Abstract
X-linked severe combined immunodeficiency (XSCID) is a life-threatening syndrome in which both cellular and humoral immunity are profoundly compromised. This disease results from mutations in theIL2RG gene, which encodes the common cytokine receptor γ chain, γc. Previously, we generated γc-deficient mice as a murine model of XSCID. We have now used lethally irradiated γc-deficient mice to evaluate a gene therapeutic approach for treatment of this disease. Transfer of the human γc gene to repopulating hematopoietic stem cells using an ecotropic retrovirus resulted in an increase in T cells, B cells, natural killer (NK) cells, and intestinal intraepithelial lymphocytes, as well as normalization of the CD4:CD8 T-cell ratio and of serum Ig levels. In addition, the restored cells could proliferate in response to interleukin-2 (IL-2). Thus, our results provide added support that gene therapy is a feasible therapeutic strategy for XSCID. Moreover, because we used a vector directing expression of human γc to correct a defect in γc-deficient mice, these data also indicate that human γc can cooperate with the distinctive cytokine receptor chains such as IL-2Rβ and IL-7R to mediate responses to murine cytokines in vivo.
X-LINKED SEVERE COMBINED immunodeficiency (XSCID) is a life-threatening disease characterized by greatly diminished numbers of T and natural killer (NK) cells.1-3 Although B cells are present in normal numbers in XSCID patients, they are nonfunctional.1-3 XSCID is caused by mutations in the common cytokine receptor γ chain, γc,4 which is a component of the receptors for interleukin-2 (IL-2), IL-4, IL-7, IL-9, and IL-15.5-11The simultaneous inactivation of these 5 different cytokine systems therefore helps to explain the severity of the immunological defects in this disease, with defective IL-7 signaling likely explaining the defective T-cell development,12 and defective IL-15 signaling explaining the defective NK-cell development.12aXSCID is the most common form of SCID, accounting for approximately half of all cases of SCID.1-3 Although most children with XSCID can be successfully treated by haploidentical bone marrow transplantation, in many of these individuals, the donor T cells engraft, but B cells do not, resulting in clinically relevant hypogammaglobulinemia that necessitates chronic intravenous gammaglobulin therapy.13 14 Therefore, the development of gene therapy for individuals with this disease could represent a substantial therapeutic advance.
Previously, our group and others have generated γc-expressing retroviral vectors, which can infect mammalian cells in vitro.15-18 More recently, investigators have used retroviral transduction of bone marrow to direct γc expression in peripheral lymphocytes in normal dogs.19 Finally, γc has been transduced into bone marrow from XSCID hematopoietic stem cells with the maturation of CD34+ bone marrow cells into double positive (CD4+CD8+) and single positive (CD4+CD8− and CD4−CD8+) lymphocytes in a hybrid human/mouse fetal thymic organ culture.20 As a logical next step towards the eventual development of gene therapy for this disease, we attempted to correct the immunological defect in mice in which γc had been deleted by homologous recombination. Such an approach has been successfully performed for mice lacking expression of Jak3.21 Jak3 deficiency is an autosomal recessive form of SCID that is clinically and immunologically similar to XSCID.22 23
Like humans with XSCID, γc-deficient mice exhibit marked T-cell and NK-cell defects.24-26 Examination of γc-deficient mice has also shown the absence of “natural” NK1.1+ T cells as well as γδ T cells, including, for example, dendritic epidermal T cells. In addition, these mice lack both αβ and γδ intestinal intraepithelial lymphocytes (IELs).24,25 A major difference between humans and mice lacking γc expression is that B cells develop in humans with XSCID (although they are dysfunctional), but B-cell development is markedly diminished in the γc-deficient mice.24-26 This latter difference appears to result from the importance of IL-7 signaling for murine B-cell development, whereas this function in humans is either served by another cytokine or is redundant.12 Thus, while the murine syndrome is somewhat different from human XSCID, the γc-deficient mice nevertheless are valuable models for determining whether a gene therapeutic approach can result in correction of the defects in lymphoid development and T-cell function. We chose to use an ecotropic retrovirus directing expression of human γc to attempt gene therapy in the γc-deficient mice. Transduction of donor γc-deficient bone marrow with this virus followed by bone marrow transplantation into γc-deficient mice increased expression of T cells, B cells, and NK cells. This establishes the potential efficacy of gene therapy and also demonstrates the ability of human γc to cooperate with the relevant murine cytokines and their distinctive receptor components.
MATERIALS AND METHODS
Retroviral producer cell lines.
The full-length human γc cDNA was excised from pCRScript using BsaI and BamHI and cloned into the NcoI and BamHI sites of the pMMP vector to generate pMMP-γc; the construction of pMMP will be reported in detail elsewhere by Drs Jeng-Shin Lee and Richard C. Mulligan (Harvard Medical School, Boston, MA), who generously provided this vector to us. Briefly, the vector uses the myeloproliferative sarcoma virus (MPSV) long terminal repeats (LTRs) and glutamine tRNA primer binding site (PBSQ) instead of the wild-type proline PBS. Both modifications were introduced to improve gene expression.27 pMMP-γc was transfected together with pCDNA3.1 (which carries the Neomycin resistance gene; Invitrogen, Carlsbad, CA) using the calcium phosphate method into the Phoenix ecotropic packaging cell line (provided by Dr Garry Nolan, Stanford University, Stanford, CA; seehttp://cmgm.stanford.edu/micro/fac/nolan.html) and the 293 SPA amphotropic packaging cell line (provided by Dr H.L. Malech, National Institutes of Health [NIH]), and stable clones were selected in G418 (Life Technologies, Grand Island, NY). Supernatants from isolated, stable producer Phoenix and 293 SPA clones were screened for their ability to induce expression of human γc in NIH3T3 cells, as assessed by flow cytometric analysis.
Bone marrow transduction and transplantation.
The γc-deficient mice in this study have been previously described25,28 29 and were back-crossed for more than 10 generations to C57BL/6 mice. Wild-type C57BL/6 or γc-deficient donor mice (6 to 10 weeks old) were injected intravenously with 5-fluorouracil (150 mg/kg body weight) 48 hours before harvesting of bone marrow. Marrow was flushed from both lower limbs and prestimulated for 48 hours in bone marrow medium (Dulbecco’s modified Eagle’s medium [DMEM] containing 15% fetal bovine serum, 4 mmol/L L-glutamine plus 20 ng/mL murine IL-3, 50 ng/mL murine IL-6, and 100 ng/mL murine stem cell factor (cytokines were all from PeproTech, Rocky Hill, NJ). Bone marrow cells were then cocultured for 48 hours with the Phoenix producer cells in the same medium with 6 μg/mL polybrene (Sigma, St Louis, MO) at 32°C. Cells were then transferred to plates coated with RetroNectin (recombinant human fibronectin fragment CH-296, 20 μg/cm2; TaKaRa Biomedicals, Shiga, Japan) and infected for 24 hours at 32°C with viral supernatant. The viral supernatant was generated by culturing a human γc virus-producing Phoenix cell clone in bone marrow medium for 16 hours. After a further 24-hour recovery period in fresh bone marrow medium, cells were collected and approximately 1 × 106 cells were injected intravenously into 6- to 8-week-old recipient C57BL/6 γc-deficient mice that had been lethally irradiated with 800 rads. Mock-transduced mice were handled identically except that bone marrow cells were cocultured with untransfected Phoenix cells that were not producing virus. Bone marrow cells from wild-type mice were just maintained in culture and were not exposed to Phoenix cells or virus.
Transduction of human Epstein-Barr virus (EBV)-transformed B-cell line from a patient with XSCID.
EBV-transformed B cells from a patient with XSCID (provided by Dr J.M. Puck, NIH, IL-2RG database cDNA number 830del 4[3] containing a deletion/frameshift at Leu 272; seehttp://www.nhgri.nih.gov/DIR/LGT/SCID/scid_query_result.hts?ExonIntronNumber=6) were maintained in RPMI 1640 medium supplemented with 2 mmol/L L-glutamine, 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were transduced with the γc-expressing amphotropic retrovirus, which was collected after 12 to 16 hours culture of the confluent 293 SPA virus-producing clone. The infection was performed during 3 24-hour cycles of incubation on RetroNectin-coated tissue culture dishes at 32°C in the presence of 6 μg/mL of polybrene. The cells were allowed to recover in growth medium for 30 hours before fluorescence-activated cell sorting (FACS) analysis or stimulation with IL-2 or IL-4.
Polymerase chain reaction (PCR) detection of transduced gene in peripheral blood.
A total of 50 μL of peripheral blood was obtained by retroorbital bleed, and DNA was isolated using the Instagene Whole Blood kit (Bio-Rad, Hercules, CA). Primers used to amplify the human γc cDNA were as follows: forward primer (M10), 5′-CTCTTATTCCTGCAGCTGCC-3′; reverse primer (M19), 5′-CGTTCCAGCCAGAAATACAC-3′. Amplification of human γc was performed using 10 μL DNA per reaction for 40 cycles of 45 seconds at 94°C, 45 seconds at 58°C, and 90 seconds at 72°C.
Flow cytometric analysis.
Fresh cells from thymus, spleen, and bone marrow were stained and analyzed on a FACSort (Becton Dickinson, San Jose, CA) using CellQuest software. Splenocyte cell suspensions were treated with ACK lysing buffer to remove red blood cells. Some splenocytes were also removed for 48-hour stimulation in 6-well plates coated with 10 μg/mL of anti-mouse CD3ε (145/2C11 monoclonal antibody [MoAb]). Cells were then stained with the following antibodies (PharMingen, San Diego, CA): fluoroscein isothiocyanate (FITC)-conjugated anti-mouse IgM, Cy-chrome–conjugated anti-mouse CD45R/B220, FITC-conjugated anti-mouse CD25 (anti–IL-2Rα, PC61), phycoerythrin (PE)-conjugated anti-mouse CD62L (L-selectin), Cy-chrome–conjugated anti-mouse CD4 (L3T4), allophycocyanin (APC)-conjugated anti-mouse CD8α (Ly-2) (53-6.7), APC-conjugated anti-mouse CD3ε (145-2C11), PE-conjugated anti-mouse NK-1.1, Cy-chrome–conjugated anti-mouse T-cell receptor (TCR) β (H57-597), and PE-conjugated TUGh4 MoAb (against human γc). To eliminate possible binding to Fc receptors, anti-mouse CD16/CD32 MoAb (2.4G2) was added during staining. EBV-transformed B cells that were not transduced or transduced with the γc retrovirus were stained with PE-conjugated mouse anti-human γc MoAb AG184 or a PE-conjugated mouse isotype-matched control MoAb (PharMingen).
Quantitation of serum Igs.
Serum was obtained from mice at the time of sacrifice by retroorbital bleed. Total serum IgA, IgG, and IgM levels were measured by sandwich enzyme-linked immunosorbent assay (ELISA) using Ig quantitation kits according to the manufacturer’s instructions (Bethyl Laboratories, Montgomery, TX). Briefly, 96-well, flat-bottom ELISA plates (Immunon 4TM; Dynex, Chantilly, VA) were coated with a polyclonal goat anti-mouse Ig Fc-specific antibody. Serum samples or a standard reference serum were serially diluted in phosphate-buffered saline (PBS) with 1% (wt/vol) bovine serum albumin (Sigma) and added to the plates. Bound Igs were detected using horseradish peroxidase (HRP)-labeled goat anti-mouse Ig Fc-specific antibodies followed by addition of the HRP-substrate o-phenylenediamine (1 mg/mL; Sigma) and H2O2 (0.03%) in phosphate-citrate buffer (pH 5.0). Serum Ig levels were determined by comparing the absorbance values (450 nm) of the samples with the values from the linear portion of the curve generated with the reference serum.
Splenocyte proliferation.
Splenocytes prepared as above were plated in triplicate in a 96-well plate at 2 × 105 cells/well in RPMI 1640 medium containing 10% fetal bovine serum, 2 mmol/L L-glutamine, and antibiotics. The cells were stimulated for 48 hours at 37°C with and without human IL-2 (2 nmol/L, kindly provided by Hoffmann LaRoche, Nutley, NJ). Cells were then pulsed with 1 μCi/well of methyl-3H-thymidine (NEN, Boston, MA) for 8 hours. 3H-thymidine uptake was then determined using a beta plate counter.
Transfection of 293T cells.
293T cells (a human embryonic kidney cell line expressing SV40 large T antigen) were transfected by the calcium phosphate method (transfection kit from 5′-3′, Inc, Boulder, CO). One million cells were plated into 100-mm tissue culture dishes 24 hours before transfection with 2 μg of murine IL-7Rα, 2 μg of Jak3, 0.5 μg each of Stat5a and Stat5b, and 2 μg of murine or human γc expression vectors. Each of these cDNAs was cloned in pME18S, a eukaryotic expression vector in which transcription is driven by the SRα promoter.30 Twenty-four hours after transfection, cells from each dish were split between 2 × 100 mm dishes and 24 hours later, cells were either stimulated or not stimulated with murine or human IL-7 for 10 minutes at 37°C, washed with cold PBS, and nuclear extracts were prepared.
Isolation of murine IL-7Rα cDNA.
The murine IL-7Rα cDNA was generated by PCR-mediated amplification of mRNA from C57BL/6 lymphocytes and from 70Z/3 cells using primers based on the published murine IL-7Rα sequence.31 DNA sequencing of our cDNAs in each case showed 5 nucleotide alterations corresponding to 4 amino acid changes, as compared with the previously reported murine IL-7Rα sequence.31 Our sequence has been submitted to GenBank (Accession no. AF078906).
Electrophoretic mobility shift assays (EMSAs).
Whole-cell or nuclear extracts were prepared as previously described.32 The β-casein, PRRIII, and FcγRI probes were labeled with 32P-deoxycytidine triphosphate (dCTP) and the Klenow fragment of DNA polymerase, as previously described.33 Samples (15 μg of whole-cell extract or 7 μg of nuclear extract) were preincubated with 2 μg of poly dI-dC for 20 to 30 minutes and then the probe was added and reaction mixtures were incubated on ice for an additional 20 minutes. Samples were then run on native 6% polyacrylamide gels in 0.5 × tris/borate/EDTA (TBE) buffer.
Histology of intestinal tissue.
Whole intestines were dissected from control or bone marrow transplanted mice and fixed in 10% buffered formalin. Ten-micrometer paraffin sections were stained with hematoxylin and eosin and evaluated by standard light microscopy. Intestinal IEL were identified as small cells with densely staining nuclei that are located above the epithelial basement membrane and below the plane of the epithelial cell nuclei. Goblet cells, the nuclei of which are in the same location as IEL, were distinguished from IEL by the presence of mucin vacuoles. Sections from different mice were evaluated blindly and 2 experiments were performed with similar results.
RESULTS
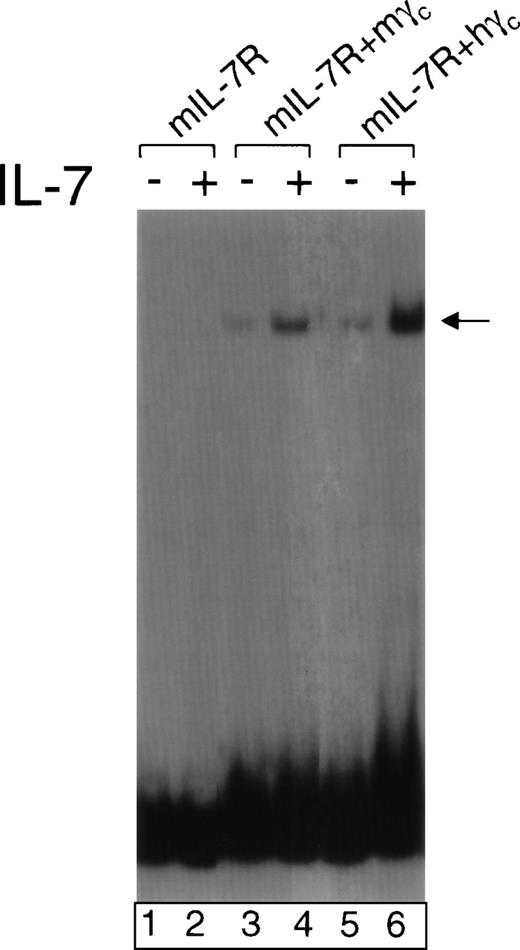
As a step toward evaluating possible gene therapy for XSCID, we assessed whether a human γc–expressing retrovirus could correct the immunological defects in γc-deficient mice. Previously, it was established that murine γc can functionally cooperate with human IL-2Rβ in mediating responses to human IL-2.34,35 Conversely, human γc can functionally cooperate with murine IL-4Rα in mediating responses to murine IL-4.7 However, the ability of human γc to cooperate with the other relevant cytokine receptor chains in response to murine γc-dependent cytokines has not been reported. Because the T-cell defect in γc-deficient mice is believed to result from defective IL-7 signaling, we investigated the ability of human γcto cooperate with murine IL-7Rα in response to murine IL-7. We transfected murine IL-7Rα together with human or murine γc into 293T cells along with Jak3, Stat5a, and Stat5b to create an in vitro reconstitution system similar to one previously developed for IL-2 signaling.36 Cells were then analyzed for the ability of murine IL-7 to induce Stat5 DNA binding activity as evaluated by EMSAs. As shown in Fig 1, like murine γc (lane 4), human γc cooperated with murine IL-7Rα to mediate a response to murine IL-7 (lane 6). This suggested that gene therapy using human γc to correct γc deficiency in mice was a rational experimental approach. Such an approach would potentially also determine the ability of human γc to cooperate with murine γc-dependent cytokines in vivo and if successful would allow the identical vector to be evaluated in murine and human cells.
Human γc can functionally cooperate with murine IL-7R in mediating the response to murine IL-7. 293T cells were transfected with expression vectors for Jak3, Stat5a, Stat5b, and the following: murine IL-7R alone (lanes 1 and 2), murine IL-7R + murine γc (lanes 3 and 4), or murine IL-7R + human γc (lanes 5 and 6). Forty-eight hours later, they were not stimulated (lanes 1, 3, and 5) or stimulated with 1 nmol/L murine IL-7 (lanes 2, 4, and 6). Nuclear extracts were prepared and EMSAs performed using the PRRIII probe corresponding to the IL-2 response element in the human IL-2R gene.37
Human γc can functionally cooperate with murine IL-7R in mediating the response to murine IL-7. 293T cells were transfected with expression vectors for Jak3, Stat5a, Stat5b, and the following: murine IL-7R alone (lanes 1 and 2), murine IL-7R + murine γc (lanes 3 and 4), or murine IL-7R + human γc (lanes 5 and 6). Forty-eight hours later, they were not stimulated (lanes 1, 3, and 5) or stimulated with 1 nmol/L murine IL-7 (lanes 2, 4, and 6). Nuclear extracts were prepared and EMSAs performed using the PRRIII probe corresponding to the IL-2 response element in the human IL-2R gene.37
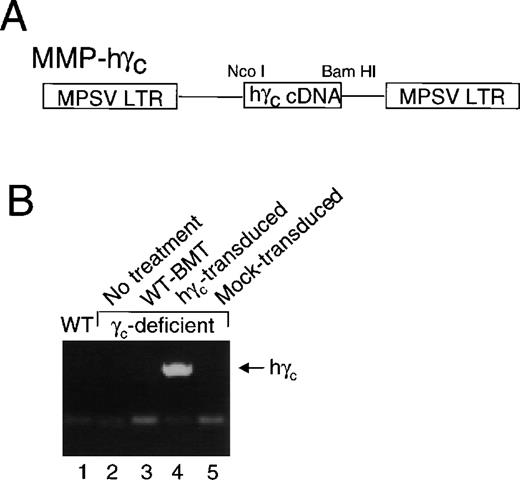
Therefore, we constructed a retroviral expression vector in which the γc cDNA was inserted into pMMP, a retroviral expression vector in which expression is under the control of the MPSV LTR. A schematic is shown in Fig 2A. We transfected Phoenix ecotropic retroviral packaging cells to make a stable clone producing the virus (see Materials and Methods).
pMMP-driven expression of γc expressed in peripheral blood of mice that received bone marrow transplantation of human γc-transduced γc-deficient bone marrow. (A) Schematic of pMMP-γc. (B) Human γc was detected by PCR of genomic DNA from peripheral blood cells from γc-deficient mice that received γc-deficient bone marrow transduced with human γc, but not from wild-type mice or γc-deficient mice that received no treatment, bone marrow from a wild-type mouse, or bone marrow from a mock-transduced γc-deficient mouse.
pMMP-driven expression of γc expressed in peripheral blood of mice that received bone marrow transplantation of human γc-transduced γc-deficient bone marrow. (A) Schematic of pMMP-γc. (B) Human γc was detected by PCR of genomic DNA from peripheral blood cells from γc-deficient mice that received γc-deficient bone marrow transduced with human γc, but not from wild-type mice or γc-deficient mice that received no treatment, bone marrow from a wild-type mouse, or bone marrow from a mock-transduced γc-deficient mouse.
Successful transduction of murine bone marrow cells with a retrovirus directing expression of human γc.
Bone marrow cells were harvested and treated as described in Materials and Methods. These cells were infected by coculture with virus-producing Phoenix cells, followed by continued exposure to viral supernatant on plates coated with RetroNectin. As a control, bone marrow cells were also cocultured with untransfected Phoenix cells (mock transduction). Approximately 1 × 106cells were injected intravenously into γc-deficient irradiated recipient mice, and the mice were returned to a standard animal facility. Six to 10 weeks later, we analyzed γc-deficient mice and wild-type mice as controls, as well as γc-deficient mice that had received bone marrow from wild-type mice or γc-transduced or mock-transduced bone marrow from γc-deficient mice. As shown in Fig 2B, human γc DNA was detected in peripheral blood cells from the γc-deficient mice that received bone marrow transduced with the human γc.
Reconstitution of T cells, B cells, and NK cells in γc-deficient mice.
Because of the greatly diminished numbers of T, B, and NK cells in γc-deficient mice,24-26 we evaluated the extent to which cellularity of these lineages was normalized by gene therapy. As shown in Fig 3A, the number of thymocytes was markedly increased in the mice receiving the γc-transduced, but not in mice receiving the mock-transduced, bone marrow. Similarly, although γc-deficient splenocytes exhibit a substantially decreased percent of CD3+ T cells with an elevated CD4:CD8 ratio as compared with wild-type mice (Fig 3B and C, second vfirst dot plots), both the percent of CD3+ T cells (Fig 3B) and the CD4:CD8 ratio (Fig 3C) were substantially corrected in the mice receiving γc-transduced bone marrow (fourth dot plot in each panel), but not in those receiving mock-transduced bone marrow (fifth dot plot in each panel). There was also partial correction of NK cells in the spleen (Fig 3D). Analysis of spleen (Fig 3B) and bone marrow (Fig 3E) in the γc-transduced mice also showed a substantial correction of the number of B cells. The numbers of reconstituted T cells, B cells, and NK cells in the spleen are shown in Table 1. Because γc-deficient mice exhibit a peripheral expansion of CD4+ T cells,25 37 it is only after irradiation that this population is diminished (in Table 1, compare mock-transduced to nontransduced mice). Thus, the reconstitution in γc-transduced mice is much better than that seen in the mock-transduced recipients and approximately equivalent to that seen in mice receiving wild-type bone marrow.
Successful reconstitution of T cells, B cells, and NK cells in γc-deficient mice by a gene therapy approach. (A) Increased thymocytes after reconstitution of γc-deficient mice with γc-deficient bone marrow that was transduced with human γc. Note that the ordinate is a log scale. (B and C) Increased splenic T cells (B) with normalization of the CD4:CD8 ratio (C) in γc-deficient mice with γc-deficient bone marrow that was transduced with human γc; (B) also indicates correction of the B-cell defect in splenocytes. (D) Increased numbers of splenic TCR−NK1.1+ cells after reconstitution of γc-deficient mice with γc-deficient bone marrow that was transduced with human γc. Note that the TCR+NK1.1− cells are partially diagonally shifted to the right (albeit still to the left of the NK1.1+ cells that are boxed in each dot plot); this is an artifact that resulted from the use of 4-color flow cytometry. (E) Increased B cells in bone marrow after reconstitution of γc-deficient mice with γc-deficient bone marrow that was transduced with human γc. In (B through E), we have selected representative dot plots for each of the cell types analyzed from our final 3 different gene therapy experiments in which all analyses were performed. Our initial experiments provided consistent information, but not all assays were included in those experiments. In the final 3 experiments, splenic cellularities (mean ± standard error of mean [SEM] × 10−6) were as follows: wild-type mice (87.0 ± 3.0, top dot plots), γc-deficient mice (49.0 ± 5.5, second dot plots), γc-deficient mice, which received wild-type bone marrow (77.8 ± 19.9, third dot plots), γc-deficient mice, which received human γc-transduced bone marrow (82.8 ± 15.6, fourth dot plots), and γc-deficient mice, which received mock-transduced bone marrow (20.8 ± 3.9, bottom dot plots). The data in (A) were from the final 2 experiments and those in (B) through (E) were representative of the final 3 experiments. The number of mice analyzed in each experiment was as follows: experiment 1 (mice analyzed at 63 days after bone marrow transplantation): 1 wild-type mouse, 2 γc KO mice (no treatment), 2 γc KO mice (WT-BMT), 3 γc KO mice (hγc-transduced), and 1 γc KO mouse (mock-transduced). Experiment 2 (mice analyzed at 62 days after bone marrow transplantation): 2 wild-type mice, 2 γc KO mice (no treatment), 1 γc KO mouse (WT-BMT), 2 γc KO mice (hγc-transduced), and 1 γc KO mouse (mock-transduced). Experiment 3 (mice analyzed at 52 days after bone marrow transplantation): 1 wild-type mouse, 2 γc KO mice (no treatment), 1 γc KO mouse (WT-BMT), 3 γc KO mice (hγc-transduced), and 2 γc KO mice (mock-transduced). Experiment 3 was not included in (A) because thymocyte numbers were not counted.
Successful reconstitution of T cells, B cells, and NK cells in γc-deficient mice by a gene therapy approach. (A) Increased thymocytes after reconstitution of γc-deficient mice with γc-deficient bone marrow that was transduced with human γc. Note that the ordinate is a log scale. (B and C) Increased splenic T cells (B) with normalization of the CD4:CD8 ratio (C) in γc-deficient mice with γc-deficient bone marrow that was transduced with human γc; (B) also indicates correction of the B-cell defect in splenocytes. (D) Increased numbers of splenic TCR−NK1.1+ cells after reconstitution of γc-deficient mice with γc-deficient bone marrow that was transduced with human γc. Note that the TCR+NK1.1− cells are partially diagonally shifted to the right (albeit still to the left of the NK1.1+ cells that are boxed in each dot plot); this is an artifact that resulted from the use of 4-color flow cytometry. (E) Increased B cells in bone marrow after reconstitution of γc-deficient mice with γc-deficient bone marrow that was transduced with human γc. In (B through E), we have selected representative dot plots for each of the cell types analyzed from our final 3 different gene therapy experiments in which all analyses were performed. Our initial experiments provided consistent information, but not all assays were included in those experiments. In the final 3 experiments, splenic cellularities (mean ± standard error of mean [SEM] × 10−6) were as follows: wild-type mice (87.0 ± 3.0, top dot plots), γc-deficient mice (49.0 ± 5.5, second dot plots), γc-deficient mice, which received wild-type bone marrow (77.8 ± 19.9, third dot plots), γc-deficient mice, which received human γc-transduced bone marrow (82.8 ± 15.6, fourth dot plots), and γc-deficient mice, which received mock-transduced bone marrow (20.8 ± 3.9, bottom dot plots). The data in (A) were from the final 2 experiments and those in (B) through (E) were representative of the final 3 experiments. The number of mice analyzed in each experiment was as follows: experiment 1 (mice analyzed at 63 days after bone marrow transplantation): 1 wild-type mouse, 2 γc KO mice (no treatment), 2 γc KO mice (WT-BMT), 3 γc KO mice (hγc-transduced), and 1 γc KO mouse (mock-transduced). Experiment 2 (mice analyzed at 62 days after bone marrow transplantation): 2 wild-type mice, 2 γc KO mice (no treatment), 1 γc KO mouse (WT-BMT), 2 γc KO mice (hγc-transduced), and 1 γc KO mouse (mock-transduced). Experiment 3 (mice analyzed at 52 days after bone marrow transplantation): 1 wild-type mouse, 2 γc KO mice (no treatment), 1 γc KO mouse (WT-BMT), 3 γc KO mice (hγc-transduced), and 2 γc KO mice (mock-transduced). Experiment 3 was not included in (A) because thymocyte numbers were not counted.
Number of Splenic T Cells, B Cells, and NK Cells in Wild-Type and Reconstituted γc KO Mice
| Mice . | BMT . | CD3+B220− (T cells) . | CD3−B220+ (B cells) . | TCRβ−NK1.1+ (NK cells) . |
|---|---|---|---|---|
| Wild-type (n = 4) | — | 25.9 ± 1.1 | 54.2 ± 2.7 | 2.1 ± 0.22 |
| γcKO (n = 6)* | — | 7.8 ± 2.5 | 0.6 ± 0.1 | 0.04 ± 0.01 |
| γcKO (n = 4) | Wild-type BM | 13.5 ± 3.8 | 40.7 ± 9.6 | 1.17 ± 0.35 |
| γcKO (n = 8) | hγc-transduced γcKO BM | 14.6 ± 2.8 | 45.5 ± 12.7 | 0.62 ± 0.1 |
| γcKO (n = 4) | Mock-transduced γcKO BM | 1.1 ± 0.2 | 0.1 ± 0.1 | 0.03 ± 0.02 |
| Mice . | BMT . | CD3+B220− (T cells) . | CD3−B220+ (B cells) . | TCRβ−NK1.1+ (NK cells) . |
|---|---|---|---|---|
| Wild-type (n = 4) | — | 25.9 ± 1.1 | 54.2 ± 2.7 | 2.1 ± 0.22 |
| γcKO (n = 6)* | — | 7.8 ± 2.5 | 0.6 ± 0.1 | 0.04 ± 0.01 |
| γcKO (n = 4) | Wild-type BM | 13.5 ± 3.8 | 40.7 ± 9.6 | 1.17 ± 0.35 |
| γcKO (n = 8) | hγc-transduced γcKO BM | 14.6 ± 2.8 | 45.5 ± 12.7 | 0.62 ± 0.1 |
| γcKO (n = 4) | Mock-transduced γcKO BM | 1.1 ± 0.2 | 0.1 ± 0.1 | 0.03 ± 0.02 |
Values are presented as mean number of cells × 10−6 ± SEM.
Abbreviations: BM, bone marrow; BMT, bone marrow transplantation.
n = 5 for NK cell numbers.
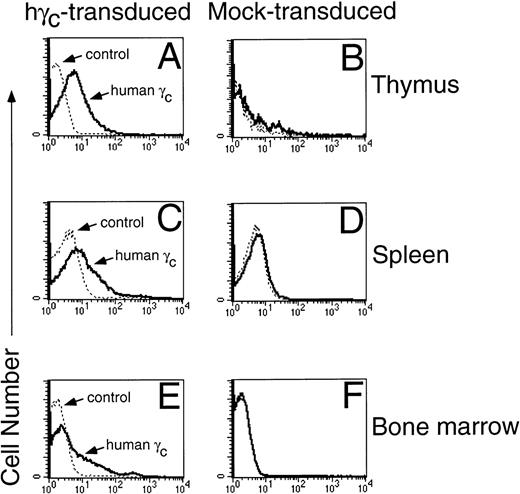
Whereas splenocytes from γc-deficient mice transplanted with mock-transduced bone marrow did not exhibit native and IL-2–induced lytic activity against the NK-sensitive target cell, YAC-1, splenocytes from γc-transduced mice had detectable cytolytic activity, suggesting that the NK cells reconstituted in these mice were functional (data not shown). The reconstituted B cells were also functional as evaluated by the augmentation of IgA, IgG, and IgM Ig levels associated with reconstitution (Table 2). In addition to correction of lymphoid populations in the thymus, spleen, and bone marrow, transduction of γc allowed the reconstitution of intraepithelial lymphocytes in the intestine (Fig 4). Consistent with the reconstitution of these lymphocyte populations, we confirmed expression of human γc in thymocytes, splenocytes, and bone marrow cells, as evaluated by flow cytometry using TUGh4 MoAb to human γc(PharMingen) (compare Fig 5A, C, and E to Fig 5B, D, and F). Note that in the thymus, it is evident that the entire peak is shifted, suggesting that most or all of the thymocytes express human γc. In the spleen, γc was expressed on CD3+B220−, CD3−B220+, and CD3−B220− cells. In the bone marrow, both B220+ and B220− populations expressed γc (data not shown). Based on forward versus side scatter, the lymphoid cells in the bone marrow expressed more γc than did the myeloid cells (data not shown).
Serum Ig Levels in Wild-Type and Reconstituted γc KO Mice
| Mice . | BMT . | Serum Ig Levels (μg/mL) . | ||
|---|---|---|---|---|
| IgG . | IgA . | IgM . | ||
| Wild-type (n = 4) | — | 5,300 ± 1,041 | 2,574 ± 873 | 540 ± 192 |
| γcKO (n = 6) | — | 198 ± 151 | 153 ± 8 | 309 ± 101 |
| γcKO (n = 4) | Wild-type BM | 6,953 ± 132 | 5,504 ± 1,615 | 1,273 ± 513 |
| γcKO (n = 8) | hγc-transduced γcKO BM | 4,554 ± 696 | 2,890 ± 1,097 | 1,610 ± 183 |
| γcKO (n = 4) | Mock-transduced γcKO BM | 151 ± 63 | 102 ± 43 | 372 ± 190 |
| Mice . | BMT . | Serum Ig Levels (μg/mL) . | ||
|---|---|---|---|---|
| IgG . | IgA . | IgM . | ||
| Wild-type (n = 4) | — | 5,300 ± 1,041 | 2,574 ± 873 | 540 ± 192 |
| γcKO (n = 6) | — | 198 ± 151 | 153 ± 8 | 309 ± 101 |
| γcKO (n = 4) | Wild-type BM | 6,953 ± 132 | 5,504 ± 1,615 | 1,273 ± 513 |
| γcKO (n = 8) | hγc-transduced γcKO BM | 4,554 ± 696 | 2,890 ± 1,097 | 1,610 ± 183 |
| γcKO (n = 4) | Mock-transduced γcKO BM | 151 ± 63 | 102 ± 43 | 372 ± 190 |
Values are presented as mean ± SEM.
Abbreviations: BM, bone marrow; BMT, bone marrow transplantation.
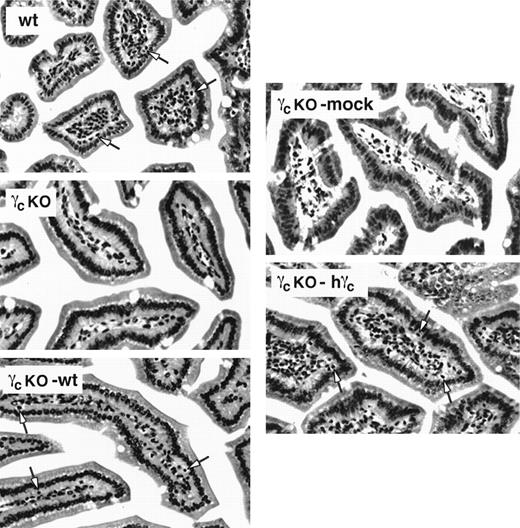
Reconstitution of IEL in γc-deficient mice that received γc-transduced bone marrow. Paraffin sections of small intestinal tissue were stained with hematoxylin and eosin and evaluated for the presence of IEL by light microscopy (40×). wt, wild-type mouse; γcKO, γc-deficient mouse that received no treatment; γcKO-mock, γc-deficient mouse that received mock-transduced γc-deficient bone marrow; γcKO-wt, γc-deficient mouse that received wild-type bone marrow; γcKO-hγc, γc-deficient mouse that received γc-deficient bone marrow transduced with human γc. Sections shown are representative of results from 2 separate experiments. Arrows indicate IEL in the wt, γcKO-wt, and γcKO-hγc mice.
Reconstitution of IEL in γc-deficient mice that received γc-transduced bone marrow. Paraffin sections of small intestinal tissue were stained with hematoxylin and eosin and evaluated for the presence of IEL by light microscopy (40×). wt, wild-type mouse; γcKO, γc-deficient mouse that received no treatment; γcKO-mock, γc-deficient mouse that received mock-transduced γc-deficient bone marrow; γcKO-wt, γc-deficient mouse that received wild-type bone marrow; γcKO-hγc, γc-deficient mouse that received γc-deficient bone marrow transduced with human γc. Sections shown are representative of results from 2 separate experiments. Arrows indicate IEL in the wt, γcKO-wt, and γcKO-hγc mice.
Expression of human γc protein in γc-transduced, but not mock-transduced mice. Human γc was expressed on the surface of thymocytes (A and B), splenocytes (C and D), and bone marrow cells (E and F). Shown are representative mice from 1 of 5 experiments. Cells were stained with TUGh4 rat anti-human γc (PharMingen) or a rat IgG2bκ isotype control.
Expression of human γc protein in γc-transduced, but not mock-transduced mice. Human γc was expressed on the surface of thymocytes (A and B), splenocytes (C and D), and bone marrow cells (E and F). Shown are representative mice from 1 of 5 experiments. Cells were stained with TUGh4 rat anti-human γc (PharMingen) or a rat IgG2bκ isotype control.
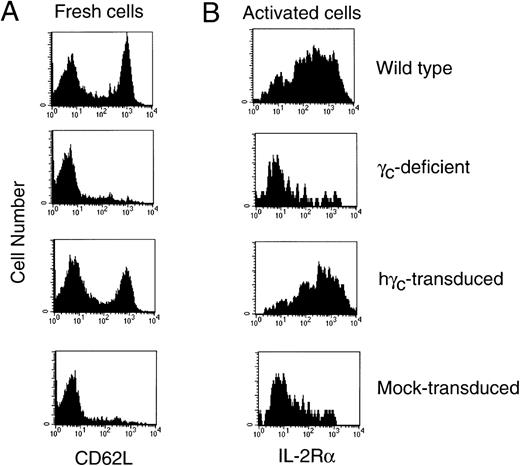
Partial correction of the CD4:CD8 T-cell ratio and IL-2–induced signaling by retroviral transduction of human γc in γc-deficient mice.
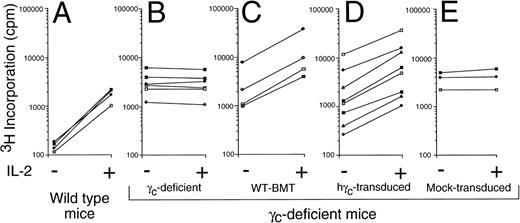
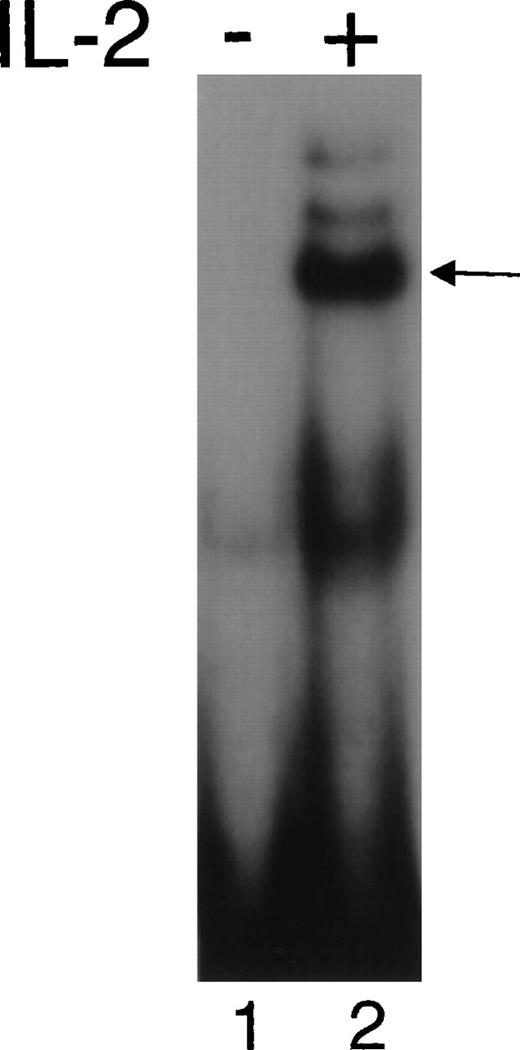
As previously reported, splenic T cells from γc-deficient mice have an activated memory phenotype, with an increase in CD62Llow cells.28 However, after retroviral transduction with the γc retrovirus, the wild-type naive pattern was restored (Fig6A). Moreover, anti-CD3 stimulation potently induced IL-2Rα (CD25) expression on the γc-transduced, but not mock-transduced CD4+ T cells (Fig 6B). As shown in Fig 7, we also evaluated the ability of the splenocytes from variously treated mice to respond to IL-2. Splenocytes from wild-type mice exhibited the greatest relative increase in proliferation in response to IL-2 and cells from γc-deficient mice receiving mock-transduced γc-deficient bone marrow did not proliferate in response to IL-2. Interestingly, splenocytes from mice receiving either γc-transduced or wild-type bone marrow proliferated to a similar extent. The absolute increase in proliferation was as high as seen with wild-type mice in 5 of 8 γc-transduced mice examined, but the relative increase was lower due to the very low basal proliferation in wild-type mice. The reason for the differences in basal proliferation levels is unclear. Note that splenocytes from γc-deficient mice (Fig 7B) have a similarly high basal proliferation in response to IL-2 as compared with splenocytes from γc-deficient mice receiving any type of bone marrow transplant (Fig 7C, D, and E). Finally, as shown in Fig 8, splenocytes from mice that received γc-transduced bone marrow could respond to IL-2 effectively, as judged by Stat5 DNA binding activity.
Normalization of T-cell activation markers after gene therapy of γc-deficient mice. (A) CD4+ T cells from γc-deficient mice transduced with human γc exhibited normalization of CD62L expression. (B) CD4+ T cells from γc-transduced mice exhibited normalization of anti-CD3–induced IL-2R expression, whereas cells from mock-transduced mice did not. The specificity of this finding was confirmed by the use of an isotype-matched antibody (data not shown). Shown are data for wild-type and γc-deficient mice or γc-deficient mice that receive γc-deficient bone marrow mock-transduced or transduced with human γc. Shown are representative mice from 1 of 5 experiments.
Normalization of T-cell activation markers after gene therapy of γc-deficient mice. (A) CD4+ T cells from γc-deficient mice transduced with human γc exhibited normalization of CD62L expression. (B) CD4+ T cells from γc-transduced mice exhibited normalization of anti-CD3–induced IL-2R expression, whereas cells from mock-transduced mice did not. The specificity of this finding was confirmed by the use of an isotype-matched antibody (data not shown). Shown are data for wild-type and γc-deficient mice or γc-deficient mice that receive γc-deficient bone marrow mock-transduced or transduced with human γc. Shown are representative mice from 1 of 5 experiments.
γc-transduced mice exhibited IL-2–induced proliferation of fresh splenocytes, whereas mock-transduced mice did not. 3H-thymidine incorporation assays were performed as described in Materials and Methods for wild-type mice (A), γc-deficient mice (B), or in γc-deficient mice that received a bone marrow transplant from wild-type mice (C) or γc-deficient bone marrow that was transduced with human γc (D) or mock-transduced (E). Shown are pooled data from 3 experiments; each pair of connected points represents an individual mouse.
γc-transduced mice exhibited IL-2–induced proliferation of fresh splenocytes, whereas mock-transduced mice did not. 3H-thymidine incorporation assays were performed as described in Materials and Methods for wild-type mice (A), γc-deficient mice (B), or in γc-deficient mice that received a bone marrow transplant from wild-type mice (C) or γc-deficient bone marrow that was transduced with human γc (D) or mock-transduced (E). Shown are pooled data from 3 experiments; each pair of connected points represents an individual mouse.
γc-transduced mice exhibited IL-2–induced STAT DNA binding activity. Whole-cell extracts were prepared from splenocytes that were cultured without or with 2 nmol/L IL-2 for 15 minutes, and EMSAs were performed using the β-casein probe.
γc-transduced mice exhibited IL-2–induced STAT DNA binding activity. Whole-cell extracts were prepared from splenocytes that were cultured without or with 2 nmol/L IL-2 for 15 minutes, and EMSAs were performed using the β-casein probe.
In view of our successful transduction of murine bone marrow, we also prepared an amphotropic version of the pMMP-human γcvirus. As expected based on previous studies,16-18 this virus could successfully direct the expression of human γc in an EBV-transformed B-cell line derived from a patient with XSCID and moreover could confer to these cells the ability to mediate IL-2–induced and IL-4–induced STAT protein activation (data not shown).
DISCUSSION
XSCID is a severe inherited disease that is uniformly fatal when bone marrow transplantation is not successful. In this study, we used γc-deficient mice as a model for human XSCID and tested whether a retroviral-mediated gene therapeutic approach could correct in vivo the defects in these mice. Whereas no correction was detected in mice that received mock-transduced γc-deficient bone marrow, substantial correction of lymphoid development of T cells, NK cells, and B cells was observed in mice receiving γc-transduced bone marrow. The T cells not only increased in number, but their cell surface phenotypic profiles also normalized. Specifically, the CD4:CD8 ratio largely was corrected, and resting T cells no longer exhibited a memory-activated phenotype (increased CD62L expression). Moreover, T-cell function was partially restored as demonstrated by responsiveness to IL-2. In addition, intestinal intraepithelial lymphocytes were reconstituted. B-cell function also improved as evaluated by the increase in serum Ig levels. Importantly, the retrovirally transduced bone marrow was almost as effective as wild-type bone marrow. As such, these studies demonstrate functional reconstitution in a murine in vivo model of XSCID. These studies complement a study in which mice deficient in Jak3 were treated by a similar gene therapy type of approach.21 Thus, for both of the two major forms of T−B+NK− SCID, gene therapy has been tested and demonstrated to be effective in murine models. Although we have demonstrated that reconstitution occurred, the latest time point at which we analyzed mice in this study was 63 days postbone marrow transplantation. Thus, it is unknown whether life-long reconstitution was achieved, and it is possible that the number of transduced clones might decrease with time. Nevertheless, it is relevant that approximately 80% of lymphocyte engraftment typically occurs within 2 months after bone marrow transplantation.38Moreover, it is reasonable to hypothesize that γc-transduced cells might exhibit a growth advantage and/or augmented survival given their ability to respond to γc-dependent cytokines, which are well-known to induce both proliferative and antiapoptotic signals.39
The fact that the retroviral transduction of human γceffectively restored T- and NK-cell development, as well as IL-2 signaling in mice, indicates that human γc successfully cooperated with murine IL-2Rβ (which is essential for IL-2–dependent proliferation and IL-15–dependent development of NK cells) and with murine IL-7Rα (which is essential for IL-7–dependent development of T cells and B cells in mice) in response to the endogenous murine cytokines. The effectiveness of human γc virus in murine stem cell transduction also suggested that an amphotropic version of the virus used in this study might have potential use for human gene therapy. As a preliminary step in this direction, we confirmed that such a virus could successfully transduce an EBV-transformed B-cell line from a patient with XSCID and confer de novo responsiveness to IL-2 and IL-4.
In XSCID, haploidentical bone marrow transplantation is highly successful even though many individuals do not successfully engraft B cells. Thus, in an investigational gene therapy clinical protocol, one would want to assure that conventional therapy was still available even if gene therapy were not successful. In this regard, it is appealing to contemplate transduction of cord blood stem cells in the perinatal period, allowing ample time for conventional bone marrow transplantation should gene therapy be unsuccessful. As haploidentical bone marrow transplantation is not uniformly successful, gene therapy is obviously a desirable eventual approach for the treatment of XSCID.
ACKNOWLEDGMENT
We thank Drs Jeng-Shin Lee and Richard C. Mulligan, Harvard Medical School, for providing the pMMP vector; Dr Garry Nolan, Stanford University, for providing Phoenix cells; Dr Harry Malech and Gilda Linton, NIAID, for providing 293 SPA cells; Dr Jennifer Puck, NHGRI, for providing the human XSCID EBV line mentioned in the results; and Dr Atsushi Miyajima for the pME18S expression vector; and Drs Cynthia Dunbar and Harry Malech for valuable conversations/critical comments.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Warren J. Leonard, MD, Bldg 10, Room 7N252, Lab of Molecular Immunology, NHLBI, NIH, Bethesda, MD 20892-1674; e-mail: wjl@helix.nih.gov.

![Fig. 3. Successful reconstitution of T cells, B cells, and NK cells in γc-deficient mice by a gene therapy approach. (A) Increased thymocytes after reconstitution of γc-deficient mice with γc-deficient bone marrow that was transduced with human γc. Note that the ordinate is a log scale. (B and C) Increased splenic T cells (B) with normalization of the CD4:CD8 ratio (C) in γc-deficient mice with γc-deficient bone marrow that was transduced with human γc; (B) also indicates correction of the B-cell defect in splenocytes. (D) Increased numbers of splenic TCR−NK1.1+ cells after reconstitution of γc-deficient mice with γc-deficient bone marrow that was transduced with human γc. Note that the TCR+NK1.1− cells are partially diagonally shifted to the right (albeit still to the left of the NK1.1+ cells that are boxed in each dot plot); this is an artifact that resulted from the use of 4-color flow cytometry. (E) Increased B cells in bone marrow after reconstitution of γc-deficient mice with γc-deficient bone marrow that was transduced with human γc. In (B through E), we have selected representative dot plots for each of the cell types analyzed from our final 3 different gene therapy experiments in which all analyses were performed. Our initial experiments provided consistent information, but not all assays were included in those experiments. In the final 3 experiments, splenic cellularities (mean ± standard error of mean [SEM] × 10−6) were as follows: wild-type mice (87.0 ± 3.0, top dot plots), γc-deficient mice (49.0 ± 5.5, second dot plots), γc-deficient mice, which received wild-type bone marrow (77.8 ± 19.9, third dot plots), γc-deficient mice, which received human γc-transduced bone marrow (82.8 ± 15.6, fourth dot plots), and γc-deficient mice, which received mock-transduced bone marrow (20.8 ± 3.9, bottom dot plots). The data in (A) were from the final 2 experiments and those in (B) through (E) were representative of the final 3 experiments. The number of mice analyzed in each experiment was as follows: experiment 1 (mice analyzed at 63 days after bone marrow transplantation): 1 wild-type mouse, 2 γc KO mice (no treatment), 2 γc KO mice (WT-BMT), 3 γc KO mice (hγc-transduced), and 1 γc KO mouse (mock-transduced). Experiment 2 (mice analyzed at 62 days after bone marrow transplantation): 2 wild-type mice, 2 γc KO mice (no treatment), 1 γc KO mouse (WT-BMT), 2 γc KO mice (hγc-transduced), and 1 γc KO mouse (mock-transduced). Experiment 3 (mice analyzed at 52 days after bone marrow transplantation): 1 wild-type mouse, 2 γc KO mice (no treatment), 1 γc KO mouse (WT-BMT), 3 γc KO mice (hγc-transduced), and 2 γc KO mice (mock-transduced). Experiment 3 was not included in (A) because thymocyte numbers were not counted.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/9/10.1182_blood.v94.9.3027/4/m_blod42111003ax.jpeg?Expires=1763483358&Signature=UzC0o1wvNhnwdZbV5TM9h3Lkoq-G1DmM8Ww6lyk-hal11va03lckt7yfg6Gnx0to82IEhUhJ2DssmqE7M24E54IV3~epclp8Ih7GKnmI4TtRxcBv~iXSJ0L0cK74D5~5C-WM792urk1yJUaAoEJtGiX8K~AO7qv19qDTzJxm-cRwmT5WY7uF~BG4qEdITRPSRub-ecYdR8WpsK45lJGTsN3Wl74auwJPyXH8cXFFLiK1ca8kybYin9rLRtlt6JNX2aFNv13DfFtHYPNL~RN9pGzZYtPnyShYsmh9QoOceeSzrNJ0n8w2AM65m0z3ShjaHOO7aHj7CBXOS3NWTULmKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Successful reconstitution of T cells, B cells, and NK cells in γc-deficient mice by a gene therapy approach. (A) Increased thymocytes after reconstitution of γc-deficient mice with γc-deficient bone marrow that was transduced with human γc. Note that the ordinate is a log scale. (B and C) Increased splenic T cells (B) with normalization of the CD4:CD8 ratio (C) in γc-deficient mice with γc-deficient bone marrow that was transduced with human γc; (B) also indicates correction of the B-cell defect in splenocytes. (D) Increased numbers of splenic TCR−NK1.1+ cells after reconstitution of γc-deficient mice with γc-deficient bone marrow that was transduced with human γc. Note that the TCR+NK1.1− cells are partially diagonally shifted to the right (albeit still to the left of the NK1.1+ cells that are boxed in each dot plot); this is an artifact that resulted from the use of 4-color flow cytometry. (E) Increased B cells in bone marrow after reconstitution of γc-deficient mice with γc-deficient bone marrow that was transduced with human γc. In (B through E), we have selected representative dot plots for each of the cell types analyzed from our final 3 different gene therapy experiments in which all analyses were performed. Our initial experiments provided consistent information, but not all assays were included in those experiments. In the final 3 experiments, splenic cellularities (mean ± standard error of mean [SEM] × 10−6) were as follows: wild-type mice (87.0 ± 3.0, top dot plots), γc-deficient mice (49.0 ± 5.5, second dot plots), γc-deficient mice, which received wild-type bone marrow (77.8 ± 19.9, third dot plots), γc-deficient mice, which received human γc-transduced bone marrow (82.8 ± 15.6, fourth dot plots), and γc-deficient mice, which received mock-transduced bone marrow (20.8 ± 3.9, bottom dot plots). The data in (A) were from the final 2 experiments and those in (B) through (E) were representative of the final 3 experiments. The number of mice analyzed in each experiment was as follows: experiment 1 (mice analyzed at 63 days after bone marrow transplantation): 1 wild-type mouse, 2 γc KO mice (no treatment), 2 γc KO mice (WT-BMT), 3 γc KO mice (hγc-transduced), and 1 γc KO mouse (mock-transduced). Experiment 2 (mice analyzed at 62 days after bone marrow transplantation): 2 wild-type mice, 2 γc KO mice (no treatment), 1 γc KO mouse (WT-BMT), 2 γc KO mice (hγc-transduced), and 1 γc KO mouse (mock-transduced). Experiment 3 (mice analyzed at 52 days after bone marrow transplantation): 1 wild-type mouse, 2 γc KO mice (no treatment), 1 γc KO mouse (WT-BMT), 3 γc KO mice (hγc-transduced), and 2 γc KO mice (mock-transduced). Experiment 3 was not included in (A) because thymocyte numbers were not counted.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/9/10.1182_blood.v94.9.3027/4/m_blod42111003bx.jpeg?Expires=1763483358&Signature=lnxo9t01sWTuc2gAfpW2YyAxj5NlsOk1LZICmn2t3MBUDgF43Uu2T~ohoB~2nInPsvs851sX~wLCjvvtMkUPivqERczs8QflO1p31uHVPd4pgc9AvN6LmwLh3gJQrSFyL7MxqvIy4rpckpdQYhxcCHpYijq--gLPKV5Zy7ao5Igr9p3wdNUZDTewbsy8zpO9maS0GVx4RFAEd4mrtEV08ZNkivOo6kN84zOns8tl0Ry5Mwo~cMYzKc3BS0EjoJFJsTQMWr6MA7PUgaEbjeEPa~MimihFUjxikz7YnonRv3CC8Y7wrNyfBIlG0nWxFuoUQ9wT1XIjQ3waLCIQFQKJ0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal