Spontaneous regression of skin lesions is characteristic of lymphomatoid papulosis (LyP), a clonal cutaneous lymphoproliferative disorder. A minority of LyP patients progress to anaplastic large cell lymphoma (ALCL) in which skin lesions no longer regress and extracutaneous dissemination often occurs. In 1 such case, we developed a tumor cell line, JK cells, and show that these cells are resistant to the growth inhibitory effects of transforming growth factor β (TGF-β) due to the loss of cell surface expression of the TGF-β type I receptor (TβR-I). Reverse transcriptase-polymerase chain reaction (RT-PCR) and sequencing of JK cell TβR-I cDNA clones identified a deletion that spanned the last 178 bp of exon 1, including the initiating methionine. Hybridization of a radiolabeled fragment internal to the deletion was detected in the genomes of TGF-β–responsive cells, but not in JK cells, indicating that they contain no wild-type TβR-I gene. PCR primers that flanked the deleted TβR-I region amplified a single band from JK cell genomic DNA that lacked the last 178 bp of exon 1 and all of the ≈ 5 kb of intron 1. This JK cell-specific genomic TβR-I PCR product was distinct from products amplified from TGF-β–responsive cells and was also readily detected in tumor biopsies obtained before the establishment of the JK cell line. Our results identify the first inactivating mutation in TβR-I gene in a human lymphoma that renders it insensitive to growth inhibition by TGF-β.
TRANSFORMING GROWTH factor-β (TGF-β) is a multifunctional cytokine, which elicits diverse physiological responses in numerous cell types, including hematopoietic cells, hepatocytes, myoblasts, adipocytes, chrondrocytes, and neurons.1-4 The initiation of transmembrane signaling and the diverse biological activities of TGF-β are mediated through the combined actions of 2 distinct receptor Ser/Thr protein kinases, termed the TGF-β type I (TβR-I) and type II (TβR-II) receptors.5-9 TGF-β–stimulated signal transduction commences following its binding to a homodimeric complex of TβR-II, which then associates with, transphosphorylates, and stimulates the protein kinase activity of a homodimeric complex of the TβR-I.4,10-13 Activated TβR-I complexes then transduce intracellular signals to the nucleus in part through the recruitment and phosphorylation of either Smad2 or Smad3 within their C-terminal Ser-Ser-X-Ser motif.14-18 Once activated, Smad2 or Smad3 associate with the shared signaling molecule Smad414,19,20; the resulting complex translocates to the nucleus and works in concert with additional transcription factors21-24 to alter the transcription of a large repertoire of genes involved in regulating a variety of biological responses, including differentiation, apoptosis, and extracellular matrix production.2-4,11 Importantly, TGF-β also inhibits the proliferation of epithelial cells by suppressing the expression of c-myc, cyclin A, and CDK4 and by inducing the expression of the CDK inhibitors p15 and p21; together these events lead to the hypophosphorylation of the retinoblastoma gene product, Rb, and the sequestration of the transcription factor, E2F-1.25 26
Cellular insensitivity to growth inhibition by TGF-β is a hallmark in the genesis and progression of human cancer, and in many instances, can be linked directly to inactivating mutations in or the loss of expression of various signaling molecules whose activities are regulated by TGF-β. For instance, mutations that inactivate Smad2 have been identified in human colorectal and lung cancers,15,27 while those leading to the inactivation or loss of expression of Smad4 have been found in human pancreatic, breast, colorectal, lung, ovarian, and head and neck cancers.14,27,28 Although not yet identified as a cause of human cancers,29,30 the gene for Smad3 functions as a tumor suppressor in mice. In particular, targeted disruption of Smad3 leads to the formation of colorectal adenocarcinomas capable of penetrating the intestinal wall and metastasizing to distant locations,31 suggesting that the gene for Smad3, like those for Smads 2 and 4, is a tumor suppressor.
The receptors for TGF-β also appear to function as tumor suppressors in vivo. Mutations in or loss of the gene for the TβR-II have been described in a variety of human malignancies, including colon, gastric, prostate, and retinal cancers, and in some T-cell lymphomas.32-39 With respect to TβR-I, several reports show a strong correlation between diminished cellular responsiveness to TGF-β and decreased expression of this receptor in B-cell chronic lymphocytic leukemia, colon, and pancreatic cancers.40-43Additionally, unknown genetic alterations in the TβR-I gene have also been reported as the cause of insensitivity to TGF-β in prostate cancer cells.44
Here we show that JK cells, a human CD30+ anaplastic large cell lymphoma (ALCL) cell line derived from and clonally related to lymphomatoid papulosis (LyP), are resistant to the growth inhibitory effects of TGF-β. Furthermore, we show that the inability of JK cells to respond to TGF-β is due to a 178-bp deletion in exon 1 of the TβR-I gene, resulting in its loss of expression. Moreover, our results have established that the TβR-I gene, like those for Smad2, Smad4, and TβR-II, is a human tumor suppressor. Our results are the first to identify a specific mutation in the TβR-I gene leading to the loss of its expression and consequently the loss of its antitumorigenic qualities in a human T-cell lymphoma cell line.
MATERIALS AND METHODS
Patient history and characteristics of tumor cells in culture.
In 1987, a 49-year old male developed LyP, a cutaneous lymphoproliferative disorder characterized by spontaneous regression of skin lesions. The patient’s lesions continued to wax and wane until 1996 when several tumor nodules developed on the right forearm, sacrum, and the left buttock. The lesions on the right forearm and buttock responded to local irradiation. A progressively enlarging ALCL lesion of the buttock was biopsied and xenografted into nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice, and the resulting explanted SCID mouse tumor used to establish the population of cells used herein, termed JK cells. A complete description of JK cells in culture will be described elsewhere (manuscript in preparation). Cultured JK cells maintained the anaplastic morphology and phenotype (CD30+, CD15−, CD3+) of the original tumor, while cytogenetics showed an aneuploid karyotype with marker chromosomes, which were present 3 years earlier in a T-cell clone isolated from a prior skin lesion of the patient’s right arm. Studies of T-cell receptor gene rearrangement showed that JK cells were clonally related to all prior LyP skin lesions analyzed dating back to 1988.45
[3H]Thymidine incorporation assay.
Mv1Lu or JK cells were plated onto 6-well plates at a density of 100,000 cells/well and were incubated in the absence or presence of increasing concentrations of TGF-β1 (0 to 20 ng/mL) for 48 hours at 37°C. During the final 4 hours of agonist treatment, cellular DNA was radiolabeled by inclusion of [3H]thymidine to the culture medium. Upon completion of agonist stimulation, the cells were harvested in phosphate-buffered saline (PBS) supplemented with 10 mmol/L EDTA and 10% fetal bovine serum before their isolation and concentration by centrifugation at 1,500 rpm for 3 minutes at room temperature. Quantitation of [3H]thymidine incorporation was measured using the SPA [3H]thymidine uptake assay system (Amersham, Arlington Heights, IL) with slight modification. Briefly, cell pellets were resuspended in 130 μL of PBS, transferred to scintillation vials containing 75 μL bead/lysis buffer mixture, and vortexed for 2 minutes at room temperature on a multitube vortexer (VWR, setting 2). Enhancer (25 μL) was added to each vial, and the samples were vortexed for 2 minutes immediately before scintillation counting in a Beckman LS6500 liquid scintillation counter (Beckman Coulter, Fullerton, CA).
Recombinant glutathione S-transferase (GST)-Smad3 phosphorylation assay.
Quiescent Mv1Lu, human HT1080 fibrosarcoma, human MDA-MB-231 breast cancer or JK cells were incubated in the absence or presence of 20 ng/mL TGF-β1 for 10 minutes at 37°C. Cytokine stimulations were terminated by washing the cells 2 times in ice-cold PBS, and cells were immediately lysed in harvesting buffer (50 mmol/L β-glycerophosphate, 150 mmol/L NaCl, 1.5 mmol/L EGTA, 1 mmol/L dithiothreitol (DTT), 0.1 mmol/L sodium vanadate, 1 mmol/L benzamidine, and 10 μg/mL leupeptin, pH 7.3) supplemented with 1% Triton X-100. Cell extracts were solubilized on ice for 60 minutes and were subsequently clarified by centrifugation for 10 minutes at 4°C. Immunoprecipitation of TGF-β receptor complexes was accomplished by rotating the clarified cell extracts (∼ 1.5 mg protein/tube) in the presence of anti-TβR–II antibody and protein A-sepharose for ∼2 hours at 4°C. The resulting immunocomplexes were recovered by brief centrifugation and were subsequently subjected to 2 washes in lysis buffer, followed by 2 washes in harvesting buffer lacking NaCl. TGF-β receptor phosphotransferase activity against recombinant GST-Smad3 was measured for 30 minutes at 30°C in a final reaction mixture of 40 μL consisting of 30 μL of TGF-β receptor immunocomplexes, 25 mmol/L β-glycerophosphate (pH 7.3), 0.5 mmol/L DTT, 1.25 mmol/L EGTA, 50 μmol/L sodium vanadate, 10 mmol/L MgCl2, and 100 μmol/L adenosine triphosphate (ATP) ([γ-32P]ATP, ≈ 2,000 cpm/pmol), and 5 μg of recombinant GST-Smad3. Protein kinase reactions were quenched by addition of 13.5 μL of 4× Laemmli sample buffer and were subsequently boiled for 5 minutes before fractionation through 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). TGF-β1–stimulated phosphorylation of recombinant GST-Smad3 was detected by autoradiography of the dried gels.
[125I]TGF-β1 radioligand binding and cross-linking assay.
Radioligand binding and cross-linking of iodinated TGF-β1 to Mv1Lu or JK cells (5 × 106 cells/tube) and the subsequent immunoprecipitation of cytokine/receptor complexes with anti-TβR–I and -TβR–II antibodies was performed as described previously.40 TGF-β1 bound to cell surface TβR-I and TβR-II was visualized by autoradiography of the dried gels.
Northern blot analysis.
Total RNA was isolated from Mv1Lu or JK cells using the RNAzol B reagent (Tel-Test, Inc, Friendswood, TX) according to the manufacturer’s recommendations. Five micrograms of total RNA was fractionated through 1% agarose/formaldehyde gels, immobilized to nylon membrane, and subsequently incubated at 68°C for 80 minutes in ExpressHyb hybridization solution (Clontech, Palo Alto, CA) containing a 32P-radiolabeled probe encoding to nucleotides 226-1536 of the TβR-I cDNA. After hybridization, the membrane was washed for 40 minutes at room temperature in 2× SSC/0.05% SDS, followed by 80 minutes of washing at 50°C in 0.1× SSC/0.1% SDS before visualization of the TβR-I mRNA by autoradiography.
cDNA synthesis and PCR of JK cell TβR-I clones.
To synthesize JK cell TβR-I cDNAs, 800 ng of JK cell total RNA was primed with oligo-dT and reverse transcribed using Superscript II (GIBCO-BRL, Rockville, MD) according to the manufacturer’s recommendations. A total of 2 μL of resulting cDNA mixture was used in 50 μL PCR reactions using the Elongase enzyme mixture (GIBCO-BRL) in the presence of 10% dimethyl sulfoxide (DMSO) to amplify full-length JK cell TβR-I cDNA. The 5′ and 3′ oligonucleotides were engineered to contain Spe I or Hind III restriction sites, respectively, to facilitate subsequent subcloning of amplified products into pBluescript. The sequences of the human TβR-I primers used were: (1) 5′ oligo; 5′-ACTAGTACTAGTGGACGCGCGTCCTCCGAGCAG, corresponding to positions −141 to −121 relative to the initiating ATG, and (2) 3′ oligo; 5′-AAGCTTAAGCTTGAGAGTTCAGGCAAAGCTGTA, corresponding to positions 1516-1536. After an initial denaturation step for 2 minutes at 94°C, the PCR amplification reaction conditions proceeded through 30 seconds at 94°C, 30 seconds at 55°C, and 2.5 minutes at 68°C for 40 cycles, followed by a final extension for 5 minutes at 68°C. The resulting ∼1.6 kb PCR fragments were subcloned into the Spe I/Hind III sites of pBluescript, propagated in Escherichia coli, and sequenced in their entirety across both strands on an Applied Biosystems 377A DNA sequencing system (Perkin Elmer, Applied Biosystems, Foster City, CA).
Genomic PCR.
Genomic DNA from TGF-β–responsive cells or JK cells was isolated using the QIAamp Blood Kit (Qiagen, Valencia, CA) according to the manufacturer’s recommendations. Amplification of exon 1 of TβR-I gene from TGF-β–responsive cells or JK cells was accomplished using the Advantage-GC genomic polymerase mix (Clontech) with the flanking intronic primer pair as described previously.46 The resulting PCR products were isolated from unincorporated nucleotides and primers before separation through a 2.5% agarose/TAE gel.
Additionally, 1 μg of genomic DNA was subjected to PCR amplification in 50-μL reaction volumes using the Elongase enzyme mixture in the presence of 10% DMSO and primers that flanked the 178-bp deletion identified in JK cell TβR-I cDNA. The sequences of the human TβR-I primers used were: (1) 5′ oligo; 5′-ACTAGTACTAGTGGACGCGCGTCCTCCGAGCAG, corresponding to positions −141 to −121 relative to the initiating methionine, and (2) 3′ oligo; 5′-ATATGTTGTAGTCACAGACCCAGT, corresponding to positions 274 to 297 relative to the initiating ATG. After an initial denaturation step for 2 minutes at 94°C, the PCR amplification reaction conditions proceeded through 30 seconds at 94°C, 30 seconds at 50°C, and 60 seconds at 68°C for 30 cycles, which was followed by a final extension for 5 minutes at 68°C. The resulting PCR products were isolated from unincorporated nucleotides and primers before separation through a 2.5% agarose/TAE gel. The products of interest were excised from the gel, purified using Qiaex II beads (Qiagen) according to recommended protocols, and subsequently sequenced in their entirety across both strands on an Applied Biosystems 377A DNA sequencing system.
Patient cutaneous tumor lesions biopsied in 1989 and 1996 were obtained from the Beth Israel Deaconess Hospital Department of Pathology archives. Genomic DNA from frozen tumor tissue (1989) was isolated using DNAzol (GIBCO-BRL) according to the manufacturer’s recommendations. Paraffin-embedded material (1996) was microdissected using a 30 gauge needle to scrape a 5-μm thick, Eosin-stained tumor sample. Tumor scrapings were placed in extraction buffer (50 mmol/L Tris, 1 mmol/L EDTA, and 0.5% Tween-20) and incubated in the presence of proteinase K for 48 hours at 37°C. Upon completion of proteinase K digestion, 3 μL of heat inactivated sample was subjected to PCR amplification in 25-μL reaction volumes using the Ready to Go PCR Beads (Pharmacia Biotech, Piscataway, NJ) and primers that flanked the 178-bp deletion identified in JK cell TβR-I cDNA. The sequences of the human TβR-I primers used were: (1) 5′ oligo; 5′-CCTCCGAGCAGTTACAAAGG, corresponding to positions −131 to −112 relative to the initiating methionine, and (2) 3′ oligo; 5′-AACGGCCTATCTCGAGGAAT, corresponding to positions 232 to 251 relative to the initiating ATG. After an initial denaturation step for 7 minutes at 94°C, the PCR amplification reaction conditions proceeded through 50 seconds at 94°C, 50 seconds at 60°C, and 90 seconds at 72°C for 35 cycles, which was followed by a final extension for 7 minutes at 72°C. The resulting PCR products were fractionated through a 2% agarose/TAE gel.
Southern blotting.
A total of 10 μg of genomic DNA was digested overnight at 37°C with Hind III and subsequently transferred to a nylon membrane. Immobilized DNA was hybridized overnight at 65°C in modified Church-Gilbert hybridization solution (500 mmol/L NaHPO4, 7% SDS, 1 mmol/L EDTA, and 1% bovine serum albumin [BSA], pH 7.2) containing a 32P-radiolabeled probe corresponding to the 178-bp sequence absent in JK cell TβR-I cDNA (ie, bases −81 to 96 relative to the initiating ATG). Upon completion of hybridization, the membrane was washed at 65°C in several changes of Church-Gilbert wash solution (40 mmol/L NaHPO4, 1 mmol/L EDTA, and 1% SDS, pH 7.2) before visualization of the TβR-I gene by autoradiography.
RESULTS
Because we had previously shown that TGF-β–mediated growth regulation can be impaired in cells that have undergone progression from LyP to ALCL,37,38 we sought to determine whether JK cells, which were derived from an ALCL lesion, remained competent to undergo growth arrest as determined by a [3H]thymidine incorporation assay in response to TGF-β administration. In contrast to the TGF-β–mediated response measured in Mv1Lu cells (Fig 1) and the expected growth inhibition of normal T-lymphocytes,47-52 incubation of JK cells in the presence of increasing concentrations of TGF-β failed to inhibit their ability to synthesize DNA (Fig 1).
JK cells fail to undergo growth arrest in response to TGF-β. Mv1Lu (•) or JK (○) cells were incubated in the absence or presence of increasing concentrations of TGF-β1 (0 to 20 ng/mL) for 48 hours at 37°C. During the final 4 hours of agonist treatment, cellular DNA was radiolabeled by addition of [3H]thymidine in the culture medium. Upon completion of agonist stimulations, the cells were harvested and prepared for scintillation counting to determine radionucleotide incorporation into cellular DNA as described in Materials and Methods. Data are the mean (± standard error of mean [SEM]) of 3 independent experiments normalized to untreated controls.
JK cells fail to undergo growth arrest in response to TGF-β. Mv1Lu (•) or JK (○) cells were incubated in the absence or presence of increasing concentrations of TGF-β1 (0 to 20 ng/mL) for 48 hours at 37°C. During the final 4 hours of agonist treatment, cellular DNA was radiolabeled by addition of [3H]thymidine in the culture medium. Upon completion of agonist stimulations, the cells were harvested and prepared for scintillation counting to determine radionucleotide incorporation into cellular DNA as described in Materials and Methods. Data are the mean (± standard error of mean [SEM]) of 3 independent experiments normalized to untreated controls.
Defects in or loss of expression of the TβR-II for TGF-β has been linked to the growth of cutaneous T-cell lymphoma.37-39 As a direct measure of JK cell TGF-β receptor functioning, we performed an in vitro protein kinase assay that assessed the ability of immunoprecipitated TGF-β receptors to phosphorylate exogenously added GST-Smad3 fusion protein. Unlike agonist-stimulated TGF-β receptors isolated from TGF-β-responsive cells (ie, Mv1Lu, HT1080 fibrosarcoma, or MDA-MB-231 breast carcinoma cells), immunocomplexes of JK cell TGF-β receptors failed to phosphorylate recombinant GST-Smad3 in an agonist-dependent manner (Fig 2A). Indeed, the inability of activated JK cell TGF-β receptors to phosphorylate GST-Smad3 is consistent with the refractoriness of JK cells to TGF-β administration and suggests that the abnormal responses of JK cells to TGF-β treatment may arise from alterations in either, or both, of the genes for the TGF-β receptors.
Analysis of JK cell TβR-I receptors. (A) Immunocomplexes of JK cell TβR-I fail to phosphorylate recombinant GST-Smad3. Mv1Lu (Mv1), human HT1080 fibrosarcoma (HT), human MDA-MB-231 breast cancer (231), or JK cells were incubated in the absence or presence of TGF-β1 for 10 minutes at 37°C. Triton X-100 solubilized cell extracts were prepared and subjected to immunoprecipitation with anti-TGF–β type II receptor antibodies, and the resulting immunocomplexes were incubated in the presence of recombinant GST-Smad3 and [γ-32P]ATP as described in Materials and Methods. Data shown is a representative autoradiograph depicting TGF-β–stimulated phosphorylation of recombinant GST-Smad3. (B) Loss of cell surface expression of TβR-I receptors in JK cells. Mv1Lu or JK cells (5 × 106 cells/tube) were incubated in the presence of 250 pmol/L [125I]TGF-β1 for 90 minutes at 4°C before addition of disuccinimidyl suberate to cross-link the cytokine/receptor complexes. Triton X-100 solubilized cell extracts were prepared and subjected to immunoprecipitation with anti-TβR–I or -TβR–II receptor antibodies as described in Materials and Methods. Data shown is a representative autoradiograph depicting iodinated TGF-β1 bound to its TβR-I and TβR-II receptors as indicated. (C) TβR-I mRNA is expressed normally in JK cells. A total of 5 μg of total mRNA was hybridized to a32P-radiolabeled probe corresponding to nucleotides 226-1536 of the TβR-I cDNA. Data shown is a representative autoradiograph of the ∼6.5 kb TβR-I mRNA transcript in Mv1Lu and JK cells.
Analysis of JK cell TβR-I receptors. (A) Immunocomplexes of JK cell TβR-I fail to phosphorylate recombinant GST-Smad3. Mv1Lu (Mv1), human HT1080 fibrosarcoma (HT), human MDA-MB-231 breast cancer (231), or JK cells were incubated in the absence or presence of TGF-β1 for 10 minutes at 37°C. Triton X-100 solubilized cell extracts were prepared and subjected to immunoprecipitation with anti-TGF–β type II receptor antibodies, and the resulting immunocomplexes were incubated in the presence of recombinant GST-Smad3 and [γ-32P]ATP as described in Materials and Methods. Data shown is a representative autoradiograph depicting TGF-β–stimulated phosphorylation of recombinant GST-Smad3. (B) Loss of cell surface expression of TβR-I receptors in JK cells. Mv1Lu or JK cells (5 × 106 cells/tube) were incubated in the presence of 250 pmol/L [125I]TGF-β1 for 90 minutes at 4°C before addition of disuccinimidyl suberate to cross-link the cytokine/receptor complexes. Triton X-100 solubilized cell extracts were prepared and subjected to immunoprecipitation with anti-TβR–I or -TβR–II receptor antibodies as described in Materials and Methods. Data shown is a representative autoradiograph depicting iodinated TGF-β1 bound to its TβR-I and TβR-II receptors as indicated. (C) TβR-I mRNA is expressed normally in JK cells. A total of 5 μg of total mRNA was hybridized to a32P-radiolabeled probe corresponding to nucleotides 226-1536 of the TβR-I cDNA. Data shown is a representative autoradiograph of the ∼6.5 kb TβR-I mRNA transcript in Mv1Lu and JK cells.
To test this hypothesis, we performed a radioligand binding and cross-linking experiment that measured the ability of iodinated TGF-β to form complexes with its receptor polypeptides. As expected, addition of iodinated TGF-β1 to Mv1Lu cells facilitated the formation of cytokine/receptor complexes comprised of the 2 signaling receptor polypeptides for TGF-β, TβR-I, and TβR-II (Fig 2B). In contrast to Mv1Lu cells and the expected TGF-β receptor binding profiles of normal T-lymphocytes,37,47 50-52 incubation of iodinated TGF-β1 with JK cells showed that the TβR-II was expressed normally to the cell surface, while expression of TβR-I capable of binding TGF-β was completely absent from the surface of JK cells (Fig 2B). This finding suggests that the insensitivity of JK cells to TGF-β may be due to defects in the gene for TβR-I.
We next subjected JK cell total RNA to Northern blot analysis using a32P-radiolabeled probe that encoded nucleotides 226-1536 of the human TβR-I. As shown in Fig 2C, a single TβR-I transcript of ∼6.5 kb was detected in the TGF-β–responsive Mv1Lu cells, as well as in the TGF-β–unresponsive JK cells. This result indicates that the loss of cell surface TβR-I expression in JK cells cannot be due to deletion of the gene for TβR-I or in its ability to be transcribed, but may instead be due to the presence of a mutation(s) within its coding sequence.
To test this hypothesis, we performed RT-PCR on JK cell total RNA to generate full-length TβR-I cDNA clones. Sequencing the complete coding region of individual JK cell TβR-I cDNA clones identified a 178-bp deletion in 12 of 13 clones. As shown in Fig 3, this deletion spanned the last 178 bp of exon 1 and eliminated the initiating methionine. No additional mutations were found within the remaining coding sequence of JK cell TβR-I cDNA clones. Consistent with the phenotype of JK cells, ectopic expression of JK TβR-I cDNA in Cos-7 cells failed to produce a protein capable of forming complexes with TβR-II upon addition of iodinated TGF-β, nor did its expression alter or reconstitute TGF-β–stimulated gene expression in Mv1Lu or R1B cells,53 respectively (data not shown). The lone remaining TβR-I clone was wild-type both in its sequence and function when ectopically expressed in mammalian cells (data not shown). It is important to note that JK cells are a population, not a clone, of the explanted SCID mouse tumor cells, and as such, it is likely that a minute quantity of wild-type cells would be present and detected by RT-PCR analysis, but would escape detection in assays designed to measure TGF-β function (Figs 1 and 2). Taken together, these results indicate that the inability of JK cells to respond to TGF-β results from the deletion of the initiating methionine contained within the deleted 178 bp of exon 1, and consequently prevents the translation of the TβR-I polypeptide.
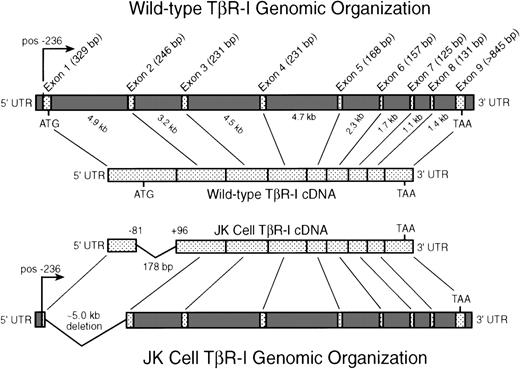
Intron-exon organization of the human TβR-I: identification and localization of an N-terminal deletion in JK cell TβR-I cDNA. JK cell total RNA was subjected to RT-PCR analysis to facilitate the cloning and isolation of full-length JK cell TβR-I cDNAs as described in Materials and Methods. Shown is the transcriptional start site (pos. −236) and the intron-exon organization of the human TβR-I as described recently by Vellucci and Reiss.63 As depicted, the TβR-I is comprised of 9 exons spanning ∼31 kb of genomic DNA sequence. Sequencing of 13 JK cell TβR-I cDNA clones generated by RT-PCR demonstrated the presence in 12 clones of a 178-bp deletion, beginning at position −81 relative to the initiating methionine and concluding at position +96. This deletion eliminates the initiating methionine and the final 178 bp of exon 1 of the TβR-I in JK cells.
Intron-exon organization of the human TβR-I: identification and localization of an N-terminal deletion in JK cell TβR-I cDNA. JK cell total RNA was subjected to RT-PCR analysis to facilitate the cloning and isolation of full-length JK cell TβR-I cDNAs as described in Materials and Methods. Shown is the transcriptional start site (pos. −236) and the intron-exon organization of the human TβR-I as described recently by Vellucci and Reiss.63 As depicted, the TβR-I is comprised of 9 exons spanning ∼31 kb of genomic DNA sequence. Sequencing of 13 JK cell TβR-I cDNA clones generated by RT-PCR demonstrated the presence in 12 clones of a 178-bp deletion, beginning at position −81 relative to the initiating methionine and concluding at position +96. This deletion eliminates the initiating methionine and the final 178 bp of exon 1 of the TβR-I in JK cells.
To exclude the possibility that the deletion identified in the TβR-I in JK cells was an artifact of PCR, we subjected JK cell genomic DNA to PCR and Southern blot analyses (Fig4A). First, use of flanking primers designed to amplify exon 1 (primers 1 and 2, Fig 4A) of TβR-I failed to elicit any signal from JK cell genomic DNA, while the expected 255-bp fragment was readily detected in genomic DNA of TGF-β–responsive cells (Fig 4B, upper panel). The inability to detect a truncated TβR-I exon 1 signal from JK cell genomic DNA implied that the 178-bp deletion identified in its cDNA may encompass part, or all, of its first intron, but would not alter the integrity of exon 2, which was wild-type in RT-PCR analyses (Fig 3). Second, and consistent with this hypothesis, we found that amplification of JK cell genomic DNA with primers that flanked the deleted TβR-I cDNA region produced a single, unique band of 260 bp that was not evident in the genomes of TGF-β–responsive cells, which exhibited an ∼500 bp fragment (Fig 4B, lower panel). Sequencing of this 260-bp fragment showed it to be identical to that found in its TβR-I cDNA, indicating that the deletion in the gene for the TβR-I in JK cells is ∼5 kb, and encompasses the last 178 bp of exon 1 and all of the first intron (Fig 3). Sequence analysis of the 500-bp PCR product amplified from TGF-β–responsive cells failed to yield TβR-I sequence, and as such, represents a nonspecific PCR product. Although we do not yet know why this nonspecific PCR product was obtained exclusively from cells harboring wild-type TβR-I genes, its appearance solely in TGF-β–responsive cells may be due to the presence of intron 1 in their TβR-I genes, a region that is absent in the TβR-I genes of JK cells. For instance, the PCR reaction conditions used in these experiments (ie, 60-second extension segments) would not be expected to amplify intron 1 in its entirety, and as such, the appearance of the nonspecific 500 bp product may arise from premature termination of PCR product elongation or from decreased fidelity of the polymerase due to the high GC-content and repetitive sequences inherent to introns. JK cells, whose TβR-I gene lacks intron 1 (Fig 3), are not subject to the intronic effects of their TGF-β–responding counterparts, and consequently fail to produce the nonspecific 500 bp product that is consistently and exclusively found in TGF-β–responsive cells.
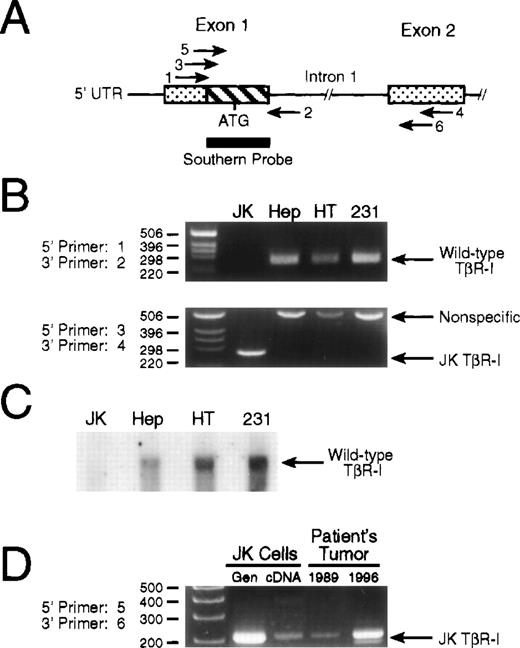
Genomic analysis of TβR-I in JK cells. (A) Schematic depicting the PCR primers and Southern blotting probe used to analyze the intron-exon structure surrounding exons 1 and 2 of the TβR-I gene. Shown is exons 1 and 2 of TβR-I (stippled boxes) and their intervening intron. The hatched box of exon 1 represents the 178-bp deletion identified by RT-PCR analysis of JK cell mRNA, and the thick bar underlying this region depicts the 32P-radiolabeled PCR fragment used in Southern blotting experiments. Amplification of exon 1 from genomic DNA was accomplished using primer pair 1 and 2 to amplify the normal 255-bp product. Exon 1 and 2 initiated amplification across intron 1 was accomplished using primer pair 3 and 4 or primer pair 5 and 6 on genomic DNA obtained from cultured cells or patient biopsies, respectively. Primer sequences and PCR reaction conditions are described in Materials and Methods. (B) PCR of genomic DNA identifies an ∼5 kb deletion in JK cell TβR-I gene. (Upper panel) A total of 100 ng of genomic DNA from JK, HepG2 hepatoma (Hep), HT1080 fibrosarcoma (HT), or MDA-MB-231 breast cancer (231) cells was subjected to PCR amplification using primer pair 1 and 2 that flanked exon 1 of the human TβR-I. The resulting PCR products were fractionated through a 2.5% agarose/TAE gel as described in Materials and Methods. Data shown is a representative picture of an ethidium bromide-stained gel demonstrating the presence of the 255-bp TβR-I product only in TGF-β–responsive cells. (Lower panel) One microgram of genomic DNA from JK, HepG2 (Hep), HT1080 (HT), or MDA-MB-231 (231) cells was subjected to PCR amplification using primer pair 3 and 4, which flanked the 178-bp deletion in the JK cell TβR-I cDNA. Shown is a representative picture of an ethidium bromide-stained gel demonstrating the presence only in JK cells of a single amplified PCR product, whose size (260 bp) and sequence was identical to that of the JK cell TβR-I cDNA. (C) Radiolabeled deletion fragment fails to hybridize with JK cell genomic DNA. Ten micrograms of genomic DNA from JK, HepG2 (Hep), HT1080 (HT), or MDA-MB-231 (231) cells was digested overnight at 37°C with Hind III, and after transfer to nylon membrane, hybridized with a 32P-radiolabeled probe corresponding to the 178-bp sequence absent in JK cell TβR-I cDNA as described in Materials and Methods. Data shown is a representative autoradiograph demonstrating the presence of a hybridizing signal in the genomes of TGF-β–responsive cells, but not in JK cells. (D) Detection of mutated TβR-I in tumor biopsies obtained before establishment of the JK cell line. JK cell genomic DNA (Gen), cDNA, or genomic DNA obtained from patient tumor biopsies performed in 1989 and 1996 were subjected to PCR amplification using primer pair 5 and 6, which flanked the 178-bp deletion in the JK cell TβR-I cDNA. Shown is a representative picture of an ethidium bromide-stained gel demonstrating the presence of mutated TβR-I gene in the patient’s primary tumor samples before the establishment of the JK cell line.
Genomic analysis of TβR-I in JK cells. (A) Schematic depicting the PCR primers and Southern blotting probe used to analyze the intron-exon structure surrounding exons 1 and 2 of the TβR-I gene. Shown is exons 1 and 2 of TβR-I (stippled boxes) and their intervening intron. The hatched box of exon 1 represents the 178-bp deletion identified by RT-PCR analysis of JK cell mRNA, and the thick bar underlying this region depicts the 32P-radiolabeled PCR fragment used in Southern blotting experiments. Amplification of exon 1 from genomic DNA was accomplished using primer pair 1 and 2 to amplify the normal 255-bp product. Exon 1 and 2 initiated amplification across intron 1 was accomplished using primer pair 3 and 4 or primer pair 5 and 6 on genomic DNA obtained from cultured cells or patient biopsies, respectively. Primer sequences and PCR reaction conditions are described in Materials and Methods. (B) PCR of genomic DNA identifies an ∼5 kb deletion in JK cell TβR-I gene. (Upper panel) A total of 100 ng of genomic DNA from JK, HepG2 hepatoma (Hep), HT1080 fibrosarcoma (HT), or MDA-MB-231 breast cancer (231) cells was subjected to PCR amplification using primer pair 1 and 2 that flanked exon 1 of the human TβR-I. The resulting PCR products were fractionated through a 2.5% agarose/TAE gel as described in Materials and Methods. Data shown is a representative picture of an ethidium bromide-stained gel demonstrating the presence of the 255-bp TβR-I product only in TGF-β–responsive cells. (Lower panel) One microgram of genomic DNA from JK, HepG2 (Hep), HT1080 (HT), or MDA-MB-231 (231) cells was subjected to PCR amplification using primer pair 3 and 4, which flanked the 178-bp deletion in the JK cell TβR-I cDNA. Shown is a representative picture of an ethidium bromide-stained gel demonstrating the presence only in JK cells of a single amplified PCR product, whose size (260 bp) and sequence was identical to that of the JK cell TβR-I cDNA. (C) Radiolabeled deletion fragment fails to hybridize with JK cell genomic DNA. Ten micrograms of genomic DNA from JK, HepG2 (Hep), HT1080 (HT), or MDA-MB-231 (231) cells was digested overnight at 37°C with Hind III, and after transfer to nylon membrane, hybridized with a 32P-radiolabeled probe corresponding to the 178-bp sequence absent in JK cell TβR-I cDNA as described in Materials and Methods. Data shown is a representative autoradiograph demonstrating the presence of a hybridizing signal in the genomes of TGF-β–responsive cells, but not in JK cells. (D) Detection of mutated TβR-I in tumor biopsies obtained before establishment of the JK cell line. JK cell genomic DNA (Gen), cDNA, or genomic DNA obtained from patient tumor biopsies performed in 1989 and 1996 were subjected to PCR amplification using primer pair 5 and 6, which flanked the 178-bp deletion in the JK cell TβR-I cDNA. Shown is a representative picture of an ethidium bromide-stained gel demonstrating the presence of mutated TβR-I gene in the patient’s primary tumor samples before the establishment of the JK cell line.
Third, as shown in Fig 4C, hybridization of a32P-radiolabeled probe encoding nucleotides −81 to 96 of human TβR-I cDNA (ie, the sequence deleted in JK cell TβR-I cDNA) to a Southern blot of genomic DNA of TGF-β–responsive cells elicited a strong hybridizing signal. Consistent with the results in Fig 4B, hybridization of JK cell genomic DNA with this same32P-radiolabeled probe was negative (Fig 4C). The absence of wild-type bands both in the JK cell genomic PCR and JK Southern blot indicates that the population of JK cells no longer contain any significant amount of wild-type TβR-I sequences within their genome.
Lastly, we asked whether the deletion in the JK cell TβR-I gene was present in the patient’s primary tumor samples. Amplification of patient genomic DNA obtained from tumor biopsies performed in 1989 and 1996 with primers that flanked the deleted region of JK cell TβR-I cDNA produced a product that was identical to that amplified from either JK cell genomic DNA or cDNA (Fig 4D). This finding demonstrates that the deletion in the JK cell TβR-I gene did not arise as a consequence of its derivation or time in culture, but rather was present during the course of the patient’s lymphoma.
DISCUSSION
Although between 10% and 20% of patients with LyP develop malignant lymphoma,54 the genetic alterations underlying this progression are currently unknown. We previously described the inability of many lymphoma cells to undergo growth arrest in response to TGF-β after their progression from LyP to malignant cutaneous tumors.37 In the present study, we sought to determine the molecular basis responsible for resistance to TGF-β–mediated growth inhibition in JK cells, a human cutaneous T-cell lymphoma cell line derived from an advanced cutaneous ALCL. We identified an ∼5 kb deletion in the gene for the TβR-I that eliminates the last 178 bp of exon 1, including the initiating methionine, and all of intron 1. Consistent with the phenotype of JK cells, expression of mutated JK cell TβR-I cDNA in mammalian cells failed to produce a polypeptide capable of interacting with TβR-II upon addition of TGF-β, nor did its expression reconstitute TGF-β–mediated signaling in R1B cells, which lack TβR-I (data not shown). Indeed, our results suggest that the insensitivity of JK cells to TGF-β arises from their inability to mediate efficient translation of TβR-I transcripts, and as such, represents the first report to identify and describe a specific mutation in the gene for the TβR-I responsible for liberating cells from the antiproliferative effects of TGF-β.
With respect to involvement in the generation and progression of human cancer, TGF-β and its intracellular signaling proteins are widely established tumor suppressors. For instance, the TGF-β–stimulated signaling molecules Smad2 and Smad4,14,15,27,28 and possibly Smad3,31 are frequently mutated or deleted in human cancers. Similarly, inactivating mutations in or loss of TβR-II expression causes insensitivity to TGF-β in a variety of human neoplasms, including some colon, gastric, prostate, and retinal cancers, and some T-cell lymphomas.32-39
Interestingly, while a number of studies have clearly established the importance of TβR-I as the initiator of TGF-β–stimulated signaling events after its activation by TβR-II,5,10,55,56 few studies have provided definitive evidence of its importance as a tumor suppressor gene in humans. Indeed, Chen et al46 have recently identified a S387Y substitution that produces only a modest inhibition in TβR-I–mediated signaling, but is nonetheless highly associated with metastatic breast cancer. Additionally, a common polymorphism localized within the signal sequence of the TβR-I gene of normal volunteers and patients with acute myeloid leukemia has also been described recently.57Although this polymorphism was detected at higher frequencies in transformed versus nontransformed cells, a complete and thorough understanding of its biological and clinical significance remains to be determined.
Insensitivity to TGF-β has, however, been attributed to reduced or lost expression of cell surface TβR-I in colon cancer or B-cell chronic lymphocytic leukemia,40,41 respectively. Diminished expression of TβR-I has also been shown to mediate resistance of pancreatic cancer cells to TGF-β,42,43 while homozygous deletion of the TβR-I gene has recently been found in 1% of all pancreatic cancers surveyed.58 Similarly, an unknown genetic alteration leading to the loss of TβR-I transcription was capable of freeing prostate cancer cells from the growth inhibitory effects of TGF-β.44 Our results identifying a TβR-I mutation in a human lymphoma, together with the above findings, indicate that the TβR-I gene is genetically unstable and highly susceptible to inactivating mutations capable of facilitating the formation of malignant cells.
Although 1 in 13 JK cell TβR-I cDNA clones sequenced after RT-PCR analysis was wild-type both in its sequence and function, this clone and its parental cell are not reflective of the population of JK cells as a whole. Because JK cells are a population, not a clone, of the explanted SCID mouse tumor cells, it is likely that the JK cell line might harbor a small percentage of wild-type cells that escape detection in TGF-β functional assays (Figs 1 and 2), but are detected in RT-PCR experiments. However, our inability to detect by Southern blotting a hybridizing signal in JK cells with a probe internal to the TβR-I deletion in JK cell cDNA, together with the absence of multiple wild-type products obtained from PCR of JK cell genomic DNA, demonstrates that the vast majority of the population of JK cells no longer harbor wild-type TβR-I genes within their genome. These results suggest that JK cells have undergone a loss of heterozygosity at the TβR-I locus, leaving only the deletion containing TβR-I gene. This hypothesis is in keeping with the paradigm of inactivation of tumor suppressor genes (ie, inactivating mutation, followed by a loss of heterozygosity), and with the recent finding that late in the development of squamous cell carcinoma, 65% of all tumors exhibit a loss of heterozygosity at chromosome position 9q22.3,59 a region to which the gene for the TβR-I has been assigned.57
Although the exact role the JK cell TβR-I deletion played during the course of the patient’s disease remains unknown, it is plausible that the loss of TβR-I expression mediated by the JK deletion after the loss of heterozygosity at the TβR-I locus conferred a selective growth advantage to the developing tumor. Although we can detect mutated TβR-I early in the clinical course of the patient’s lymphoma, we cannot ascertain exactly when homozygosity at the TβR-I locus occurred; however, as the loss of TβR-I heterozygosity occurred before the establishment of the JK cell line, it is tempting to speculate that this event may have taken place during the progression from LyP to ALCL. Studies of TGF-β1–deficient mice showed that although approximately 50% of all pups died in utero, the remaining littermates developed normally and survived to term, only to succumb to severe inflammatory responses within 3 to 4 weeks after parturition.3,60,61 These findings suggest that TGF-β plays a vital role in suppressing immune cell activation and proliferation in vivo. With respect to the patient’s developing lymphoma, the loss of TGF-β sensitivity would have removed the immunosuppressive properties of TGF-β and enhanced cell proliferation. Furthermore, because malignant cells typically upregulate their production and secretion of TGF-β, ancestral cells homozygous for the JK TβR-I deletion would also be expected to experience increased protection against immune system surveillance by inhibiting the clonal expansion of tumor-infiltrating lymphocytes.62 Clinically, the presence of TβR-I, not TβR-II, was found to be a highly predictive marker of prostate cancer treatability and patient survival, with loss of TβR-I expression resulting in higher Gleason scores (ie, more poorly differentiated), more advanced clinical stages, decreased survival rates, and increased serological recurrence after radical prostatectomy.34Although studies in more patients are clearly needed, it is tempting to speculate that the integrity of TβR-I in human lymphomas may be used as a prognostic indicator for patient treatability and survival.
In summary, our results are the first to identify a specific mutation in the TβR-I gene leading not only to the loss of its expression, but also to the loss of its tumor suppressive properties in human T-cell lymphoma. More studies are clearly needed, and are currently ongoing, to further our understanding of the role of the TβR-I during the development and progression of cancer, particularly those involving T- and B-cell lymphomas, where alterations in receptor signaling appear to play a role in the development of the malignant phenotype.
ACKNOWLEDGMENT
The authors thank Dr Christina Rhodes for help and suggestions with the RT-PCR reactions, Dr Xeudong Liu for providing the GST-Smad3 construct, and Dr Rebecca Wells for providing iodinated TGF-β1 and anti-TβR–I and -TβR–II antibodies. Members of the Lodish laboratory are gratefully acknowledged for providing useful technical suggestions and numerous helpful discussions. Drs Ralph Lin and Allen Sirotkin are thanked for critical reading of the manuscript.
Supported by Grant No. CA73161-01 from the National Cancer Institute (to W.P.S.), Grant No. CA63260 from the National Institutes of Health (to H.F.L.), by the Leukemia Society of America (M.E.K.), Grant No. ROG-125-01 from the American Cancer Society (to M.E.K.), a grant from Berlex, Inc (to M.E.K), and by the Deutsche Krebshilfe (W.M.P.).
Address requests for JK cell line to Marshall E. Kadin, MD, Department of Pathology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Harvey F. Lodish, PhD, Whitehead Institute for Biomedical Research, Nine Cambridge Center, Cambridge, MA 02142; e-mail: lodish@wi.mit.edu.

![Fig. 1. JK cells fail to undergo growth arrest in response to TGF-β. Mv1Lu (•) or JK (○) cells were incubated in the absence or presence of increasing concentrations of TGF-β1 (0 to 20 ng/mL) for 48 hours at 37°C. During the final 4 hours of agonist treatment, cellular DNA was radiolabeled by addition of [3H]thymidine in the culture medium. Upon completion of agonist stimulations, the cells were harvested and prepared for scintillation counting to determine radionucleotide incorporation into cellular DNA as described in Materials and Methods. Data are the mean (± standard error of mean [SEM]) of 3 independent experiments normalized to untreated controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2854.420k07_2854_2861/7/m_blod42007001x.jpeg?Expires=1767810629&Signature=sW96WrY42XOO0~MWAunH7ig2N708F1qj3ZvuGl7px1MqFr~EPperBc~tPBMJeggbVEdZXMCrEKQrXPApQBQq4~wczhx3w24pwcADEfu-6tCILtnRGbnDKYg~3wsDhz2SLbbzZU4iKrEWhBxl5S0p62tXEU8512W0PW4GyuRHEUvhmkAT6Wj9CMsjfucMkI-6FalSgIEmuXj-3d9TaFQ8F968LQQ3QzlYFpGuUrAJsRqczPagrLK3nt1lzs4t6angZio7OuEoO42O9LXfTPwSshFQSZPlKT-Fk4DA4uQjhBU0tt4pI4Ph~gsnAD94XcNXyRsDzqZiIculxJIhDSMjIA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Analysis of JK cell TβR-I receptors. (A) Immunocomplexes of JK cell TβR-I fail to phosphorylate recombinant GST-Smad3. Mv1Lu (Mv1), human HT1080 fibrosarcoma (HT), human MDA-MB-231 breast cancer (231), or JK cells were incubated in the absence or presence of TGF-β1 for 10 minutes at 37°C. Triton X-100 solubilized cell extracts were prepared and subjected to immunoprecipitation with anti-TGF–β type II receptor antibodies, and the resulting immunocomplexes were incubated in the presence of recombinant GST-Smad3 and [γ-32P]ATP as described in Materials and Methods. Data shown is a representative autoradiograph depicting TGF-β–stimulated phosphorylation of recombinant GST-Smad3. (B) Loss of cell surface expression of TβR-I receptors in JK cells. Mv1Lu or JK cells (5 × 106 cells/tube) were incubated in the presence of 250 pmol/L [125I]TGF-β1 for 90 minutes at 4°C before addition of disuccinimidyl suberate to cross-link the cytokine/receptor complexes. Triton X-100 solubilized cell extracts were prepared and subjected to immunoprecipitation with anti-TβR–I or -TβR–II receptor antibodies as described in Materials and Methods. Data shown is a representative autoradiograph depicting iodinated TGF-β1 bound to its TβR-I and TβR-II receptors as indicated. (C) TβR-I mRNA is expressed normally in JK cells. A total of 5 μg of total mRNA was hybridized to a32P-radiolabeled probe corresponding to nucleotides 226-1536 of the TβR-I cDNA. Data shown is a representative autoradiograph of the ∼6.5 kb TβR-I mRNA transcript in Mv1Lu and JK cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2854.420k07_2854_2861/7/m_blod42007002w.jpeg?Expires=1767810629&Signature=ciRWOkWe8y28kf-Q~c8uhYq-6MrfX6eHv2Lh3dGJNLVM8TUzM9xd~PkYsZ2S93e0keTZ0vsBQJbbkzQU62fV2TNZYY9cJukJDAmN1VHWKoGgAO0J-0IJx0mV~1P9xisHbAY8dnXVigt08qZrP7o9rv6nBgixdNNmM440nfOIpQP~r15~m90-LH5p73jYnX2UkZvq~SH6n9Wv9ECtFzj37f572H1KJVBOuyb3DfqGZebRC1ya3caxrgfLE0WHukpbMWT85cg7F0B~xI7enrFzYqoNRZ1R0k4YWVKAkkNATMv3cChRQ9CmvX4ZrglDpYNccYBP7j9AbXur~zpqOo8dNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal