B-chronic lymphocytic leukemia (B-CLL) is characterized by the accumulation of long-lived CD5+ B lymphocytes. We have analyzed the effect in vitro of the combination of fludarabine with cyclophosphamide and/or mitoxantrone on cells from 20 B-CLL patients. Mafosfamide, the active form of cyclophosphamide in vitro, increased the cytotoxicity of fludarabine in all of the patients studied and produced a significant synergistic effect (P < .01) after 48 hours of incubation. The addition of mitoxantrone to this combination increased the cytotoxic effect in cells from 8 patients, but in the remaining 12 patients no significant increase was observed. The effect of fludarabine and mafosfamide was dose-dependent. Mafosfamide and fludarabine had a synergistic effect in inducing apoptosis of B-CLL cells as determined by DNA staining with propidium iodide and analysis of phosphatidylserine exposure. Mafosfamide significantly increased the apoptosis induced by fludarabine on CD19+ cells (P = .007), but not on CD3+ cells (P= .314). Cell viability was correlated with a decrease in Mcl-1 levels and an increase in p53 levels. These results support that fludarabine in combination with cyclophosphamide and/or mitoxantrone can be highly effective in the treatment of B-CLL.
B-CELL CHRONIC lymphocytic leukemia (B-CLL) is characterized by the accumulation of long-lived, functionally inactive, mature appearing neoplastic B lymphocytes.1 Most circulating cells appear to be arrested at the G0 phase of the cell cycle and the clonal excess of B cells is mainly caused by defects that prevent programmed cell death rather than by alterations in cell cycle regulation.2
Patients with B-CLL in early clinical stages (Binet A, Rai 0) and stable disease should not be treated unless the disease progresses. In contrast, most patients with poor prognostic features, such as an advanced clinical stage, diffuse bone marrow infiltration, or rapidly increasing blood lymphocyte levels, require therapy. For many years, chlorambucil, either alone or in combination with prednisone, has been the treatment of choice for B-CLL.3 In the last few years, purine analogs, particularly fludarabine, have changed the treatment possibilities of B-CLL. Fludarabine has demonstrated high efficacy in the treatment of this form of leukemia.4-8 However, although fludarabine produces the highest response rate ever reported for a single agent in B-CLL, responses are not sustained and all patients eventually relapse. In addition, fludarabine does not prolong survival of individuals with B-CLL in comparison with chlorambucil and CAP (cyclophosphamide, doxorubicin, and prednisone).9,10For all these reasons, there is increasing interest in assessing whether the results obtained with fludarabine alone could be improved by combining it with other drugs.11,12 Recently, pilot studies have analyzed the use of fludarabine in combination with prednisone, doxorubicin, cyclophosphamide, epirubicin, chlorambucil, and mitoxantrone in the treatment of B-CLL.6,11,13-19 Among these regimens, those who have given the most promising results are the combination of fludarabine and cyclophosphamide11,14-16 and these 2 with mitoxantrone.19
The aim of this study was to analyze the chemosensitivity of B-CLL cells in vitro to the combination of fludarabine, mafosfamide (the active form of cyclophosphamide in vitro), and mitoxantrone, alone and in combination.
MATERIALS AND METHODS
Patients.
Twenty patients (9 men and 11 women) with B-CLL who had not received treatment for the previous 6 months, with a median age of 72 years (range, 42 to 86 years), were studied. B-CLL was diagnosed according to standard clinical and laboratory criteria. The median peripheral blood leukocytosis was 102 × 109 leukocytes/L (range, 19 to 510 × 109/L). Leukemic cells phenotyped for cell surface markers by flow cytometry were positive in all cases for CD5 and CD19. According to Binet’s classification,20 at the time of inclusion, 12 patients were at stage A, 3 patients were at stage B, and 5 patients were at stage C (Table 1).
Characteristics of B-CLL Patients
| Patient No. . | Age/ Sex . | Stage* . | WBC . | CD5 . | CD19 . | CD22 . | CD23 . | FMC7 . | κ/λ . | CD79b . | β2M . | +12 . | Previous Treatment . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 72/M | A | 180 | 99 | 90 | 93+ | 50 | 2 | 0/93++ | 84++ | 6.4 | Yes | CLB |
| 2 | 61/M | A | 68 | 95 | 84 | 77+ | 85 | 4 | 79+/0 | 27dim | 2 | ND | No |
| 3 | 67/F | C | 163 | 68 | 90 | 15 dim | 87 | 0 | 0/88 | 19 | ND | No | No |
| 4 | 77/F | A | 107 | 85 | 59 | 38+ | 43 | 14 | 17/0 | ND | 2 | ND | No |
| 5 | 63/F | A | 87 | 97 | 90 | 84+/++ | 64 | 5 | 63/0 | ND | ND | ND | No |
| 6 | 69/F | A | 19 | 94 | 69 | 74 | 25 | 6 | 45+/0 | 53+ | ND | No | No |
| 7 | 47/M | B | 425 | 96 | 95 | 70+ | 95 | 7 | 0/93+/++ | ND | 5.4 | ND | 2-CDA |
| 8 | 70/M | A | 31 | 91 | 87 | 88+ | 78 | 14 | 86+/0 | ND | 6.2 | No | CLB/PDN; FLU |
| 9 | 47/M | B | 18 | 97 | 92 | 54+ | 90 | 16 | 54+/0 | ND | 2.5 | ND | CLB; FLU |
| 10 | 84/F | A | 154 | 96 | 93 | 93+ | 41 | 0 | 91+/0 | 90+ | 4.1 | ND | No |
| 11 | 76/M | C | 84 | 93 | 93 | 92+ | 93 | 65 | 71+/0 | 20 | 5 | No | No |
| 12 | 80/M | C | 198 | 99 | 97 | 0 | 0 | 3 | 1/75+ | 2 | 5.5 | ND | No |
| 13 | 83/F | A | 170 | 99 | 97 | 49+ | 51 | 0 | 71+/0 | 60+ | 9.8 | ND | No |
| 14 | 42/F | B | 141 | 97 | 94 | 65+ | 45 | 15 | 83+/0 | 34 | 1.4 | No | No |
| 15 | 85/F | A | 98 | 97 | 93 | 40+ | 46 | ND | 47+/0 | ND | 5.9 | ND | No |
| 16 | 76/F | A | 194 | 90 | 87 | 74+ | 51 | 4 | 93+/++/0 | 87++ | 5.9 | Yes | No |
| 17 | 65/M | C | 38 | 99 | 95 | 69+ | 69 | 2 | 0/80+/++ | 4 | 12.8 | No | CLB |
| 18 | 74/F | C | 510 | 93 | 94 | 74 | 50 | 20 | 20+/0 | 55+ | 7 | ND | No |
| 19 | 73/M | A | 29 | 80 | 83 | 80+ | 67 | 0 | 0/60+ | 53 | 6 | Yes | CLB |
| 20 | 86/F | A | 18 | 97 | 88 | 91+ | 91 | 20 | 59+/0 | 68+ | 4 | Yes | No |
| Patient No. . | Age/ Sex . | Stage* . | WBC . | CD5 . | CD19 . | CD22 . | CD23 . | FMC7 . | κ/λ . | CD79b . | β2M . | +12 . | Previous Treatment . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 72/M | A | 180 | 99 | 90 | 93+ | 50 | 2 | 0/93++ | 84++ | 6.4 | Yes | CLB |
| 2 | 61/M | A | 68 | 95 | 84 | 77+ | 85 | 4 | 79+/0 | 27dim | 2 | ND | No |
| 3 | 67/F | C | 163 | 68 | 90 | 15 dim | 87 | 0 | 0/88 | 19 | ND | No | No |
| 4 | 77/F | A | 107 | 85 | 59 | 38+ | 43 | 14 | 17/0 | ND | 2 | ND | No |
| 5 | 63/F | A | 87 | 97 | 90 | 84+/++ | 64 | 5 | 63/0 | ND | ND | ND | No |
| 6 | 69/F | A | 19 | 94 | 69 | 74 | 25 | 6 | 45+/0 | 53+ | ND | No | No |
| 7 | 47/M | B | 425 | 96 | 95 | 70+ | 95 | 7 | 0/93+/++ | ND | 5.4 | ND | 2-CDA |
| 8 | 70/M | A | 31 | 91 | 87 | 88+ | 78 | 14 | 86+/0 | ND | 6.2 | No | CLB/PDN; FLU |
| 9 | 47/M | B | 18 | 97 | 92 | 54+ | 90 | 16 | 54+/0 | ND | 2.5 | ND | CLB; FLU |
| 10 | 84/F | A | 154 | 96 | 93 | 93+ | 41 | 0 | 91+/0 | 90+ | 4.1 | ND | No |
| 11 | 76/M | C | 84 | 93 | 93 | 92+ | 93 | 65 | 71+/0 | 20 | 5 | No | No |
| 12 | 80/M | C | 198 | 99 | 97 | 0 | 0 | 3 | 1/75+ | 2 | 5.5 | ND | No |
| 13 | 83/F | A | 170 | 99 | 97 | 49+ | 51 | 0 | 71+/0 | 60+ | 9.8 | ND | No |
| 14 | 42/F | B | 141 | 97 | 94 | 65+ | 45 | 15 | 83+/0 | 34 | 1.4 | No | No |
| 15 | 85/F | A | 98 | 97 | 93 | 40+ | 46 | ND | 47+/0 | ND | 5.9 | ND | No |
| 16 | 76/F | A | 194 | 90 | 87 | 74+ | 51 | 4 | 93+/++/0 | 87++ | 5.9 | Yes | No |
| 17 | 65/M | C | 38 | 99 | 95 | 69+ | 69 | 2 | 0/80+/++ | 4 | 12.8 | No | CLB |
| 18 | 74/F | C | 510 | 93 | 94 | 74 | 50 | 20 | 20+/0 | 55+ | 7 | ND | No |
| 19 | 73/M | A | 29 | 80 | 83 | 80+ | 67 | 0 | 0/60+ | 53 | 6 | Yes | CLB |
| 20 | 86/F | A | 18 | 97 | 88 | 91+ | 91 | 20 | 59+/0 | 68+ | 4 | Yes | No |
Immunophenotype is expressed in percentage of positive cells: +, less intense than normal; ++, normal intensity.
Abbreviations: WBC, white blood cell count (109/L); β2M, β2microglobulin (mg/L); +12, trisomy 12; CLB, chlorambucil; PDN, prednisone; 2-CDA, 2-chlorodesoxiadenosine; FLU, fludarabine; ND, not determined.
According to Binet’s classification.
Isolation of B-CLL cells.
Mononuclear cells were isolated from peripheral blood samples by centrifugation on a Ficoll/Hypaque (Seromed, Berlin, Germany) gradient and cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide (DMSO).
Reagents.
Fludarabine monophosphate was obtained from Schering AG (Berlin, Germany). Mafosfamide was obtained from ASTAMedica AG (Frankfurt, Germany). Mitoxantrone was obtained from Lederle Laboratories (Gosport, Hampshire, UK). 3,(4,5-Dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) and propidium iodide (PI) were obtained from Sigma Chemicals Co (St Louis, MO).
Cell culture.
B-CLL lymphocytes were cultured immediately after thawing at a concentration of 2 to 5 × 106 cells/mL in RPMI 1640 culture medium (GIBCO BRL, Paisley, Scotland) supplemented with 10% heat-inactivated fetal calf serum (Bio Whittaker, Verviers, Belgium), 2 mmol/L glutamine, and 0.04 mg/mL gentamicin at 37°C in a humidified atmosphere containing 5% carbon dioxide.21 Factors were added at the beginning of the culture.
Cell viability assay.
Cell viability was determined by the MTT assay.22 B-CLL lymphocytes (5 × 105 cells/well) were incubated in 96-well plates in the absence or in the presence of factors in a final volume of 100 μL. After 48 hours, 10 μL of MTT (5 mg/mL in phosphate-buffered saline [PBS]) was added to each well for a further 6 hours. The blue MTT formazan precipitated was dissolved in 100 μL of isopropanol:1 mol/L HCl (24:1) and the absorbance values at 550 nm were determined on a multiwell plate reader.
PI DNA staining.
Quantification of apoptosis by PI staining and fluorescence-activated cell sorting (FACS) analysis was performed as described previously.23 Briefly, cells were harvested and fixed in 70% ethanol. Cells were centrifuged, washed in PBS, and resuspended in 0.5 mL PBS containing PI (5 μg/mL) and RNase (100 μg/mL). Tubes were incubated for 30 minutes at 37°C and placed at 4°C in the dark overnight before flow cytometry analysis to identify the sub-G0 peak corresponding to apoptosis.
Analysis of apoptosis by annexin binding.
Exposure of phosphatidylserine was quantified by surface annexin V staining as described previously.24 One million cells were incubated for 24 hours with the indicated factors. Cells were then washed in PBS and incubated with phycoerythrin (PE)-conjugated anti-CD19 (DAKO, Glostrup, Denmark) or PE-conjugated anti-CD3 (Caltag Laboratories, Burlingame, CA) for 15 minutes in the dark. Cells were then washed, resuspended in 200 μL of binding buffer (10 mmol/L HEPES, pH 7.4, 2.5 mmol/L CaCl2, 140 mmol/L NaCl), and incubated with 0.5 μg/mL of Annexin V-fluorescein isothiocyanate (FITC; Bender MedSystems, Vienna, Austria) for 5 to 15 minutes in the dark. Cells were washed again and resuspended in binding buffer. PI (5 μg/mL) was added to each sample before flow cytometric analysis (FACScan; Becton Dickinson, Mountain View, CA). Samples were acquired using Lysis-II software and data were analyzed with the Paint-a-gate Pro software (Becton Dickinson). To analyze a sufficient number of cells, a live-gate in side scatter (SSC) versus CD19 or SSC versus CD3 was drawn and at least 5,000 CD19+ cells or CD3+ cells were acquired.
Western blot.
Cells were lysed in 80 mmol/L Tris HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 10% glycerol, and 0.1 mol/L dithiothreitol (DTT), and equal amounts of protein were separated by electrophoresis on 12% polyacrylamide gel and transferred to Immobilon-P (Millipore, Bedford, MA) membranes. The membranes were incubated with monoclonal antibodies against p53 (Oncogene Science Inc, Uniondale, NY), Bcl-2 (DAKO), or polyclonal antibodies against Mcl-1 (Santa Cruz Biotechnology Inc, Santa Cruz, CA) or Bax (Pharmingen, San Diego, CA). Antibody binding was detected using a secondary antibody (swine antimouse or mouse antirabbit Ig; DAKO) conjugated to horseradish peroxidase and an enhanced chemiluminescence (ECL) detection kit (Amersham, Buckinghamshire, UK).
Statistical analysis.
The interactions and synergisms between drugs were analyzed by a Multilevel regression model.25 Levels of significance between samples were determined using the t-test for nonpaired samples and the analysis of variance (ANOVA), Fisher’s Protected Least Significant Difference (PLSD). Synergy between drugs for individual patients was determined by comparing the viability obtained when B-CLL cells were incubated with the combination of the drugs and the viability expected that was calculated by multiplying the viability of the cells when incubated with each factor alone. Correlations between Mcl-1 levels, p53 levels, and cell viability were determined by the Pearson method.
RESULTS
Cytotoxic effect of the combination of fludarabine with mitoxantrone and mafosfamide.
B-CLL cells from 20 patients were incubated for 48 hours with pharmacological concentrations of fludarabine (F; 1 μg/mL), mitoxantrone (M; 0.5 μg/mL), and mafosfamide (Mf; 1 μg/mL) alone or in combination (Table 2).
Cytotoxic Effect of Fludarabine, Mafosfamide, and Mitoxantrone on B-CLL Lymphocytes
| Patient No. . | Viability (%) . | |||||||
|---|---|---|---|---|---|---|---|---|
| Control . | Fludarabine . | Mitoxantrone . | Mafosfamide . | M + Mf . | F + Mf . | F + M . | F + M + Mf . | |
| 1 | 80 ± 4 | 46 ± 3 | 57 ± 1 | 71 ± 2 | 46 ± 2 | 20 ± 3 | 18 ± 2 | 11 ± 2 |
| 2 | 92 ± 6 | 71 ± 3 | 80 ± 7 | 87 ± 3 | 75 ± 0.8 | 39 ± 1 | 51 ± 1 | 33 ± 2 |
| 3 | 100 ± 6 | 83 ± 0.6 | 88 ± 1 | 94 ± 1 | 82 ± 4 | 30 ± 0.5 | 42 ± 5 | 21 ± 1 |
| 4 | 91 ± 3 | 67 ± 2 | 87 ± 8 | 90 ± 5 | 65 ± 2 | 43 ± 0.9 | 57 ± 6 | 36 ± 3 |
| 5 | 59 ± 8 | 29 ± 0.3 | 17 ± 1 | 38 ± 6 | 16 ± 0.2 | 11 ± 1 | 11 ± 0.1 | 9 ± 0.1 |
| 6 | 79 ± 2 | 66 ± 3 | 65 ± 4 | 79 ± 2 | 67 ± 1 | 51 ± 2 | 50 ± 4 | 43 ± 2 |
| 7 | 75 ± 2 | 57 ± 5 | 56 ± 3 | 76 ± 3 | 56 ± 3 | 31 ± 0.9 | 37 ± 4 | 24 ± 0.8 |
| 8 | 82 ± 2 | 50 ± 1 | 69 ± 5 | 76 ± 4 | 64 ± 0.5 | 38 ± 2 | 38 ± 0.6 | 31 ± 1 |
| 9 | 81 ± 3 | 69 ± 3 | 62 ± 0.2 | 77 ± 2 | 58 ± 6 | 47 ± 2 | 44 ± 2 | 35 ± 3 |
| 10 | 100 ± 5 | 81 ± 10 | 37 ± 4 | 100 ± 3 | 34 ± 2 | 35 ± 1 | 22 ± 3 | 16 ± 2 |
| 11 | 93 ± 2 | 64 ± 2 | 87 ± 0 | 69 ± 1 | 60 ± 3 | 30 ± 1 | 60 ± 1 | 27 ± 1 |
| 12 | 96 ± 13 | 60 ± 6 | 56 ± 9 | 74 ± 7 | 38 ± 2 | 10 ± 2 | 18 ± 4 | 8 ± 0.4 |
| 13 | 71 ± 18 | 31 ± 5 | 35 ± 4 | 68 ± 4 | 27 ± 3 | 10 ± 3 | 13 ± 3 | 7 ± 2 |
| 14 | 69 ± 13 | 27 ± 6 | 33 ± 5 | 42 ± 3 | 29 ± 2 | 19 ± 2 | 20 ± 2 | 16 ± 1 |
| 15 | 48 ± 1 | 16 ± 0.5 | 17 ± 0.5 | 34 ± 1 | 16 ± 1 | 14 ± 1 | 11 ± 1 | 13 ± 1 |
| 16 | 94 ± 3 | 72 ± 1 | 96 ± 2 | 86 ± 2 | 85 ± 6 | 46 ± 5 | 61 ± 2 | 46 ± 0.4 |
| 17 | 100 ± 0.4 | 81 ± 6 | 90 ± 1 | 91 ± 6 | 80 ± 0.2 | 35 ± 1 | 56 ± 10 | 28 ± 1 |
| 18 | 73 ± 3 | 45 ± 1 | 62 ± 6 | 55 ± 2 | 49 ± 1 | 16 ± 1 | 33 ± 5 | 16 ± 4 |
| 19 | 99 ± 2 | 53 ± 8 | 65 ± 2 | 80 ± 4 | 56 ± 4 | 28 ± 2 | 34 ± 3 | 26 ± 2 |
| 20 | 68 ± 4 | 53 ± 7 | 61 ± 4 | 64 ± 2 | 50 ± 1 | 40 ± 2 | 41 ± 1 | 37 ± 1 |
| Mean ± SD | 82 ± 16 | 56 ± 19* | 62 ± 23* | 73 ± 19* | 53 ± 21 | 29 ± 13†,‡ | 36 ± 17† | 24 ± 122-153 |
| Patient No. . | Viability (%) . | |||||||
|---|---|---|---|---|---|---|---|---|
| Control . | Fludarabine . | Mitoxantrone . | Mafosfamide . | M + Mf . | F + Mf . | F + M . | F + M + Mf . | |
| 1 | 80 ± 4 | 46 ± 3 | 57 ± 1 | 71 ± 2 | 46 ± 2 | 20 ± 3 | 18 ± 2 | 11 ± 2 |
| 2 | 92 ± 6 | 71 ± 3 | 80 ± 7 | 87 ± 3 | 75 ± 0.8 | 39 ± 1 | 51 ± 1 | 33 ± 2 |
| 3 | 100 ± 6 | 83 ± 0.6 | 88 ± 1 | 94 ± 1 | 82 ± 4 | 30 ± 0.5 | 42 ± 5 | 21 ± 1 |
| 4 | 91 ± 3 | 67 ± 2 | 87 ± 8 | 90 ± 5 | 65 ± 2 | 43 ± 0.9 | 57 ± 6 | 36 ± 3 |
| 5 | 59 ± 8 | 29 ± 0.3 | 17 ± 1 | 38 ± 6 | 16 ± 0.2 | 11 ± 1 | 11 ± 0.1 | 9 ± 0.1 |
| 6 | 79 ± 2 | 66 ± 3 | 65 ± 4 | 79 ± 2 | 67 ± 1 | 51 ± 2 | 50 ± 4 | 43 ± 2 |
| 7 | 75 ± 2 | 57 ± 5 | 56 ± 3 | 76 ± 3 | 56 ± 3 | 31 ± 0.9 | 37 ± 4 | 24 ± 0.8 |
| 8 | 82 ± 2 | 50 ± 1 | 69 ± 5 | 76 ± 4 | 64 ± 0.5 | 38 ± 2 | 38 ± 0.6 | 31 ± 1 |
| 9 | 81 ± 3 | 69 ± 3 | 62 ± 0.2 | 77 ± 2 | 58 ± 6 | 47 ± 2 | 44 ± 2 | 35 ± 3 |
| 10 | 100 ± 5 | 81 ± 10 | 37 ± 4 | 100 ± 3 | 34 ± 2 | 35 ± 1 | 22 ± 3 | 16 ± 2 |
| 11 | 93 ± 2 | 64 ± 2 | 87 ± 0 | 69 ± 1 | 60 ± 3 | 30 ± 1 | 60 ± 1 | 27 ± 1 |
| 12 | 96 ± 13 | 60 ± 6 | 56 ± 9 | 74 ± 7 | 38 ± 2 | 10 ± 2 | 18 ± 4 | 8 ± 0.4 |
| 13 | 71 ± 18 | 31 ± 5 | 35 ± 4 | 68 ± 4 | 27 ± 3 | 10 ± 3 | 13 ± 3 | 7 ± 2 |
| 14 | 69 ± 13 | 27 ± 6 | 33 ± 5 | 42 ± 3 | 29 ± 2 | 19 ± 2 | 20 ± 2 | 16 ± 1 |
| 15 | 48 ± 1 | 16 ± 0.5 | 17 ± 0.5 | 34 ± 1 | 16 ± 1 | 14 ± 1 | 11 ± 1 | 13 ± 1 |
| 16 | 94 ± 3 | 72 ± 1 | 96 ± 2 | 86 ± 2 | 85 ± 6 | 46 ± 5 | 61 ± 2 | 46 ± 0.4 |
| 17 | 100 ± 0.4 | 81 ± 6 | 90 ± 1 | 91 ± 6 | 80 ± 0.2 | 35 ± 1 | 56 ± 10 | 28 ± 1 |
| 18 | 73 ± 3 | 45 ± 1 | 62 ± 6 | 55 ± 2 | 49 ± 1 | 16 ± 1 | 33 ± 5 | 16 ± 4 |
| 19 | 99 ± 2 | 53 ± 8 | 65 ± 2 | 80 ± 4 | 56 ± 4 | 28 ± 2 | 34 ± 3 | 26 ± 2 |
| 20 | 68 ± 4 | 53 ± 7 | 61 ± 4 | 64 ± 2 | 50 ± 1 | 40 ± 2 | 41 ± 1 | 37 ± 1 |
| Mean ± SD | 82 ± 16 | 56 ± 19* | 62 ± 23* | 73 ± 19* | 53 ± 21 | 29 ± 13†,‡ | 36 ± 17† | 24 ± 122-153 |
B-CLL lymphocytes were incubated at 37°C for 48 hours in the absence of any factor (control), with mitoxantrone (M; 0.5 μg/mL), with fludarabine (F; 1 μg/mL), with mafosfamide (Mf; 1 μg/mL), or with the combinations of these drugs. Cell viability was determined by the MTT assay as described in Materials and Methods and is expressed as the percentage with respect to the viability of control cells at the beginning of the culture. Data are shown as the mean value ± SD of triplicate cultures.
P < .01 v control.
P < .01 v fludarabine.
P = .172 v fludarabine + mitoxantrone.
P = .08 v fludarabine + mafosfamide.
The statistical analysis of the values obtained for these 20 patients shows that the viability of control cells was 82% ± 16% (95% confidence interval [CI], 75 to 89). Incubation of B-CLL cells with fludarabine, mafosfamide, or mitoxantrone alone produced a significant decrease in cell viability (P < .01). The strongest effect was produced by fludarabine, which reduced viability by 25 units (U) (95% CI, 22 to 28.9), followed by mitoxantrone (20 U; 95% CI, 16.7 to 23.7) and mafosfamide (9 U; 95% CI, 5.6 to 12.5). All of the combinations of 2 drugs were more cytotoxic than any drug alone. The combination of mitoxantrone with either fludarabine or mafosfamide produced a significant additive effect (P < .01) and the mean value viability was 36% ± 17% and 53% ± 21%, respectively. Interestingly, the combination of mafosfamide with fludarabine produced a significant synergistic effect (P < .01) and induced an additional loss of 17 U (95% CI, 12.47 to 22.33) to the expected viability that would have been obtained if the combination of these 2 drugs had produced only an additive effect.
Although the comparison of fludarabine + mitoxantrone versus fludarabine + mafosfamide showed no statistical significance (P= .172), in cells from 8 patients (patients no. 2, 3, 4, 7, 11, 16, 17, and 18) a higher effect was observed when the cells were incubated with the combination of fludarabine + mafosfamide (P < .05 in all cases).
When considering the mean values of the 20 patients studied, the addition of mitoxantrone to the combination of fludarabine and mafosfamide did not induce a significant increase in the cytotoxic effect obtained by these 2 drugs (P = .08). However, the analysis of individual patients showed a significant increase in cytotoxicity in cells from 8 patients (patients no. 1, 3, 6, 7, 8, 9, 10, and 17) when the cells were incubated with the 3 drugs (P < .05 in all cases). We analyzed whether there was a correlation between this greater sensitivity and any biological or clinical characteristics of the patients (Table 1). No correlation was found with clinical stage, peripheral blood leukocyte count, trisomy of chromosome 12, or immunophenotype. Interestingly, a significant correlation was found with previous treatment. Thus, whereas in 5 of 6 (83.3%) previously treated patients (3 of whom had received purine analogues) the combination of fludarabine, mafosfamide, and mitoxantrone was necessary to achieve the highest cytotoxic effect. This was the case in only 3 of 14 patients (21.4%) with no prior therapy (P = .036; χ2 with continuity correction).
Correlation with in vivo response.
Five of the 20 patients included in this study received treatment with the combination of fludarabine, cyclophosphamide, and mitoxantrone (FCM). Two patients achieved partial response (patients no. 8 and 9) and 2 patients achieved complete response (patients no. 7 and 14). The remaining patient (patient no. 17) could not be evaluated, because the treatment had to be discontinued due to toxicity. However, a decrease in the peripheral blood leukocyte count was observed (from 40 to 6.4 × 109/L).
An additional patient who was eventually excluded from the analysis because she was receiving chlorambucil at the time of the study did not respond to FCM. Interestingly, the cells from this patient had not responded in vitro to any of the combinations assayed.
Although the number of patients is too small to make statistical studies, it appears that there is a tendency for better clinical responses in patients who showed higher rates of apoptosis in vitro when treated with the combination of all 3 drugs.
Dose-dependence of the cytotoxic effect of fludarabine and mafosfamide.
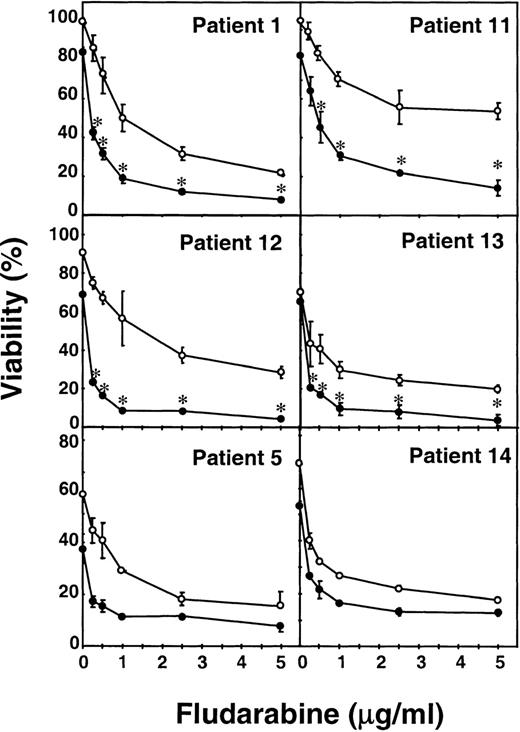
To study the synergism between fludarabine and mafosfamide in more detail, B-CLL cells from 6 patients were incubated for 48 hours with various concentrations of fludarabine, ranging from 0.25 to 5 μg/mL, in the presence or absence of 1 μg/mL mafosfamide. As seen in Fig 1, fludarabine produced a dose-dependent cytotoxic effect in all of the cases studied, although the sensitivity differed from one patient to another. The addition of mafosfamide increased fludarabine-induced cytotoxicity and, in patients no. 1, 11, 12, and 13, the combination produced a significant (P < .05) synergistic effect. In all patients studied, the effect of the combination of 0.25 μg/mL fludarabine and 1 μg/mL mafosfamide was greater than the effect of 1 μg/mL fludarabine alone.
Potentiation of the cytotoxic effect of fludarabine by mafosfamide. Cells from 6 patients were incubated for 48 hours with various concentrations of fludarabine ranging from 0.25 to 5 μg/mL, without (○) or with 1 μg/mL mafosfamide (•). Cell viability was determined by the MTT assay as described in Materials and Methods and is expressed as the percentage with respect to control cells at the beginning of the culture. Data are shown as the mean value ± SD of triplicate cultures. Statistical significance of the synergism between fludarabine and mafosfamide was assayed by ANOVA (Fisher’s PLSD). *P < .05.
Potentiation of the cytotoxic effect of fludarabine by mafosfamide. Cells from 6 patients were incubated for 48 hours with various concentrations of fludarabine ranging from 0.25 to 5 μg/mL, without (○) or with 1 μg/mL mafosfamide (•). Cell viability was determined by the MTT assay as described in Materials and Methods and is expressed as the percentage with respect to control cells at the beginning of the culture. Data are shown as the mean value ± SD of triplicate cultures. Statistical significance of the synergism between fludarabine and mafosfamide was assayed by ANOVA (Fisher’s PLSD). *P < .05.
Dose-dependence was also found when B-CLL cells were incubated with increasing doses of mafosfamide (0.25 to 2.5 μg/mL) in the presence of a constant dose of fludarabine (1 μg/mL; Fig 2). These results indicate that 0.25 μg/mL mafosfamide is sufficient to increase the cytotoxic effect of fludarabine in most patients. Furthermore, synergy was produced by the addition of the various concentrations of mafosfamide to the cells incubated with fludarabine in 4 of the 6 patients studied (patients no. 1, 11, 12, and 13).
Potentiation of the cytotoxic effect of mafosfamide by fludarabine. B-CLL lymphocytes from 6 patients were incubated for 48 hours with various concentrations of mafosfamide ranging from 0.25 to 2.5 μg/mL, without (○) or with 1 μg/mL fludarabine (•). Cell viability was determined by the MTT assay as described in Materials and Methods and is expressed as the percentage with respect to control cells at the beginning of the culture. Data are shown as the mean value ± SD of triplicate cultures. Statistical significance of the synergism between fludarabine and mafosfamide was assayed by ANOVA (Fisher’s PLSD). *P < .05.
Potentiation of the cytotoxic effect of mafosfamide by fludarabine. B-CLL lymphocytes from 6 patients were incubated for 48 hours with various concentrations of mafosfamide ranging from 0.25 to 2.5 μg/mL, without (○) or with 1 μg/mL fludarabine (•). Cell viability was determined by the MTT assay as described in Materials and Methods and is expressed as the percentage with respect to control cells at the beginning of the culture. Data are shown as the mean value ± SD of triplicate cultures. Statistical significance of the synergism between fludarabine and mafosfamide was assayed by ANOVA (Fisher’s PLSD). *P < .05.
Induction of apoptosis by fludarabine, mafosfamide, and mitoxantrone in B-CLL cells.
To analyze whether the cytotoxic effect produced by the different combinations of these 3 drugs was due to potentiation of apoptosis, we studied the effect of these combinations on the percentage of apoptotic cells by flow cytometry. Figure 3 shows the histograms obtained by FACS analysis of the hypodiploid peak obtained by PI staining of cells from patient no. 17 incubated with fludarabine, mitoxantrone, and mafosfamide alone and in combination. When the cells were incubated with just 1 drug, the greatest effect was produced by fludarabine. The use of 2 drugs produced a higher number of apoptotic cells, especially with the combinations of fludarabine + mafosfamide and fludarabine + mitoxantrone. Finally, the combination of all 3 drugs produced the highest number of apoptotic cells. Similar results were obtained with cells from 5 additional patients (results not shown).
Induction of apoptosis by fludarabine, mafosfamide, and mitoxantrone on B-CLL cells. Cells from patient no. 17 were incubated for 48 hours with fludarabine (F; 1 μg/mL), mitoxantrone (M; 0.5 μg/mL), and/or mafosfamide (Mf; 1 μg/mL). DNA content was quantified by PI staining and flow cytometry analysis as described in Materials and Methods.
Induction of apoptosis by fludarabine, mafosfamide, and mitoxantrone on B-CLL cells. Cells from patient no. 17 were incubated for 48 hours with fludarabine (F; 1 μg/mL), mitoxantrone (M; 0.5 μg/mL), and/or mafosfamide (Mf; 1 μg/mL). DNA content was quantified by PI staining and flow cytometry analysis as described in Materials and Methods.
The apoptotic effect induced by the combination of fludarabine + mafosfamide was analyzed in cells from 13 patients by the same method. Fludarabine and mafosfamide alone increased the number of apoptotic cells (control, 34% ± 18%; fludarabine, 53% ± 22%; mafosfamide, 49% ± 23%), and the combination of the 2 drugs significantly increased the number of apoptotic cells (77% ± 21%) when compared with fludarabine (P = .009) or mafosfamide (P = .003) alone.
Furthermore, apoptosis was also quantified by flow cytometry analysis of phosphatidylserine exposure. Annexin V-FITC binding was studied in CD19+ and CD3+ cells from 11 B-CLL patients after 24 hours of incubation. At this time, incubation of B-CLL lymphocytes with fludarabine induced apoptosis of CD3+(P = .017) cells, but not of CD19+ cells (P= .67; Fig 4). No significant effect was produced by incubation of cells with mafosfamide alone on either type of cell. Interestingly, mafosfamide significantly increased the apoptosis induced by fludarabine on CD19+ cells (P= .007), but not on CD3+ cells (P = .314).
Comparison between the induction of apoptosis in B cells and T cells from 11 B-CLL patients. Cells were incubated with fludarabine (F) and/or mafosfamide (Mf) for 24 hours and phosphatidylserine exposure was measured by binding of annexin V-FITC to CD19+ or CD3+ cells as described in Materials and Methods. Statistical significance was determined using the t-test for nonpaired samples: *versus control; §versus fludarabine.
Comparison between the induction of apoptosis in B cells and T cells from 11 B-CLL patients. Cells were incubated with fludarabine (F) and/or mafosfamide (Mf) for 24 hours and phosphatidylserine exposure was measured by binding of annexin V-FITC to CD19+ or CD3+ cells as described in Materials and Methods. Statistical significance was determined using the t-test for nonpaired samples: *versus control; §versus fludarabine.
Effect on the expression of apoptosis-regulatory proteins.
Using antibodies specific for Bcl-2, Bax, Bcl-X, and Mcl-1, we studied whether the combinations of fludarabine with mafosfamide and/or mitoxantrone produced changes in the levels of these apoptosis-regulatory proteins. No modification of Bcl-2 nor Bax levels was observed. Bcl-X was not detected in control cells, and its presence was not induced by any of the drug combinations (data not shown). Analysis of Mcl-1 in 10 patients showed a decrease in the levels of this protein when cells were incubated with the 3 drugs in combination (data not shown). The effect of the drugs alone and of all the combinations was studied in cells from 3 patients (Fig 5). Fludarabine alone produced a decrease in patients no. 2 and 3, but mitoxantrone or mafosfamide did not affect Mcl-1. Lower levels of protein were detected after incubation with fludarabine + mitoxantrone or fludarabine + mafosfamide, whereas incubation with mitoxantrone + mafosfamide only produced a decrease in cells from patient no. 3. Finally, the combination with the 3 drugs produced the lowest levels of Mcl-1. The timing of the decrease in Mcl-1 protein was parallel to the decrease in cell viability (data not shown). A correlation was found between Mcl-1 levels and cell viability in response to the different treatments for 48 hours (r = .8; P < .001).
Effect of the combination of fludarabine with mitoxantrone and/or mafosfamide on p53 and Mcl-1 levels. Cells from patients no. 2, 3, and 17 were incubated for 48 hours with fludarabine (F; 1 μg/mL), mitoxantrone (M; 0.5 μg/mL), and/or mafosfamide (Mf; 1 μg/mL). Cell viability was determined by the MTT assay as described in Materials and Methods and is expressed as the percentage with respect to control cells. Western blots of p53 and Mcl-1 were quantified and the values are expressed as the percentage with respect to control cells (Mcl-1) or cells incubated with the 3 drugs (p53). Similar p53 levels were obtained for the 3 patients.
Effect of the combination of fludarabine with mitoxantrone and/or mafosfamide on p53 and Mcl-1 levels. Cells from patients no. 2, 3, and 17 were incubated for 48 hours with fludarabine (F; 1 μg/mL), mitoxantrone (M; 0.5 μg/mL), and/or mafosfamide (Mf; 1 μg/mL). Cell viability was determined by the MTT assay as described in Materials and Methods and is expressed as the percentage with respect to control cells. Western blots of p53 and Mcl-1 were quantified and the values are expressed as the percentage with respect to control cells (Mcl-1) or cells incubated with the 3 drugs (p53). Similar p53 levels were obtained for the 3 patients.
DNA-damaging agents increase p53 levels26,27 and B-CLL cells with p53 gene mutations are more resistant to chlorambucil and fludarabine.28 29 Therefore, we analyzed the effect of the combinations of these drugs on p53 protein. First, p53 levels were determined in cells from 10 patients. In all cases, the levels of p53 in untreated cells were very low or undetectable, and the incubation of the cells with the combination of the 3 drugs induced the accumulation of p53 (data not shown). The effect of the drugs alone and combined was studied in cells from 3 patients. As shown in Fig 5, after 48 hours of incubation with fludarabine, mitoxantrone, or mafosfamide alone, an increase in p53 levels was observed, except for 1 patient for whom no modification was detected in response to mafosfamide. A higher increase was observed when cells were incubated with fludarabine + mafosfamide or fludarabine + mitoxantrone, and the combination of the 3 drugs produced the highest p53 levels of all conditions. This increase in p53 levels correlated with the decrease in cell viability in the patients analyzed (r = −.82; P < .001) and with the decrease in Mcl-1 (r = −.65; P = .001). Furthermore, the increase in p53 protein was detectable after 12 hours of incubation, preceding the loss of viability (data not shown).
DISCUSSION
The results of this study demonstrate the synergism of the combination of fludarabine and cyclophosphamide on neoplastic cells from B-CLL and that the cytotoxic effect of this combination can be increased in some cases by mitoxantrone. Several studies have found a correlation between the response to different treatments in vitro and the effect of therapy on the patient’s clinical response.29-32 In this regard, our results are consistent with the promising clinical results obtained with the combination of fludarabine and cyclophosphamide11,14-16 and the combination of fludarabine with cyclophosphamide and mitoxantrone.19
The basis for the treatment of cancer with combinations of drugs is their additive or synergistic effects. A number of studies have analyzed the effect of the combination of fludarabine with other drugs in vitro on B-CLL cells.33-36 However, neither the effect of the combination of fludarabine with cyclophosphamide nor the effect of these 2 combined with mitoxantrone has been reported.
The dose-response assays show that doses of fludarabine less than 1 μg/mL (0.25 to 0.5 μg/mL) in combination with 1 μg/mL mafosfamide produce a higher effect than that obtained with 1 μg/mL fludarabine alone. Furthermore, our results also show that lower doses of mafosfamide (0.25 to 0.5 μg/mL) when combined with fludarabine could produce a similar effect to that obtained with 1 μg/mL mafosfamide. These results support the use of these drugs together.
The mechanism of the synergism between fludarabine and mafosfamide in B-CLL cells is not known. Cyclophosphamide is an alkylating agent that induces DNA damage and fludarabine inhibits DNA and RNA synthesis as well as DNA repair.13,37 The effectiveness of these drugs in B-CLL cells may be attributed to the apparent requirement for continual DNA housekeeping, even in nonproliferating cells.38 Inhibition of DNA interstrand cross-link removal by fludarabine may account for the synergistic cytotoxicity of this combination.39
DNA-damaging agents increase p53 levels by posttranslational stabilization and induce p53-dependent cell death.26,27 The importance of the p53 pathway in B-CLL was demonstrated by the finding of p53 mutations.40 Importantly, B-CLL cells from patients with p53 gene mutations were more resistant to chlorambucil or fludarabine,28,29 and p53 mutations or deletions are associated with drug resistance and short survival.28,41,42We found a high correlation between the cytotoxicity of the different combinations of drugs and the increase in p53 protein. Furthermore, this increase precedes loss of viability. These results suggest that 1 of the mechanisms by which the combination of these drugs increases its cytotoxicity is the potentiation of DNA damage and stabilization and activation of p53. One of the mechanisms involved in p53 stabilization in response to DNA damage is its phosphorylation by ataxia telangiectasia mutated (ATM).43 Very interestingly, ATM has been found inactivated in B-CLL cells.44 45
The levels of Mcl-1 protein were decreased by all the combinations that induced apoptosis and a high correlation between levels of Mcl-1 and viability was found. However, the levels of Bcl-2 and Bax were not modified. These results are consistent with those reported by Kitada et al.46 Our results indicate that higher cytotoxic effects correlate with concurrent low levels of Mcl-1 and high levels of p53. The timing of the decrease in Mcl-1 protein is parallel to the decrease in viability, and so it is very difficult to demonstrate a causal effect, but the decrease in Mcl-1 protein may contribute to the potentiation of apoptosis.
The results obtained by PI staining of cells with a subdiploid content of DNA and by analysis of phosphatidylserine exposure show that the synergistic effect of fludarabine and mafosfamide is due to potentiation of apoptosis. Consistent with Consoli et al,47fludarabine was cytotoxic to CD3+ cells of B-CLL patients. However, the addition of mafosfamide to this drug produces a higher increase in the apoptosis of B lymphocytes than of T lymphocytes.
In conclusion, this in vitro study shows that cyclophosphamide synergizes with fludarabine in inducing cytotoxicity and apoptosis in B-CLL cells. In addition, the combination of mitoxantrone with these 2 drugs significantly increases the cytotoxic effect in some cases, especially in previously treated patients. These results provide experimental support to clinical trials assessing the effect of fludarabine combined with cyclophosphamide11,14-16 and these 2 with mitoxantrone.19
ACKNOWLEDGMENT
The authors thank L. Quintó from the “Unidad de Epidemiologı́a y Bioestadı́stica” for his help in statistical analysis. We thank Dr F. Bosch, Dr J. Esteve, Dr A. Carrió, M. Perales, O. Casanovas, and C. Pastor for their help and suggestions and thank R. Rycroft for language assistance.
B.B. is a recipient of a research fellowship from “Fundación de la Asociación Española Contra el Cáncer.” Supported by “Fondo de Investigaciones Sanitarias de la Seguridad Social” (FIS 95/0873, FIS 94/0665), “Comisión Interministerial de Ciencia y Tecnologia” (SAF 98-0100), “Generalitat de Catalunya” (97 SGR/74), and Schering AG (Berlin, Germany).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Joan Gil, PhD, Departament de Ciències Fisiològiques II, Campus de Bellvitge, Universitat de Barcelona, 08907 L’Hospitalet, Spain; e-mail:joangil@bellvitge.bvg.ub.es.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal