Abstract
Heparin-binding epidermal growth factor–like growth factor (HB-EGF) is a widely expressed EGF superfamily member that induces mitogenic and/or chemotactic activities toward different cell types through binding to EGF receptors 1 or 4. Membrane-bound HB-EGF exerts growth activity and adhesion capabilities and possesses the unique property of being the receptor for diphtheria toxin (DT). Using molecular and functional techniques, we show that human polymorphonuclear granulocytes (PMN), which did not express HB-EGF in resting conditions, expressed it at mRNA and protein level, following incubation with granulocyte-macrophage colony-stimulating factor (GM-CSF). Other classic agonists for PMN (including lipopolysaccharide, phagocytable particles, tumor necrosis factor-, or G-CSF) failed to induce HB-EGF. The effects of GM-CSF on HB-EGF mRNA levels were concentration-dependent, reached a plateau after 1 to 2 hours of stimulation, and did not require protein synthesis. After GM-CSF treatment, membrane-bound HB-EGF was detected by flow cytometry. At the same time, PMN acquired sensitivity to the apoptosis-promoting effect of DT, which, moreover, specifically suppressed the GM-CSF–induced priming of formyl-methionyl-leucyl-phenylalanine–stimulated superoxide anion release. Finally, soluble HB-EGF was detected in the PMN culture medium by a specific enzyme-linked immunosorbent assay. Thus, we provide evidence that HB-EGF is specifically inducible by GM-CSF in PMN and represents a novel peptide to be included in the repertoire of PMN-derived cytokines.
NEUTROPHIL POLYMORPHONUCLEAR granulocytes (PMN) act as the first line of defense against invading micro-organisms, representing the predominant infiltrating cell type in the cellular phase of the acute inflammatory response.1Although mature PMN are terminally differentiated cells and have generally been considered as lacking RNA/protein synthesis capacity, convincing studies have clearly shown that PMN are capable of producing a variety of cytokines under appropriate circumstances.2Human PMN may release pro- and anti-inflammatory cytokines, including the interleukin-1α (IL-1α) and IL-1β, tumor necrosis factor-α (TNF-α), IL-1 receptor antagonist (IL-1ra), and chemokines such as IL-8, macrophage inflammatory protein-1α (MIP-1α) and MIP-1β, growth-related gene product-α (GROα), and others.3Remarkably, PMN have also been shown to express cytokines involved in processes such as angiogenesis, including vascular endothelial growth factor (VEGF),4-6 GROβ,7 and interferon-γ inducible protein-10 (IP-10),8 as well as in cell proliferation and fibrosis, such as transforming growth factor-α (TGFα),9 and TGFβ1,10,11 less conventionally related to PMN functions. On the whole, these and other data present PMN as candidate regulatory cells in conditions such as wound healing, neoplastic growth, and even degenerative lesions.3
Heparin-binding EGF-like growth factor (HB-EGF) is a heavily glycosylated EGF superfamily member of approximately 22 kD, originally identified in human macrophages and U937 monocytic cell line conditioned medium,12,13 and expressed in a wide range of cell types, including monocytes,13,14CD4+ lymphocytes,15 eosinophils,16myeloid leukemia blasts,17 vascular smooth muscle cells (SMC),18 and endothelial19 and normal20 or neoplastic epithelial cells.12,21Although HB-EGF can be released from the cell membrane through proteolytic mechanisms,22 multiple mRNA species for HB-EGF are produced,23 including transcripts corresponding to a short HB-EGF form lacking intramembrane and intracytoplasmic domains.24 Membrane-bound and soluble HB-EGF bind to EGF receptors 1 (HER-1) and 4 (HER-4),13,14,25 eliciting different biological responses,12,25 including adhesion activities,12 both mitogenic and chemotactic effects on fibroblasts15 and SMC,12,26,27 chemotaxis on endothelial cells27 and astrocytes,28 and growth activity for some epithelial cells.12,20,21Interestingly, membrane-bound HB-EGF has the unique property of acting as the receptor for the diphtheria toxin (DT),29 a protein synthesis inhibitor capable of triggering apoptotic death on target cells.30 CD9 coexpression enhances the mitogenic activity of membrane-bound HB-EGF31 as well as the sensitivity to DT.32
In this study, we analyzed whether human PMN express HB-EGF. We show that, after incubation with GM-CSF, PMN express amounts of HB-EGF mRNA, synthesize the related protein, and produce both the membrane-bound and soluble HB-EGF forms. We also provide evidence for a functional significance of PMN-derived HB-EGF.
MATERIALS AND METHODS
Cell purification and culture.
Highly purified granulocytes (>99.5%) and peripheral blood mononuclear cells (PBMC) were isolated under endotoxin-free conditions from buffy coats of healthy donors, as previously described.33 The granulocyte populations usually contained less than 4% eosinophils, as shown by May-Grünwald-Giemsa staining. In selected experiments, granulocytes were depleted of eosinophils according to the method described by Koenderman et al.34 Immediately after purification, cells were suspended in RPMI-1640 medium supplemented with 10% low-endotoxin (<6 pg/mL) fetal calf serum (FCS) (Seromed; Biochrom KG, Berlin, Germany) and treated with one of the following: 25 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (Genetics Institute, Boston, MA), 100 ng/mL lipopolysaccharide (LPS) (fromEscherichia coli, serotype 026:B6; Sigma, St Louis, MO), 5 ng/mL TNF-α (Peprotech, Rocky Hill, NJ), heat-killed yeast particles opsonized with IgG (Y-IgG) at a particle/cell ratio of 2:1, 10 nmol/L formyl-methionyl-leucyl-phenylalanine (fMLP; Sigma), 1,000 U/mL G-CSF (Granulokine; Hoffmann-La Roche, Basel, Switzerland), 100 U/mL interferon-γ (IFN-γ) (Hoffmann-La Roche), 1,000 U/mL IFN-α (Peprotech), 15 ng/mL IL-3 (Peprotech), 10 ng/mL IL-5 (Peprotech), 100 U/mL IL-10 (kindly provided by Dr K. Moore, DNAX and Schering-Plough, Palo Alto, CA), 100 ng/mL IL-12 (Peprotech), 100 ng/mL IL-15 (Genzyme, Cambridge, MA), 300 nmol/L staurosporin (Sigma), 100 μmol/L dexamethasone (DEX) (Sigma), or 20 μg/mL cycloheximide (CHX) (Sigma). In many experiments we also stimulated PMN with optimal concentrations (0.5 ng/mL) of GM-CSF purchased from Peprotech. All reagents used were the highest available grade and were dissolved in pyrogen-free water for clinical use. Cells were plated either in 24-well tissue culture plates (Nunc, Roskilde, Denmark) at 3 × 106/mL, or at 5 to 8 × 106/mL in polystyrene flasks (Greiner, Nurtingen, Germany), or at 3 × 106/mL in 96-well tissue culture plates (Greiner) and subsequently incubated at 37°C, 5% CO2 atmosphere. After culture for the appropriate times (see below), cells were either extracted for total RNA or used for functional assays. Cell-free supernatants (SN) were harvested and stored at −20°C. In selected experiments, the U937 cell line (monocytic leukemia–derived)35 was also used.
RNA isolation and Northern blot analysis.
Total RNA from PMN and PBMC was extracted and analyzed by Northern blot (10 μg of RNA per lane) as previously described.17 36Filters were hybridized using an HB-EGF cDNA probe obtained as described below, IL-1ra, IL-6, and β-actin cDNA fragments labeled with 32P using a Ready-to-go DNA labeling kit (Pharmacia, Uppsala, Sweden).
HB-EGF and HER-4 RT-PCR. HB-EGF probe generation.
A quantity of 4 μg of RNA from the cells of interest was reverse transcribed as previously described.17,37 cDNA was amplified using the following primers (Genenco, M-medical, Florence, Italy). (1) HB-EGF sense 5′-TGGTGCTGAAGCTCTTTCTGG-3′ and antisense 5′-GTGGGAATTAGTCATGCCCAA-3′; these primers were designed to give a fragment of 605 bp (complete form of HB-EGF)23 or a fragment of 605 + 94 bp (short form of HB-EGF).24 (2) HER-4 sense 5′-AGATGGAGGTTTTGCTGCTGAACA-3′ and antisense 5′-TTACACCACAGTATTCCGGTGTCT-3′ (726-bp fragment).38 (3) Vimentin sense 5′-GCTCAGATTCAGGAACAGCAT-3′ and antisense 5′-TAAGGGCATCCACTTCACAGG-3′ (266-bp fragment). The cDNA was denatured for 5 minutes at 94°C before 35 runs in a thermal cycler (GeneAmp PCR System 2400; Perkin Elmer, Norwalk, CT) using 1.25 U of Taq polymerase (Perkin Elmer, Branchburg, NJ) in 50 μL (94°C 40 seconds, 57°C 40 seconds, 72°C 50 seconds) followed by 5 minutes at 72°C. The PCR products were separated by electrophoresis on 1.5% agarose gel. HB-EGF cDNA amplified from the U937 cell line using the primers specified above was analyzed for the SmaI (Life Technologies, Rockville, MD) restriction site (which gave the expected HB-EGF fragments of 388 and 217 bp), and was sequenced (Sequenase 2.0 sequencing kit; USB, Cleveland, OH) as a plasmid insert (TA cloning kit; Invitrogen, San Diego, CA), from which the HB-EGF probe was generated for Northern blot analysis.
Flow cytometric analysis.
A quantity of 1 × 106/mL PMN cultured for 21 hours in the presence or absence of GM-CSF or LPS was washed and incubated in 100 μL of phosphate-buffered saline (PBS) for 30 minutes at 4°C with 5% human serum and stained with 10 μL of 100 μg/mL purified rabbit anti–HB-EGF polyclonal H6 antibody (kindly provided by Dr S. Higashiyama, Osaka, Japan)32 for 1 hour at 4°C followed by a biotinylated second antibody [goat F(ab′)2anti-rabbit IgG (Caltag, Burlingame, CA) preadsorbed with human IgG] for 30 minutes at 4°C and, after washing, by phycoerythrin (PE)-conjugated streptavidin (Becton Dickinson, Sunnyvale, CA) for 15 minutes at 4°C. Freshly isolated PMN (3 × 106/mL) were incubated with 10 μL PE-conjugated anti-CD9 (SBA, Birmingham, AL) or fluorescein isothiocyanate (FITC)-conjugated anti–HER-1 (Medac, Hamburg, Germany) monoclonal antibodies (MoAbs) for 30 minutes at 4°C. Irrelevant purified rabbit Ig or isotype FITC- or PE-conjugated (Immunotech, Westbrook, MA) MoAbs were used as controls. Flow cytometry analysis was performed on a FACScan (Becton Dickinson, Mountain View, CA).
DT-induced apoptosis.
Aliquots of 1 × 106/mL PMN were incubated for the times indicated with or without GM-CSF in the presence or absence of 10−11 to 10−8 highly purified DT (kindly provided by Dr E. Papini, CNR Center for Biomembranes, Padua, Italy) and then examined for cell apoptosis by two distinct methods. (1) Analysis of apoptotic (hypodiploid) nuclei by flow cytometry, as described by others.39 PMN were harvested, washed twice with PBS, and suspended in 1.5 mL hypotonic fluorochrome solution (propidium iodide 50 μg/mL in 0.1% sodium citrate and 0.1% Triton X-100). The mixture was placed in the dark overnight at 4°C. The fluorescence of each nucleus was measured using an XL-Coulter flow cytometer (Coulter, Hialeah, FL). (2) Analysis of apoptotic cell morphology. A quantity of 0.5 × 106 PBS-washed PMN were centrifuged for cytospin preparations and stained using May-Grünwald-Giemsa. Cells were scored as apoptotic versus nonapoptotic, based on diminution of cell volume and chromatin condensation yielding fragmented homogeneously stained nuclei.40
Measurement of superoxide anion (O2−) generation.
This was performed as previously described.41 Briefly, 100 μL of PMN suspension (3 × 106/mL) containing or not GM-CSF or LPS in the presence or absence of 10−8mol/L DT were added to tissue-culture polystyrene 96-well plates. After a 2- or 21-hour incubation, 100 μL of Hanks’ Balanced Salt Solution (HBSS), containing 1 mmol/L CaCl2, 10 mmol/L glucose, 4 mmol/L NaN3, and 160 μmol/L cytochrome C, with or without stimulus (100 nmol/L fMLP) in the presence or not of superoxide dismutase (SOD), were added on top to each well. Plates were then incubated at 37°C in an automated EL34 microplate reader (Biotec Instruments, Highland Park, UT) to record absorbance at 550 and 468 nm. Nanomoles of O2− were calculated using an extinction coefficient of 24.5 mmol/L.42 In selected experiments for neutrophil-derived superoxide anion, 0.5 to 1 ng/mL HB-EGF (R&D System, Minneapolis, MN) was used either as a direct triggering agonist or as a priming agent.
Enzyme-linked immunosorbent assay (ELISA) for HB-EGF.
Soluble HB-EGF protein was measured in the cell-free SN using a specific ELISA developed in our laboratory, according to a method recently published.43 Briefly, flat-bottomed 96-well plates (MaxiSorp; Nunc) were coated with 50 μL/well of 2 μg/mL polyclonal anti-HB-EGF antibody (R&D System) in 50 mmol/L sodium bicarbonate buffer, pH 8.5 for 8 hours at room temperature, and incubated overnight at 4°C with 100 μL/well of blocking buffer (20 mmol/L TBS, pH 7.4, 3% bovine serum albumin [BSA]). After extensive washings with TBS, pH 7.4, 0.05% Tween 20 (washing buffer), 50 μL/well of either HB-EGF standards (R&D System) or cell-free culture SN were added, followed by a 2-hour incubation at 37°C. SN collected after a 21-hour culture of U937 cells or PBMC and PMN stimulated or not with GM-CSF or LPS were used undiluted. SN of PMN previously cultured in medium supplemented with 1% low-endotoxin FCS were used after approximately 80-fold concentration (by the Centricon Plus 20 device from Amicon Inc, Beverly, MA). Plates were rinsed with washing buffer before addition of 50 μL/well of biotinylated anti–HB-EGF antibody (R&D System) (0.5 μg/mL in TBS, 0.05% Tween 20, 0.1% BSA) and incubated for 1.5 hours at room temperature. After extensive washings of the plates, streptavidin-conjugated alkaline phosphatase, diluted 1:10,000 (Life Technologies) with TBS containing 0.5% BSA and 0.05% Tween 20, was added and incubated for 1 hour at room temperature. After washing, a chromogenic reaction was performed using the ELISA Amplification System (Life Technologies), according to the manufacturer’s protocol. Assays were carried out in duplicate. The reaction was stopped with 0.3 mol/L H2SO4 and the absorbance at 490 nm was measured. This ELISA had a detection limit of 6.25 pg/mL, and did not cross-react with 100 ng/mL IP-10, 100 ng/mL IL-8, 1 ng/mL MIP-1α, 10 ng/mL GROα, 10 ng/mL IFN-γ, 5 ng/mL IL-10, 10 ng/mL IL-1β, 10 ng/mL TNF-α, or 10 ng/mL GM-CSF other than EGF, TGFα, and HGF.43
Statistical analysis.
Student’s t-test, the Mann-Whitney U test, and analysis of variance according to Kruskall-Wallis ANOVA by ranks were used. Differences were considered statistically significant when theP value was <.05.
RESULTS
GM-CSF–induced HB-EGF mRNA in PMN.
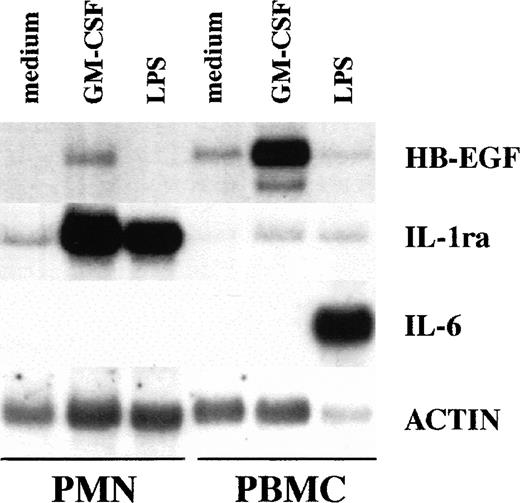
To investigate whether amounts of HB-EGF mRNA were expressed in PMN, these cells were initially cultured for 2 hours in the presence or absence of 100 ng/mL LPS and 25 ng/mL GM-CSF, and then total RNA was processed for Northern blot analysis. Autologous PBMC were also stimulated under identical experimental conditions, for the purposes of comparison. Figure 1 shows that resting and LPS-treated PMN did not express detectable HB-EGF mRNA. The effectiveness of LPS-stimulation was confirmed by its ability to upregulate the IL-1ra mRNA levels.44 Other PMN agonists, listed in Table 1, also failed to induce HB-EGF steady-state mRNA levels in PMN, even if PMN were cultured for up to 21 hours in the presence or absence of IFN-γ (data not shown), a cytokine which usually primes PMN for an enhanced gene expression.3 By contrast, GM-CSF–treated PMN exhibited a considerable accumulation of HB-EGF transcripts as well as IL-1ra mRNA.45
Comparative ability of PMN and PBMC to express HB-EGF mRNA when stimulated with GM-CSF. GM-CSF induced HB-EGF transcripts in PMN and, more dramatically, in PBMC, while LPS, which upregulated IL-1ra in PMN and IL-6 in PBMC, was ineffective on HB-EGF mRNA production. PBMC presented also a costitutive, low production of HB-EGF mRNA. Purified populations of PMN and PBMC from the same donor were cultured with or without 25 ng/mL GM-CSF and 100 ng/mL LPS for 2 hours and then total RNA was extracted and Northern blot analysis for HB-EGF, IL-1ra, IL-6, and actin mRNA was performed. Ten micrograms of total RNA was loaded on each gel lane. The experiment depicted is representative of 4.
Comparative ability of PMN and PBMC to express HB-EGF mRNA when stimulated with GM-CSF. GM-CSF induced HB-EGF transcripts in PMN and, more dramatically, in PBMC, while LPS, which upregulated IL-1ra in PMN and IL-6 in PBMC, was ineffective on HB-EGF mRNA production. PBMC presented also a costitutive, low production of HB-EGF mRNA. Purified populations of PMN and PBMC from the same donor were cultured with or without 25 ng/mL GM-CSF and 100 ng/mL LPS for 2 hours and then total RNA was extracted and Northern blot analysis for HB-EGF, IL-1ra, IL-6, and actin mRNA was performed. Ten micrograms of total RNA was loaded on each gel lane. The experiment depicted is representative of 4.
Effect of Various Agonists on HB-EGF mRNA Induction in PMN
| Medium | − |
| LPS | − |
| TNF-α | − |
| IL-3 | − |
| IL-5 | − |
| IL-10 | − |
| IL-12 | − |
| IL-15 | − |
| G-CSF | − |
| GM-CSF | + |
| IFN-α | − |
| IFN-γ | − |
| fMLP | − |
| IgG-opsonized yeasts | − |
| Staurosporin | − |
| Dexamethasone | − |
| Cycloheximide | − |
| Medium | − |
| LPS | − |
| TNF-α | − |
| IL-3 | − |
| IL-5 | − |
| IL-10 | − |
| IL-12 | − |
| IL-15 | − |
| G-CSF | − |
| GM-CSF | + |
| IFN-α | − |
| IFN-γ | − |
| fMLP | − |
| IgG-opsonized yeasts | − |
| Staurosporin | − |
| Dexamethasone | − |
| Cycloheximide | − |
HB-EGF accumulation pattern in PMN and PBMC.
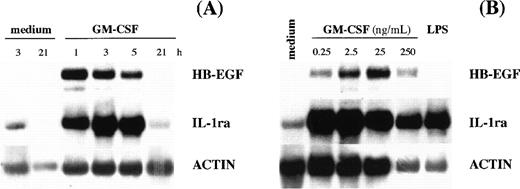
Kinetic experiments showed that maximal levels of HB-EGF mRNA occurred after 1 to 2 hours of GM-CSF stimulation (Fig 2A), whereas dose-response studies indicated that the highest HB-EGF mRNA accumulation in PMN could be obtained after stimulation with 25 ng/mL of GM-CSF (Fig 2B). A different pattern of HB-EGF mRNA accumulation was observed in PBMC, which not only presented low but constitutive HB-EGF transcripts in all donors examined (n = 4), but dramatically responded to GM-CSF stimulation (Fig 1), producing, as observed for other cytokines,2 more HB-EGF transcripts than PMN did.
HB-EGF mRNA levels in GM-CSF–treated PMN. (A) Time-course. PMN were incubated with 25 ng/mL GM-CSF. At the time-points indicated, total mRNA was extracted and analyzed for HB-EGF, IL-1ra, and actin mRNA expression. The experiment depicted is representative of 2. (B) Dose-dependence. PMN were stimulated with increasing doses of GM-CSF for 2 hours and then total RNA was extracted and analyzed for HB-EGF, IL-1ra, and actin mRNA expression. The experiment depicted is representative of 2.
HB-EGF mRNA levels in GM-CSF–treated PMN. (A) Time-course. PMN were incubated with 25 ng/mL GM-CSF. At the time-points indicated, total mRNA was extracted and analyzed for HB-EGF, IL-1ra, and actin mRNA expression. The experiment depicted is representative of 2. (B) Dose-dependence. PMN were stimulated with increasing doses of GM-CSF for 2 hours and then total RNA was extracted and analyzed for HB-EGF, IL-1ra, and actin mRNA expression. The experiment depicted is representative of 2.
Genuine expression of HB-EGF by PMN.
PBMC expressed several HB-EGF mRNA species, in agreement with earlier studies.23,46 As illustrated in Figs 2 and3, at least 2 HB-EGF mRNA species were induced by GM-CSF in PMN, the most abundant being the 2.7-kb size transcript, a pattern matching that observed in PBMC. Furthermore, IL-6 mRNA was expressed only in LPS-stimulated PBMC and not in PMN, according to published data,47,48 thus excluding contamination with mononuclear cells (Fig 1). Also consistent with the genuine ability of PMN to express HB-EGF mRNA in response to GM-CSF were 3 additional experiments in which complete depletion of eosinophils, which have also been reported to express HB-EGF in a rat experimental model,16 did not influence the accumulation of HB-EGF mRNA in GM-CSF–treated PMN (data not shown). Taken together, our data rule out the possibility that the results obtained in PMN with respect to HB-EGF mRNA levels can be attributed to contamination with mononuclear cells or eosinophils.
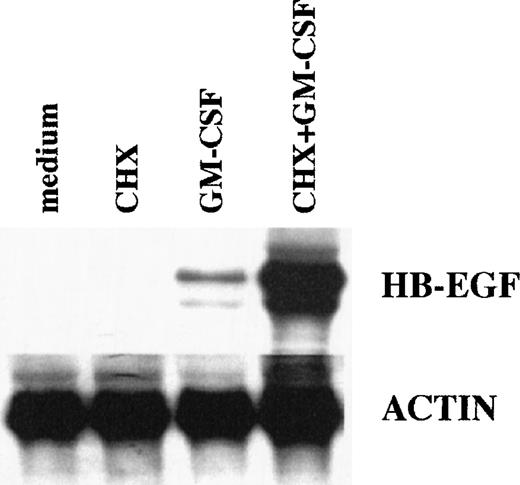
Effects of CHX on HB-EGF mRNA levels in GM-CSF–treated PMN. CHX did not inhibit, but overinduced, the GM-CSF–dependent upregulation of HB-EGF mRNA, showing that protein synthesis was not required. PMN were pretreated with 20 μg/mL CHX before stimulation with 25 ng/mL GM-CSF for 2 hours. Total RNA was then extracted and analyzed for HB-EGF and actin mRNA levels. The experiment depicted is representative of 2.
Effects of CHX on HB-EGF mRNA levels in GM-CSF–treated PMN. CHX did not inhibit, but overinduced, the GM-CSF–dependent upregulation of HB-EGF mRNA, showing that protein synthesis was not required. PMN were pretreated with 20 μg/mL CHX before stimulation with 25 ng/mL GM-CSF for 2 hours. Total RNA was then extracted and analyzed for HB-EGF and actin mRNA levels. The experiment depicted is representative of 2.
Protein-synthesis-independent induction of HB-EGF mRNA in PMN.
We also examined whether de novo protein synthesis was necessary for the GM-CSF–driven induction of HB-EGF mRNA in PMN. As shown in Fig 3, this was not the case. The inhibitor of protein synthesis CHX not only did not inhibit, but actually superinduced the GM-CSF–dependent upregulation of HB-EGF mRNA, in a similar way to previous observations in monocytes stimulated with LPS for 1 hour.46
Membrane-bound HB-EGF demonstration by flow cytometry on GM-CSF–treated PMN.
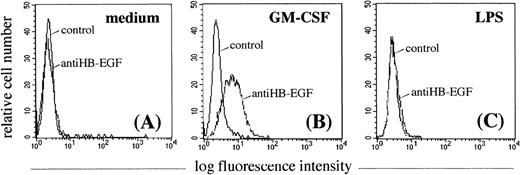
To determine whether GM-CSF–treated PMN expressed the membrane-bound HB-EGF molecule, they were investigated by flow cytometry using a specific purified anti–HB-EGF polyclonal antibody. As shown in Fig 4A, resting PMN were negative for HB-EGF. GM-CSF proved to be an efficient inducer of membrane-bound HB-EGF surface expression in cultured PMN (Fig 4B), while LPS was not (as expected from the Northern blot data) (Fig 4C). Surface HB-EGF expression, however, was detectable when PMN were cultured with GM-CSF for longer than 6 hours. GM-CSF also efficiently upregulated HB-EGF surface expression in monocytes (data not shown).
Effect of GM-CSF and LPS on the surface expression of HB-EGF molecule in PMN. PMN were cultured in the absence (A) or presence of 25 ng/mL GM-CSF (B) or 100 ng/mL LPS (C) for 21 hours. Only GM-CSF–treated PMN acquired membrane expression of the HB-EGF molecule (B). Membrane-bound HB-EGF expression was examined by indirect immunofluorescence analysis using the polyclonal H6 antibody (kindly provided by Dr S. Higashiyama), followed by a human-IgG preadsorbed biotinylated second antibody and by PE-conjugated streptavidin. Cells were also stained with irrelevant antibodies as controls. The expression patterns presented in this figure were reproduced in 5 independent experiments.
Effect of GM-CSF and LPS on the surface expression of HB-EGF molecule in PMN. PMN were cultured in the absence (A) or presence of 25 ng/mL GM-CSF (B) or 100 ng/mL LPS (C) for 21 hours. Only GM-CSF–treated PMN acquired membrane expression of the HB-EGF molecule (B). Membrane-bound HB-EGF expression was examined by indirect immunofluorescence analysis using the polyclonal H6 antibody (kindly provided by Dr S. Higashiyama), followed by a human-IgG preadsorbed biotinylated second antibody and by PE-conjugated streptavidin. Cells were also stained with irrelevant antibodies as controls. The expression patterns presented in this figure were reproduced in 5 independent experiments.
DT-induced apoptosis in GM-CSF–treated PMN.
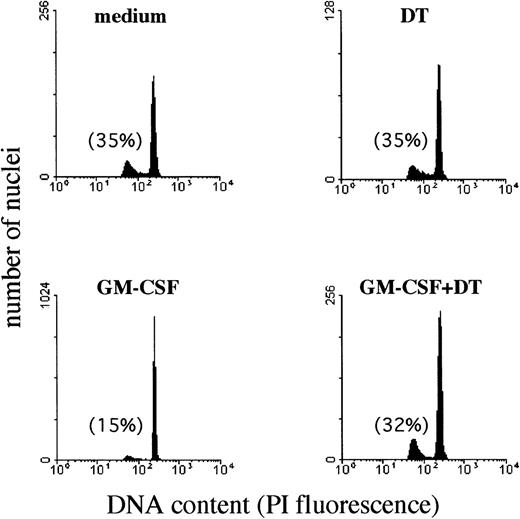
To evaluate whether the membrane-bound HB-EGF molecule expressed on GM-CSF–treated PMN was functionally active, we tested PMN sensitivity to DT. DT represents a ligand of membrane-bound HB-EGF and induces apoptosis in a dose-dependent manner on HB-EGF–expressing cells.29,30 Although PMN undergo constitutive apoptosis when aged ex vivo, evidence suggests that this process may be substantially delayed if cells are cultured in the presence of GM-CSF, LPS, IFN-γ, and other cytokines, or, conversely, that it may be accelerated if cells are incubated, for instance, with anti-CD95 antibodies.49 Therefore, we sought to determine whether the presence of DT in the cultures of GM-CSF–treated PMN inhibited the protective effect of GM-CSF on PMN apoptosis50 as a consequence of the GM-CSF–induced surface expression of HB-EGF acting as a DT receptor. To test this hypothesis, we measured the rates of PMN apoptosis by analyzing apoptotic (hypodiploid) nuclei and cell morphology. Figure 5illustrates a representative experiment performed using flow cytometry to quantify the apoptotic nuclei of PMN, while Table 2 summarizes the data from all our experiments. Apoptosis of PMN after a 44-hour culture period was 34% ± 9% (n = 5), and this percentage was not significantly affected by the presence of DT (35.7% ± 15%, n = 5) in the culture medium. IFN-γ and, more effectively, GM-CSF were found to exert a significant protective effect on PMN apoptosis. By contrast, 10−8 mol/L DT completely suppressed (P < .05) the protective effect of GM-CSF (Fig 5 and Table 2), but not that of IFN-γ (Table 2). Preliminary dose-response experiments in the range of 10−11 to 10−8 mol/L DT concentrations showed that 10−8 mol/L DT displayed maximal suppression of the protective GM-CSF–mediated effect on PMN apoptosis (data not shown). Therefore, 10−8 mol/L DT was used in all subsequent experiments. GM-CSF–treated PMN also displayed sensitivity to the apoptosis-promoting effects of DT at morphological examination (Fig 6).
Effect of DT on the development of apoptotic nuclei in PMN maintained in culture with GM-CSF. The presence of DT (kindly provided by Dr E. Papini) in the cultures inhibited specifically the protective effect of GM-CSF on PMN apoptosis. PMN suspensions, cultured for 44 hours alone or with 25 ng/mL GM-CSF in the presence or absence of 10−8 mol/L DT, were processed for DNA content analysis by propidium iodide staining and flow cytometry analysis. Data were plotted as red fluorescence intensity versus number of nuclei with a given DNA content as determined in each experimental condition. Numbers reported indicate the percentage of hypodiploid (apoptotic) nuclei. Similar results were observed in PMN isolated from 3 independent donors.
Effect of DT on the development of apoptotic nuclei in PMN maintained in culture with GM-CSF. The presence of DT (kindly provided by Dr E. Papini) in the cultures inhibited specifically the protective effect of GM-CSF on PMN apoptosis. PMN suspensions, cultured for 44 hours alone or with 25 ng/mL GM-CSF in the presence or absence of 10−8 mol/L DT, were processed for DNA content analysis by propidium iodide staining and flow cytometry analysis. Data were plotted as red fluorescence intensity versus number of nuclei with a given DNA content as determined in each experimental condition. Numbers reported indicate the percentage of hypodiploid (apoptotic) nuclei. Similar results were observed in PMN isolated from 3 independent donors.
Effect of DT (10−8 mol/L) on the Apoptosis Rate of GM-CSF–Treated PMN
| . | −DT . | . | +DT . |
|---|---|---|---|
| medium | 34 ± 9 | NS | 35.7 ± 15 |
| (n = 5) | (n = 5) | ||
| GM-CSF | 21.5 ± 10 | P < .05 | 33.5 ± 15 |
| (n = 5) | (n = 5) | ||
| IFN-γ | 22.2 ± 11.2 | NS | 15.7 ± 13 |
| (n = 3) | (n = 3) |
| . | −DT . | . | +DT . |
|---|---|---|---|
| medium | 34 ± 9 | NS | 35.7 ± 15 |
| (n = 5) | (n = 5) | ||
| GM-CSF | 21.5 ± 10 | P < .05 | 33.5 ± 15 |
| (n = 5) | (n = 5) | ||
| IFN-γ | 22.2 ± 11.2 | NS | 15.7 ± 13 |
| (n = 3) | (n = 3) |
Data are expressed as percentage of fragmented nuclei reflecting the proportion of apoptotic cells after a 44-hour period of culture in the presence or absence of the listed factors. Means ± SD for the number of experiments indicated in parentheses.
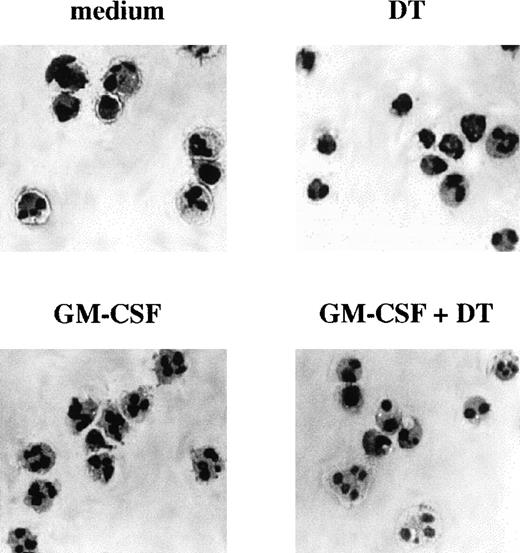
Morphologic features of PMN cultured with GM-CSF in the presence or absence of DT. Only after GM-CSF treatment, PMN acquired sensitivity to DT, which induced typical apoptotic features. Cytospin preparations of PMN were stained using the May-Grünwald-Giemsa method after incubation in vitro for 21 hours in the presence or absence of 0.5 ng/mL GM-CSF with or without 10−8 mol/L DT. The experiment depicted is representative of 2.
Morphologic features of PMN cultured with GM-CSF in the presence or absence of DT. Only after GM-CSF treatment, PMN acquired sensitivity to DT, which induced typical apoptotic features. Cytospin preparations of PMN were stained using the May-Grünwald-Giemsa method after incubation in vitro for 21 hours in the presence or absence of 0.5 ng/mL GM-CSF with or without 10−8 mol/L DT. The experiment depicted is representative of 2.
DT-mediated suppression of GM-CSF priming on PMN respiratory burst capacity.
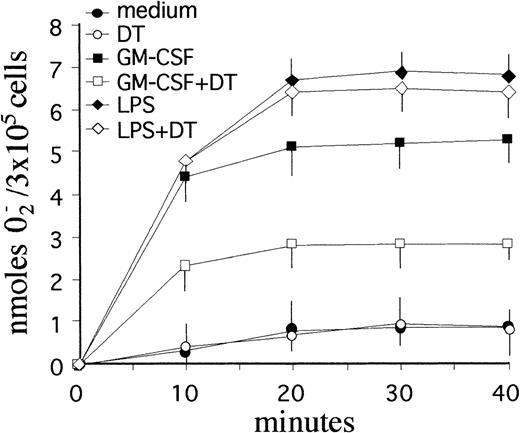
To determine whether the apoptosis-promoting effects of DT were selective or accompanied by other biological effects, additional experiments were performed to assess the respiratory burst capacity of PMN cultured with GM-CSF. Like IFN-γ or LPS,33 GM-CSF is known to greatly potentiate the ability of PMN to release reactive oxygen intermediates (ROI).51 To this end, PMN were incubated with GM-CSF or LPS for 2 and 21 hours in the presence or absence of 10−8 mol/L DT, and then stimulated with 100 nmol/L fMLP, a bacteria-derived chemotactic peptide routinely used to stimulate ROI release in PMN.41 Both GM-CSF and LPS-treatment resulted in a dramatic upregulation of fMLP-stimulated, SOD-inhibitable superoxide anion (O2−) release from PMN, at both 2- (not shown) and 21-hour incubation (Fig 7). While DT failed to influence the constitutive ability of PMN to produce O2− in response to fMLP at both 2- (not shown) and 21-hour incubation, it significantly suppressed (by approximately 60%) the priming effect of GM-CSF at 21 hours, but was largely ineffective toward LPS (Fig 7). Interestingly, DT did not affect the priming effect of GM-CSF at 2-hour incubation (data not shown), which is consistent with a lack of surface expression of membrane HB-EGF at that time-point (see above).
Effect of DT on the respiratory burst of PMN maintained in culture with GM-CSF. DT specifically suppressed the priming effect of GM-CSF, but was largely ineffective toward LPS (P < .05). PMN (3 × 105/well) were cultured for 21 hours in the presence or absence of 0.5 ng/mL GM-CSF with or without 10−8 mol/L DT before stimulation with 100 nmol/L fMLP. Data represent the mean values (±SD) of SOD-inhibitable O2− release from triplicate assays for each condition. Similar results were obtained in 3 separate experiments.
Effect of DT on the respiratory burst of PMN maintained in culture with GM-CSF. DT specifically suppressed the priming effect of GM-CSF, but was largely ineffective toward LPS (P < .05). PMN (3 × 105/well) were cultured for 21 hours in the presence or absence of 0.5 ng/mL GM-CSF with or without 10−8 mol/L DT before stimulation with 100 nmol/L fMLP. Data represent the mean values (±SD) of SOD-inhibitable O2− release from triplicate assays for each condition. Similar results were obtained in 3 separate experiments.
Release of membrane-bound HB-EGF into SN.
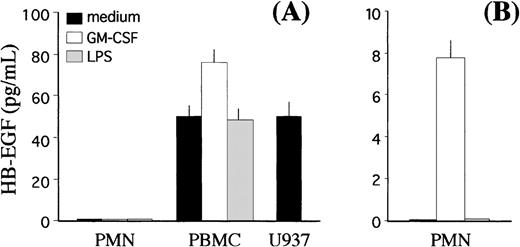
Finally, we investigated whether GM-CSF–treated PMN, in addition to expressing the membrane-bound form, could also release HB-EGF protein into the SN. To this end, we developed a specific ELISA, according to the indications by Yamada et al.43 The threshold sensitivity of this ELISA was 6.25 pg/mL and its validity was demonstrated by the fact that it was able to detect substantial amounts of soluble HB-EGF in U937 cell-conditioned medium12 14(Fig 8A). No extracellular production of soluble HB-EGF was detected in cell-free SN harvested from either resting or GM-CSF– and LPS-treated PMN, whereas significant levels of soluble antigenic HB-EGF were measured in SN harvested from autologous PBMC in resting conditions, which increased (P < .05) after treatment with GM-CSF (Fig 8A). If SN from GM-CSF–treated PMN were previously concentrated up to 80-fold, then detectable yields of soluble HB-EGF could be specifically measured (Fig 8B).
Release of the soluble form of HB-EGF by PMN and PBMC. GM-CSF treatment induces release of the HB-EGF molecule into the culture medium (P < .05). (A) PMN and PBMC were cultured alone or in the presence of either 0.5 ng/mL GM-CSF or 100 ng/mL LPS. Cell-free SN were collected after 21 hours, and the levels of soluble HB-EGF protein were measured by a specific ELISA. The levels of soluble HB-EGF spontaneously released by the U937 cell line were also determined for comparison. (B) In selected experiments, PMN-derived SN were concentrated approximately 80-fold before ELISA detection. Values represent means (±SD) of duplicate determinations calculated from 3 independent experiments.
Release of the soluble form of HB-EGF by PMN and PBMC. GM-CSF treatment induces release of the HB-EGF molecule into the culture medium (P < .05). (A) PMN and PBMC were cultured alone or in the presence of either 0.5 ng/mL GM-CSF or 100 ng/mL LPS. Cell-free SN were collected after 21 hours, and the levels of soluble HB-EGF protein were measured by a specific ELISA. The levels of soluble HB-EGF spontaneously released by the U937 cell line were also determined for comparison. (B) In selected experiments, PMN-derived SN were concentrated approximately 80-fold before ELISA detection. Values represent means (±SD) of duplicate determinations calculated from 3 independent experiments.
Lack of HER-1, HER-4, and CD9 expression in PMN: Inefficacy of HB-EGF.
Because HB-EGF binds to HER-1 or HER-4,13,14,25 and CD9 behaves as a coreceptor of membrane-bound HB-EGF,31 32 we investigated whether these molecules were expressed by PMN. We failed to detect the expression of either HER-1 or CD9 molecules, or of HER-4 mRNA in PMN, as investigated by flow cytometry analysis and reverse transcriptase-polymerase chain reaction (RT-PCR), respectively. In line with the lack of the related receptors, stimulatory experiments using HB-EGF did not induce any modifications in PMN. Interestingly, HER-4 mRNA was detectable in U937 cells in basal conditions (data not shown).
DISCUSSION
HB-EGF is a member of the EGF superfamily, which also includes epidermal growth factor, TGFα, amphiregulin, betacellulin, epiregulin, neuregulin-1 and -2, and vaccinia growth factor.12,52 These are growth and differentiation factors, some of which, especially HB-EGF, have been shown to actively participate in important tissue-modeling phenomena, involving autocrine or paracrine regenerative and neoplastic growth and highly complex activities such as angiogenesis and blastocyst implantation.12,52 Although PMN have been found to infiltrate a number of proliferative or degenerative lesions, their ability to release cytokines of the EGF family has rarely been considered. For instance, attention has only recently focused on PMN-produced TGFα.9 In general, the role of PMN infiltrating tissue lesions is far from clear and there is currently a great deal of interest in studying PMN-derived cytokines and especially the role they play in cell proliferation and angiogenesis.3
This study provides the first demonstration that human PMN, under particular stimulatory conditions, are capable of producing, bearing on their membrane, and releasing the important EGF superfamily cytokine, HB-EGF. Our findings, therefore, document a mechanism whereby PMN might directly influence tissue regeneration and even cancer progression.
Although negative in basal conditions, PMN expressed HB-EGF mRNA upon treatment with GM-CSF, as shown by both Northern blot analysis (Fig 1) and RT-PCR (data not shown). Classic agonists (listed in Table 1) for PMN all failed to induce HB-EGF, despite the presence in the culture medium of IFN-γ, which usually acts as a PMN priming factor for gene expression.3 The effects of GM-CSF on HB-EGF mRNA levels were concentration-dependent, reached a plateau after 1 to 2 hours of stimulation, and were independent of protein synthesis. Because it has been shown that HB-EGF may be induced via the Raspathway12,53,54 and that GM-CSF actually activates theRas and Raf-1 and the MAP-kinase signaling pathways by binding to the beta subunit of its receptor,55,56 theRas pathway may therefore be a likely candidate for the GM-CSF–mediated induction of HB-EGF in PMN. By contrast, NFkB are unlikely to play a role in PMN HB-EGF upregulation. Although putative binding sites for NFkB have been identified in the HB-EGF promoter12,23 and NFkB can be mobilized through the GM-CSF receptor in many cell types,55,56 recent studies of ours have failed to demonstrate activation of NFkB in GM-CSF–stimulated PMN.57
The surface expression and structural integrity of membrane-bound HB-EGF were detected by flow cytometry and by the demonstration that PMN acquired sensitivity to the apoptosis-promoting effect of DT29,30 after GM-CSF treatment. DT-induced PMN cytolysis was associated with the development of hypodiploid nuclei and apoptotic cellular morphology. We did not, however, find complete killing of PMN after exposure to DT. DT receptor density or expression only in a subset of cells, lack of coreceptors such as CD9,32efficiency of DT internalization, and, in general, the equilibrium status of apoptotic pathways in terms of overexpression of anti-apoptotic factors, possibly induced by GM-CSF itself, may influence PMN sensitivity to DT at any given time. The suppression of the priming effect of GM-CSF on fMLP-stimulated, SOD-inhibitable superoxide anion release was another effect of DT internalization. This effect coincided with the surface expression of membrane-bound HB-EGF, because it was not observed at 2 hours of incubation with GM-CSF. Whether the suppressive action on the respiratory burst is attributable to DT-mediated inhibition of protein synthesis,22 and specifically to the synthesis of the various NADPH oxidase components,33 is an intriguing possibility, whose further investigation may help to clarify the molecular basis of cytokine enhancement of the phagocyte respiratory burst capability.
Finally, we have also shown that GM-CSF–treated PMN may release the HB-EGF molecule into the culture medium. Because neither HER-1 nor HER-4 expression was detected and HB-EGF did not induce any modifications in PMN, we are inclined to rule out the possibility that soluble HB-EGF might act in an autocrine manner.
In conclusion, HB-EGF is a novel protein that can now be included in the repertoire of PMN-derived cytokines. It is not expressed in resting conditions, but is specifically inducible by GM-CSF, a factor involved in modulating a number of PMN functions.1,3 The role, if any, played by HB-EGF expressed in GM-CSF–stimulated PMN is less intuitive. Nevertheless, the fact that PMN produce HB-EGF is of interest, considering that a relationship has been detected between HB-EGF and pivotal biological activities12,53 which, up until very recently, were rarely or never associated with PMN. HB-EGF has been reported as playing a role in reproductive biology,58,59 wound healing,60 atheromatous phenomena,31,61 angiogenesis,27 and epithelial neoplastic growth.21 Remarkably, a number of epithelial neoplasias, for which HB-EGF is an autocrine growth factor and, possibly, an angiogenetic factor through VEGF induced in vascular SMC,27 are capable of producing and releasing GM-CSF62-64 and are often infiltrated by PMN, especially when necrosis occurs.65 Thus, although the relationship between infiltrating PMN and neoplastic cells is fairly elusive, PMN may provide an unexpected source of a proliferative, angiogenetic factor such as HB-EGF, thus participating in the progression of cancer.21 Similar findings have been reported with regard to neoplasia-infiltrating T lymphocytes that have been shown to produce HB-EGF, when, as has been reported, exposed to a nonsupporting environment.21 In PMN, however, we have documented a specific mechanism of activation for HB-EGF that may match the specific biological properties of some cancers.62-65 Anyway, the fact that PMN can synthesize, store, and release a substantial array of cytokines, including HB-EGF, lends further support to the suggestion that the role of PMN in physiopathology needs to be redefined.
ACKNOWLEDGMENT
The authors thank Dr E. Papini for kindly providing highly purified DT, Dr S. Higashiyama for his generous gift of anti–HB-EGF antibodies, and F. Calzetti for her excellent technical assistance.
Supported by grants from MURST (60% funds and “cofinanziamento MURST-Università 40%”), Associazione Italiana per la Ricerca sul Cancro (AIRC, Milano, Italy) and Progetto Sanità 96/97, Fondazione Cassa di Risparmio VR-VI-BL-AN (Verona, Italy).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Fabrizio Vinante, MD, Cattedra di Ematologia, Policlinico GB Rossi, 37134 Verona, Italy; e-mail:VINANTE@borgoroma.univr.it; Marco A. Cassatella, MD, Patologia Generale, 37134 Verona, Italy; e-mail: MCNCSS@BORGOROMA.UNIVR.IT.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal