The mechanisms for induction of eosinophil apoptosis remain uncertain. The role of oxidative stress has not been investigated. The present study was undertaken to determine the role of reactive oxygen species (ROS) and selective antioxidants in eosinophil apoptosis. Eosinophils were cultured with sodium arsenite (SA) known to induce intracellular oxidative metabolites. There was a significant increase in the rate of eosinophil apoptosis with low concentrations of arsenite, whereas high concentrations showed rates of apoptosis similar to control medium. Investigating the role of intracellular oxidants by flow cytometry, we found that while inducing apoptosis, SA more than anti-Fas resulted in a significant dose-dependent production of intracellular H2O2. In contrast, the extracellular release of superoxide decreased after stimulation with SA or anti-Fas as assessed by lucigenin-dependent chemiluminescence. Coincubation experiments demonstrated that arsenite-induced apoptosis can be nearly completely prevented by selective antioxidants such as glutathione (GSH) and N-acetyl-cysteine (NAC), but not dimethyl sulfoxide (DMSO) or taurine (TAUR). Moreover, GSH and NAC significantly reduced eosinophil apoptosis mediated by a monoclonal antibody directed to Fas antigen. Next it was shown that GSH and NAC, but not DMSO or TAUR, were able to significantly delay spontaneous apoptosis in unstimulated eosinophils. Taken together, these data point to an important role of oxygen-dependent mechanisms in the regulation of eosinophil survival and apoptosis. We propose that eosinophil apoptosis may be related to the ability of the cell to maintain an appropriate oxidant-antioxidant balance.
THE LAST FEW YEARS have provided increasing evidence that apoptosis plays a major role in promoting resolution of the acute and chronic inflammatory response. The eosinophil granulocyte is able to release a variety of proinflammatory mediators such as basic proteins (eosinophil cationic protein, major basic protein), enzymes (eosinophil peroxidase), several cytokines, and potent reactive oxygen species (ROS), and it is widely accepted that eosinophils contribute to tissue destruction during allergic inflammation.1-3 In contrast to necrotic eosinophils, apoptotic eosinophils are recognized by macrophages by a specific process without disgorgement of their histotoxic contents.4,5 Thus, to prevent tissue injury and inflammation, it is important to determine the mechanisms by which eosinophils undergo apoptosis. Known major triggers involved in eosinophil apoptosis are glucocorticosteroids,6,7theophylline,8 transforming growth factor-β,9,10 abrogation of Fas/CD95 by its ligand or a monoclonal antibody (MoAb),6,11 or CD69 perturbation with MoAb.12 However, the cellular mechanisms involved in eosinophil apoptosis are far from being clear. Recently, it was suggested that bcl-2 family members and interleukin-1β converting enzyme (ICE)-like proteases may be implicated in the regulation of eosinophil apoptosis.13,14 The role of ROS has not been investigated, although there are emerging data indicating that ROS can serve as potent intracellular second messengers.15-17 ROS are highly reactive metabolites generated during normal cell metabolism; the cell contains many systems to limit their damaging effects.18 Many agents, which induce apoptosis, are either oxidants or stimulators of cellular oxidative metabolisms, whereas many inhibitors of apoptosis show antioxidant activities.19 For example, thiol antioxidants such as N-acetylcysteine (NAC) and glutathione (GSH) completely block the activation-induced cell death of T-cell hybridomas.20 Antioxidants, such as dimethyl sulfoxide (DMSO) and o-phenanthroline, inhibited endotoxin-mediated endothelial cell apoptosis.21 In human monocytes, Fas, a potent mediator of apoptosis in many cell types, was shown to mediate its apoptotic effect by a ROS-dependent pathway.22Recently, it was shown that in naturally Fas resistant tumor cell lines a reduction in intracellular O2−concentration induced sensitivity to Fas, whereas an increased intracellular superoxide anion concentration abrogated fas-mediated cell death.23 All of these data point to a major role of intracellular oxidative metabolites in the regulation of apoptosis. Therefore, we studied the role of the heavy metal salt sodium arsenite (SA), which represents not only a tumor enhancer, but also a potent inducer of stress responses.24 SA is known to disturb the oxygen metabolism in mitochondria, which are major sites of ROS production. Previous studies demonstrated that SA-induced oxidative stress resulted in apoptosis mediated by ROS in several cell systems.25-27 Apart from the induction of intracellular oxidative metabolites, SA also regulates intracellular GSH levels.28 Furthermore, the effect of several antioxidants in SA-, Fas-mediated, and spontaneous eosinophil apoptosis was assessed, and we analyzed the possibility that SA and anti-Fas may modulate the production of cellular oxidants.
In the present report, we provide clear evidence that antioxidants such as NAC and GSH, but not taurine (TAUR) or DMSO, can completely block SA- and Fas-induced apoptosis of eosinophils. In addition, these antioxidants also inhibited spontaneous eosinophil apoptosis implicating a major role for oxidative stress in eosinophil apoptosis. Induction of apoptosis by SA and anti-Fas MoAb correlated with enhanced production of intracellular ROS and decreased release of extracellular ROS. These results point to oxidative stress as a central mechanism regulating eosinophil apoptosis.
MATERIALS AND METHODS
Purification of eosinophils.
Peripheral blood eosinophils were separated by Ficoll density gradient centrifugation and an improved immunomagnetic negative selection procedure using anti-CD16 antibody-coated Dynabeads (Dynal A.S., Oslo, Norway) as described in detail.29 30 Purity and viability were 96% or greater after isolation, as assessed by Kimura staining and trypan blue dye exclusion, respectively.
Cultures for eosinophil survival assay.
Eosinophils were resuspended in culture medium RPMI-1640 with 10% fetal calf serum (FCS), 1% penicillin/streptomycin (Life Technologies, Eggenstein, Germany) to 1 × 106/mL, and 1-mL aliquots were added to 24-well flat-bottomed tissue culture plates and stimulated with an equal volume of culture medium or the test factors at 37°C, 5% CO2. SA, GSH, NAC, DMSO, and TAUR (all from Sigma Chemicals, Deisenhofen, Germany) were diluted in phosphate-buffered saline (PBS) in concentrations as indicated. Anti-Fas MoAb (2 μg/mL), known to significantly induce apoptosis in eosinophils,6 served as positive control (Immunotech, Marseille, France), and interleukin-3 (IL-3) (100 U/mL) was used to significantly inhibit eosinophil apoptosis (Genzyme Corp, Cambridge, MA).
Eosinophil viability.
Viability of cultured eosinophils was assessed at different time points by flow cytometric analysis monitoring cell size and ethidium bromide (Ethbr; Molecular Probes, Eugene, OR) uptake. Briefly, after indicated incubation times, cells received Ethbr (1 μg/mL), were incubated at room temperature for 5 minutes, and analyzed on a FACScan flow cytometer (Becton Dickinson Immunocytometry System, San Jose, CA). Ethbr penetrates and intercalates into the DNA of nonviable cells, causing them to fluoresce red with ultraviolet (UV) light (620 nm).
Identification of cells undergoing apoptosis by light and fluorescence microscopy.
Light microscopic appearance of apoptotic eosinophils induced by SA and anti-Fas and its modification by antioxidants and IL-3 was studied on cytocentrifuge smears stained with Mayer’s 0.1% hematoxylin. Apoptotic eosinophils are smaller than normal eosinophils with condensed nuclei, which are round in shape, but frequently the nuclear structures become undiscernible among the numerous granules and the cell diameter was strongly reduced as previously described.31 32 Fluorescence microscopic appearance of apoptotic eosinophils was studied on cytocentrifuge smears stained with propidium iodide (PI) solution (100 μg/mL in PBS).
Cytofluorometric analysis of eosinophil apoptosis.
The proportion of apoptotic eosinophils displaying a hypodiploid DNA peak was determined using a modification of the protocol of Nicoletti et al.33-35 In brief, 1 × 105 eosinophils were gently resuspended in 200 μL of hypotonic fluorochrome solution (prodium iodide, 50 μg/mL in 0.1% sodium citrate plus 0.1% Triton X-100) and incubated for 2 hours at 4°C. The red fluorescence of PI of individual nuclei was measured using a FACScan flow cytometer. The forward scatter and side scatter of cells were measured simultaneously. Cell debris was excluded from analysis by appropriately raising the forward scatter threshold, but no gates were set. The PI fluorescence of individual nuclei with an acquisition of FL2 was plotted against forward scatter, and the data were registered on a logarithmic scale. The minimum number of 5,000 events was collected. Apoptotic eosinophil nuclei were distinguished by their hypodiploid DNA content from the diploid DNA content of normal eosinophil nuclei.
DNA fragmentation assay.
The classical biochemical method for demonstrating apoptosis is the presence of oligonucleosome-sized fragments of DNA, which when run on agarose gels, produce a “ladder” pattern. DNA fragmentation assay was performed as described.29 Electrophoresis of the nucleosomal DNA fragments was performed in 1.8% agarose gels using 0.5 × Tris borate/EDTA electrophoresis buffer containing 0.1 μg/mL ethidium bromide. After electrophoresis, fragments were visualized by UV light and photographed.
Detection of hydrogen peroxide (H2O2).
To measure changes in intracellular H2O2, we used the oxidation-sensitive fluorescent probe dihydrorhodamine (DHR). DHR (Molecular Probes) is nonfluorescent, uncharged, and accumulates within cells, whereas R123, the product of DHR oxidation, is fluorescent, positively charged, and trapped within cells (emission at 525 nm).36 Eosinophils (1 × 106/mL) were incubated with control medium or SA (50 μmol/L), anti-Fas (2 μg/mL), IL-3 (100 U/mL), or phorbol myristate acetate (PMA) (10−7 mol/L) for 20 hours at 37°C. Thereafter, the cells were labeled with 1 μmol/L DHR for another 10 minutes at 37°C. Probes were placed on ice and green fluorescence (FL 1) was measured immediately using a FACScan flow cytometer (Becton Dickinson Immunocytometry System).
Chemiluminescence.
Highly sensitive lucigenin-dependent chemiluminescence representing a reliable measurement for the release of ROS, which is independent from the release of peroxidase, was quantitated by a single-photon imaging system (MTP reader; Hamamatsu Photonics, Herrsching, Germany) as described previously.37 38 After stimulation (in RPMI-1640 as indicated), eosinophils were gently pelleted and resuspended at 5 × 104/mL in Hanks’ Balanced Salt Solution (HBSS), pH 7.4, containing 200 μmol/L lucigenin and 1 mg/mL bovine serum albumin (BSA). One hundred–microliter aliquots were placed in flat-bottom, white microtiter plates (Microfluor; Dynatech Deutschland, Denkendorf, Germany). Measurements were performed in triplicate at 37°C. Integral counts were obtained from a 0- to 60-minute incubation interval immediately after addition of stimuli and were indicated as intensity counts in the figure.
Statistical analysis.
Unless otherwise stated, all data are presented as mean ± standard error of mean (SEM). Analysis of variance (one-way ANOVA) was used for comparing experimental group with control value. If global test for differences was significant, pair-wise tests for differences between groups were applied (Student’s t-test for paired data or Mann-Whitney rank sum test), a statistical software package (SigmaStat for Windows; Jandel Scientific, Erkrath, Germany) being used. AP value < .05 was considered statistically significant.
RESULTS
Effects of SA on eosinophil apoptosis.
To study the potential involvement of ROS in eosinophil apoptosis, we assessed the effect of the heavy metal salt, SA, known to induce intracellular oxygen metabolites and to regulate glutathione levels.39
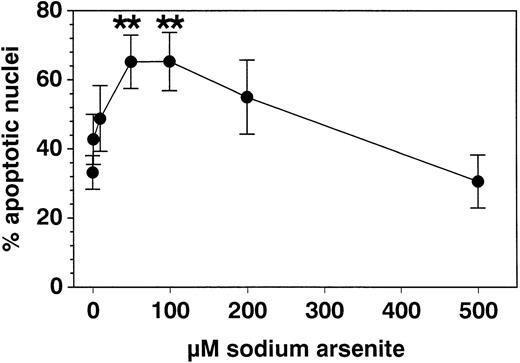
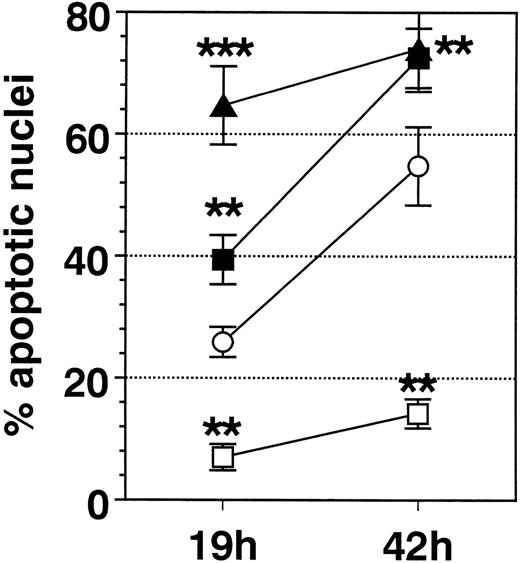
SA significantly promoted apoptosis in a dose-dependent manner from 50 μmol/L up to 100 μmol/L, whereas higher concentrations (>200 μmol/L) were ineffective (Fig 1). Anti-Fas MoAb consistently enhanced eosinophil apoptosis, whereas IL-3 stimulation resulted in a significant inhibition (not shown). After a 19-hour incubation, enhancement of apoptosis by SA (50 μmol/L) was significantly higher compared with Fas-mediated apoptosis, whereas after 42 hours, both stimuli resulted in similar numbers of apoptotic nuclei (Fig 2). Moreover, time kinetic studies showed that SA-mediated apoptosis occurred after about 8 hours (Fig 3), similar to Fas-mediated apoptosis (not shown). This time-delayed effect suggests that both arsenite and anti-Fas trigger a series of events leading to DNA fragmentation.
Effect of SA incubation with eosinophils for 19 hours on percent apoptotic nuclei compared with medium control. The data were determined by PI uptake (modified Nicoletti’s protocol) and are given as mean ± SEM values of 8 separate experiments with eosinophils of different subjects. Fifty and 100 μmol/L SA significantly enhanced eosinophil apoptosis, whereas at higher concentrations (200 to 500 μmol/L), this apoptosis inducing effect was reversed. **P < .01 versus control medium.
Effect of SA incubation with eosinophils for 19 hours on percent apoptotic nuclei compared with medium control. The data were determined by PI uptake (modified Nicoletti’s protocol) and are given as mean ± SEM values of 8 separate experiments with eosinophils of different subjects. Fifty and 100 μmol/L SA significantly enhanced eosinophil apoptosis, whereas at higher concentrations (200 to 500 μmol/L), this apoptosis inducing effect was reversed. **P < .01 versus control medium.
Comparison of the effect of IL-3 (100 U/mL, □), anti-Fas MoAb (FAS, 1 μg/mL, ▪), SA (50 μmol/L, ▴), and medium control (Med, ○) on eosinophil apoptosis. Data are presented as mean ± SEM of 8 separate experiments. Whereas IL-3 significantly inhibited eosinophil apoptosis, FAS and SA significantly enhanced it. After 19 hours of incubation, SA resulted in a significantly higher enhancement of apoptosis compared with Fas. **P < .01, ***P< .001 versus Med.
Comparison of the effect of IL-3 (100 U/mL, □), anti-Fas MoAb (FAS, 1 μg/mL, ▪), SA (50 μmol/L, ▴), and medium control (Med, ○) on eosinophil apoptosis. Data are presented as mean ± SEM of 8 separate experiments. Whereas IL-3 significantly inhibited eosinophil apoptosis, FAS and SA significantly enhanced it. After 19 hours of incubation, SA resulted in a significantly higher enhancement of apoptosis compared with Fas. **P < .01, ***P< .001 versus Med.
(A) Side scatter and forward scatter characteristics of unstimulated eosinophils. (B) Time course for the SA-mediated eosinophil apoptosis. Eosinophils were incubated with medium control or 50 μmol/L SA for 2 to 19 hours as indicated. Original flow cytometric histograms demonstrating hypodiploid DNA peaks representing apoptotic eosinophil nuclei are demonstrated. SA-mediated apoptosis (unfilled graph) occurred after about 8 hours similar to Fas-mediated eosinophil apoptosis (not shown) when compared with spontaneous apoptosis (medium control, shown by the filled graph).
(A) Side scatter and forward scatter characteristics of unstimulated eosinophils. (B) Time course for the SA-mediated eosinophil apoptosis. Eosinophils were incubated with medium control or 50 μmol/L SA for 2 to 19 hours as indicated. Original flow cytometric histograms demonstrating hypodiploid DNA peaks representing apoptotic eosinophil nuclei are demonstrated. SA-mediated apoptosis (unfilled graph) occurred after about 8 hours similar to Fas-mediated eosinophil apoptosis (not shown) when compared with spontaneous apoptosis (medium control, shown by the filled graph).
To confirm that apoptosis is the mechanism of SA-mediated death, eosinophils were analyzed by light microscopy of cytospin preparations, by flow cytometry after incubation with Ethbr, by fluorescence microscopy, and by DNA fragmentation formation (not shown). All of these assays showed that SA-mediated cell death was due to apoptosis and not to necrosis.
Effects of SA and anti-Fas on intracellular release of ROS.
We then analyzed the possibility that production of cellular oxidants may be controlled by Fas or SA using the DHR method. Intracellular ROS, primarily hydrogen peroxide H2O2, oxidate DHR, which rapidly accumulates within the cells to the fluorescent R123. After 19 hours, the intracellular R123 fluorescence of eosinophils was increased by eosinophil treatment with SA in a dose-dependent manner (Fig 4A). The optimum concentration of SA was 50 μmol/L, whereas higher concentrations resulted in a decrease of the fluorescence. The dose response of oxidation using the DHR method was similar to the dose-response effects of SA on apoptosis (Fig4B).
(A) Dose response of intracellular oxidation by SA. Eosinophils were incubated with medium control or the indicated concentration of SA (μmol/L). The mean green fluorescence representing the intracellular release of H2O2as determined by augmented R123 fluorescence (FL 1-H) is indicated. (B) Intracellular H2O2 increase (A) was associated with increased percentages of apoptotic nuclei. Eosinophils were incubated for 19 hours with medium control or the indicated concentration of SA and the hypodiploid DNA peak was measured using the PI method. Nuclei were flow cytometrically analyzed and data plotted on log histograms as red fluorescence intensity (x-axis) versus relative cell number (y-axis). Percentages of hypodiploid DNA peaks corresponding to apoptotic nuclei are indicated.
(A) Dose response of intracellular oxidation by SA. Eosinophils were incubated with medium control or the indicated concentration of SA (μmol/L). The mean green fluorescence representing the intracellular release of H2O2as determined by augmented R123 fluorescence (FL 1-H) is indicated. (B) Intracellular H2O2 increase (A) was associated with increased percentages of apoptotic nuclei. Eosinophils were incubated for 19 hours with medium control or the indicated concentration of SA and the hypodiploid DNA peak was measured using the PI method. Nuclei were flow cytometrically analyzed and data plotted on log histograms as red fluorescence intensity (x-axis) versus relative cell number (y-axis). Percentages of hypodiploid DNA peaks corresponding to apoptotic nuclei are indicated.
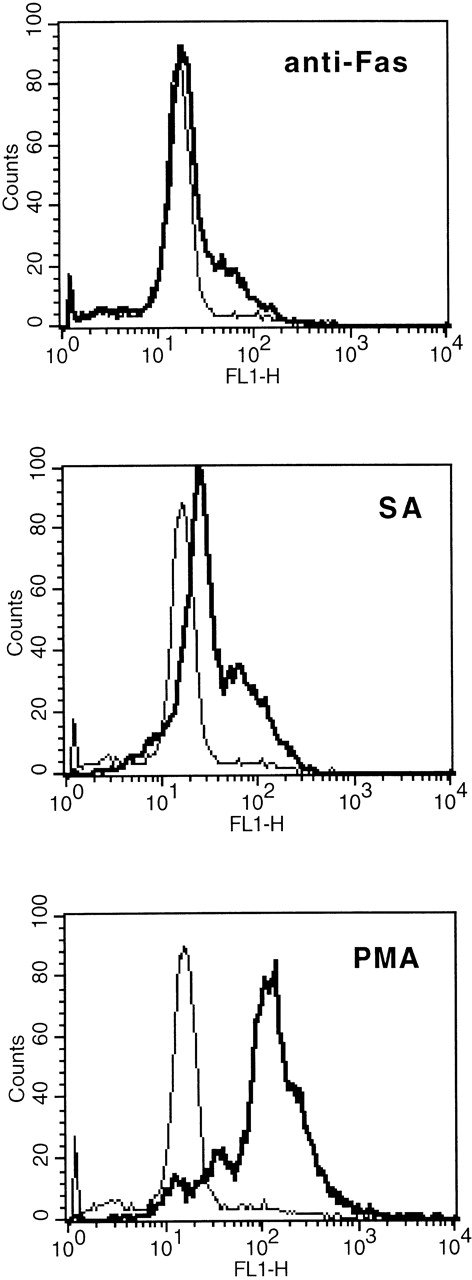
In comparison to anti-Fas (2 μg/mL), 50 μmol/L SA resulted in a significant higher augmentation of the cellular R123 fluorescence, indicating intracellular production of H2O2(Fig 5).
Comparison of SA-, anti-Fas–, and PMA-mediated intracellular release of H2O2 as determined by augmented R123 fluorescence (FL 1). Eosinophils were stimulated with medium control or SA (50 μmol/L) or anti-Fas (2 μg/mL) for 19 hours, thereafter the cells were labeled with DHR (for details see Materials and Methods). Stimulation with PMA (10−7 mol/L) served as positive control. One representative of 4 experiments is shown. The thin line represents the medium control, whereas the bold line represents the stimulus indicated.
Comparison of SA-, anti-Fas–, and PMA-mediated intracellular release of H2O2 as determined by augmented R123 fluorescence (FL 1). Eosinophils were stimulated with medium control or SA (50 μmol/L) or anti-Fas (2 μg/mL) for 19 hours, thereafter the cells were labeled with DHR (for details see Materials and Methods). Stimulation with PMA (10−7 mol/L) served as positive control. One representative of 4 experiments is shown. The thin line represents the medium control, whereas the bold line represents the stimulus indicated.
Effects of SA and Fas on eosinophil respiratory burst.
Stimulation of eosinophils with anti CD95 MoAb at concentrations of 0.1 to 2 μg/mL for 20 hours resulted in a significant reduction of respiratory burst (Fig 6A). This inhibition was similar when the eosinophils were activated with f-Met-Leu-Phe (fMLP, 10−7 mol/L, Fig 6) or PMA (10−7 mol/L, not shown). SA alone both inhibited (10, 50, and 100 μmol/L) and potentiated (500 μmol/L, not shown) superoxide generation after 10 minutes (Fig 6B).
(A) Stimulation with anti-CD95 (anti-Fas) MoAb (0.1 to 2 μg/mL) for 19 hours resulted in a significant reduction of respiratory burst in unstimulated (▪) and fMLP (10−7mol/L) activated (•) eosinophils as determined by lucigenin-dependent chemiluminescence. (B) Stimulation with 10 to 100 μmol/L SA (⧫) for 10 minutes significantly blocked fMLP (10−7mol/L)-induced eosinophil respiratory burst, whereas 500 μmol/L resulted in a significant enhancement (not shown). Data are presented as mean ± SEM intensity counts of 8 different experiments. *P< .05, **P < .01.
(A) Stimulation with anti-CD95 (anti-Fas) MoAb (0.1 to 2 μg/mL) for 19 hours resulted in a significant reduction of respiratory burst in unstimulated (▪) and fMLP (10−7mol/L) activated (•) eosinophils as determined by lucigenin-dependent chemiluminescence. (B) Stimulation with 10 to 100 μmol/L SA (⧫) for 10 minutes significantly blocked fMLP (10−7mol/L)-induced eosinophil respiratory burst, whereas 500 μmol/L resulted in a significant enhancement (not shown). Data are presented as mean ± SEM intensity counts of 8 different experiments. *P< .05, **P < .01.
Inhibition of SA-, anti-Fas MoAb-mediated, and spontaneous eosinophil apoptosis by antioxidants.
To address a possible involvement of ROS in SA- and Fas-mediated apoptosis, eosinophils were incubated with selective antioxidants such as NAC, GSH, TAUR, and DMSO. NAC can directly reduce ROS and increase the intracellular level of glutathione, GSH is an endogenous antioxidant reducing the intracellular H2O2load, TAUR plays a functional role in the regulation of the oxidative pathway, and the cell-membrane permeable DMSO represents a potent scavenger .OH.
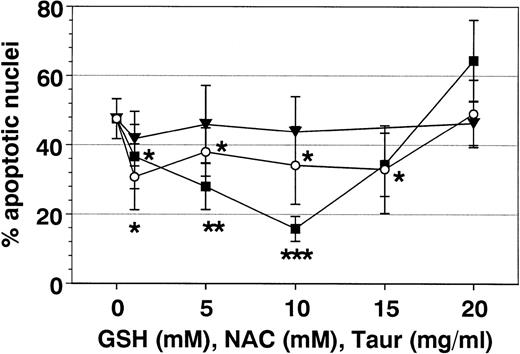
As shown in Figs 7 and8, NAC and GSH inhibited SA- and Fas-mediated apoptosis in a dose-dependent manner. Optimal inhibition was obtained at 10 mmol/L of each, demonstrating a role for cellular oxidants in SA- and FAS-mediated apoptosis.
Effect of antioxidants on SA-induced eosinophil apoptosis. Eosinophils were coincubated for 19 hours with SA (50 μmol/L) and GSH (○), NAC (▪), or TAUR (▾) at the indicated concentrations. Data are presented as mean ± SEM of 11 separate experiments. NAC more than GSH inhibited SA-mediated eosinophil apoptosis. Optimal concentration of GSH and NAC were 10 mmol/L each. In contrast, TAUR and DMSO (not shown) showed no effect on SA-mediated eosinophil apoptosis. *P < .05, **P < .01, ***P < .001 versus control (without stimulus).
Effect of antioxidants on SA-induced eosinophil apoptosis. Eosinophils were coincubated for 19 hours with SA (50 μmol/L) and GSH (○), NAC (▪), or TAUR (▾) at the indicated concentrations. Data are presented as mean ± SEM of 11 separate experiments. NAC more than GSH inhibited SA-mediated eosinophil apoptosis. Optimal concentration of GSH and NAC were 10 mmol/L each. In contrast, TAUR and DMSO (not shown) showed no effect on SA-mediated eosinophil apoptosis. *P < .05, **P < .01, ***P < .001 versus control (without stimulus).
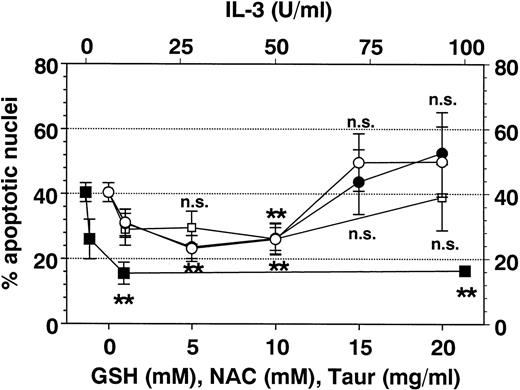
Effect of antioxidants on Fas-mediated eosinophil apoptosis. Eosinophils were coincubated for 19 hours with anti-Fas MoAb (2 μg/mL) and IL-3 (▪), GSH (○), NAC (•), or TAUR (□) at the concentrations indicated. Data are presented as mean ± SEM of percent apoptotic nuclei as compared with eosinophils incubated with control medium (n = 8 separate experiments). NAC and GSH inhibited Fas-mediated eosinophil apoptosis in a similar manner (optimal concentrations 5 to 10 mmol/L). Higher concentrations of GSH and NAC (15 to 20 mmol/L) induced apoptosis (not significant). Interestingly, TAUR at 10 mg/mL also significantly blocked Fas-mediated apoptosis. DMSO (0.5% to 4%) had no inhibitable effect (not shown). **P< .01 versus control (without stimulus). n.s., not significant.
Effect of antioxidants on Fas-mediated eosinophil apoptosis. Eosinophils were coincubated for 19 hours with anti-Fas MoAb (2 μg/mL) and IL-3 (▪), GSH (○), NAC (•), or TAUR (□) at the concentrations indicated. Data are presented as mean ± SEM of percent apoptotic nuclei as compared with eosinophils incubated with control medium (n = 8 separate experiments). NAC and GSH inhibited Fas-mediated eosinophil apoptosis in a similar manner (optimal concentrations 5 to 10 mmol/L). Higher concentrations of GSH and NAC (15 to 20 mmol/L) induced apoptosis (not significant). Interestingly, TAUR at 10 mg/mL also significantly blocked Fas-mediated apoptosis. DMSO (0.5% to 4%) had no inhibitable effect (not shown). **P< .01 versus control (without stimulus). n.s., not significant.
Additional metabolic inhibitors such as DMSO and TAUR did not alter SA- or Fas-mediated eosinophil apoptosis, although they were reported to block apoptosis in other cell types.21,40 41 However, after 19 hours (Fig 8), but not after 42 hours, taurine at 10 mg/mL reversed Fas-mediated eosinophil apoptosis.
Finally, it was shown that NAC and GSH were also able to block spontaneous apoptosis in unstimulated eosinophils (Fig 9). This effect was most evident after incubation for 42 hours in vitro. Thus, ROS may play a major role in the regulation of eosinophil apoptosis.
Effect of antioxidants on spontaneous eosinophil apoptosis in vitro. Eosinophils were incubated with control medium, GSH, NAC, DMSO, or TAUR for 19 hours (○) and 42 hours (•) at the concentrations indicated. Data are presented as mean ± SEM of 8 separate experiments. GSH (optimal concentration, 5 to 10 mmol/L) more than NAC (optimal concentration, 10 mmol/L) inhibited spontaneous eosinophil apoptosis. In contrast, higher concentrations of NAC and GSH (20 mmol/L) and DMSO (>1%) significantly induced eosinophil apoptosis. With TAUR, only 5 mg/mL significantly inhibited spontaneous eosinophil apoptosis after 19 hours, but this effect was not confirmed after incubation for 42 hours and was not dose-dependent. *P < .05, **P < .01, ***P < .001 versus unstimulated control. n.s., not significant.
Effect of antioxidants on spontaneous eosinophil apoptosis in vitro. Eosinophils were incubated with control medium, GSH, NAC, DMSO, or TAUR for 19 hours (○) and 42 hours (•) at the concentrations indicated. Data are presented as mean ± SEM of 8 separate experiments. GSH (optimal concentration, 5 to 10 mmol/L) more than NAC (optimal concentration, 10 mmol/L) inhibited spontaneous eosinophil apoptosis. In contrast, higher concentrations of NAC and GSH (20 mmol/L) and DMSO (>1%) significantly induced eosinophil apoptosis. With TAUR, only 5 mg/mL significantly inhibited spontaneous eosinophil apoptosis after 19 hours, but this effect was not confirmed after incubation for 42 hours and was not dose-dependent. *P < .05, **P < .01, ***P < .001 versus unstimulated control. n.s., not significant.
DISCUSSION
In contrast to necrosis, apoptosis represents a kind of cell death in which cells are phagocytosed without provocation of any inflammatory response. The eosinophil represents a major proinflammatory cell, particularly in the allergic response. Parasitic infections and allergic diseases, such as allergic rhinitis, bronchial asthma, and atopic dermatitis, are often associated with peripheral blood and/or tissue eosinophilia. Previously, we have shown that regulation of eosinophil apoptosis may be pivotal in atopic diseases such as inhalant allergy and atopic dermatitis.29 Moreover, it was shown that eosinophil apoptosis is significantly delayed in eosinophil-rich nasal polyps of nonatopic subjects with aspirin-sensitive bronchial asthma,42 and that eosinophil apoptosis may be clinically relevant in asthma.43 However, the cellular mechanisms of eosinophil apoptosis are not clear, and only a few promoters with potential therapeutical use have been described so far.
Herein we showed that apoptosis is significantly induced in human eosinophils by the heavy metal salt, SA, known to generate cellular oxidative metabolites. Previous studies demonstrated that oxidative stress induced by SA resulted in apoptosis in neutrophils, endothelial cells, and Chinese hamster ovary cells by generation of ROS.25-27 In this study at low concentrations (10 to 100 μmol/L), SA induced apoptosis, whereas at high concentrations, apoptosis was inhibited (>200 μmol/L). The demonstration of such paradoxical actions is characteristic, even predictable, of systems sensitive to redox regulation.
Analyzing the role of SA and Fas with regard to the production of intracellular ROS in eosinophils, we found that induction of apoptosis was associated with a dose-dependent increase in the intracellular production of H2O2. A similar increase in intracellular H2O2 was demonstrated previously in myeloid cells treated with oxidants, but not in myeloid cells treated with cytokines.44
In contrast, spontaneous, receptor-dependent (by FMLP), and receptor independent stimulation (by PMA) of the extracellular release of ROS, namely superoxide anion, was significantly reduced during apoptosis. In this context, it may be noted that cytokines known to enhance eosinophil survival and delay apoptosis such as granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3, or IL-5 were described to significantly enhance the release of O2−.45-47
That SA-induced eosinophil apoptosis may be mediated through an oxygen-dependent mechanism was confirmed by the fact that it could be prevented by low concentrations (5 to 10 mmol/L) of selective antioxidants such as NAC and GSH. Interestingly, higher concentrations of NAC and GSH (>15 mmol/L) resulted in apoptosis. This effect may be explained by the fact that antioxidants not only function as antioxidants, but that intrinsically they have prooxidant action as well.
Moreover, comparing SA-mediated apoptosis with apoptosis induced by the MoAb to Fas antigen (anti-Fas), our data suggest similar mechanisms. Both stimuli resulted in apoptosis after 8 hours, implying a time-delayed mechanism resulting in DNA fragmentation. The antioxidants, GSH and NAC, displayed at similar concentrations a dose-dependent ability to reverse SA- and Fas-mediated apoptosis.
Furthermore, we clearly showed that ROS may represent key signals not only in SA- and Fas-mediated, but also in spontaneous eosinophil apoptosis. Spontaneous eosinophil apoptosis in culture was significantly inhibited by the antioxidants NAC and GSH. This effect became statistically significant after 19 hours and most evident after 42 hours. Interestingly, only NAC and GSH prevented spontaneous and SA- as well as Fas-mediated eosinophil apoptosis, whereas DMSO and TAUR were ineffective. In contrast, in neutrophils, not only GSH and NAC, but also TAUR, inhibited SA-mediated apoptosis27 and it was demonstrated that Escherichia coli (E coli) induces neutrophil apoptosis through an oxygen-dependent mechanism inhibitable by antioxidants such as DMSO (1%), GSH (25 mmol/L), and NAC (25 mmol/L).41 Moreover, TAUR and DMSO prevented apoptosis in human endothelial cells.21,40 Interestingly, in neutrophils, NAC and GSH have been shown to be ineffective in delaying spontaneous apoptosis.48 49 In contrast, our data show significant inhibition of spontaneous eosinophil apoptosis by NAC and GSH. Whereas in neutrophils, 25 mmol/L of GSH and NAC were protective in SA-mediated apoptosis, in eosinophils similar concentrations induced apoptosis. These data implicate a species- or cell-specific sensitivity to different oxidant species even between different granulocyte types.
TAUR is a semiessential amino acid contained in eosinophils, but in lower concentrations compared with neutrophils.50 Our data indicate that in eosinophils, TAUR represents an antioxidant not modulating apoptosis. The cell-membrane permeable antioxidant, DMSO, specifically removes .OH, but was not protective in eosinophil apoptosis. GSH and NAC, which clearly blocked apoptosis, represent thiol antioxidants modulating the intracellular level of GSH. Thus, a thiol-dependent redox state may define if the eosinophil survives or if it undergoes apoptosis or necrosis. This is confirmed by the fact that SA, which significantly induced apoptosis, regulates intracellular GSH levels.28 A role for intracellular GSH in apoptosis has been recently demonstrated in T cells and in neural cells.51-53
The major source of ROS in most cell types is probably the single electron reduction of molecular oxygen to superoxide ions. Superoxide ions are subsequently converted to hydrogen peroxide (H2O2) by the action of Cu2+/Zn2+-dependent or Mn2+-dependent superoxide dismutases (Cu/ZnSOD or MnSOD), and H2O2 is then detoxified by catalase or GSH peroxidase. However, H2O2can also react to generate the highly damaging hydroxyl radical by the Fe2+-dependent Fenton reaction or the Fe2+-catalyzed Haber-Weiss reaction.18 The oxygen metabolites .OH and H2O2have been shown to induce apoptosis in a variety of cells. However, ROS may not result in apoptosis per se, rather an oxidative shift in the cellular redox state may modulate a stimulatory signal resulting in apoptosis. It should be noted that eosinophils have various endogenous enzymes, which can detoxify ROS such as catalase, GSH peroxidase, and eosinophil peroxidase. Different contents of these enzymes may define the intracellular concentration of ROS, thereby resulting in survival, apoptosis, or necrosis.
Taken together, our data show that SA, which generates intracellular oxidative metabolites, induces eosinophil apoptosis. It was shown that spontaneous Fas- and SA-mediated eosinophil apoptosis can be blocked by specific antioxidants modulating the GSH content. Our data suggest that eosinophil apoptosis may be regulated by an intracellular thiol-sensitive redox system. Moreover, we demonstrated that eosinophil apoptosis is associated with a decrease in the releasability of superoxide anion and with an increase in intracellular hydrogen peroxide. Hebestreit et al34 showed that nitric oxide (NO) inhibits Fas-mediated apoptosis in eosinophils, indicating that reactive nitrogen species (RNS) may block apoptosis. Thus, we hypothesize that ROS may induce and RNS may inhibit apoptosis. Emerging data suggest that a balance between NO and superoxide generation may be a critical determinant in the etiology of many human diseases including chronic inflammation, atherosclerosis, neurodegenerative disorders, and cancer54; therefore, it is not astonishing that a balance between these radicals may regulate eosinophil survival and cell death.
Supported by Grants No. We-1912/3-1 and Ka-578/7-1 from the German Research Foundation (DFG).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Bettina Wedi, MD, Department of Dermatology and Allergology, Hannover Medical University, Ricklinger Str 5, D-30449 Hannover, Germany.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal