3 + MIP-1α + CK, 2.5 ± 0.4-fold LTC-IC expansion was seen at week 2, but LTC-IC recovery at week 5 (23 ± 6%) was no better than with cytokines alone.
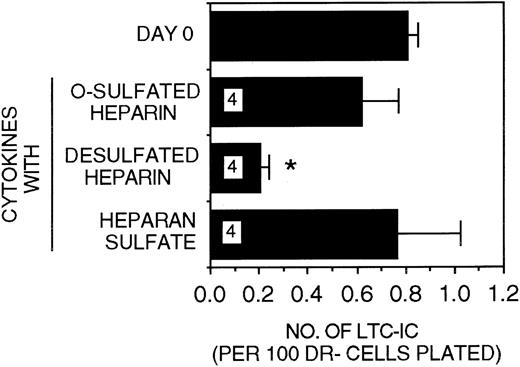
When PF4 (200 ng/mL) was substituted for MIP-1α (10 ng/mL) in stroma-free cultures, LTC-IC maintenance was similar to that seen in the presence of MIP-1α (Figure 3). Most of the LTC-IC were maintained in cultures supplemented with IL-3 + PF4 + CK in the presence of either HS or O-sulfated heparin (LTC-IC supportive GAGs) but not in the presence of desulfated heparin (LTC-IC nonsupportive GAG). Thus, both the heparin-binding chemokines MIP-1α and PF4 require the sequences present in O-sulfated HS to support LTC-IC maintenance.
Efficacy of differentially sulfated GAGs to modulate LTC-IC maintenance in the presence of PF4.
A total of 10 000 to 20 000 DR− cells were plated in 0.4-μm transwell inserts in 6-well tissue culture clusters. Medium in the lower chambers of the wells was replaced daily by LTBMC medium supplemented with or without a combination of cytokines (500 pg/mL G-CSF, 50 pg/mL GM-CSF, 200 pg/mL SCF, 50 pg/mL LIF, 2 ng/mL IL-6, and 200 pg/mL MIP-1α) with 5 ng/mL IL-3 and 200 ng/mL PF4 and with or without 5 μg/mL each of GAGs. Cultures were harvested after 5 weeks and replated at limiting dilutions for estimation of LTC-IC frequency, as described in Methods. Numbers within the bars indicate the number of experiments. Comparison between day 0 and other conditions: *P < .002.
Efficacy of differentially sulfated GAGs to modulate LTC-IC maintenance in the presence of PF4.
A total of 10 000 to 20 000 DR− cells were plated in 0.4-μm transwell inserts in 6-well tissue culture clusters. Medium in the lower chambers of the wells was replaced daily by LTBMC medium supplemented with or without a combination of cytokines (500 pg/mL G-CSF, 50 pg/mL GM-CSF, 200 pg/mL SCF, 50 pg/mL LIF, 2 ng/mL IL-6, and 200 pg/mL MIP-1α) with 5 ng/mL IL-3 and 200 ng/mL PF4 and with or without 5 μg/mL each of GAGs. Cultures were harvested after 5 weeks and replated at limiting dilutions for estimation of LTC-IC frequency, as described in Methods. Numbers within the bars indicate the number of experiments. Comparison between day 0 and other conditions: *P < .002.
The generation of CFC from primitive progenitors is induced by cytokines and does not require GAGs
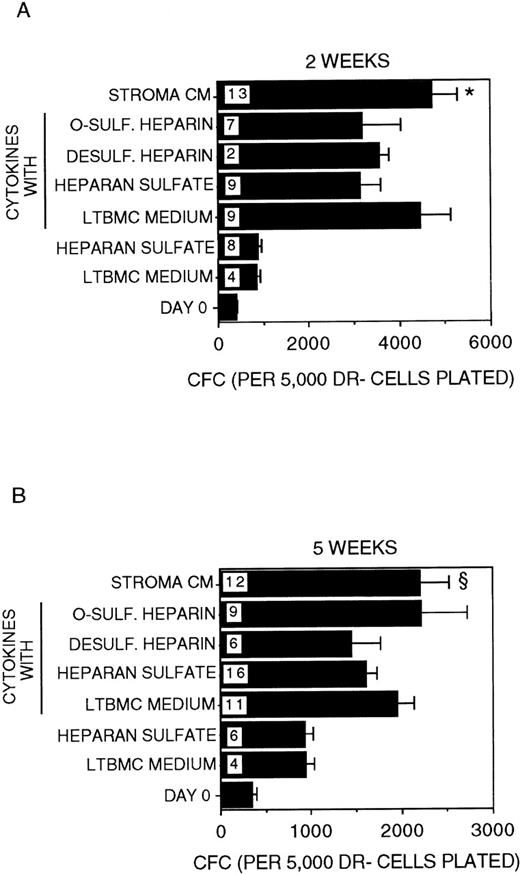
A 2-fold expansion in the numbers of CFC generated was sustained for 2 to 5 weeks in cultures supplemented with IL-3 + MIP-1α (Figure4). The addition of HS to IL-3 + MIP-1α did not affect CFC numbers. However, CFC generation was significantly higher in the presence of stroma-conditioned medium + IL-3 + MIP-1α at both 2 and 5 weeks (P < .001 and P = .02, respectively, vs HS + IL-3 + MIP-1α). This suggests that the increased CFC generation in the latter condition is due to the presence of low concentrations of cytokines present in stroma-conditioned medium. Indeed, when low-dose cytokines (known to be present in stroma-conditioned medium) were added to IL-3 + MIP-1α in stroma-free cultures, the generation of CFC at 2 to 5 weeks became comparable to that seen in the presence of stroma-conditioned medium + IL-3 + MIP-1α, although the size of the colonies generated in the presence of stroma-conditioned medium was significantly larger. This may be due to the presence in stroma-conditioned medium of additional cytokines, such as flt-3 ligand, IL-11, and thrombopoietin, which were not added to the stroma-free cultures. Overall, the presence of CK + IL-3 + MIP-1α induced a 10- to12-fold increase in CFC at 2 weeks and 6-fold increase in CFC at 5 weeks, compared to CFC present in the input population at day 0. CFC numbers were not affected by the further addition of HS, O-sulfated heparin, or desulfated heparin to CK + IL-3 + MIP-1α.
Generation of CFC.
Number of CFC in the starting DR− population at day 0 and after 2 weeks (A) or 5 weeks (B) in culture. All cultures were supplemented with 5 ng/mL IL-3 and 10 ng/mL MIP-1α. The indicated cultures were additionally supplemented with the low-dose cytokine combination. Numbers within the bars indicate the number of experiments. Comparison between HS and Stroma CM: *P < .001 (at 2 weeks); §P = .02 (at 5 weeks).
Generation of CFC.
Number of CFC in the starting DR− population at day 0 and after 2 weeks (A) or 5 weeks (B) in culture. All cultures were supplemented with 5 ng/mL IL-3 and 10 ng/mL MIP-1α. The indicated cultures were additionally supplemented with the low-dose cytokine combination. Numbers within the bars indicate the number of experiments. Comparison between HS and Stroma CM: *P < .001 (at 2 weeks); §P = .02 (at 5 weeks).
As for CFC generation, the addition of HS or O-sulfated heparin to IL-3 + MIP-1α + CK did not affect cell expansion (the total numbers of terminally differentiated mature cells present in culture) at 2 to 5 weeks (data not shown). Cell expansion in cultures supplemented with IL-3 + MIP-1α + CK was comparable to that seen in cultures supplemented with stroma-conditioned medium + IL-3 + MIP-1α (500- to 800-fold expansion at 5 weeks).
Cytokine-binding profiles of hematopoietically supportive GAGs
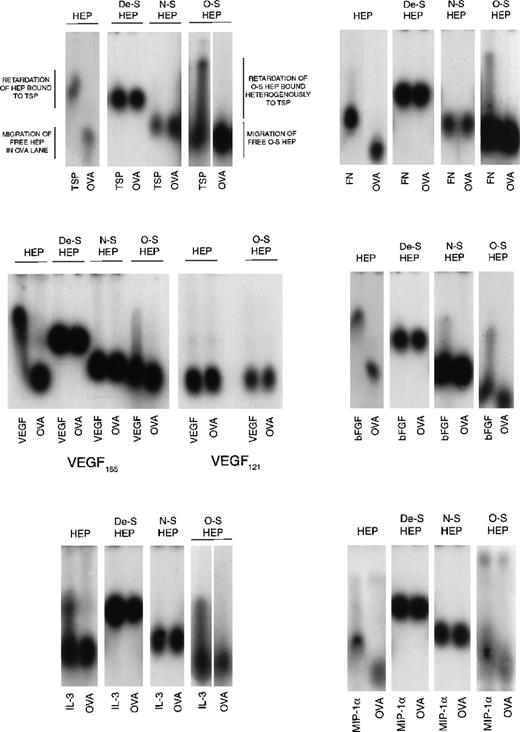
Because O-sulfated heparin but not the other modified heparins or unmodified heparin supported LTC-IC, we next examined the cytokine- and matrix component–binding capabilities of these heparins to examine if differences in this capability correlated with their ability to support LTC-IC maintenance. Desulfated heparin and N-sulfated heparin, which did not support LTC-IC maintenance, showed no binding to MIP-1α, IL-3, VEGF, FN, or TSP by ACE (Figure 5). N-sulfated heparin, but not desulfated heparin, bound bFGF to a small extent. O-sulfated heparin, which supports LTC-IC maintenance, bound to IL-3, bFGF, FN, TSP and, to a lesser extent, to VEGF and MIP-1α. Unmodified heparin, which possesses a high degree of both N- and O-sulfation, also bound to all these proteins. We have already shown,26 using surface plasmon resonance, that O-sulfated heparin and unmodified heparin, but not desulfated heparin or N-sulfated heparin, bind PF4.
Binding of modified heparins to proteins by affinity coelectrophoresis (ACE).
125I-labeled GAGs (HEP, unmodified heparin; De-S HEP, completely desulfated heparin; N-S HEP, N-sulfated heparin; O-S HEP, O-sulfated heparin) were electrophoresed through the proteins (cytokines and matrix components) cast in agarose gels. Results are shown for binding of the GAGs to 100 nM TSP, 25 nM bFGF, and 500 nM each of VEGF, FN, IL-3, and MIP-1α. Ovalbumin (500 nM) was used as a negative control protein. The NaCl electrophoresis buffer was used for TSP, bFGF, and IL-3, and the sodium acetate buffer was used for VEGF, FN, and MIP-1α. Migration of unbound GAGs that are not bound to proteins is dependent on both size and charge. The average size of all 4 GAGs was comparable (8 to 12 kd). Because the negative charge on De-S HEP is the least, its migration was slowest. N-S HEP has an intermediate charge and migrated faster than De-S HEP. O-S HEP and HEP, which are the most highly charged, migrated at comparable rates. Binding to proteins was seen as retardation of the relative rate of migration of the GAGs through the protein lanes, compared to its migration through ovalbumin.
Binding of modified heparins to proteins by affinity coelectrophoresis (ACE).
125I-labeled GAGs (HEP, unmodified heparin; De-S HEP, completely desulfated heparin; N-S HEP, N-sulfated heparin; O-S HEP, O-sulfated heparin) were electrophoresed through the proteins (cytokines and matrix components) cast in agarose gels. Results are shown for binding of the GAGs to 100 nM TSP, 25 nM bFGF, and 500 nM each of VEGF, FN, IL-3, and MIP-1α. Ovalbumin (500 nM) was used as a negative control protein. The NaCl electrophoresis buffer was used for TSP, bFGF, and IL-3, and the sodium acetate buffer was used for VEGF, FN, and MIP-1α. Migration of unbound GAGs that are not bound to proteins is dependent on both size and charge. The average size of all 4 GAGs was comparable (8 to 12 kd). Because the negative charge on De-S HEP is the least, its migration was slowest. N-S HEP has an intermediate charge and migrated faster than De-S HEP. O-S HEP and HEP, which are the most highly charged, migrated at comparable rates. Binding to proteins was seen as retardation of the relative rate of migration of the GAGs through the protein lanes, compared to its migration through ovalbumin.
However, the binding profile of O-sulfated heparin was different than that of heparin. At the concentrations examined, unmodified heparin bound homogeneously to TSP, bFGF, VEGF, and FN, whereas O-sulfated heparin bound heterogeneously to these proteins, resulting in a smear in the protein lanes. Binding of both heparin and O-sulfated heparin to IL-3 was seen as a smear. When labeled heparin was eluted from the upper and lower portions of the smear and reelectrophoresed into a separate IL-3–containing gel (see ref. 14 for method), labeled heparin from the upper portion bound to IL-3, whereas that from the lowest portion did not bind to IL-3 (data not shown). Thus, the uppermost portion of the smear (most retarded) is indicative of the most avidly bound subset of labeled molecules in the O-sulfated heparin, whereas the lowest portion of the smear (least retarded) is indicative of unbound O-sulfated heparin molecules, because it migrated at the same rate as O-sulfated heparin in the ovalbumin lane. Thus, O-sulfated heparin may contain GAG chains that bind to proteins over a range of affinities, unlike the highly sulfated unmodified heparin, and suggests that at least part of the binding requirements are provided by N-sulfate groups.
The VEGF165 isoform contains a heparin-binding domain in exon 7. VEGF121 isoform lacks this exon.18,48 49 Both O-sulfated heparin and unmodified heparin bound to VEGF165 but not to VEGF121. This indicates that the binding of both GAGs to VEGF165, as seen on ACE, is dependent on the presence of the heparin-binding domain in this protein.
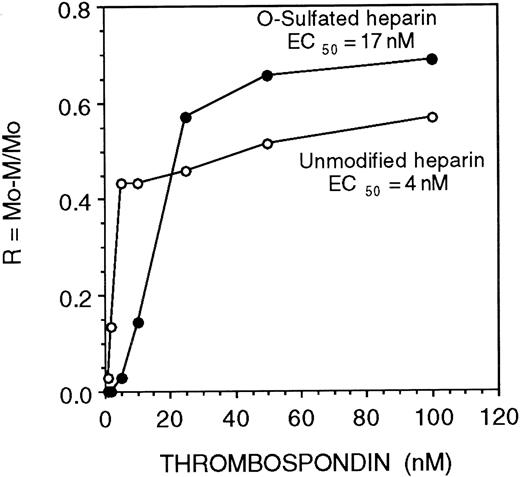
Recent studies demonstrate that the modulation of the biological activity of cytokines by HS depends on the affinity and kinetics of association (“on” rate) between cytokines and HS.50 We observed that both O-sulfated heparin and heparin bound all the proteins examined above, but only O-sulfated heparin supported LTC-IC maintenance. We therefore examined the relative affinity and kinetics of binding of these GAGs for proteins required for HPC localization and maintenance. The affinity of heparin for TSP, a matrix protein, was 4-fold higher than the affinity of O-sulfated heparin (half maximal binding {EC50} 4 nM vs 17 nM, respectively; Figure 6).
Affinity of unmodified and O-sulfated heparins for thrombospondin.
The affinity of unmodified or O-sulfated heparin for TSP was examined using affinity coelectrophoresis. 125I-labeled GAGs were electrophoresed through varying concentrations of TSP (0 to 100 nM). Half-maximal binding (EC50; a measure of affinity) was calculated as the concentration of TSP (in nM) at which retardation of migration of the GAGs (R; a function of binding) was 50%.
Affinity of unmodified and O-sulfated heparins for thrombospondin.
The affinity of unmodified or O-sulfated heparin for TSP was examined using affinity coelectrophoresis. 125I-labeled GAGs were electrophoresed through varying concentrations of TSP (0 to 100 nM). Half-maximal binding (EC50; a measure of affinity) was calculated as the concentration of TSP (in nM) at which retardation of migration of the GAGs (R; a function of binding) was 50%.
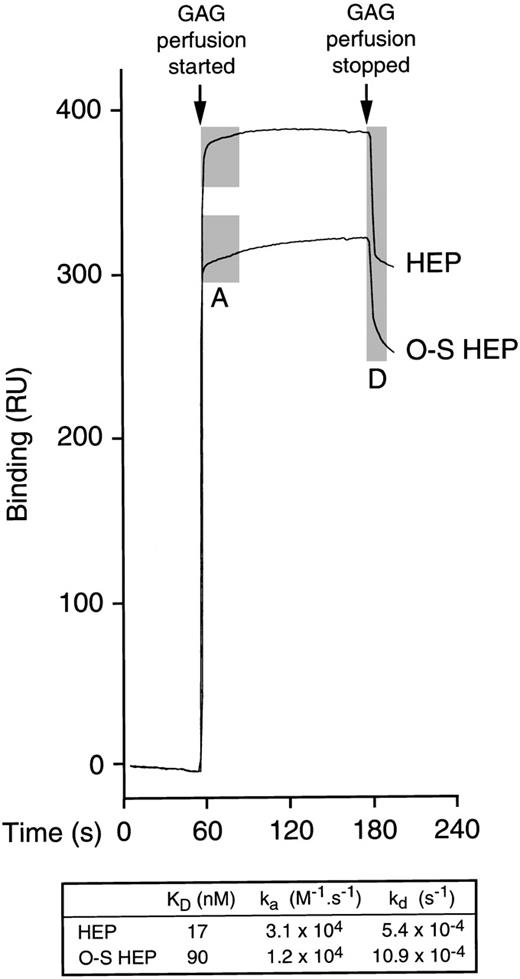
Because the chemokine PF4 is effective in supporting maintenance of LTC-IC in vitro, its binding was also investigated. By ACE, the affinity of heparin for PF4 was higher than the affinity of O-sulfated heparin (not shown). We therefore further examined the real-time binding kinetics between PF4 and unmodified or O-sulfated heparin using surface plasmon resonance (Figure 7). These studies demonstrated that the affinity of heparin for PF4 was 5-fold higher than the affinity of O-sulfated heparin (KD, 17 nM vs 90 nM). Moreover, the rates of association (“on” rate; ka) and dissociation (“off” rate; kd) of heparin were 2- to 2.5-fold higher than those of O-sulfated heparin, for PF4.
Binding of unmodified and O-sulfated heparins to PF4.
After a stable baseline tracing was established by perfusion of unsupplemented buffer over biotinylated PF4 immobilized on a streptavidin chip in a BIAcore® biosensor equipment, a range of concentrations of unmodified heparin (HEP) or O-sulfated heparin (O-S HEP) were perfused over the chip. Binding of each GAG was measured in resonance units (1 RU = 1 pg/mm2) in triplicate at each concentration at 5 different concentrations. Representative binding curves are shown for the following concentrations of GAGs: 20.8 μmol/L HEP and 31.3 μmol/L O-S HEP. The perfusion of GAGs resulted in a shift in the tracing due to binding of the GAGs to PF4 (association phase; shaded area labeled A). When GAG perfusion was stopped and perfusion with unsupplemented buffer resumed, the tracing returned downward as the bound GAGs dissociated from PF4 (dissociation phase; shaded area labeled D). Perfusion of heparin resulted in a sharp up-slope, indicating rapid association, and a sharp down-slope following cessation of heparin perfusion, indicating rapid dissociation. In contrast, perfusion of O-sulfated heparin resulted in more gradual up-slope and down-slope, indicating slower association and dissociation from PF4. The table shows the median values for KD, ka (association rate) and kd(dissociation rate).
Binding of unmodified and O-sulfated heparins to PF4.
After a stable baseline tracing was established by perfusion of unsupplemented buffer over biotinylated PF4 immobilized on a streptavidin chip in a BIAcore® biosensor equipment, a range of concentrations of unmodified heparin (HEP) or O-sulfated heparin (O-S HEP) were perfused over the chip. Binding of each GAG was measured in resonance units (1 RU = 1 pg/mm2) in triplicate at each concentration at 5 different concentrations. Representative binding curves are shown for the following concentrations of GAGs: 20.8 μmol/L HEP and 31.3 μmol/L O-S HEP. The perfusion of GAGs resulted in a shift in the tracing due to binding of the GAGs to PF4 (association phase; shaded area labeled A). When GAG perfusion was stopped and perfusion with unsupplemented buffer resumed, the tracing returned downward as the bound GAGs dissociated from PF4 (dissociation phase; shaded area labeled D). Perfusion of heparin resulted in a sharp up-slope, indicating rapid association, and a sharp down-slope following cessation of heparin perfusion, indicating rapid dissociation. In contrast, perfusion of O-sulfated heparin resulted in more gradual up-slope and down-slope, indicating slower association and dissociation from PF4. The table shows the median values for KD, ka (association rate) and kd(dissociation rate).
Discussion
The present studies demonstrate that, in the absence of stroma, a combination of specific HS GAGs with IL-3 and MIP-1α or PF4 is sufficient and necessary for the maintenance of the majority of input numbers of human LTC-IC for up to 5 weeks. LTC-IC expansion requires the presence of additional growth-promoting cytokines that are detectable in stroma-conditioned medium.
Results presented here are consistent with our recent observation that stroma-derived, O-sulfated HS GAGs improve LTC-IC maintenance in stroma-free cultures.26,34 In support of the importance of such O-sulfated GAGs for hematopoiesis, HS from the AFT024 stromal cell line, which supports multipotential and transplantable progenitors,2-4,51-53 is also enriched in such O-sulfated HS.54 Use of either HS or HS coupled to ovalbumin or human serum albumin in combination with IL-3 + MIP-1α ± CK resulted in equivalent LTC-IC maintenance, CFC generation, and cell expansion (data not shown). This indicates that (i) the LTC-IC maintaining activity is attributable to the HS side chains of HS proteoglycans and (ii) that the multimeric structure formed by coupling of HS chains to a “core” protein may not be more active than single HS chains in solution. Consistent with previous studies from our group, GAGs were not required for the cytokine-induced generation of mature blood cells and committed progenitors, or for short-term expansion of more primitive LTC-IC.
Both 2-week expansion and 5-week maintenance of LTC-IC were slightly superior in stroma-conditioned medium + IL-3 + MIP-1α cultures, compared to stroma-free cultures supplemented with cytokines and GAGs. This suggests that additional cytokines, such as flt-3 ligand or thrombopoietin, or additional unidentified stromal factors may further enhance support of primitive hematopoietic progenitors.3,51 52
Of note, we demonstrate that IL-3, MIP-1α, and PF4 require O-sulfated GAGs for their LTC-IC–maintaining capability. Other investigators have shown that the reconstituting ability of stem cells is lost when cultured in vitro with high concentrations of IL-3 in the absence of stroma.55,56 In contrast, microenvironmentally presented IL-3 produced by genetically engineered stromal cells does not exhaust primitive progenitors.57 Thus, whereas IL-3 alone may be detrimental to the in vitro maintenance of primitive stem cells, in the presence of O-sulfated GAGs and/or other matrix molecules (added to the cultures or produced by stromal feeders), IL-3 supports HPC in vitro.
It is still not completely clear how GAGs modulate LTC-IC maintenance in vitro. We have previously hypothesized that GAGs may colocalize cytokines and matrix proteins with HPC and/or modulate the activity of cytokines.26,34 Although IL-3 is not detectable in soluble form in stromal supernatants,58 stromal HS proteoglycans bind and present IL-3 in a biologically active form to hematopoietic cells9,35,59 and augment the localization of clonogenic progenitors with IL-3.37 These studies, and our observation that HPC-supportive O-sulfated heparin directly binds IL-3 and MIP-1α, support our colocalization hypothesis. GAGs may also modulate the activity of localized cytokines on target cells.8,21,22,60 Previous studies have shown that heparin-induced dimerization of FGF molecules induces receptor activation and cell proliferation61 and that dimers of IL-3 may be required for high-affinity receptor binding.62 The interaction of IL-3 with HPC-supportive GAGs may thus promote or modulate the biological activity of the cytokine via diverse mechanisms. Highly sulfated heparin also directly inhibits expression of cytokines and cell cycle proteins in target cells,63suggesting yet another mechanism for GAG-mediated modulation of the proliferation and differentiation-inducing effects of exogenously added cytokines on HPC.
Structural features of O-sulfated heparin and unmodified heparin (Table) may account for the observed differences in their affinity, kinetics, and profiles of protein binding. These differences may be responsible for our observation that O-sulfated heparin, but not unmodified heparin, supports LTC-IC maintenance, even though both GAGs bind the same cytokines and matrix molecules. Nearly all the individual GAG chains in unmodified heparin possess an adequate number of highly sulfated (“default” sulfated)64 disaccharide units with sulfation at N-, 2-O- and 6-O- positions {-IdoA (2−OSO3)-GlcNS (6−OSO3)-} (Table) for the various proteins to bind to the GAG chains at the concentrations examined. In contrast, O-sulfated heparin chains infrequently possess such “default” sulfated disaccharides (Table). The binding of proteins to O-sulfated heparin chains is likely to occur to more “selective” protein-binding regions related to the O-sulfated disaccharides, which are 6-sulfated, with or without 2-O sulfation: The majority of disaccharides in O-sulfated heparin are identical to such units and resemble sulfation pattern at the “transition points” of HS. The variability in the ability of subsets of O-sulfated heparin chains to bind proteins, as seen on ACE, might be indicative of the presence or absence of such binding regions on individual O-sulfated heparin chains. Consistent with this notion, the slow “on” time (association rate) seen for binding of PF4 suggests that proteins may take time to find such binding sites on O-sulfated heparin, and the slow “off” time (dissociation rate) suggests that, once bound, proteins may not come off rapidly.
Recent studies have demonstrated that HS possessing fast “on” rate and high-affinity binding sites for bFGF do not activate the biological activity of bFGF on target cells, whereas HS with a slow “on” rate, low-affinity binding sites have this capability.50 Separation of the 2 types of binding sites in microheterogenous populations of HS, by enzymatic digestion with heparinase that digests the high-affinity sites, restored the ability of the HS to activate bFGF. We demonstrate that the O-sulfated heparin, which optimally supports HPC maintenance and expansion, binds relevant growth factors and matrix molecules with a lower affinity and a slower “on” rate than unmodified heparin. This suggests that the higher degree of sulfation in heparin64 is detrimental to the LTC-IC–maintaining ability of GAGs. O-sulfated heparin represents an “average” structure and sulfation pattern, required for binding of multiple cytokines and matrix proteins. The lower protein-binding affinity of O-sulfated heparin than of the more highly sulfated unmodified heparin may be essential for optimal delivery of proteins to cell surface GAGs or receptors, and the slow dissociation rate may help mediate accumulation of bound proteins in close proximity to the progenitors.50 65 Taken together, differences between unmodified heparin and O-sulfated heparin are consistent with the concept that GAG-mediated support of human HPC in vitro (and likely other target cells) depends not only on the ability of GAGs to bind proteins, but also on optimal binding affinity and kinetics of interactions with early-acting cytokines and matrix components.
Acknowledgments
The authors acknowledge the excellent technical assistance of Brad Anderson and of the Biomedical Imaging and Processing Laboratory, University of Minnesota, and thank Dr James D. San Antonio (Jefferson Medical College, Philadelphia, PA) for helpful discussions.
Supported by the US Department of Veterans Affairs, Washington, DC; grants R01-HL-48738, P01-CA-65493, and AR-32372 from the National Institutes of Health, Bethesda, MD; the Minnesota Medical Foundation, the University of Minnesota Bone Marrow Transplant Research Fund, and the University of Minnesota Hospital and Clinic, Minneapolis, MN.
C.M.V. is a scholar of the Leukemia Society of America.
Reprints:Pankaj Gupta, Hematology/Oncology Section 111E, VA Medical Center, 1 Veterans Drive, Minneapolis, MN 55417; e-mail: gupta013@gold.tc.umn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal