We examined the expression of messenger RNA (mRNA) of the human inducible nitric oxide synthase (hiNOS) gene in a panel of human T-cell lines. Reverse transcriptase-polymerase chain reaction showed that human T-cell leukemia virus type-I (HTLV-I)–infected T-cell lines (MT-1, SLB-1, and C5/MJ) expressed mRNA for the hiNOS, but TL-Om1 or uninfected Jurkat, H9, and CCRF-CEM did not. The MT-1, SLB-1, and C5/MJ cell lines are infected with HTLV-I and express the viral transactivator Tax, whereas TL-Om1 cells, although derived from adult T-cell leukemia (ATL) leukemic cells, do not express Tax. There was, thus, a correlation between Tax and hiNOS mRNA expression. The transcriptional regulatory region of the hiNOS gene was activated by Tax in Jurkat, in which endogenous hiNOS is induced by Tax. Deletion analysis showed that the region of hiNOS encompassing nucleotides −159 to −111 contained the minimum Tax-responsive elements. Mutations in the NF-κB element at position −115 and −106 bp in the hiNOS promoter were still activated by Tax, and a Tax mutant defective for activation of the NF-κB pathway retained the ability to activate the hiNOS promoter. In addition, overexpression of the dominant-negative mutants of IκB and I κBβ failed to reduce Tax-induced activation of hiNOS gene. Furthermore, hiNOS mRNA was detected in leukemic cells from ATL patients. Our results show that the hiNOS promoter contains a minimum Tax-responsive element located between nucleotides −159 and −111, and imply that the expression of the hiNOS gene is involved in the pathogenesis of HTLV-I–associated diseases.
HUMAN T-CELL LEUKEMIA virus type-I (HTLV-I) is the etiologic agent of adult T-cell leukemia (ATL).1,2 HTLV-I may also be associated with several chronic inflammatory diseases of presumed autoimmune etiology, such as HTLV-I–associated myelopathy/tropical spastic paraparesis,3,4 HTLV-I–associated arthropathy,5 uveitis,6polymyositis,7 and Sjögren’s syndrome.8The possible roles of abnormal immune activation and cytokine overproduction in the pathogenesis of diseases caused by HTLV-I has been suggested, but the mechanism by which they cause these diseases remains unknown.
Nitric oxide (NO) is an intercellular and intracellular messenger engaged in the function of virtually every mammalian organ system. Its functional repertoire includes inflammation and antimicrobial defense, in addition to neurotransmission and vascular homeostasis.9The enzyme, NO synthase (NOS), synthesizes NO from L-arginine. Three isoforms of NOS have been identified, which conform to two biochemical profiles. The neuronal and endothelial isoforms are calcium/calmodulin-dependent and are expressed constitutively (cNOS), whereas the macrophage isoform is calcium-independent and is expressed after transcriptional induction by several stimuli (iNOS).10,11 Induction of iNOS has been reported in human cells, including macrophages,12 neutrophils,13hepatocytes,14 vascular smooth muscles,15megakaryoblasts,16 chondrocytes,17keratinocytes,18 and the human colonic adenocarcinoma cell line.19 In human T cells, however, little is known about the expression of human iNOS (hiNOS) mRNA.
hiNOS may contribute to the pathogenesis of HTLV-I–associated diseases if it were expressed in HTLV-I–infected T cells. In the present study, we showed that hiNOS mRNA is expressed in leukemic cells of ATL patients as well as in some HTLV-I–infected T-cell lines. We have previously shown that interleukin (IL)-1β induces hiNOS gene expression via NF-κB activation in human tumor cell lines.20 The Tax protein encoded by the pX region of HTLV-I is essential for viral gene expression by driving its transcription through the cAMP-responsive element (CRE) in the viral long terminal repeat.21 Furthermore, Tax is known to transactivate many cellular genes through a number of cis-acting DNA elements including NF-κB and serum responsive element (SRE).21 The hiNOS promoter contains consensus binding sequences for several of these factors, implying but not confirming that they contribute to Tax-induced hiNOS activation. The studies described here were designed to identify the molecular mechanism of hiNOS activation after HTLV-I infection in T cells. We report for the first time that Tax induces hiNOS promoter in T cells.
MATERIALS AND METHODS
Cell lines.
Human T-cell lines Jurkat, H9, CCRF-CEM, and HTLV-I–infected T-cell lines MT-1, SLB-1, C5/MJ, TL-Om1 were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin G (50 U/mL), and streptomycin (50 μg/mL) in a humidified incubator containing 5% CO2 in air. JPX-9 and JPX-9/M (kindly provided by Dr M. Nakamura, Tokyo Medical and Dental University, Tokyo, Japan) are subclones of Jurkat cells, expressing Tax and nonfunctional Tax, respectively, under the control of metallothionein promoter.22
Patient samples.
The study protocol was approved by the Human Ethics Review Committees of the participating institutions. Leukemic cells from eight patients diagnosed with acute-type (n = 5; patients 3, 5 to 8) and chronic-type ATL (n = 3; patients 1, 2, and 4) were analyzed. The diagnosis was based on clinical features, hematological findings, and serum anti–HTLV-I antibodies. Monoclonal HTLV-I provirus integration into the DNA of leukemic cells was confirmed by Southern blot hybridization in all cases (data not shown). Mononuclear cells were isolated by Ficoll/Hypaque (Pharmacia LKB, Uppsala, Sweden) with density gradient centrifugation, washed with phosphate-buffered saline (PBS), and further incubated at 37°C for 2 hours in plastic culture dishes to remove adherent cells. This solution was then removed from the dishes, and cells were washed with PBS.
Determination of nitrite and nitrate concentrations.
To assess the amount of NO produced, the culture supernatants were assayed by measuring the accumulated stable degradation products, nitrite, and nitrate, using the 2,3-diaminonaphthalene kit (Dojin Chemicals, Kumamoto, Japan). Fluorescence intensity was measured with a Fluoroscan II (Labsystems, Helsinki, Finland).
Reverse transcriptase-polymerase chain reaction (RT-PCR).
Total RNA was extracted by using Trizol (GIBCO-BRL, Gaithersburg, MD), according to the protocol provided by the manufacturer. First-strand complementary DNA (cDNA) was synthesized in a 20 μL reaction volume using an RNA-PCR kit (Takara Shuzo, Kyoto, Japan) with random primers. Thereafter, cDNA was amplified for 30, 28, and 28 cycles for hiNOS, Tax, and β-actin, respectively. The oligonucleotide primers used were as follows: for hiNOS, sense 5′-CGGTGCTGTATTTCCTTACGAGGCGAAGAAGG-3′, antisense 5′-GGTGCTGCTTGTTAGGAGGTCAAGTAAAGGGC-3′; for Tax, sense 5′-CCCACTTCCCAGGGTTTGGACAGA-3′, antisense 5′-CTGTAGAGCTGAGCCGATAACGCG-3′; for β-actin, sense 5′-GTGGGGCGCCCCAGGCACCA-3′, antisense 5′-CTCCTTAATGTCACGCACGATTTC-3′. Product sizes were: 259, 203, and 548 bp, respectively. Cycling conditions were as follow: denaturing at 94°C (45 seconds for hiNOS and 30 seconds for Tax and β-actin), annealing at 60°C (45 seconds for hiNOS and 30 seconds for Tax and β-actin), and extension at 72°C (120 seconds for hiNOS and 90 seconds for Tax and β-actin). The PCR products were fractionated on 2% agarose gel and visualized by ethidium bromide staining.
Detection of iNOS protein by fluorescence-activated cell sorting (FACS) analysis.
The presence of iNOS was analyzed by flow cytometry of permeabilized cells. Briefly, 106 cells were fixed with cold 4% paraformaldehyde for 20 minutes, then washed with PBS containing 0.1% sodium azide and suspended in 50 μL of permeabilization buffer (PBS with 0.1% sodium azide, 1% heat-inactivated FBS, and 0.1% saponin). Cells were incubated at 4°C with an fluorescein isothiocyanate (FITC)-labeled anti-iNOS monoclonal antibody (MoAb) (FITC-macNOS, clone 6; Transduction Laboratories, Lexington, KY) and IgG2a-FITC as control isotype for 45 minutes, washed with permeabilization buffer, suspended in PBS, and analyzed by flow cytometry. The anti-iNOS MoAb (clone 6) is directed against a protein fragment corresponding to the amino acids 961 to 1,144 of murine iNOS and recognize the human enzyme.
Western blot analysis.
Cellular lysates were fractionated by 10% sodium dodecyl sulfate-polyacrylamide gel, electrophoretically transferred to polyvinylidine difluoride membranes (Immobilon-P; Millipore Corp, Bedford, MA), and then analyzed for immunoreactivity with a mouse anti-Tax antibody, Lt-4 (provided by Dr Y. Tanaka, University of the Ryukyus, Okinawa, Japan),23 and horseradish peroxidase-conjugated sheep antimouse IgG (Amersham Life Science Inc, Arlington Heights, IL) with an enhanced chemiluminescence detection system (ECL; Amersham Life Science Inc).
Plasmids.
To construct the hiNOS reporter gene constructs, the 3.2 kb putative promoter region cloned into pUC118 was liberated as a KpnI/Xba I fragment and ligated into the Kpn I/NheI site of a pGL3-basic plasmid (Promega, Madison, WI), which contained the luciferase gene as a reporter and the gene for the poly(A) signal sequence of late SV40 (pGLNOSa).20 A series of deletion constructs were prepared by cloning various restriction fragments of the hiNOS promoter into the pGL3-basic plasmid described previously.20 The four shortest constructs (pGLNOS-0.37, 0.33, 0.16, 0.11) were constructed by PCR using upstream primers that begin at specific nucleotides within the promoter, and a common downstream primer containing Nco I site of the pGL3-basic. The PCR fragments were cloned into Kpn I/Nco I site within the plasmid. The forward upstream primers containing the Kpn I site were designed as follows: 5′-GGGAGGTACCAGAGCTCCCTGCTGAGGAAA-3′ for pGLNOS-0.37, 5′-GGGAGGTACCAAGTGAGGCCATGTGGCTT-3′ for pGLNOS-0.33, 5′-GGGAGGTACCCCTGGCAGTCACAGTCATA-3′ for pGLNOS-0.16, 5′-GGGAGGTACCACTCCCTTTGGAAACC-3′ for pGLNOS-0.11. The sequence of the common downstream primer was 5′-TGGCGTCTTCCATGGTGGCTTTACCAAC-3′. The mutated construct in the putative NF-κB binding site was generated from the pGLNOSa plasmid using a site-directed mutagenesis kit.20 The pH2R40M (+Tax) plasmid (kindly provided by Dr M. Hatanaka, Shionogi Institute for Medical Science, Osaka, Japan) was a Tax expression plasmid containing the SV40 sequences including the SV40 promoter and the SV40 polyadenylation signal, an R fragment of HTLV-I long-terminal repeat, and a neomycin-resistant gene under control of the SV40 promoter.24 The pH2Rneo (−Tax) plasmid was used as a negative control. Tax wild-type (TaxMT-2) and mutant expression vectors (TaxM22 and Tax703) were generously provided by Dr K. Matsumoto (Osaka Red Cross Blood Center, Osaka, Japan). These genes were cloned into pH βPr0.1-neo, which has a β-actin promoter for protein expression. The Tax mutants TaxM22 and Tax703 have been characterized previously.25 NF-κB p65 expression vector was reported previously.26 Deletion mutants of IκBα (IκBαΔN)27 and IκBβ (IκBβΔN)28lacking the NH2-terminal 36 amino acids and 23 amino acids, respectively, were cloned into the pCMV4 polylinker (kindly provided by Dr D.W. Ballard, Vanderbilt University School of Medicine, Nashville, TN). κB-LUC, WT-LUC (provided by Dr J. Fujisawa, Kansai Medical University, Osaka, Japan), and pCArG-LUC (provided by Dr K. Shimotohno, Kyoto University, Kyoto, Japan) are luciferase expression plasmids regulated by five copies of the κB element from the IL-2 receptor (IL-2R) α gene, five copies of the 21-bp viral enhancer (CRE), a monomer of the CArG box from the c-fos gene, respectively.
Cell transfection and luciferase assays.
Electroporation of cells was performed as previously described.29 After 24 hours of incubation, cell lysates were prepared and luciferase activity was measured. Relative luciferase activities were normalized to the amount of intracellular protein. Results were confirmed by at least three independent transfections.
DNA fragmentation.
106 cells were pelleted and lysed for 10 minutes at 4°C in 10 mmol/L Tris-HCl, pH 7.4, 0.5% Triton X-100, 10 mmol/L EDTA. After centrifugation at 15,000 rpm for 20 minutes, the supernatant was treated for 1 hour at 37°C with RNase and then treated with 0.4 mg/mL of proteinase K for 1 hour at 37°C. DNA was precipitated with 0.5 mol/L NaCl and 1 vol of isopropanol at −20°C overnight. Purified DNA was dissolved in 20μL of TE (10 mmol/L Tris-HCl, 1 mmol/L EDTA, pH 8.0) buffer, loaded on a 2% agarose gel and separated by electrophoresis. Separated DNA was then visualized by staining with 0.5 μg/mL ethidium bromide.
RESULTS
Constitutive expression of hiNOS mRNA in Tax-expressing T-cell lines infected with HTLV-I.
To determine whether hiNOS expression in T cells correlates with HTLV-I infection, RNA was extracted from HTLV-I–infected or uninfected human T-cell lines, and expression of hiNOS gene was analyzed by RT-PCR using hiNOS-specific primers. Three HTLV-I–infected cell lines expressed the viral transactivator Tax (Fig1, lane 4, C5/MJ; lane 5, SLB-1; lane 6, MT-1). The hiNOS transcript was also detected in these three cell lines, whereas it was undetectable in the uninfected lines, Jurkat, H9, and CCRF-CEM (lanes 1 to 3). The other infected cell line, TL-Om1, without Tax transcript, also did not express hiNOS (lane 7). These results show a plausible correlation between hiNOS expression and the viral transactivator Tax expression, suggesting that Tax is responsible for the stimulated transcription of hiNOS gene similar to other Tax-responsive cellular genes.
Detection of hiNOS mRNA in Tax-expressing T-cell lines infected with HTLV-I by RT-PCR analysis. RT of cellular RNA (1 μg total RNA) and PCR were performed. One tenth of the PCR mixture was analyzed by ethidium bromide staining of 2% agarose gel. Agarose gel electrophoresis of DNA amplified by hiNOS, Tax, and β-actin primers. M: size markers. The band of specific mRNA for hiNOS is 259 bp, Tax of HTLV-I is 203 bp, and β-actin as internal control is 548 bp. The three T-cell lines, Jurkat, H9, and CCRF-CEM, are negative for HTLV-I. Other T-cell lines are positive for HTLV-I. Arrows indicate the position of the specifically amplified DNA.
Detection of hiNOS mRNA in Tax-expressing T-cell lines infected with HTLV-I by RT-PCR analysis. RT of cellular RNA (1 μg total RNA) and PCR were performed. One tenth of the PCR mixture was analyzed by ethidium bromide staining of 2% agarose gel. Agarose gel electrophoresis of DNA amplified by hiNOS, Tax, and β-actin primers. M: size markers. The band of specific mRNA for hiNOS is 259 bp, Tax of HTLV-I is 203 bp, and β-actin as internal control is 548 bp. The three T-cell lines, Jurkat, H9, and CCRF-CEM, are negative for HTLV-I. Other T-cell lines are positive for HTLV-I. Arrows indicate the position of the specifically amplified DNA.
Detection of iNOS protein and NO oxidation products nitrite and nitrate in Tax-expressing T-cell lines infected with HTLV-I. Cell lines detected hiNOS transcript were analyzed for the intracytoplasmic expression of iNOS proteins by direct immunofluorescence FACS analysis on permeabilized cells. Tax-expressing T-cell lines infected with HTLV-I were significantly labeled with the anti-iNOS MoAb (clone 6) directly conjugated to FITC. The percentage of positive cells was 68%, 43%, and 13% in C5/MJ, SLB-1, and MT-1 cells, respectively. Furthermore, the supernatants from C5/MJ, SLB-1, and MT-1 cells cultured for 48 hours contained readily detectable levels of the NO oxidation products nitrite and nitrate release (9.6, 6.4, and 3.1 μmol/L, respectively).
Expression of hiNOS mRNA in ATL.
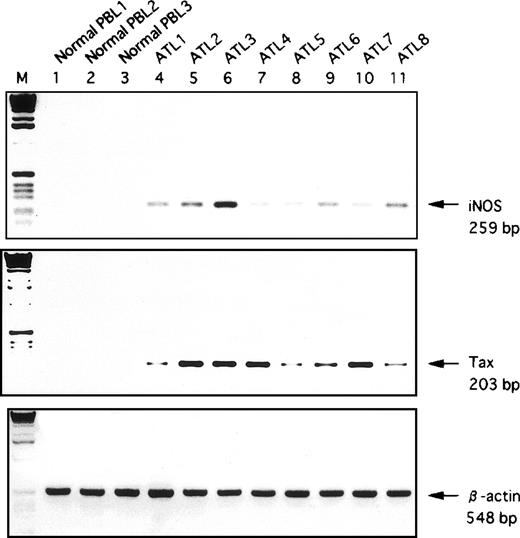
RT-PCR assay was performed to evaluate the expression of hiNOSgene on leukemic T cells derived from patients with ATL. In eight of eight cases, we observed a significant expression of hiNOS mRNA (Fig2), indicating that this gene is abnormally expressed in ATL. Furthermore, similar results were obtained with fresh leukemic cells in the absence of any culture (data not shown), indicating that hiNOS mRNA expression was not a result of stimulation by components in the culture medium. In contrast, hiNOS was not expressed in peripheral blood lymphocytes (PBL) from three normal donors. The intensity of the RT-PCR product of hiNOS did not correlate with the level of expression of Tax in ATL.
Detection of hiNOS and Tax mRNA in fresh ATL samples by RT-PCR analysis. Lanes 1-3, normal PBL RNA; Lanes 4-11, PBL RNA samples from patients with ATL. Bottom, ethidium bromide staining of the RT-PCR performed with β-actin primers. Arrows indicate the position of the specifically amplified DNA.
Detection of hiNOS and Tax mRNA in fresh ATL samples by RT-PCR analysis. Lanes 1-3, normal PBL RNA; Lanes 4-11, PBL RNA samples from patients with ATL. Bottom, ethidium bromide staining of the RT-PCR performed with β-actin primers. Arrows indicate the position of the specifically amplified DNA.
Tax induces expression of hiNOS gene.
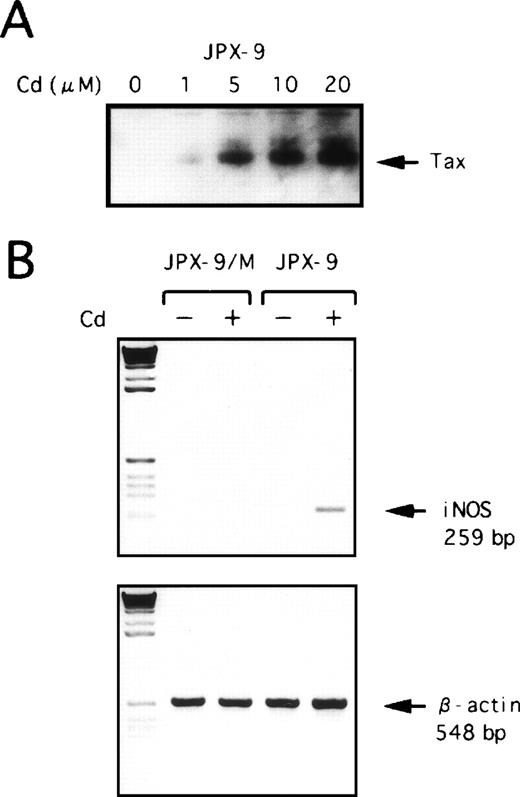
Expression of hiNOS in HTLV-I–infected T cells strongly suggested that its expression might be directly transactivated by thetax gene product of HTLV-I, because Tax can activate the transcription of cellular genes as well as the HTLV-I genome. To further evaluate the effect of Tax on the expression of hiNOS, we next used two inducible Jurkat Tax transfectants, JPX-9, which generates wild-type Tax, and JPX-9/M, which produces a nonfunctional Tax mutant after the addition of CdCl2.22Western blot analysis showed that the addition of CdCl2 to the culture medium induced Tax protein in JPX-9 cells (Fig3A). Expression of hiNOS gene was assayed by RT-PCR performed on total RNA extracted after a 48-hour culture of cells either in medium alone or in medium containing 20 μmol/L CdCl2. As shown in Fig 3B, hiNOS mRNA expression was detected in JPX-9 treated with CdCl2 but not in the mutant JPX-9/M. These data suggest that the Tax protein is involved in the high levels of hiNOS transcript observed in HTLV-I–infected T cells.
Effect of Tax expression on hiNOS mRNA induction. (A) JPX-9 cells were incubated with various concentrations of CdCl2 for 48 hours and then collected for preparation of whole-cell extracts. Whole-cell extracts isolated from JPX-9 cells were subjected to immunoblotting with anti-Tax antibody. (B) PCR analysis of reverse-transcribed hiNOS mRNA in JPX-9 and JPX-9/M cells cultured in medium alone (−) or medium containing 20 μmol/L CdCl2(+) was performed with the hiNOS oligonucleotide primer pair. Amplified products were electrophoresed.
Effect of Tax expression on hiNOS mRNA induction. (A) JPX-9 cells were incubated with various concentrations of CdCl2 for 48 hours and then collected for preparation of whole-cell extracts. Whole-cell extracts isolated from JPX-9 cells were subjected to immunoblotting with anti-Tax antibody. (B) PCR analysis of reverse-transcribed hiNOS mRNA in JPX-9 and JPX-9/M cells cultured in medium alone (−) or medium containing 20 μmol/L CdCl2(+) was performed with the hiNOS oligonucleotide primer pair. Amplified products were electrophoresed.
Analysis of hiNOS promoter activity in HTLV-I–infected T-cell lines.
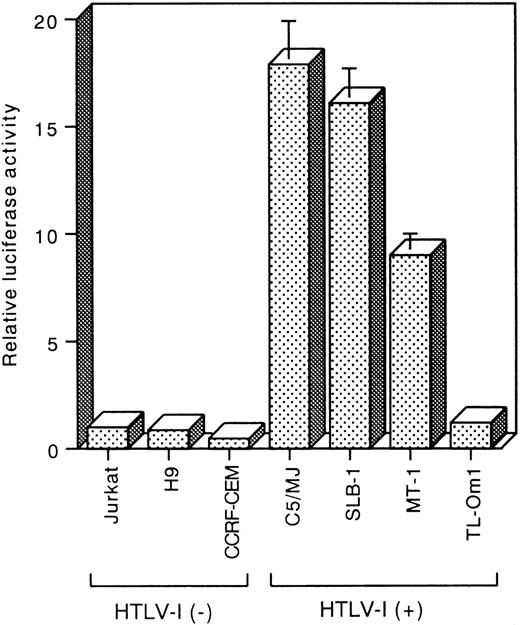
To determine the promoter activity of hiNOS, we transiently transfected the pGLNOSa plasmid, which contains the 3.2 kb putative promoter of hiNOS, into T-cell lines. The promoter activity was expressed as the fold induction compared with that of HTLV-I negative control Jurkat. The reporter activity of the pGLNOSa construct was 17.9-fold higher in C5/MJ, 16.1-fold higher in SLB-1, and 9-fold higher in MT-1, compared with Jurkat (Fig 4). In contrast, when the same construct was transfected into another HTLV-I negative T-cell lines (H9 and CCRF-CEM) and an HTLV-I–infected T-cell line, TL-Om1, which does not express Tax, the level of reporter activity was similar to that seen in Jurkat (Fig 4). These results are in agreement with the data of hiNOS mRNA expression shown in Fig 1.
Reporter assays of the hiNOS promoter construct pGLNOSa in HTLV-I–infected and uninfected T-cell lines. Five micrograms of the pGLNOSa construct were transfected. Luciferase activity was normalized to protein content. The promoter activity is represented as fold induction relative to that of the HTLV-I negative control Jurkat. Data represent the mean ± SEM of three experiments.
Reporter assays of the hiNOS promoter construct pGLNOSa in HTLV-I–infected and uninfected T-cell lines. Five micrograms of the pGLNOSa construct were transfected. Luciferase activity was normalized to protein content. The promoter activity is represented as fold induction relative to that of the HTLV-I negative control Jurkat. Data represent the mean ± SEM of three experiments.
Transactivation of hiNOS promoter by HTLV-I Tax.
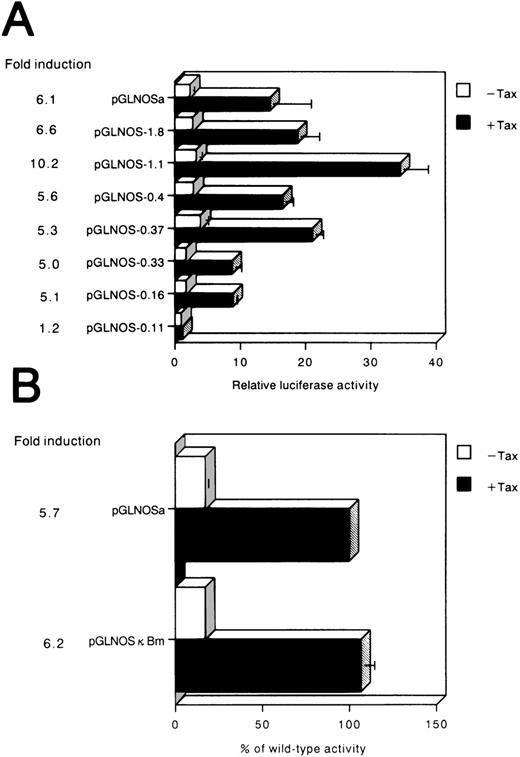
To examine whether the hiNOS promoter responds to Tax, we assayed luciferase activity in the cells. After transfecting the pGLNOSa plasmid with or without a Tax expression plasmid, we measured the luciferase activity expressed in cells. Luciferase expression was enhanced 6.1-fold by the Tax plasmid (Fig5A). Therefore, the promoter of hiNOS was transactivated by the viral transactivator Tax. This could be one mechanism by which HTLV-I–infected cells express the hiNOSgene.
(A) Activation of the hiNOS promoter by HTLV-Itax gene. The described hiNOS promoter constructs (1 μg) were transiently cotransfected with 5 μg of pH2R40M (+Tax) or with 5 μg of pH2Rneo (−Tax) into Jurkat cells. Luciferase activity normalized by protein content was expressed as the mean ± SEM of at least three experiments. The relative luciferase activity was calculated from the luciferase activity of each reporter plasmid relative to the value of pGLNOS-0.11, cotransfected with pH2Rneo and assigned a value of 1.0. The induction ratio represented the average stimulated value divided by average unstimulated value. (B) Effect of point mutation on the inducibility of luciferase activity. pGLNOSa or pGLNOSa bearing a 3-bp mutation in the NF-κB site (pGLNOSκBm) were cotransfected into Jurkat cells with 5 μg of pH2R40M (+Tax) or 5 μg of pH2Rneo (−Tax). The NF-κB site (−115 to −106; GGGACACTCC) in the hiNOS promoter was mutated to CTCACACTCC. Tax-dependent increase in reporter gene activity was expressed as a percentage of the value of pGLNOSa cotransfected with pH2R40M. Data represent the mean ± SEM of three experiments.
(A) Activation of the hiNOS promoter by HTLV-Itax gene. The described hiNOS promoter constructs (1 μg) were transiently cotransfected with 5 μg of pH2R40M (+Tax) or with 5 μg of pH2Rneo (−Tax) into Jurkat cells. Luciferase activity normalized by protein content was expressed as the mean ± SEM of at least three experiments. The relative luciferase activity was calculated from the luciferase activity of each reporter plasmid relative to the value of pGLNOS-0.11, cotransfected with pH2Rneo and assigned a value of 1.0. The induction ratio represented the average stimulated value divided by average unstimulated value. (B) Effect of point mutation on the inducibility of luciferase activity. pGLNOSa or pGLNOSa bearing a 3-bp mutation in the NF-κB site (pGLNOSκBm) were cotransfected into Jurkat cells with 5 μg of pH2R40M (+Tax) or 5 μg of pH2Rneo (−Tax). The NF-κB site (−115 to −106; GGGACACTCC) in the hiNOS promoter was mutated to CTCACACTCC. Tax-dependent increase in reporter gene activity was expressed as a percentage of the value of pGLNOSa cotransfected with pH2R40M. Data represent the mean ± SEM of three experiments.
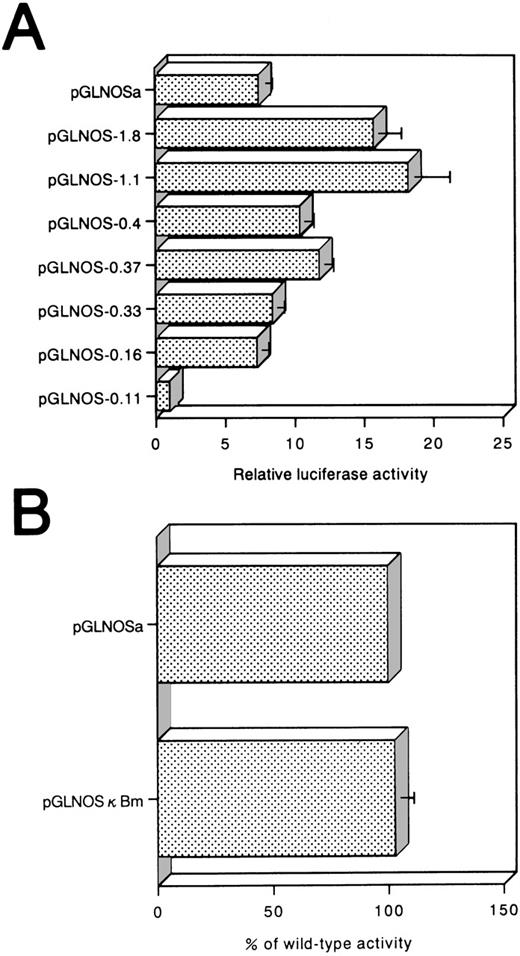
To identify the regions of hiNOS promoter that are important for the Tax-induced activity in Jurkat, a series of deletion constructs were established with progressively smaller fragments of the 5′ flanking sequence, and then transiently transfected into Jurkat cells (Fig 5A). The luciferase activity fluctuated between −1,088 and −419: the expression level reached the maximum with construct pGLNOS-1.1 and decreased about twofold with construct pGLNOS-0.4. These results suggested that there are several positive regulatory elements present between −1,088 and −419. The pGLNOS-1.8, 1.1, 0.4, 0.37, 0.33, and 0.16 deletion constructs manifested Tax-induced luciferase activity. With pGLNOS-0.11, in which the region between nucleotides −159 to −111 of pGLNOS-0.16 construct is deleted, no induction of luciferase activity by Tax was observed, indicating that this region of the hiNOS promoter is essential for Tax-induced transcriptional activity. Furthermore, these serial deletions at the 5′ end of the hiNOS construct were transfected into the C5/MJ cells as a representative of HTLV-I–positive cell lines (Fig 6A). Removal of the region between nucleotides −159 and −111 in the pGLNOS-0.16 construct reduced the reporter activity (Fig 6A), indicating that this region of the hiNOS promoter is required for its basal transcriptional activity in HTLV-I–positive cells.
(A) Reporter assay of hiNOS promoter deletion constructs in C5/MJ, an HTLV-I–infected T-cell line. The relative luciferase activity was calculated from the luciferase activity of each reporter plasmid relative to the value of pGLNOS-0.11 and assigned a value of 1.0. (B) Effect of point mutation on hiNOS basal transcriptional activity in C5/MJ cells. Values represent the mean ± SEM of three experiments.
(A) Reporter assay of hiNOS promoter deletion constructs in C5/MJ, an HTLV-I–infected T-cell line. The relative luciferase activity was calculated from the luciferase activity of each reporter plasmid relative to the value of pGLNOS-0.11 and assigned a value of 1.0. (B) Effect of point mutation on hiNOS basal transcriptional activity in C5/MJ cells. Values represent the mean ± SEM of three experiments.
NF-κB binding site is not essential for activation by Tax.
Between −115 and −106 bp of the hiNOS promoter fragment, there is a consensus sequence for the NF-κB binding site, 5′-GGGACACTCC-3′, which is reportedly essential for the cytokine-induced expression of hiNOS gene.20 Since the Tax-responsive cellular enhancer also contains an NF-κB–responsive element, this site may be a Tax-responsive element. Furthermore, because the NF-κB binding site at −115 and −106 bp is destroyed in the reporter construct pGLNOS-0.11, the results of the deletion analysis suggested that the NF-κB site was involved in Tax transactivation of the hiNOS promoter. Substitution mutations were introduced into this site of the pGLNOSa plasmid to examine its role on Tax transactivation. However, the substitution in the NF-κB site (Fig 5B, pGLNOSκBm) did not affect Tax-induced activation. To evaluate that the NF-κB site within the hiNOS promoter is functional, the DLD-1 cells, a human colorectal adenocarcinoma cell line, were transfected with the wild-type pGLNOSa construct. The transfected cells showed the inducibility of the reporter activity after IL-1β stimulation (2.1-fold induction). These cells transfected with the mutant pGLNOSκBm construct completely failed to respond to IL-1β. These results show that the NF-κB binding site is dispensable for Tax-induced expression. Furthermore, we transfected pGLNOSκBm into C5/MJ cells. However, mutation in NF-κB site did not abrogate hiNOS promoter activity (Fig 6B).
Tax mutants that are defective for activation of the NF-κB, CRE, and SRE pathways activate the hiNOS promoter.
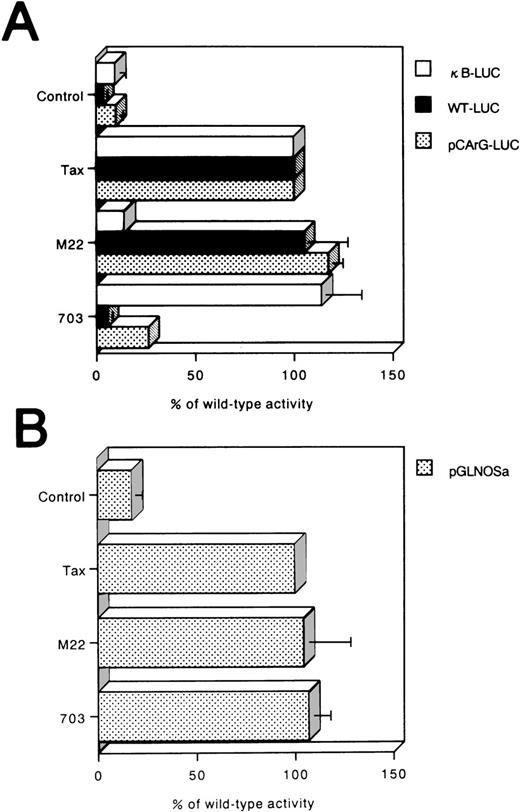
Tax is known to activate promoters containing CRE, SRE, or NF-κB elements. To confirm that NF-κB binding site within the hiNOS promoter was not required for Tax activation, Tax mutants that failed to activate NF-κB (TaxM22)-CRE, (Tax703)-, or SRE (Tax703)-dependent promoters25 were analyzed to examine their ability to activate the pGLNOSa promoter construct (Fig7B). Both TaxM22 and Tax703 activated the pGLNOSa promoter to levels similar to those observed with the wild-type Tax (Fig 6B). Controls for these experiments showed that Tax703, but not TaxM22, transactivated κB-LUC (Fig 7A). Conversely, Tax mutant M22, but not 703, induced luciferase expression from WT-LUC and pCArG-LUC (Fig 7A).
Tax transactivation of the hiNOS promoter is independent of NF-κB element. (A) Reporter luciferase plasmids with κB (κB-LUC), CRE element (WT-LUC), or CArG box (pCArG-LUC) were used as control promoters for Tax mutants. Luciferase activity was determined after incubation for 24 hours and normalized for protein content. The activity of Tax mutants, M22 and Tax703, relative to that of the wild-type Tax, was calculated by comparing luciferase activity in the presence of M22 or Tax703 with that in the presence of wild-type Tax. (B) Jurkat cells were transfected with pGLNOSa together with expression plasmids encoding Tax, its indicated mutants, or the parental expression vector with no insert (Control). Data represent the mean ± SEM of three experiments.
Tax transactivation of the hiNOS promoter is independent of NF-κB element. (A) Reporter luciferase plasmids with κB (κB-LUC), CRE element (WT-LUC), or CArG box (pCArG-LUC) were used as control promoters for Tax mutants. Luciferase activity was determined after incubation for 24 hours and normalized for protein content. The activity of Tax mutants, M22 and Tax703, relative to that of the wild-type Tax, was calculated by comparing luciferase activity in the presence of M22 or Tax703 with that in the presence of wild-type Tax. (B) Jurkat cells were transfected with pGLNOSa together with expression plasmids encoding Tax, its indicated mutants, or the parental expression vector with no insert (Control). Data represent the mean ± SEM of three experiments.
Overexpression of dominant-negative mutants of IκBα and IκBβ fail to reduce Tax-induced activation of hiNOS gene.
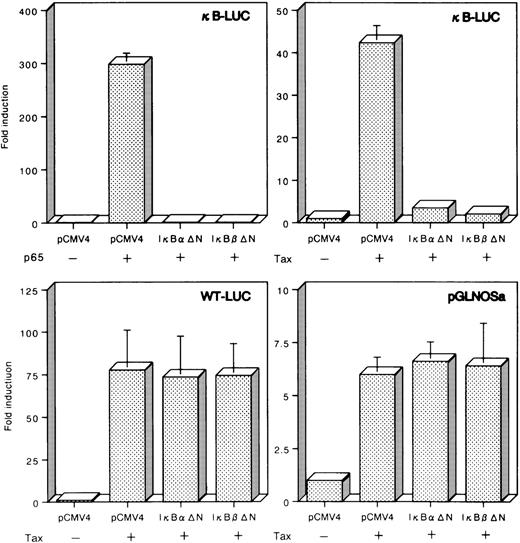
The cytoplasmic inhibitor IκB is responsible for sequestering NF-κB subunits in the cytoplasm by masking the nuclear localization signals. Previous studies showed that both IκBα and IκBβ mutants in their NH2-terminal region escape from proteolytic breakdown and prevent NF-κB activation in Tax-expressing cells.27 28 To further confirm the role of NF-κB in the regulation of hiNOS, transient cotransfections were performed with a mutant of IκBα (IκBαΔN) or IκBβ (IκBβΔN) lacking the NH2-terminal amino acids. As shown in Fig8, both IκBαΔN and IκBβΔN abolished p65-dependent transcription of κB-LUC, containing aluciferase gene under the control of the NF-κB binding site of IL-2Rα gene. Furthermore, both IκBαΔN and IκBβΔN also inhibited Tax-induced transcription from κB-LUC (Fig 8). To evaluate the specificity of this interaction, either IκBαΔN or IκBβΔN effector plasmid was cotransfected with Tax and a distinct Tax-responsive reporter construct. In sharp contrast to κ-LUC, both IκBαΔN and IκBβΔN failed to significantly downregulate transcription directed from WT-LUC, which responds to Tax by five repeats of a 21-bp viral enhancer (CRE). Tax-induced transcription from the hiNOS promoter was not reduced in cells expressing both IκBαΔN and IκBβΔN (Fig 8). Considered together, these results show that Tax activation of the hiNOS promoter does not depend on the NF-κB pathway. Thus, Tax activation of the hiNOS promoter is likely to involve a previously uncharacterized Tax-responsive element.
Failure of repression of Tax-dependent transcription from the hiNOS promoter by the dominant-negative mutants of IκB and IκBβ. Jurkat cells were transfected with 5 μg of a p65 or Tax expression vector, 5 μg of IκB▵N or IκBβ▵N effector plasmid, and 5 μg of a luciferase reporter construct under transcriptional control of either the κB-LUC, WT-LUC, or pGLNOSa. Input DNA for all transfections was normalized by addition of a blank pCMV4 expression vector. The resulting activities were plotted as fold induction relative to that with the corresponding reporter in the absence of p65 or Tax. Data represent the mean ± SEM of three experiments.
Failure of repression of Tax-dependent transcription from the hiNOS promoter by the dominant-negative mutants of IκB and IκBβ. Jurkat cells were transfected with 5 μg of a p65 or Tax expression vector, 5 μg of IκB▵N or IκBβ▵N effector plasmid, and 5 μg of a luciferase reporter construct under transcriptional control of either the κB-LUC, WT-LUC, or pGLNOSa. Input DNA for all transfections was normalized by addition of a blank pCMV4 expression vector. The resulting activities were plotted as fold induction relative to that with the corresponding reporter in the absence of p65 or Tax. Data represent the mean ± SEM of three experiments.
Effect of NO on apoptosis in an HTLV-I–infected T-cell line.
Human Epstein-Barr virus-infected B lymphocytes and Burkitt lymphoma cell lines constitutively express hiNOS.30 NO appears to play a role in the inhibition of apoptosis of lymphocytes and prevention of viral reactivation in these cells. In addition, spontaneous expression of a functional hiNOS that has an antiapoptotic role has been reported in B-cell chronic lymphocytic leukemia cells.31 In agreement with previous reports, we have shown that the addition of NG-monomethyl-L-arginine (L-NMMA), a NOS inhibitor, enhanced DNA fragmentation in C5/MJ, HTLV-I–infected T-cell line (Fig 9). These results suggest that NO released by HTLV-I–infected T cells may contribute to their survival in vivo. Failure of cells to undergo apoptosis is a factor contributing to the development of some forms of cancer. Thus, elevated NO production mediated by Tax may inhibit apoptotic death of leukemic cells in ATL patients, thereby contributing to leukemogenesis.
Effect of NO on apoptosis in an HTLV-I–infected T-cell line. C5/MJ cells were incubated for 72 hours in the presence (+) or absence (−) of L-NMMA (1 mmol/L). Cellular DNA was prepared and resolved by electrophoresis on a 2% agarose gel.
Effect of NO on apoptosis in an HTLV-I–infected T-cell line. C5/MJ cells were incubated for 72 hours in the presence (+) or absence (−) of L-NMMA (1 mmol/L). Cellular DNA was prepared and resolved by electrophoresis on a 2% agarose gel.
DISCUSSION
The major finding of the present study was the demonstration of overexpression of hiNOS gene in HTLV-I–infected T-cell lines and primary ATL cells compared with uninfected T-cell lines and normal PBL. The expression in HTLV-I–infected cells suggests that the gene might play a role in the pathogenesis of HTLV-I–associated diseases. Recently, Tax has been shown to induce hiNOS mRNA expression and NO production in a human monoblast cell line.32 In the present study, we investigated the ability of Tax to transactivate the hiNOS promoter in T cells. Our results showed that Tax can induce hiNOS mRNA and activate the hiNOS promoter, and the minimum Tax-responsive region of this promoter was narrowed to a 48-bp sequence located between nucleotides −159 and −111. Because Tax has previously been shown to function through NF-κB element, we suggest that the NF-κB element located between nucleotide −115 and −106 is involved in Tax activation. However, Tax can transactivate promoters containing a mutation in the NF-κB site (pGLNOSκBm). To confirm this, we showed that a Tax mutant defective for activation of NF-κB–dependent promoter was capable of activating the hiNOS promoter. We have also shown that overexpression of the dominant-negative mutants of IκBα and IκBβ failed to reduce Tax-induced activation of hiNOS gene. These results show that Tax activation of the hiNOS promoter does not depend on the NF-κB pathway.
Studies elucidating the molecular mechanisms involved in the transcriptional regulation of iNOS have been reported for murine macrophages. Two regions in the murine macrophage iNOS promoter are essential for conferring inducibility of iNOS to lipopolysaccharide and interferon (IFN)-γ.33,34 An NF-κB element, which exists within 0.1-kb from the transcriptional start site of murineiNOS gene, was found to bind members of the NF-κB/Rel family of proteins in response to lipopolysaccharide.35 Further upstream (positions −923 to −913), an IFN-γ–responsive element, was shown to bind IRF-1 upon stimulation of RAW 264.7 cells with IFN-γ.36 On the other hand, the putative promoter region of hiNOS has been cloned.37,38 There are, however, major differences in the sequence and potential cis-acting elements between human and rodent iNOS 5′ flanking regions.37,38 We have previously shown that the promoter regions respond to IL-1β via NF-κB activation in a human colorectal adenocarcinoma cell line.20 In contrast, de Vera et al39 reported that the functional promoter of hiNOS does not exist within the 3.8 kb DNA sequence in human liver epithelial cell line. More recently, the same group reported multiple NF-κB elements responsible for cytokine-induced transcriptional activation of the gene that were all localized in the 5′ flanking region upstream of −4.7 kb.40 In the present study, we showed that Jurkat cells transfected with a series of hiNOS-luciferase gene constructs exhibited significant activity in the first 3.2 kb of the 5′ flanking region upon stimulation with Tax similar to the human tumor cell line stimulated with IL-1β. Transient expression of the luciferase reporter gene linked to serially deleted sequences of the 5′ flanking region of the hiNOS gene has shown several positive regulatory elements involved in the Tax-inducible expression of the hiNOS gene in Jurkat cells. A distal region between −1,088 and −419 was shown to be involved in the Tax-inducible expression of the hiNOS gene. Through computer search, several DNA sequences within −1,088 to −419 were found to be similar to the binding sites of certain known transcription factors. These sites included six IFN-γ–responsive elements at −1,074 to −1,067, −977 to −970, −881 to −874, −864 to −857, −840 to −833, and −474 to −467. A proximal region between −159 and −111 was identified as the minimum sequence for the Tax-inducible expression of the hiNOS gene. Interestingly, a unique NF-κB element in the hiNOS promoter is not involved in Tax activation. Homology search showed the presence of some putative elements between nucleotides −159 and −111, including a tumor necrosis factor-responsive element and IFN-γ–responsive elements.37 38 Whereas our initial studies could not identify the specific elements required for transcriptional activation by Tax, the present results showed that the hiNOS promoter contains important Tax-responsive regions located between nucleotides −159 and −111.
Apparently, there is no doubt that the viral transactivator Tax can transactivate the hiNOS promoter. However, the question is whether Tax is the only mechanism for constitutive expression of hiNOS gene in ATL. The intensity of the RT-PCR product of hiNOS did not correlate with the level of expression of Tax in ATL in vivo. Although the accumulation of hiNOS mRNA was obviously induced by Tax in JPX-9, the level appeared to be much lower than those in the cell lines infected with HTLV-I, such as C5/MJ and SLB-1. These findings indicate that factors of either viral or cellular origin other than Tax are also involved in the constitutive expression of hiNOS in HTLV-I–infected cells.
Our experiments point to an antiapoptotic role for NO in HTLV-I–infected T cells. The inhibition of apoptosis is potentially deleterious in supporting tumor cell growth and survival. It is also interesting to note that transgenic animals for the tax gene show chronic inflammatory arthropathy.41 Furthermore, increased iNOS expression and NO production have been noted in induced and spontaneous animal models of arthritis, whereas inhibitors of NOS can reduce arthritis in these conditions.42 Other studies have shown high levels of NO catabolites in serum and synovial fluid of patients with inflammatory arthritis.43 In addition, synovial tissue and peripheral blood mononuclear cells from humans with inflammatory arthritis express iNOS mRNA and protein, and generate NO.44 Further investigation of hiNOS gene expression using Tax transgenic animals could provide the basis for our understanding the precise role of hiNOS in the pathogenesis of HTLV-I–associated diseases. NO-related therapies may be useful in the treatment of ATL and HTLV-I–associated diseases.
ACKNOWLEDGMENT
We are grateful to Drs W.C. Greene, D.W. Ballard, M. Hatanaka, J. Fujisawa, K. Shimotohno, and K. Matsumoto for providing plasmids, Dr Y. Tanaka for providing anti-Tax MoAb, Dr M. Nakamura for providing JPX-9 and JPX-9/M, and Fujisaki Cell Center, Hayashibara Biochemical Laboratories, Inc (Okayama, Japan) for providing Jurkat, MT-1, and C5/MJ cell lines. We also thank Dr Y. Yamasaki for providing the blood samples of patients and M. Yamamoto and M. Sasaki for the excellent technical assistance.
Supported in part by a Grant-in-Aid for Encouragement of Young Scientists from the Ministry of Education, Science, Sports and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal