To elucidate the pathways by which nitric oxide (NO) influences macrophage iron metabolism, the uptake, release, and intracellular distribution of iron in the murine macrophage cell line J774 has been investigated, together with transferrin receptor (TfR) expression and iron-regulatory protein (IRP1 and IRP2) activity. Stimulation of macrophages with interferon-γ (IFN-γ) and/or lipopolysaccharide (LPS) decreased Fe uptake from transferrin (Tf), and there was a concomitant downregulation of TfR expression. These effects were mediated by NO-dependent and NO-independent mechanisms. Addition of the NO synthase (NOS) inhibitor N-monomethyl arginine (NMMA) partially restored Fe uptake but either had no effect on or downregulated TfR expression, which suggests that NO by itself is able to affect iron availability. Analysis of the intracellular distribution of incorporated iron revealed that in IFN-γ/LPS-activated macrophages there was a decreased amount and proportion of ferritin-bound iron and a compensatory increase in insoluble iron, which probably consists mainly of iron bound to intracellular organelles. Finally, although NO released by IFN-γ/LPS-activated macrophages increased the iron-responsive element (IRE)-binding activity of both IRP1 and IRP2, IFN-γ treatment decreased IRP2 activity in an NO-independent manner. This study demonstrates that the effect of IFN-γ and/or LPS on macrophage iron metabolism is complex, and is not entirely due to either NO-or to IRP-mediated mechanisms. The overall effect is to decrease iron uptake, but not its utilization.
IRON METABOLISM and macrophage physiology are closely connected. Macrophages, through processing of hemoglobin-iron from senescent erythrocytes, are responsible for iron supply to peripheral tissues, including the bone marrow.1Moreover, changes in macrophage iron content can affect the function of these cells in the inflammatory response. For example, iron plays a critical role in macrophage-mediated cytotoxicity by contributing to the production of highly toxic hydroxyl radicals via the Fenton reaction2 and by controlling the production of nitric oxide (NO) after activation by immunologic stimuli.3
Intracellular iron homeostasis is controlled by cytoplasmic iron regulatory proteins (IRP1 and IRP2), which regulate several mRNAs containing iron-responsive elements (IREs) in their untranslated regions.4 IRP binding to the IREs in the 5′ untranslated regions of ferritin (Ft) and erythroid-specific 5-aminolaevulinate synthase mRNAs represses their translation,5 whereas binding of IRP to multiple IREs in the 3′ untranslated region of transferrin receptor (TfR) mRNA confers stability against targeted endonucleolytic degradation.6
IRP1 is a bifunctional protein that can act either as a cytoplasmic aconitase or as an IRE-binding protein.7 In iron-replete cells, IRP1 bears a 4Fe-S cluster and shows aconitase activity, but in iron-depleted cells reversible disassembly of the cluster converts IRP1 to its RNA-binding form. IRP2, despite conservation of the cluster-ligating cysteines at the active site, is unable to assemble an Fe-S cluster in vitro and therefore is unable to exhibit aconitase activity.8 Unlike the regulation of IRP1 by iron, loss of IRE binding of IRP2 in iron-replete cells is due to iron-dependent oxidation, ubiquitinylation, and degradation by the proteasome.9
In addition to iron availability, other signals such as NO and oxidative stress (ie, H2O2 and peroxynitrite) can modulate the activity of both IRPs and thus influence cellular iron metabolism.10-12 Stimulation of macrophages with IFN-γ and lipopolysaccharide (LPS) induces NO synthesis and was originally reported to activate IRE-binding by IRP1 and IRP2.10,13However, other investigators have recently reported that stimulation of macrophages with IFN-γ and LPS results in an increase of IRP1 activity, but a strong reduction of IRP2 activity.12,14 A further point of controversy is that while Bouton et al14reported that the decrease of IRP2 activity is not due to NO, Recalcati et al12 showed that inhibition of IRP2 activity was NO-dependent.
Although the effect of NO on IRP activity has been well studied, no data regarding the effect of NO on iron uptake, release, and distribution after macrophage stimulation with cytokines are available. Such information is important if the role of inflammatory stimuli and NO in macrophage iron metabolism is to be understood. In the present study, we examined cellular iron uptake, release, and distribution, as well as TfR expression and IRP1/2 activities in stimulated murine J774 macrophages to better clarify the regulation of iron metabolism in activated macrophages. We found that treatment of these cells with IFN-γ and LPS strongly inhibits iron uptake from transferrin despite the fact that IRP1 is upregulated, and that both NO-dependent and -independent mechanisms are involved.
MATERIALS AND METHODS
Reagents.
RPMI 1640 culture medium and fetal calf serum (FCS) were purchased from GIBCO (Paisley, UK). Human apotransferrin (apoTf) was obtained from Sigma (Poole, UK) and when required saturated with59Fe using Fe-nitrilotriacetate (FeNTA; Fe:NTA molar ratio, 1:4)15 prepared from Na-NTA and59FeCl3 (Amersham, Amersham, UK). Human serum albumin (HSA; Tf-free), NMMA, LPS, leupeptin, phenylmethylsulfonyl fluoride (PMSF), benzamidine, and pepstatin A were all obtained from Sigma; desferrioxamine (DFO) was from Novartis (Horsham, UK); (α-32P) uridine triphosphate (UTP) was from Amersham; and RNAsin ribonuclease inhibitor, XbaI restriction enzyme, and T7 RNA polymerase were from Promega (Southampton, UK). R-phycoerythrin (R-PE)–conjugated rat IgG1,κ anti-mouse CD71 (TfR) monoclonal antibody and R-PE–conjugated rat IgG1,κ monoclonal immunoglobulin isotype standard were obtained from Pharmingen (Oxford, UK). Rabbit anti-mouse ferritin was produced in our laboratory.15 Mouse recombinant IFN-γ was kindly provided by Dr E. Esfandiari, Department of Immunology, University of Glasgow.
Cell cultures and treatments.
The mouse macrophage cell line J774 was kindly supplied by Dr G.-J. Feng (Department of Immunology, University of Glasgow) and grown in RPMI-1640 supplemented with 10% heat-inactivated FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in 5% CO2. To avoid problems of variability in levels of iron and transferrin caused by the presence of serum, all of the treatments were performed in serum-free RPMI 1640 containing 1 mg/mL HSA and 50 μg/mL human apoTf unless otherwise indicated. Cells were incubated overnight with additives as required, then washed twice with ice-cold phosphate-buffered saline (PBS) and subjected to further procedures as indicated below. To ensure that equal numbers of cells were used, cultures were always seeded with 106 viable cells per milliliter, monitored daily by microscopic observation to check for uniform cell number and viability, and experiments performed when the cultures had reached near-confluence.
Iron uptake assay.
After overnight incubation with appropriate additives, cells were pulsed for 8 hours with 50 μg/mL 59Fe-Tf to allow incorporation of 59Fe, then washed and lysed with 2% sodium dodecyl sulfate (SDS). The radioactivity in cell lysates and culture supernatants was determined and 59Fe uptake calculated.
Iron release assay.
After overnight stimulation, cells were pulsed for 4 hours with 50 μg/mL 59Fe-Tf, washed twice with prewarmed PBS, and then incubated for 2 hours in the presence of “cold” Fe-Tf. Thereafter, the cells were washed, lysed in 2% SDS and the radioactivity present in cell lysates and supernatants determined. Alternatively, cells were exposed to 59Fe-Tf for 24 hours, washed, and then incubated overnight with cold Fe-Tf in the presence of the different treatments. After stimulation the radioactivity was determined as above.
Intracellular distribution of iron.
Cells were treated as described for iron uptake assays, then lysed in 100 μL cytoplasmic lysis buffer (1% Triton X-100, 40 mmol/L KCl, 25 mmol/L Tris-HCl pH 7.4, 50 μg/mL leupeptin, 200 μg/mL PMSF, 1 mmol/L benzamidine, and 50 μg/mL pepstatin A) and the intracellular iron distribution determined as described by Alvarez-Hernández et al16 with slight modifications. Briefly, this consisted of centrifugation at 10,000g for 5 minutes and immunoprecipitation of ferritin with a rabbit anti-ferritin antibody. This allows separation of intracellular iron into 3 compartments containing predominantly insoluble material (consisting mainly of hemosiderin and iron bound to intracellular organelles), ferritin-bound iron, and nonferritin cytoplasmic iron, respectively. Separation of the soluble nonferritin cytoplasmic iron into high- and low-molecular-weight fractions was omitted.
TfR expression.
Aliquots of approximately 5 × 105 cells were washed in PBS containing 2% FCS and 0.05% sodium azide, and stained for 20 minutes at 4°C with saturating amounts of R-PE–conjugated rat anti-mouse TfR monoclonal antibody. Cells were washed twice and then fixed in PBS containing 1% formaldehyde. The percentage of fluorescent cells and the mean fluorescence intensity were measured by flow cytometry using a fluorescence-activated cell sorter (Becton Dickinson, Franklin Lakes, NJ). Specificity of staining was checked by using a monoclonal immunoglobulin isotype standard.
IRP activity.
IRP activity was determined by mobility shift assay, using a32P-labeled probe containing the ferritin IRE sequence obtained by in vitro transcription of an IRE-CAT plasmid (kindly provided by Dr N. Gray, European Molecular Biology Laboratory [EMBL], Heidelberg) linearized with XbaI. Cell extracts in cytoplasmic lysis buffer were freshly prepared as described above and aliquots (20 μg of protein) loaded with a molar excess of transcript (15,000 cpm) and subjected to nondenaturing gel electrophoresis.17 Total IRP1 was determined by incubating samples before the addition of the probe with 2% 2-mercaptoethanol (2ME), which converts inactive IRP1 to the RNA binding form.18 Autoradiographs were densitometrically scanned and the proportion of spontaneously active IRP calculated.
Measurement of nitrite.
Nitrite was determined in the culture medium by the Griess reagent.19
Protein determination.
The protein concentration of cell lysates was estimated by the BCA protein assay reagent (Pierce, Chester, UK) using bovine serum albumin as a standard.
Statistical analysis.
Data were analyzed by 1-way analysis of variance (ANOVA) and unpaired Student’s t-test to determine difference between each group. Differences were considered statistically significant when P< .05.
RESULTS
Iron uptake and release.
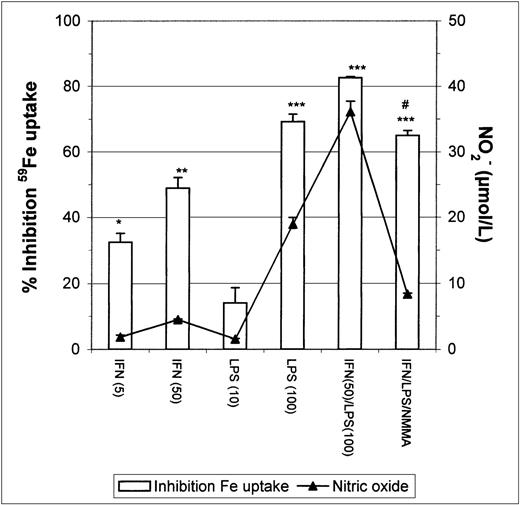
Stimulation of J774 macrophages with IFN-γ or LPS caused a strong dose-dependent inhibition of Fe-Tf uptake (Fig1), there being a correlation between nitric oxide release by macrophages (assayed as NO2− accumulation in the medium) and inhibition of Fe uptake. To confirm whether the observed inhibition of Fe uptake was mediated by the nitric oxide released after macrophage activation by IFN-γ/LPS, we stimulated macrophages in the presence of NMMA, an inhibitor of NOS. The results show that NMMA partially restored Fe incorporation (Fig 1).
Inhibition of 59Fe uptake in J774 macrophages by IFN-γ/LPS stimulation. Macrophages were incubated overnight with the indicated concentrations of IFN-γ (U/mL), LPS (ng/mL), and/or NMMA (500 μmol/L) and then exposed to 50 μg/mL 59Fe-Tf for 8 hours. Nitrite production was assayed in the culture medium by the Griess reaction. Results are expressed as percent inhibition of iron uptake compared with the control, and are representative of 5 separate experiments. Values are given as the mean ± SE of triplicate cultures. Versus control: *P < .01, **P < .001, ***P < .0001; versus IFN/LPS: #P< .001.
Inhibition of 59Fe uptake in J774 macrophages by IFN-γ/LPS stimulation. Macrophages were incubated overnight with the indicated concentrations of IFN-γ (U/mL), LPS (ng/mL), and/or NMMA (500 μmol/L) and then exposed to 50 μg/mL 59Fe-Tf for 8 hours. Nitrite production was assayed in the culture medium by the Griess reaction. Results are expressed as percent inhibition of iron uptake compared with the control, and are representative of 5 separate experiments. Values are given as the mean ± SE of triplicate cultures. Versus control: *P < .01, **P < .001, ***P < .0001; versus IFN/LPS: #P< .001.
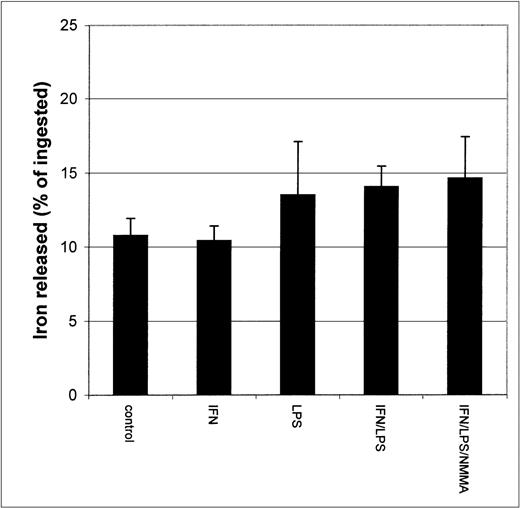
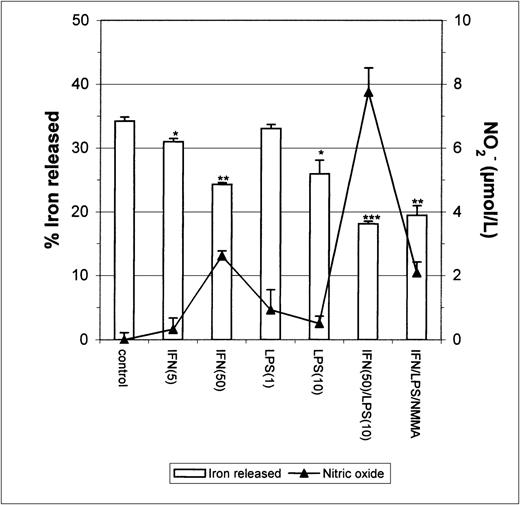
Since earlier studies indicated that NO causes iron release from cells,20 21 it seemed possible that the apparent inhibition of iron uptake might be due to increased efflux of iron from IFN-γ/LPS-activated J774 cells during the incubation. To test this possibility, we performed two different experiments. First, cells were stimulated with IFN-γ/LPS immediately before the addition of59Fe-Tf. This had no significant impact on 59Fe released by the cells during a subsequent chase with unlabeled Fe-Tf (Fig 2), indicating that these mediators did not cause increased release of Fe during the acquisition process, for example, by preventing intracellular release of Fe from transferrin and allowing it to be recycled out of the cell. Addition of59Fe-Tf followed by a chase in the presence of IFN-γ/LPS actually caused a modest but significant decrease in iron release, which was not affected by NMMA (Fig 3). Thus, the mediators did not provoke release of iron already incorporated into the cell. Therefore, these results confirm that the main effect of IFN-γ/LPS treatment was to decrease macrophage Fe uptake rather than to increase its release.
Iron released by IFN-γ/LPS-activated J774 macrophages. Cells were stimulated overnight with IFN-γ (50 U/mL), LPS (10 ng/mL), and/or NMMA (500 μmol/L), pulsed for 4 hours with 50 μg/mL59Fe-Tf, and then incubated 2 more hours in the presence of unlabeled Fe-Tf. Nitrite production was assayed in the culture medium by the Griess reaction. Results shown are representative of 3 separate experiments. Values are given as the mean ± SE of triplicate cultures.
Iron released by IFN-γ/LPS-activated J774 macrophages. Cells were stimulated overnight with IFN-γ (50 U/mL), LPS (10 ng/mL), and/or NMMA (500 μmol/L), pulsed for 4 hours with 50 μg/mL59Fe-Tf, and then incubated 2 more hours in the presence of unlabeled Fe-Tf. Nitrite production was assayed in the culture medium by the Griess reaction. Results shown are representative of 3 separate experiments. Values are given as the mean ± SE of triplicate cultures.
Iron released by IFN-γ/LPS-activated J774 macrophages. Macrophages were incubated for 24 hours with 50 μg/mL59Fe-Tf and then stimulated overnight with the indicated concentrations of IFN-γ (U/mL), LPS (ng/mL), and/or NMMA (500 μmol/L) in the presence of unlabeled Fe-Tf. Nitrite production was assayed in the culture medium by the Griess reaction. Results shown are representative of 3 separate experiments. Values are given as the mean ± SE of triplicate cultures. Versus control: *P < .05, **P < .001, ***P < .0001.
Iron released by IFN-γ/LPS-activated J774 macrophages. Macrophages were incubated for 24 hours with 50 μg/mL59Fe-Tf and then stimulated overnight with the indicated concentrations of IFN-γ (U/mL), LPS (ng/mL), and/or NMMA (500 μmol/L) in the presence of unlabeled Fe-Tf. Nitrite production was assayed in the culture medium by the Griess reaction. Results shown are representative of 3 separate experiments. Values are given as the mean ± SE of triplicate cultures. Versus control: *P < .05, **P < .001, ***P < .0001.
Intracellular distribution of iron.
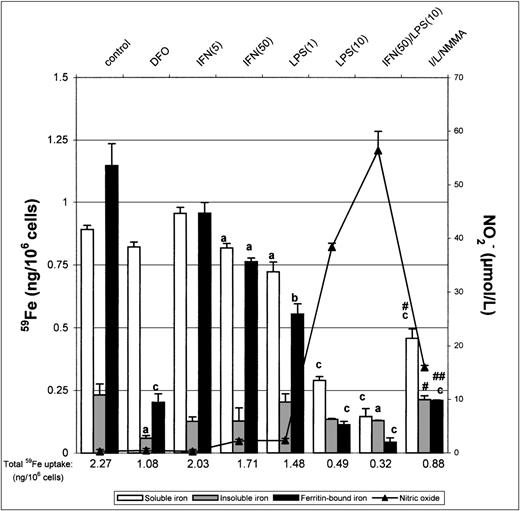
We next investigated the distribution of incorporated iron in nonactivated and IFN-γ/LPS-activated macrophages. J774 macrophages were pulsed for 8 hours with 50 μg/mL 59Fe-Tf to allow incorporation of 59Fe, lysed, and the lysates analyzed by a fractionation method, which provides a useful means of making comparative analysis of intracellular iron distribution.15After the 8-hour pulse with 59Fe-Tf, nonactivated macrophages contained approximately 40% soluble iron, 10% insoluble iron, and 50% ferritin-bound iron (Fig 4). In contrast, DFO-treated macrophages had approximately 80% of total incorporated iron as soluble nonferritin iron and very little insoluble iron. Activation with IFN-γ/LPS downregulated the total iron incorporated into all 3 fractions because of the previously observed inhibition of iron uptake. However, there was also a noticeable decrease in the proportion of ferritin-bound iron and relatively more insoluble iron, the effects being mediated at least in part by nitric oxide as they were to some extent reversed by NMMA. The soluble iron pool was proportionally much greater in DFO-treated cells, due probably to iron complexed with DFO itself, but unchanged upon activation with IFN/LPS. Thus, treatment of J774 cells with IFN-γ/LPS not only decreased total iron uptake, but also altered intracellular distribution, with ferritin-iron being particularly decreased and insoluble iron least so. NMMA increased the proportion of ferritin-iron, but had less effect on insoluble iron, which suggests that the former but not the latter compartment was downregulated by NO.
Intracellular iron distribution in J774 macrophages. Macrophages were treated as described in the legend to Fig 1 and then lysed in cytoplasmic lysis buffer containing protease inhibitors and the intracellular iron distribution determined as described in the Methods. Nitrite production was assayed in the culture medium by the Griess reaction. Results shown are representative of 5 separate experiments. Values are given as the mean ± SE of triplicate cultures. Versus control: aP < .05,bP < .01, cP < .001; versus IFN/LPS: #P < .01, ##P < .001.
Intracellular iron distribution in J774 macrophages. Macrophages were treated as described in the legend to Fig 1 and then lysed in cytoplasmic lysis buffer containing protease inhibitors and the intracellular iron distribution determined as described in the Methods. Nitrite production was assayed in the culture medium by the Griess reaction. Results shown are representative of 5 separate experiments. Values are given as the mean ± SE of triplicate cultures. Versus control: aP < .05,bP < .01, cP < .001; versus IFN/LPS: #P < .01, ##P < .001.
TfR expression.
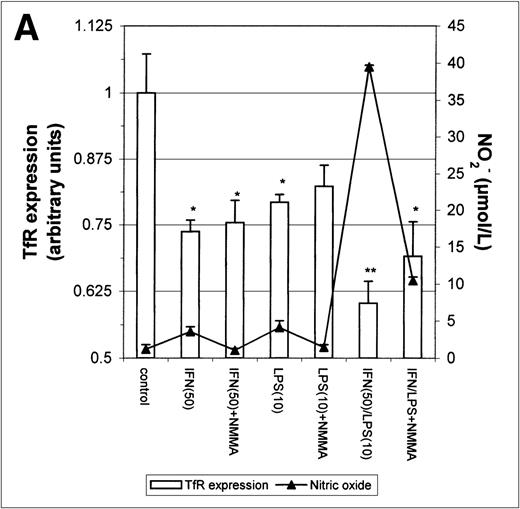
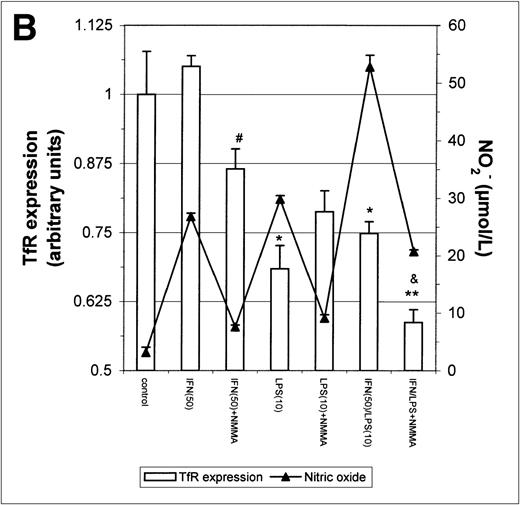
The above results prompted us to examine the effect of IFN-γ and LPS on TfR expression. Incubation with either IFN-γ or LPS for 16 hours downregulated macrophage TfR expression, even though NO production was extremely low (Fig 5A). In cultures containing both IFN-γ and LPS, TfR expression was further inhibited, but NMMA produced only a small and statistically insignificant reversal of inhibition. Thus, the inhibition of TfR expression 16 hours after stimulation of the cells appeared to be largely NO-independent. However, when the macrophages were stimulated for 24 hours instead of 16 hours with IFN-γ alone or with IFN-γ plus LPS, a less strong TfR downregulation was found and this correlated with a greater NO production. Furthermore, in the case of cells activated with IFN-γ plus LPS, downregulation of TfR was enhanced by addition of NMMA (Fig5B). This suggests that stimulation of macrophages with IFN-γ/LPS has 2 opposing effects on TfR expression: an NO-independent inhibitory effect, and an opposing upregulation by NO when the latter is produced in sufficient quantity. The changes in TfR expression between 16 hours and 24 hours, though modest, were consistently observed, and even modest changes in TfR expression are likely to have significant effects on iron uptake given the efficiency and rapidity with which the TfR delivers iron to the cell.
Modulation of TfR expression by IFN-γ/LPS in J774 macrophages. Cells were stimulated for 16 hours (A) or 24 hours (B) with the indicated concentrations of IFN-γ (U/mL), LPS (ng/mL), and/or NMMA (500 μmol/L) and the expression of TfR determined by flow cytometry as described in the Methods. Nitrite production was assayed in the culture medium by the Griess reaction. Results shown are representative of 5 separate experiments. Values are given as the mean ± SE of triplicate cultures. Versus control: *P < .05, **P < .01; versus IFN: #P < .05; versus IFN/LPS: &P < .001.
Modulation of TfR expression by IFN-γ/LPS in J774 macrophages. Cells were stimulated for 16 hours (A) or 24 hours (B) with the indicated concentrations of IFN-γ (U/mL), LPS (ng/mL), and/or NMMA (500 μmol/L) and the expression of TfR determined by flow cytometry as described in the Methods. Nitrite production was assayed in the culture medium by the Griess reaction. Results shown are representative of 5 separate experiments. Values are given as the mean ± SE of triplicate cultures. Versus control: *P < .05, **P < .01; versus IFN: #P < .05; versus IFN/LPS: &P < .001.
IRP1 and IRP2 activity.
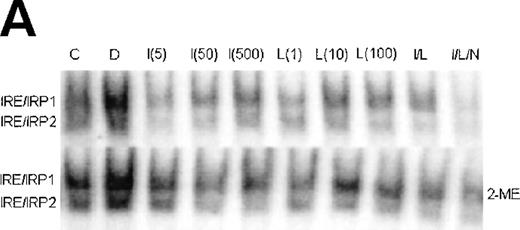
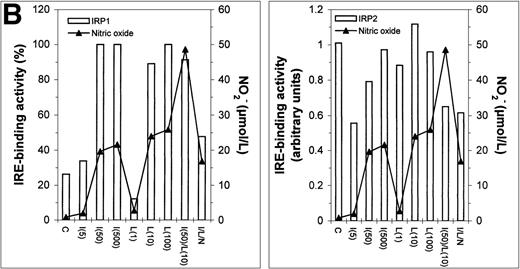
Since uptake and utilization of cellular iron is regulated by the iron regulatory proteins IRP1 and IRP2,4 it was of interest to determine whether the activity of these proteins in stimulated J774 cells could explain the effects of IFN-γ/LPS on iron uptake and distribution in J774 cells. Figure 6 shows a representative gel retardation assay using extracts prepared from nonactivated control (C), DFO-treated (D), and IFN-γ (I)- and/or LPS (L)-activated cells, with or without NMMA (N). DFO, included as a positive control, activated both IRP1 and IRP2 as expected. Stimulation with either IFN-γ or LPS, or both together, caused an increase in IRE-binding activity of IRP1 that appeared to depend on NO, as it was largely reversed by NMMA. The effect on activity of IRP2 was more complex; a low dose of IFN-γ (5 U/mL), which caused minimal NO production, decreased activity of IRP2, but at higher IFN-γ doses, at which significant amounts of NO were produced, IRP2 activity was progressively regained. LPS alone had no effect on IRP2, even at doses sufficient to cause significant NO production, while IFN-γ plus LPS caused a decrease in IRP2 activity that was not reversed by NMMA. Downregulation of IRE-binding activity of IRP2 was still observed when the cells were treated with a combined low dose of IFN-γ plus LPS that was insufficient to cause significant NO production (data not shown). These observations provide evidence that while IRP1 is upregulated by NO, IRP2 is downregulated by IFN-γ alone, in an NO-independent manner.
Regulation of IRE-binding activity of IRPs by IFN-γ/LPS in J774 macrophages. (A) Cells were treated overnight in RPMI culture medium containing 10% FCS with 100 μmol/L DFO (D), 5 to 500 U/mL IFN-γ (I), 10 to 1,000 ng/mL LPS (L), and/or 500 μmol/L NMMA (N), or remained untreatred (control, C). Twenty micrograms of detergent cell extract was assayed for IRE-binding activity of IRP1 and IRP2 by gel-shift assay in the absence or presence of 2% 2-ME. Nitrite production was assayed in the culture medium by the Griess reaction. Results shown are representative of 4 separate experiments. (B) Autoradiographs were densitometrically scanned and the proportion of spontaneous IRP1 activity was expressed as a percentage of the value obtained after exposure to 2% 2-ME, which allows calculation of total IRE binding activity of IRP1. IRP2 activity was plotted in arbitrary units.
Regulation of IRE-binding activity of IRPs by IFN-γ/LPS in J774 macrophages. (A) Cells were treated overnight in RPMI culture medium containing 10% FCS with 100 μmol/L DFO (D), 5 to 500 U/mL IFN-γ (I), 10 to 1,000 ng/mL LPS (L), and/or 500 μmol/L NMMA (N), or remained untreatred (control, C). Twenty micrograms of detergent cell extract was assayed for IRE-binding activity of IRP1 and IRP2 by gel-shift assay in the absence or presence of 2% 2-ME. Nitrite production was assayed in the culture medium by the Griess reaction. Results shown are representative of 4 separate experiments. (B) Autoradiographs were densitometrically scanned and the proportion of spontaneous IRP1 activity was expressed as a percentage of the value obtained after exposure to 2% 2-ME, which allows calculation of total IRE binding activity of IRP1. IRP2 activity was plotted in arbitrary units.
DISCUSSION
Iron homeostasis is of central importance for the regulation of macrophage functions.22 Increased intracellular free iron decreases the effectiveness of IFN-γ action on monocytic cells23,24 and reduces the transcription of inducible NO synthase (iNOS) mRNA and subsequent NO formation in J774 macrophages.3 Nevertheless, adequate iron levels are required for macrophage-mediated cytotoxicity to catalyze the production of highly toxic hydroxyl radicals via the Fenton reaction.2
Intracellular iron levels are maintained by regulating the uptake, utilization, and storage of iron. The most important molecules involved in this process are TfR, which mediates cellular uptake of Tf-bound iron,25 and Ft, which stores iron acquired in excess.26 Two cytoplasmic protein, IRP1 and IRP2, interact with both TfR and Ft mRNAs during iron deficiency or in cells exposed to oxidative stress or NO, thereby blocking Ft translation and increasing TfR mRNA stability.10-12 In addition to this, several cytokines influence iron metabolism by IRP-independent transcriptional and posttrancriptional regulation of TfR and Ft expression.22
Although the effect of NO on IRP activity has been well studied, the combined effects of NO and cytokine stimulation on the actual handling of iron by macrophages has not been investigated in any detail. In this study, we have shown that in J774 macrophages, cell activation by IFN-γ and/or LPS inhibits Fe uptake with a concomitant downregulation of TfR expression. This inhibition of iron uptake was partially but not completely reversed by NMMA, which indicates that NO, despite its previously reported ability to activate IRP1,10 can actually inhibit iron uptake by macrophages. The failure of NMMA to completely reverse inhibition of iron uptake may indicate that the effect is not entirely NO-mediated, or may simply reflect that fact that, as found by others,3,12,14 NMMA does not completely inhibit NO synthesis. Incubation of J774 cells with the NO generator S-nitroso-N-acetyl-penicillamine (SNAP) also inhibited iron uptake, but this result is difficult to interpret because penicillamine itself, which does not generate NO, also caused some inhibition (data not shown), perhaps because penicillamines can chelate iron. Iron release by IFN-γ/LPS-activated cells was not greater than from control cells, indicating that the effect is actually caused by inhibition of Fe uptake from Tf, rather than accelerated release of iron during the incubation period. This contrasts with earlier work with K562 erythroleukemia cells, in which NO (produced exogenously from SNAP) did cause an increase in iron release.21,27 Although inhibition of NO production in IFN-γ/LPS-treated cells by NMMA increased Fe incorporation, it did not cause a corresponding increase in TfR expression, and if incubation was extended to 24 hours, TfR expression was actually further reduced by NMMA. These apparently contradictory findings may indicate that NO directly affects iron availability to the cell. One possible explanation is that NO removes iron from transferrin at the cell membrane immediately before endocytosis of the transferrin-TfR complex, thus reducing the efficiency of iron uptake but not the expression of TfR. However, a previous study found that NO could not remove iron from Tf at neutral pH.27 A further possibility is that NO reduces the rate of recycling of TfR without reducing surface expression, as has been reported in spleen cells exposed to oxidative stress in vivo.28 Our results clearly indicate that NO by itself affects iron uptake independently of TfR expression, and this needs further investigation.
The effect of IFN-γ and LPS on TfR expression by J774 macrophages appears to be complex and regulated by both NO-dependent and -independent mechanisms. IFN-γ can by itself downregulate TfR expression, this being NO-independent, but correlating with a concomitant decrease in IRE-binding activity of IRP2. However, if incubation is continued long enough to allow significant production of NO, TfR expression can be at least partially restored, accompanied by activation of IRP1 and, to a lesser extent, of IRP2. In contrast, LPS by itself decreases TfR expression, even though NO is produced and IRP1 is activated. Costimulation of macrophages with both IFN-γ and LPS also leads to a downregulation of TfR expression that is initially NO-independent, but may be modulated by NO after a longer (24 hours) incubation period. Previous reports have also suggested that the regulation of TfR expression in activated macrophages is complex and may depend on experimental differences among groups.22Thus, cells exposed to NO-releasing drugs or overexpressing NOS exhibit higher TfR mRNA expression,10,21,29,30 whereas IFN-γ/LPS-stimulated macrophages show decreased TfR mRNA levels, despite production of NO and subsequent activation of IRP1 and IRP2.8,12,13,31 These apparently contradictory data can be reconciled by the fact that IFN/LPS can decrease TfR expression via an IRE/IRP-independent mechanism,13,32 as well as by the NO-independent IFN-γ–mediated downregulation of IRP2 described in this study and by others.14 Overall, it is clear that the major effect of IFN-γ/LPS on both TfR expression and iron uptake by J774 macrophages is downregulatory. Although NO activates IRP1, this is only able to compensate for the other inhibitory effects on TfR expression when high concentrations of NO are present.
The regulation of ferritin mRNA expression and protein content in IFN-γ/LPS-activated macrophages is also controversial. Weiss et al33,34 reported that IFN-γ/LPS treatment increases ferritin mRNA expression in J774 macrophages but decreases ferritin translation, indicating that IRP activation mediated by NO overcomes the increased mRNA expression. In contrast Recalcati et al12 more recently demonstrated increased ferritin synthesis and accumulation in IFN-γ/LPS-stimulated macrophages, accompanied by an NO-dependent increase in IRP1 and decrease in IRP2 activity. However, the effect on iron incorporation into ferritin has not been previously reported. In the present study, we found that IFN-γ/LPS treatment results in a strong decrease in both the amount and the proportion of iron incorporated into ferritin, together with a corresponding compensatory increase in insoluble iron (consisting mainly of hemosiderin and/or iron bound to intracellular organelles). These findings probably indicate an increase in iron bound to mitochondria in the more highly metabolic activated macrophages, rather than increased formation of hemosiderin, and the overall picture is one of iron being used for metabolic activity rather than being diverted to an enlarged ferritin compartment. Nevertheless, an NO-dependent downregulation of ferritin-bound iron in activated macrophages, perhaps as a consequence of NO-mediated iron release from ferritin,35 should not be discounted, as NMMA partially increased this intracellular iron fraction.
The cytoplasmic proteins IRP1 and IRP2 have a key role in the regulation of iron metabolism by binding specifically to stem-loop structures (IRE) of several mRNAs. Recent evidence indicates that these two proteins respond preferentially to different immunologic stimuli.12,14 In this work, we found that IFN-γ/LPS-stimulated macrophages showed an increase in IRP1 activity and a strong reduction of IRP2 activity, as also reported by others.12,14 Although both of these previous studies demonstrated that upregulation of IRP1 was NO-mediated, Bouton et al14 showed that the decrease of IRP2 activity is not mediated by NO, whereas Recalcati et al12 reported it to be NO-dependent. Our results accord with those of Bouton et al,14 as we found that NO released by IFN-γ/LPS-activated macrophages increased IRE-binding activity of IRP1, whereas low levels of IFN-γ, insufficient to cause significant NO production, were able to decrease IRP2 activity. These results strongly suggest that (1) NO is not involved in the IFN-γ/LPS-induced downregulation of IRP2 activity despite significant NO production, as LPS treatment is unable to downregulate IRP2 activity even when NO is produced; and (2) NO can mediate the activation of both IRP1 and IRP2, probably by affecting iron availability.
In conclusion, this study demonstrates that activation of IRPs, expression of TfR, and iron uptake and utilization in IFN-γ/LPS-activated macrophages is complex, and that a model based solely on NO-mediated activation of IRP1 is not tenable as it appears to affect TfR expression positively only at high NO concentrations. The overall picture is one of reduced iron uptake but increased cellular utilization for metabolic activity, mediated partly by an NO-independent downregulation of IRP2, and partly by IRP-independent mechanisms, some of which may also involve NO. Such a scenario would allow activated macrophages to fine-tune their iron uptake to a level which permits optimal activation of antimicrobial and cytotoxic activities but without accumulating excess iron that might facilitate intracellular microbial growth.
ACKNOWLEDGMENT
We thank Dr C. Guillén for her helpful advice with the band shift assay.
V.M. was supported by a grant from CajaMurcia (Spain).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Jeremy H. Brock, PhD, Department of Immunology and Bacteriology, Western Infirmary, Glasgow G11 6NT, UK; e-mail: jhb1h@clinmed.gla.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal