Human mast cells are known to arise from a CD34+/c-kit+ progenitor cell population that also gives rise to neutrophils, eosinophils, basophils, and monocytes. To further characterize cells within the CD34+/c-kit+ population that yield mast cells, this progenitor was additionally sorted for CD13, a myeloid marker known to appear early on rodent mast cells and cultured human mast cells, but not expressed or expressed at low levels on human tissue mast cells; and cultured in recombinant human (rh) stem cell factor (rhSCF), rh interleukin-3 (rhIL-3; first week only), and rhIL-6. Initial sorts revealed that although the majority of cells in culture arose from the CD34+/c-kit+/CD13−cell population, mast cells arose from a CD34+/c-kit+/CD13+progenitor cell that also gave rise to a population of monocytes. Sequential sorting confirmed that CD34+/c-kit+/CD13+cells in CD34+/c-kit+/CD13−sorts gave rise to the few mast cells observed in CD13−sorted cells. CD34+/c-kit+/CD13+cells plated as single cells in the presence of various cytokine combinations gave rise to pure mast cell, monocyte, or mixed mast cell/monocyte progeny. Addition of either rh granulocyte-macrophage colony-stimulating factor (rhGM-CSF) or rhIL-5 to the CD34+/c-kit+/CD13+progenitor cell population cultured in rhSCF, rhIL-3, and rhIL-6 did increase the number of total cells cultured and in the case of rhIL-5, did increase total mast cell numbers. Neither rhGM-CSF or rhIL-5 led to additional cell populations, ie, even with the addition of rhGM-CSF or rhIL-5, only mast cells and monocytes grew from CD34+/c-kit+/CD13+cells. Thus, human mast cells and a population of monocytes arise from precursor cells that express CD34, c-kit, and CD13; and within which, are mast cell, monocyte, and mast/monocyte (bipotential) precursors.
HUMAN MAST cells originate from CD34+ progenitor cells when these cultures are maintained in stem cell factor (SCF), thus defining the mast cell precursor as CD34+/c-kit+.1-5 However, other cells including monocytes, basophils, eosinophils, and neutrophils may also be cultured from this cell population. To further define the mast cell precursor, we noted that CD34+ myeloid progenitor cells have been reported to variably express CD13, CD33, CD44, CD45, or CD117 (c-kit).6-10 Of particular interest is CD13, detected on rodent mast cells11 and human mast cells cultured from liver,12 but which is not expressed or expressed at low levels by mast cells digested from human tissues.13-16 CD13, a broadly distributed or myeloid marker additionally known as aminopeptidase N and gp150, is a type II integral membrane protein composed of 967 amino acids, and is expressed on the cell surface as a homodimer.17 This membrane-bound zinc-binding metalloprotease is known to be expressed during hematopoiesis at several different stages of myeloid differentiation.18
Because of its expression on cultured human mast cells and on murine mast cells, we hypothesized that CD13 might help define an early human mast cell progenitor. To explore this question, we sorted cells expressing various combinations of CD34, c-kit, and CD13; cultured them in selected growth factors; and examined the resulting cell cultures over 8 weeks. As will be shown, CD34+/c-kit+/CD13+ cells preferentially gave rise to mast cells. The remaining cells were monocytes. CD34+/c-kit+/CD13−and CD34+/c-kit−/CD13+cells in culture produced monocytes, eosinophils, basophils, and neutrophils, but not mast cells. It thus seems that when CD13 is expressed on CD34+/c-kit+ cells, it becomes a marker of a progenitor cell population that includes mast cell, monocyte, and mast cell/monocyte (bipotential) precursors.
MATERIALS AND METHODS
CD34+ immunoselection.
Human bone marrow (BM) from mastocytosis patients and peripheral blood (PB) mononuclear cells from normal volunteers (collected by leukapheresis) were obtained and processed, after informed consent was given.19 Twenty milliliters of BM aspirates was collected for study. Progenitor cells were purified by positive immunomagnetic selection19 or using commercially available affinity columns.20 CD34+ cells were 95% to 99% pure and used immediately or were aliquoted and frozen in liquid nitrogen until ready for use.
Cell selection and fluorescence-activated cell sorting (FACS) analysis.
CD34+/c-kit+/CD13+progenitor cells were further sorted from BM or PB CD34+cells and analyzed for surface antigens.19 For cell sorting, 1 to 10 × 106 CD34+ cells were first incubated in 1X phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) and 1% milk for 1 hour at 37°C. Cells were then incubated with 1 μg/mL R-phycoerythrin (PE)–conjugated mouse antihuman c-kit (1 mg/mL; Ancell, Bayport, MN), 1 μg/mL PE Cyanine 5 (PECy5)–conjugated mouse antihuman CD13 (50 μg/mL; Immunotech, Westbrook, ME), and 10 μL of fluorescein isothiocyanate (FITC)–conjugated mouse antihuman CD34 (anti-HPCA2; Becton Dickinson, San Jose, CA) for 1 hour at 37°C. Cells examined for FcεRI were incubated overnight at 37°C with 2 μg/mL FITC-conjugated human IgEPS (see below). Cells were then washed and resuspended in cold PBS containing 0.1% BSA. Control cells were unstained or stained with an irrelevant mouse IgG1. Selection of CD34+ subpopulations and cell analysis were performed using a FACStarPlus (Becton Dickinson) and CellQuest software (Becton Dickinson). Monocytes present in mast cell cultures were characterized for surface expression of CD11b, CD14, CD15 (Becton Dickinson), c-kit, and FcεRI. For lysozyme staining, paraformaldehyde-fixed slides were blocked for endogenous peroxidase, incubated for 30 minutes in Tris-buffered saline containing 3% goat serum, rinsed, and then incubated for 2 hours with rabbit antihuman lysozyme (DAKO, Carpinteria, CA). Secondary staining and color development were performed on an automated immunostainer (Ventana Medical Systems, Tucson, AZ).
Cell culture.
To assess hematopoietic potential, BM and PB CD34+/c-kit+/CD13+, CD34+/c-kit+/CD13−, CD34+/c-kit−/CD13+, and CD34+/c-kit−/CD13−cells were placed at a concentration of 5 × 104cells/mL in serum-free media (StemPro-34 SFM; Life Technologies, Grand Island, NY) supplemented with 2 mmol/L L-glutamine, 100 IU/mL penicillin, 50 μg/mL streptomycin, 100 ng/mL recombinant human stem cell factor (rhSCF), 100 ng/mL recombinant human interleukin-6 (rhIL-6), and 30 ng/mL rhIL-3 (first week only; Peprotech, Rocky Hill, NJ). Hemidepletions were performed weekly with media containing 100 ng/mL rhSCF and 100 ng/mL rhIL-6.19 In some experiments, cultures were supplemented with 10 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) or 10 ng/mL rhIL-5, with hemidepletions performed as above. Cell aliquots were taken weekly for determination of total cell and mast cell numbers, and for histochemical and immunohistochemical staining. Acidic toluidine blue (pH 1.0) was used to stain mast cell cytopreparations (Cytospin 3; Shandon, Pittsburgh, PA) or sections fixed in a mixture of 2% paraformaldehyde and 1.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer, pH 7.3, after sectioning for electron microscopy. Wright-Giemsa staining and qualitative tryptase enzyme determinations on cytocentrifuged cell preparations were performed as described.1 19 For clonogenic cultures, cells in each well were gently pipetted and placed on slides treated with 3-aminopropylethoxysilane (Digene, Beltsville, MD). The slides were incubated in 5% CO2 in air at 37°C in a humidified incubator for 30 to 40 minutes, centrifuged at 100g for 15 minutes, and stained with Wright-Giemsa.
Clonogenic cultures.
Single-cell liquid cultures were prepared as described.21,22 Briefly, sequentially sorted and enriched CD34+/c-kit+/CD13+ cells were individually seeded into 96-well plates using the automated cell deposition unit (ACDU; Becton Dickinson), which permits single-cell sorting with an accuracy of greater than 99%. To allow for the cloning of most cell phenotypes, 3 liquid culture cytokine mixture systems were prepared using triplicate 96-well plates: rhIL-3 30 ng/mL, rhSCF 100 ng/mL, and rhIL-6 100 ng/mL; rhIL-3 30 ng/mL, rhSCF 100 ng/mL, rhIL-6 100 ng/mL, rh erythropoietin (rhEpo) 2 U/mL, and rh thrombopoietin (rhTPO) 50 ng/mL (Peprotech)21; and rhIL-3 30 ng/mL, rhSCF 100 ng/mL, rhIL-6 100 ng/mL, rhEpo 2.5 U/mL, rhGM-CSF 10 ng/mL, rh insulin-like growth factor-1 (rhIGF-1) 10 ng/mL, and rh basic fibroblast growth factor (rhbFGF) 2.5 ng/mL (Peprotech).22All cultures were incubated in 5% CO2 in air at 37°C. Cell growth was checked on days 3, 5, 7, and 10. On day 14, cells were scored for the presence of nonadherent cells, adherent cells, or a mixture of both, and harvested.
FITC conjugation of IgEPS.
IgEPS was conjugated with FITC.23 Protein concentration (mg/mL) was calculated using the formula:
Moles of FITC per mole of IgE was calculated using the formula:
Conjugated IgEPS was 2.66 mg/mL with 7.3 mol FITC per mole IgEPS.
Immunomagnetic selection of FcεRI+ cells.
2 × 106 cells were removed from CD34+/c-kit+/CD13+ 2-week cultures and incubated overnight at 37°C with 2 μg/mL biotinylated human IgEPS. Cells were washed and resuspended in 1 mL of PBS with 0.1% BSA and 100 ng/mL rhSCF, to which was added 25 μL (107 beads) of washed superparamagnetic, polystyrene beads coated with recombinant streptavidin (CELLection Kit; Dynal, Lake Success, NY). Cells were gently tilted and rotated for 15 minutes at 4°C, recovered with a magnet (Dynal MPC), and washed 3 times. Cell counts and flow cytometric analysis of rosetted cells revealed 50% to 60% recovery of >97% FcεRI+ cells. Cells were cultured in serum-free media, as above, containing either 30 ng/mL rhIL-3 alone or 100 ng/mL rhSCF and 100 ng/mL rhIL-6.
Histamine release and analysis.
Four-week-old c-kit+/CD13+/FcεRI+mast cells were harvested, incubated (5,000 cells/0.5 mL) with 2 μg/mL human IgEPS overnight at 37°C, washed, and incubated with 10 μg/mL affinity-purified antihuman IgE (1 mg/mL; Kirkegaard and Perry, Gaithersburg, MD) for 30 minutes at 37°C. After incubation with 2 μg/mL human IgEPS, control cells were incubated with either media alone or 1 μmol/L A23187. In all cases, supernatants and cell pellets were examined for histamine using an enzyme-linked immunosorbent assay (ELISA; Immunotech).19 Percentage histamine release was expressed as the histamine measured in the supernatant divided by the sum of the histamine in the supernatant and cell pellet.
Electron microscopy.
Intracytoplasmic staining for tryptase and chymase.
Two-and 8-week-old c-kit+/CD13+/FcεRI+mast cells were permeabilized and stained for tryptase and chymase as reported.24 Briefly, 50 to 100 × 103cells were fixed with 4% paraformaldehyde, washed, and incubated with a blocking solution of 1X PBS-Saponin (PBS-S) containing 0.1% BSA and 1% milk for 1 hour at 37°C. For tryptase staining only, cells were incubated with 3 μg/mL mouse antihuman tryptase (1.14 mg/mL; Chemicon, Temecula, CA) for 1 hour at 37°C, washed, and incubated with 30 μg/mL PE-conjugated goat antimouse IgG (1 mg/mL; Southern Biotechnology, Birmingham, AL) for 30 minutes at 37°C. For chymase staining, cells first stained for tryptase were washed and incubated with 3 μg/mL biotinylated mouse antihuman chymase (3 mg/mL; Chemicon) for 1 hour at 37°C, washed, and incubated with 2 μg/mL allophycocyanine (APC)-conjugated streptavidin for 30 minutes at 37°C. After staining, all cells were washed and resuspended in cold 1X PBS containing 0.1% BSA. Control cells were unstained or stained with an irrelevant mouse IgG1. Cell analysis was performed using a FACScan and CellQuest software.
RESULTS
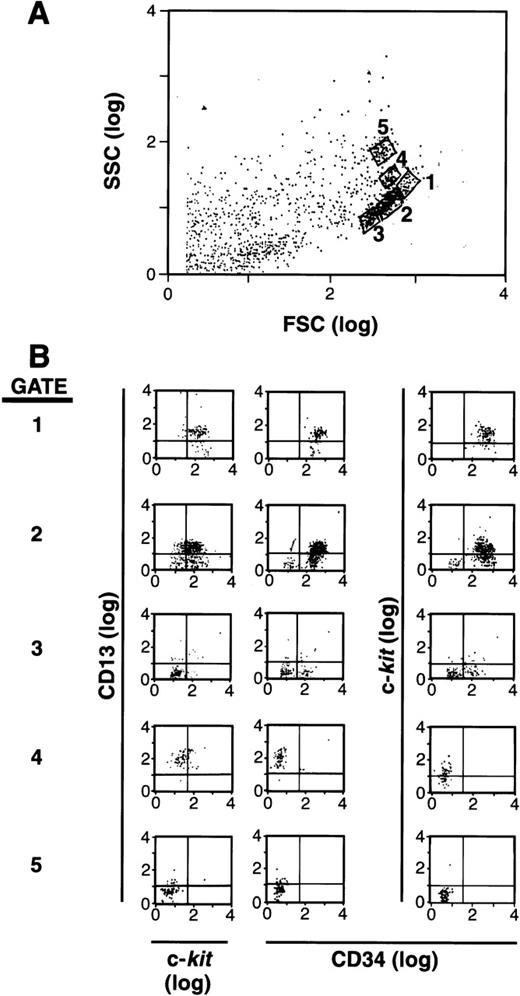
It has been documented that mast cells arise from an SCF-dependent (c-kit+) CD34+ population of cells in human BM and PB.1-5,19 25 Based on the reports that CD13+ is expressed early in hematopoiesis as well as on rodent mast cells, we hypothesized that this marker might help define the subset of cells within the CD34+ population that is committed to the mast cell lineage. As a first step, we separated CD34+ cells and labeled these cells with antibodies to CD34, c-kit, and CD13. We then analyzed these cells by forward (FSC) and side (SSC) scatter using flow cytometry (Fig 1A), and examined gated subpopulations of cells for their expression of CD34, c-kit, and CD13. As can be seen in Fig 1B, a subpopulation of cells was observed that was CD34+, c-kit+, and CD13+ in gates 1 and 2, whereas other CD34+ cells expressed various combinations of CD34, c-kit, and CD13 in gates 3, 4, and 5. Nonviable or cell fragments (lowest forward and side scatter parameters) were gated and eliminated before sorting, and therefore, were not cultured. The remaining viable CD34+ population was 95% to 99% pure. A series of cell subpopulations could thus be identified within the CD34+ population that variously expressed c-kit, CD13, or both.
FSC, SSC, and surface antigen expression of BM-and PB-derived CD34+ cells. (A) FSC and SSC display with 5 populations gated for further study. (B) Expression of CD13 versus c-kit and CD34 in each gate.1-5 The population of cells in gate 1 is CD34+/c-kit+/CD13+; gate 2 is mixed CD34+/c-kit+/c-kit−/CD13+/CD13−; gate 3 is mixed CD34+/CD34−/c-kit−/CD13−; gate 4 is CD34−/c-kit+/CD13+; and gate 5 is CD34−/c-kit−/CD13−. Results shown are representative of 6 experiments separately performed, using either BM (n = 3) or PB (n = 3) cells, and in which the FSC by SSC patterns resemble one another.
FSC, SSC, and surface antigen expression of BM-and PB-derived CD34+ cells. (A) FSC and SSC display with 5 populations gated for further study. (B) Expression of CD13 versus c-kit and CD34 in each gate.1-5 The population of cells in gate 1 is CD34+/c-kit+/CD13+; gate 2 is mixed CD34+/c-kit+/c-kit−/CD13+/CD13−; gate 3 is mixed CD34+/CD34−/c-kit−/CD13−; gate 4 is CD34−/c-kit+/CD13+; and gate 5 is CD34−/c-kit−/CD13−. Results shown are representative of 6 experiments separately performed, using either BM (n = 3) or PB (n = 3) cells, and in which the FSC by SSC patterns resemble one another.
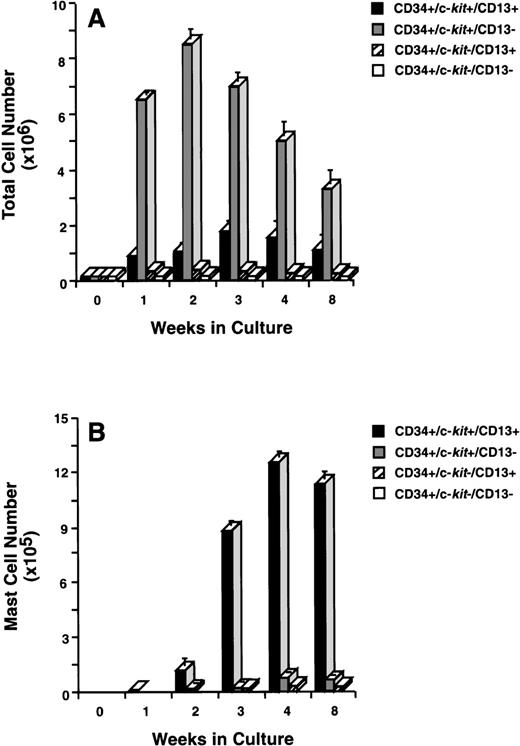
To determine which of these subpopulations included committed mast cell progenitors, we sorted CD34+ cells (Fig 2A) by flow cytometry into 4 distinct populations of cells that were CD34+/c-kit+/CD13+ (Fig2B), CD34+/c-kit+/CD13−(Fig2C), CD34+/c-kit−/CD13+(Fig 2D), and CD34+/c-kit−/CD13−(Fig 2E). In 6 CD34+ sorts, the purity of c-kit+/CD13+ cells averaged 85% ± 5%, c-kit+/CD13− cells 89% ± 3%, c-kit−/CD13+ cells 68% ± 4%, and c-kit−/CD13− cells 86% ± 4%. Sorted cells were then placed in culture, and total and mast cell numbers were determined over 8 weeks. CD34+/c-kit−/CD13+and CD34+/c-kit−/CD13−cells did not appreciably expand in culture, and through 8 weeks, few, if any, mast cells developed from these subpopulations (Fig 3A and B). The most marked cell expansion was seen from the CD34+/c-kit+/CD13−subpopulation. Cell numbers increased 60-fold by 2 weeks and declined in number thereafter. At all time points, mast cells constituted <3% of the cells cultured from the CD13− subpopulation. Basophils were detected at 2 weeks in cultures derived from CD34+/c-kit+/CD13−and CD34+/c-kit−/CD13+, but not CD34+/c-kit+/CD13+– sorted cells. The CD34+/c-kit+/CD13+ cell culture expanded 12-fold, peaked at 3 weeks, and declined thereafter. In contrast with other subpopulations at 2 weeks, approximately 15% of these cells could be identified as mast cells by morphologic criteria. By 3 weeks, approximately 60% had the morphologic appearance of mast cells, and by 4 weeks, approximately 85% were mast cells. Thus, the CD34+/c-kit+/CD13+subpopulation contained the mast cell progenitors that gave rise to approximately 90% of the mast cells cultured from the 4 CD34+ subpopulations. Because of the starting purity of these cell populations, the data supports the hypothesis that mast cell progenitors are CD34+/c-kit+/CD13+.
CD34+ cells sorted into CD13+and CD13− progenitor cells. (A) Presorted CD34+ cells. CD34+/c-kit+/CD13+cells averaged 10% to 15%. (B) Postsorted CD34+/c-kit+/CD13+cells. (C) Postsorted CD34+/c-kit+/CD13−cells. (D) Postsorted CD34+/c-kit−/CD13+cells. (E) Postsorted CD34+/c-kit−/CD13−cells. Similar results were obtained in 6 experiments separately performed, using either BM (n = 3) or PB (n = 3) cells in which the percentage of cells in quadrants of interest did not significantly differ between BM and PB.
CD34+ cells sorted into CD13+and CD13− progenitor cells. (A) Presorted CD34+ cells. CD34+/c-kit+/CD13+cells averaged 10% to 15%. (B) Postsorted CD34+/c-kit+/CD13+cells. (C) Postsorted CD34+/c-kit+/CD13−cells. (D) Postsorted CD34+/c-kit−/CD13+cells. (E) Postsorted CD34+/c-kit−/CD13−cells. Similar results were obtained in 6 experiments separately performed, using either BM (n = 3) or PB (n = 3) cells in which the percentage of cells in quadrants of interest did not significantly differ between BM and PB.
Total and mast cell numbers after 8 weeks in culture. (A) Total cell numbers, and (B) mast cell numbers derived from CD34+/c-kit+/CD13+cells, CD34+/c-kit+/CD13−cells, CD34+/c-kit−/CD13+cells, and CD34+/c-kit−/CD13−cells. Results are shown as the mean ± SEM of 3 separate experiments. PB cells were used and are representative of data from BM.
Total and mast cell numbers after 8 weeks in culture. (A) Total cell numbers, and (B) mast cell numbers derived from CD34+/c-kit+/CD13+cells, CD34+/c-kit+/CD13−cells, CD34+/c-kit−/CD13+cells, and CD34+/c-kit−/CD13−cells. Results are shown as the mean ± SEM of 3 separate experiments. PB cells were used and are representative of data from BM.
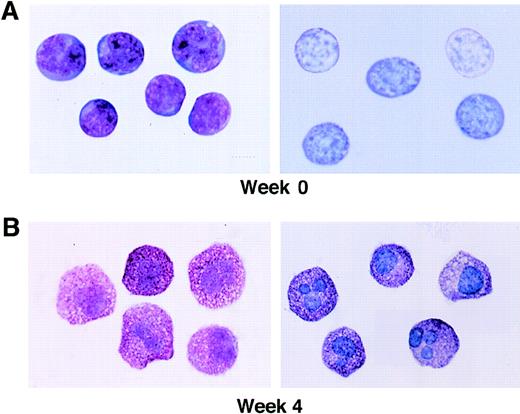
The morphologic appearance of the initial CD34+/c-kit+/CD13+ cell population is shown in Fig 4, as well as mast cells at 4 weeks. Sorted CD34+/c-kit+/CD13+ cells at day 0 were agranular and lymphoid in appearance with a large nucleus/cytoplasm ratio and basophilic-staining cytoplasm, consistent with immature hematopoietic progenitors (Fig 4A). The appearance of mast cells containing metachromatic granules is shown for comparison at 4 weeks (Fig 4B).
Wright-Giemsa staining and metachromasia of CD34+/ c-kit+/CD13+cells at 0 weeks (A), as well as mast cells at 4 weeks (B). Left panels show light microscopy of Wright-Giemsa–stained cells. Right panels show acid toluidine blue–positive cells (original magnification × 1,000).
Wright-Giemsa staining and metachromasia of CD34+/ c-kit+/CD13+cells at 0 weeks (A), as well as mast cells at 4 weeks (B). Left panels show light microscopy of Wright-Giemsa–stained cells. Right panels show acid toluidine blue–positive cells (original magnification × 1,000).
Ultrastructural examination of cultured CD34+/c-kit+/CD13+ progeny revealed cells with characteristics typical of cultured human mast cells and monocytes, but not basophils.1 19 Four-week-old mast cells had numerous surface projections and contained cytoplasm with electron-dense lipid bodies and cytoplasmic granules with partial scrolls, particles, dense cores, and homogeneously dense material consistent with more mature cultured mast cells. Granules stained strongly positive for tryptase. Monocytes were larger cells with abundant cytoplasm and few granules with nonspecific electron-dense material.
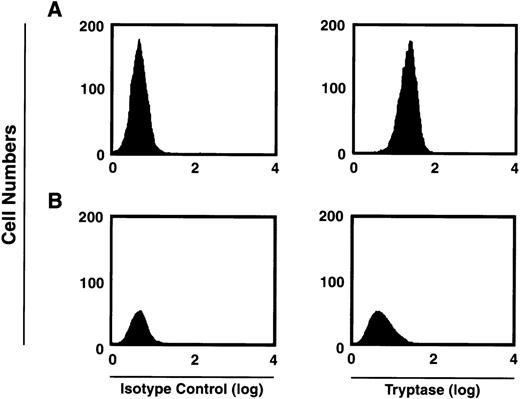
Human mast cells variably express chymase, with most if not all mast cells expressing tryptase.26 We first verified that the majority of tryptase-containing cells were present within the CD34+/c-kit+/CD13+–cultured cell subpopulation. As can be seen in Fig5, at 2 weeks approximately 59% of the total CD34+/c-kit+/CD13+ progeny in culture were tryptase-positive, whereas approximately 2% of cells derived from CD34+/c-kit+/CD13−cells in culture were tryptase-positive. This data is consistent with previous observations that tryptase expression is observed during mast cell differentiation.1 2 Eight-week-old mast cells were further characterized by flow cytometry as having tryptase without chymase (MCT), or both enzymes (MCTC). In these cultures, 85% of the mast cells were found to exhibit both enzymes (MCTC); 15% were only positive for tryptase (MCT).
FACS analysis of intracytoplasmic tryptase expression by 2-week-old c-kit+/CD13+ (A) and c-kit+/CD13− (B) progeny. Right panels show tryptase expression. Isotype controls are shown in left panels. Results shown are representative of 4 experiments separately performed, using either BM (n = 2) or PB (n = 2) cells.
FACS analysis of intracytoplasmic tryptase expression by 2-week-old c-kit+/CD13+ (A) and c-kit+/CD13− (B) progeny. Right panels show tryptase expression. Isotype controls are shown in left panels. Results shown are representative of 4 experiments separately performed, using either BM (n = 2) or PB (n = 2) cells.
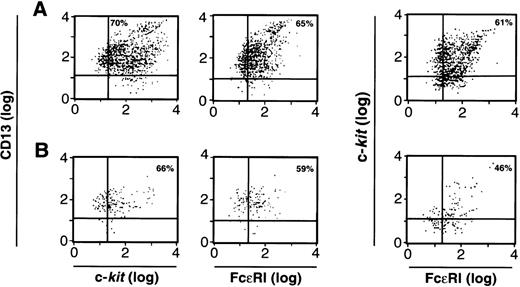
We next examined the cells cultured from CD34+/c-kit+/CD13+(Fig 6A) and the CD34+/c-kit+/CD13−(Fig 6B) cell subpopulations at 2 weeks for the expression of FcεRI, c-kit, and CD13. As can be seen, greater than 60% of cell progeny in the CD34+/c-kit+/CD13+ sort, and greater than 40% of the cell progeny in the CD34+/c-kit+/CD13−sort simultaneously expressed FcεRI, c-kit, and CD13. Because there are substantially more cells in Fig 6A, the majority of mast cells were thus cultured from CD34+/c-kit+/CD13+ cells and, as expected, expressed FcεRI and c-kit and remained CD13+. FcεRI+ progeny derived from CD34+/c-kit+/CD13+ cells at 2 weeks also behaved as mast cells rather than basophils when purified and recultured. When immunomagnetically purified (>97%) FcεRI+ cells at 2 weeks were recultured in either rhIL-3 alone or rhIL-6 and rhSCF for an additional 4 weeks, only those FcεRI+ cells cultured in rhIL-6 plus rhSCF survived, as would be predicted for mast cells. No basophils were observed.
FACS analysis of 2-week-old c-kit+/CD13+ (A) and c-kit+/CD13− (B) progeny for CD13, c-kit, and FcɛRI expression; or c-kit and FcɛRI expression on the cell population gated by FSC and SSC to identify mast cells. Results shown are representative of 4 experiments separately performed, using either BM (n = 2) or PB (n = 2) cells.
FACS analysis of 2-week-old c-kit+/CD13+ (A) and c-kit+/CD13− (B) progeny for CD13, c-kit, and FcɛRI expression; or c-kit and FcɛRI expression on the cell population gated by FSC and SSC to identify mast cells. Results shown are representative of 4 experiments separately performed, using either BM (n = 2) or PB (n = 2) cells.
Histamine was not detected in CD34+/c-kit+/CD13+ cells on day 0, but was approximately 2 to 3 pg/cell at 4 weeks of cell culture. Cells cultured from CD34+/c-kit+/CD13+ cells were functionally competent, as shown by the capacity to release histamine. Using 4-week-old mast cells (Fig 4B), histamine release at 30 minutes after addition of 1 μmol/L A23187 averaged 90%. Histamine release at 30 minutes from mast cells sensitized with IgEPSand incubated with goat antihuman IgE averaged 23%.
The CD34+/c-kit+/CD13+–sorted cells, which in culture gave rise to mast cells, initially expressed HLA-DR (80%), CD33 (90%), and CD38 (98%). By 2 to 3 weeks in culture, the c-kit+/CD13+/FcεRI+mast cells observed within the progeny of the CD34+/c-kit+/CD13+ cells no longer expressed CD34 and HLA-DR; none of the mast cell progeny of CD34+/c-kit+/CD13+ cells expressed CD3, CD11b, CD15, CD16, CD19, CD36, or erythrocyte markers at 2, 4, and 8 weeks. Monocytes expressed CD14 and CD15, but were negative for detectable CD11b, FcεRI and c-kit. Monocytes present in cultures also stained positive for lysozyme.
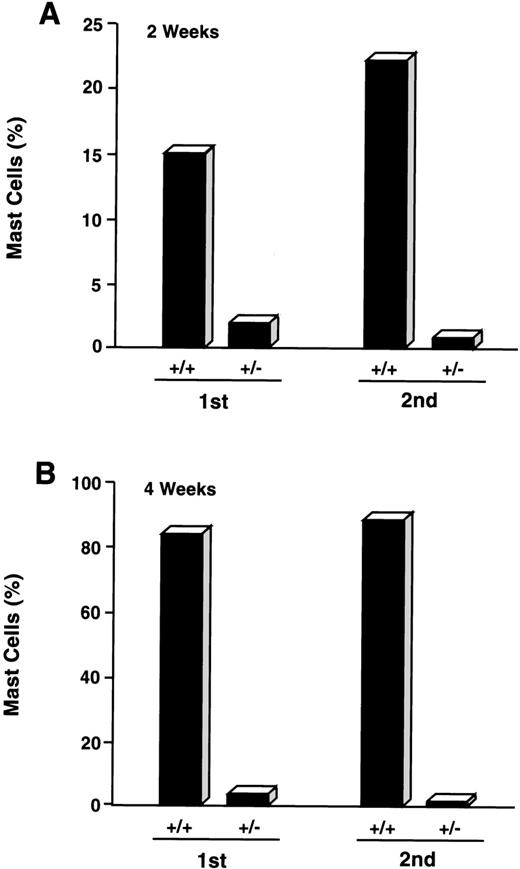
CD34+/c-kit+/CD13−cultures were consistently noted to give rise to small percentages (≤5%) of mast cells by 2 to 4 weeks. Because cell selection by fluorescent cell sorting has technical limitations and therefore did not allow for 100% pure CD34+/c-kit+/CD13−cell sorting, we next determined if mast cells arising in CD34+/c-kit+/CD13− cell cultures might be derived from CD34+/c-kit+/CD13+ cells that contaminated these sorts. CD34+/c-kit+/CD13+ cells were therefore first sorted into CD34+/c-kit+/CD13+ and CD34+/c-kit+/CD13−cell populations. CD34+/c-kit+/CD13−cells then underwent a second sort for CD13, yielding double-sorted CD34+/c-kit+/CD13+– and CD34+/c-kit+/CD13−–sorted cells. These double-sorted cells were also then cultured. As shown in Fig 7, the majority of mast cells observed at 2 weeks (15%) and 4 weeks (85%) were again noted in cell cultures derived from CD34+/c-kit+/CD13+ (+/+) sorts. Mast cells (≤5%) observed in CD34+/c-kit+/CD13−(+/−) cultures were reduced by greater than 50% after sequential sorting, confirming that mast cells within the first +/− sort originated from CD34+/c-kit+/CD13+progenitor cells.
Effect of sequential sorting of CD34+/c-kit+/CD13−progenitor cells on the appearance of mast cells at 2 (A) and 4 (B) weeks of culture. Equal numbers (1.5 × 105 cells) of c-kit+/CD13+ (+/+) and c-kit+/CD13− (+/−) cells from the first sort were placed in culture (1st). A portion of CD34+/c-kit+/CD13−cells underwent a second sort (2nd) into c-kit+/CD13+ (+/+) and c-kit+/CD13− (+/−) cells that were also placed in culture. Equal numbers (1.5 × 105cells) were again plated. Results are presented as percentage of mast cells and are representative of 2 experiments separately performed, using PB cells.
Effect of sequential sorting of CD34+/c-kit+/CD13−progenitor cells on the appearance of mast cells at 2 (A) and 4 (B) weeks of culture. Equal numbers (1.5 × 105 cells) of c-kit+/CD13+ (+/+) and c-kit+/CD13− (+/−) cells from the first sort were placed in culture (1st). A portion of CD34+/c-kit+/CD13−cells underwent a second sort (2nd) into c-kit+/CD13+ (+/+) and c-kit+/CD13− (+/−) cells that were also placed in culture. Equal numbers (1.5 × 105cells) were again plated. Results are presented as percentage of mast cells and are representative of 2 experiments separately performed, using PB cells.
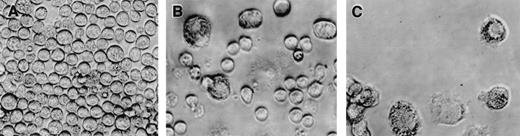
The CD34+/c-kit+/CD13+progenitor cell population thus gives rise to the mast cells that develop in culture. This CD34+/c-kit+/CD13+progenitor cell population also gives rise to monocytes. To determine whether mast cells and monocytes were derived from the same or different progenitors, single cell clonal assays were performed and evaluated at 2 weeks. As shown in Table 1, single CD34+/c-kit+/CD13+cells seeded in wells using 3 different cytokine mixtures produced similar results and gave rise to either pure mast cells (11-18 clones/480 seeded wells), pure monocytes (2-6 clones/480 seeded wells), or mixtures of mast cells and monocytes (10-16 clones/480 seeded wells). No basophils or other cell phenotypes were seen. Mast cells tended to be rounded (Fig 8A and B), whereas monocytes adhered to the well bottom (Fig 8B and C).
Effects of Cytokines on Clonal Expansion of CD34+/c-kit+/CD13+Cells at 2 Weeks
| . | MC . | MC/Monocyte . | Monocyte . | Other . |
|---|---|---|---|---|
| IL-3, SCF, IL-6 | 11/480 | 10/480 | 2/480 | 0/480 |
| IL-3, SCF, IL-6, TPO, Epo | 18/480 | 16/480 | 2/480 | 0/480 |
| IL-3, SCF, IL-6, Epo, GM-CSF, IGF-1, bFGF | 16/480 | 12/480 | 6/480 | 0/480 |
| . | MC . | MC/Monocyte . | Monocyte . | Other . |
|---|---|---|---|---|
| IL-3, SCF, IL-6 | 11/480 | 10/480 | 2/480 | 0/480 |
| IL-3, SCF, IL-6, TPO, Epo | 18/480 | 16/480 | 2/480 | 0/480 |
| IL-3, SCF, IL-6, Epo, GM-CSF, IGF-1, bFGF | 16/480 | 12/480 | 6/480 | 0/480 |
Cells were individually deposited per well in 96-well plates containing serum-free media with designated cytokines. Incubations were performed for 14 days at 37°C in a humidified atmosphere of 5% CO2/95% air. Each well was scored positive for proliferation when ≥5 cells were identified. Data represent the number of positive wells/total number of wells seeded.
Abbreviations: MC, mast cells; Other, includes erythrocytes, myeloid cells, lymphocytes, and megakaryocytes.
Phenotypic appearance of single cell clonal assays at 2 weeks by inverted scope. (A) Mast cell colony. (B) Mixed mast cell/monocyte colony. (C) Monocyte colony.
Phenotypic appearance of single cell clonal assays at 2 weeks by inverted scope. (A) Mast cell colony. (B) Mixed mast cell/monocyte colony. (C) Monocyte colony.
Mast cells and monocytes were again the only cell types present in liquid suspension cultures examined later than 2 weeks. In 4-week liquid suspension cultures containing only rhSCF and rhIL-6 (single-sorted CD34+/c-kit+/CD13+ cells; Table 2), mast cells constituted 1.50 of 1.82 or 82.4% of the cells in culture, and monocytes constituted 0.32 of 1.82 or 17.6%. Similarly, the double-sorted CD34+/c-kit+/CD13+ cells gave rise again only to mast cells at 4 weeks (1.00 of 1.21 or 83.3%) and monocytes (0.21 of 1.21 or 17.4%). Thus, the CD34+/c-kit+/CD13+progenitor cell population that gives rise to mast cells also gives rise to a population of monocytes.
Growth of Mast Cells From Single- and Double-Sorted CD34+/c-kit+/CD13+(+/+) and CD34+/c-kit+/CD13−(+/−) Cells
| First Sort | First Sort | |||||||
| +/+ | +/− | |||||||
| Week | Tot | MC | M | O | Tot | MC | M | O |
| ×106 | ×106 | |||||||
| 4 | 1.82 | 1.50 | 0.32 | 0 | 5.00 | 0.25 | 4.65 | 0.10 |
| Second Sort | Second Sort | |||||||
| +/+ | +/− | |||||||
| Week | Tot | MC | M | O | Tot | MC | M | O |
| ×106 | ×106 | |||||||
| 4 | 1.21 | 1.00 | 0.21 | 0 | 3.80 | 0.07 | 3.61 | 0.12 |
| First Sort | First Sort | |||||||
| +/+ | +/− | |||||||
| Week | Tot | MC | M | O | Tot | MC | M | O |
| ×106 | ×106 | |||||||
| 4 | 1.82 | 1.50 | 0.32 | 0 | 5.00 | 0.25 | 4.65 | 0.10 |
| Second Sort | Second Sort | |||||||
| +/+ | +/− | |||||||
| Week | Tot | MC | M | O | Tot | MC | M | O |
| ×106 | ×106 | |||||||
| 4 | 1.21 | 1.00 | 0.21 | 0 | 3.80 | 0.07 | 3.61 | 0.12 |
Flasks were initially seeded with 1.5 × 105 cells (50,000 cells/mL). CD34+/c-kit+/CD13−cells recovered after first sort were resorted into CD34+/c-kit+/CD13+(+/+) and CD34+/c-kit+/CD13−(+/−) populations and cultured.
Abbreviations: Tot, total cells; MC, mast cells; M, monocytes; O, other cells.
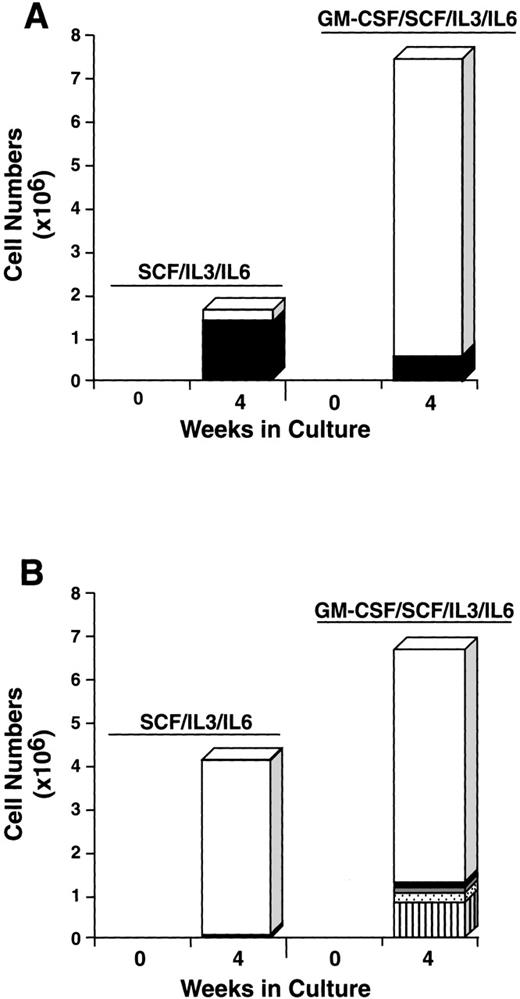
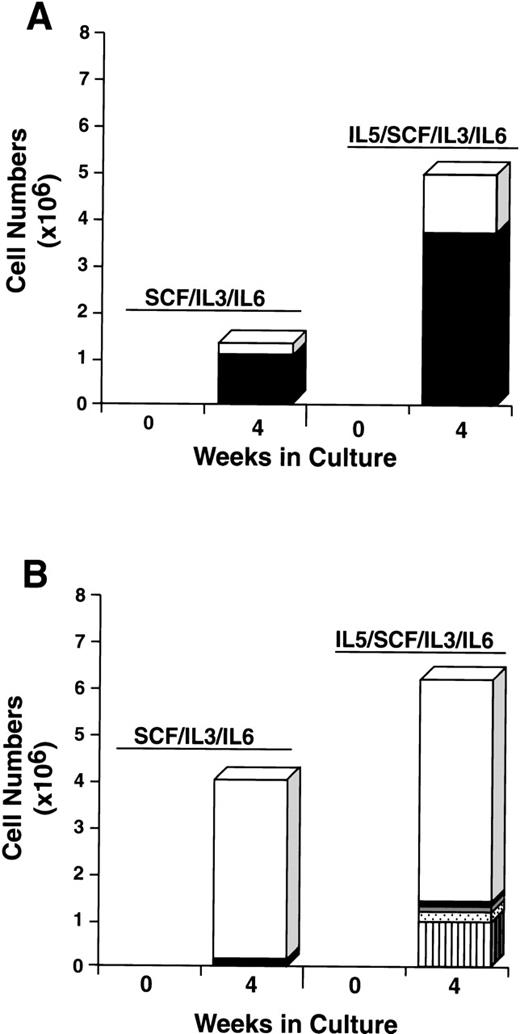
To further explore the characteristics of the starting CD34+/c-kit+/CD13+ and CD34+/c-kit+/CD13−cell subpopulations, we next cultured these cells for 4 weeks in the usual conditions of rhSCF, rhIL-3 (first week only), and rhIL-6, but with and without rhGM-CSF or rhIL-5. The CD34+/c-kit+/CD13+ cells cultured in rhSCF, rhIL-3, and rhIL-6 at 4 weeks consisted of 80% mast cells and 20% monocytes (Fig 9A), in agreement with previous data. In contrast, CD34+/c-kit+/CD13−cells cultured under these conditions consisted largely of monocytes. The addition of rhGM-CSF to the culture of CD34+/c-kit+/CD13+ cells increased the overall cell number, but resulted in fewer mast cells being observed, the remainder being monocytes (Fig 9A). This inhibition of mast cell growth by GM-CSF has been reported in murine27and human28 systems. CD34+/c-kit+/CD13−cells cultured with the addition of rhGM-CSF gave rise to a variety of cell types in addition to monocytes, including basophils, eosinophils, and neutrophils (Fig 9B). Mast cells consisted of <5% of cells.
Effect of rhGM-CSF on the differentiation of CD34+/c-kit+/CD13+(A) and CD34+/c-kit+/CD13−(B) cells cultured in rhSCF/rhIL-3/rhIL-6 over 4 weeks. Results shown are the average of 3 experiments separately performed, using PB cells. (□), Monocytes; (▪), mast cells; ( ), neutrophils; (), basophils; (▥), eosinophils.
), neutrophils; (), basophils; (▥), eosinophils.
Effect of rhGM-CSF on the differentiation of CD34+/c-kit+/CD13+(A) and CD34+/c-kit+/CD13−(B) cells cultured in rhSCF/rhIL-3/rhIL-6 over 4 weeks. Results shown are the average of 3 experiments separately performed, using PB cells. (□), Monocytes; (▪), mast cells; ( ), neutrophils; (), basophils; (▥), eosinophils.
), neutrophils; (), basophils; (▥), eosinophils.
The addition of rhIL-5 to cultures of CD34+/c-kit+/CD13+ cells increased both the total number of cells and the mast cell number approximately 2-fold to 4-fold. Approximately 70% of cells cultured were mast cell and 30% were monocytes under both conditions (Fig 10A). The addition of rhIL-5 to CD34+/c-kit+/CD13−cells, as expected, increased the eosinophil number (Fig 10B). Mast cells consisted of <5% of cells under both conditions. Thus, the single-sorted CD34+/c-kit+/CD13+ cell subpopulation again gave rise to greater than 95% of mast cells cultured, and this result was not altered by the addition of rhIL-5 or rhGM-CSF. In addition, the data clearly show that rhIL-5 increases the mast cell number in rhSCF/rhIL-3/rhIL-6–dependent cell cultures.
Effect of rhIL-5 on the differentiation of CD34+/c-kit+/CD13+(A) and CD34+/c-kit+/CD13−(B) cells cultured in rhSCF/rhIL-3/rhIL-6 over 4 weeks. Results shown are the average of 3 experiments separately performed, using PB cells. (□), Monocytes; (▪), mast cells; ( ), neutrophils; (), basophils; (▥), eosinophils.
), neutrophils; (), basophils; (▥), eosinophils.
Effect of rhIL-5 on the differentiation of CD34+/c-kit+/CD13+(A) and CD34+/c-kit+/CD13−(B) cells cultured in rhSCF/rhIL-3/rhIL-6 over 4 weeks. Results shown are the average of 3 experiments separately performed, using PB cells. (□), Monocytes; (▪), mast cells; ( ), neutrophils; (), basophils; (▥), eosinophils.
), neutrophils; (), basophils; (▥), eosinophils.
DISCUSSION
A progenitor cell population, capable of giving rise only to mast cells and monocytes, has not been described. Such progenitor cells were identified and sorted using FACS analysis for CD34, c-kit, and CD13 surface antigen expression (Fig 1B), in addition to size (FSC) and density (SSC) (Fig 1A). Cell-sorting parameters used to isolate c-kit+/CD13+ progenitors from other c-kit−– or CD13−–committed progenitors were relatively easy to establish once the larger, more dense BM and PB mast cell progenitor cells with the CD34+/c-kit+/CD13+ surface antigen phenotype were identified (Fig 2B). The most marked cell expansion (60-fold) was seen from the CD34+/c-kit+/CD13−subpopulation (Fig 3A), in which mast cells constituted <3% of the cells cultured (Fig 3B). In contrast, CD34+/c-kit+/CD13+cells expanded 12-fold, but were 85% mast cells by 4 weeks (Figs 3B and 4). The remainder of the cells were monocytes (Table 2). Some mast cells had an indented or segmented nucleus (Fig 4B). Similar cells have been observed in human marrow,29 and rodent mast cells have exhibited lobed nuclei.30
In the present work, cultured human mast cells were shown to express FcεRI at 2 weeks, similar to FcεRI expression on cultured human basophils and rodent mast cells.31 Flow cytometric analysis of intracytoplasmic tryptase with 2-week-old c-kit+/CD13+/FcεRI+cell progeny identified a greater number of probable mast cells than could be identified by acidic toluidine blue staining and tryptase immunohistochemistry (Fig 5). Of interest, cultured mast cells at 8 weeks by flow cytometry were approximately 85% MCTC and 15% MCT, documenting that the CD34+/c-kit+/CD13+progenitor cell population gives rise to both MCT and MCTC. The discrepancy between the larger number of tryptase and chymase–positive (MCTC) mast cells observed with flow cytometry rather than with Carnoy’s-fixed cytopreparations and immunohistochemistry may be a result of the negative effect on chymase-positive mast cells by Carnoy’s fixative32 and the increased sensitivity of flow cytometry. BM and PB c-kit+/CD13+/FcεRI+mast cell progeny at 8 weeks, derived from sorted progenitors, also had phenotypic FACS profiles closely resembling mast cell cultures derived from unsorted CD34+ progenitors that were allowed to mature in culture over 8 weeks (data not shown).
We speculated that, because of the limitations of fluorescent cell sorting, mast cells noted at 4 weeks in the CD34+/c-kit+/CD13−cell cultures were a result of CD34+/c-kit+/CD13+ cells contaminating the starting cell population (Fig 3B). To prove this hypothesis, we performed sequential cell sorting of CD34+/c-kit+/CD13−cells before cell culture to remove additional CD34+/c-kit+/CD13+ (+/+) cells from the CD34+/c-kit+/CD13−(+/−) subpopulation. This reduced by greater than 50% the number of mast cells arising in CD13− sorts at 4 weeks (Fig7A and B and Table 2).
A close lineage relationship between mast cells and monocytes is suggested by clinical findings in mastocytosis. In this disease of mast cell neoplasia, monocytosis is frequently observed as the disease progresses,33 and chronic myelogenous leukemia is observed to arise in some patients.34,35 Thus, the observation that the CD34+/c-kit+/CD13+precursor cell population gives rise both to mast cells and monocytes (Tables 1 and 2; Figs 8, 9A, and 10A) under similar conditions is consistent with these clinical observations of a close lineage relationship between mast cells and monocytes. Note that basophil neoplasia, for instance, is not observed in mastocytosis.33 35
To verify the potential of CD34+/c-kit+/CD13+ cells and confirm the absence of other cell types at 2 weeks, single cell clonal assays were performed. In addition, both rhGM-CSF and rhIL-5 were added to some liquid suspension cultures to see if other cell lineages might be observed from these starting cells at 4 weeks. Single CD34+/c-kit+/CD13+ cells cultured for 2 weeks with combinations of rhIL-3, rhIL-6, rhSCF, rhTPO, rhEpo, rhGM-CSF, rhIGF-1, and rhbFGF gave rise only to pure mast cell, monocyte, or mixed mast cell/monocyte clones (Table 1). No erythroid precursors, myeloid cells, lymphocytes, or megakaryocytes were noted. In liquid suspension cultures, both rhGM-CSF and rhIL-5 increased the total number of cells cultured (Figs 9 and 10). As expected, rhGM-CSF inhibited mast cell outgrowth (Fig 9A), whereas monocytes increased in number. In contrast, rhIL-5 increased mast cell numbers, but again, few, if any, mast cells originated from CD34+/c-kit+/CD13−cells (Fig 10B). These observations using liquid suspension cultures would suggest that rhIL-5 may act as a growth and maturation and/or survival factor for mast cells, an observation especially relevant in inflammatory conditions known to be associated with increased IL-5 levels, such as in asthma. Taken together, the data confirms that the CD34+/c-kit+/CD13+ cell population gives rise to only mast cells and monocytes, even in the presence of rhIL-3, rhTPO, rhEpo, rhGM-CSF, rhIL-5, rhIGF-1, and rhbFGF. It should be noted that monocytes are also observed to grow from CD34+/c-kit+/CD13−cells, suggesting that an earlier cell population that is CD13− may be the ultimate monocyte precursor and that it is possible that the bipotential cell and monocyte precursor cell herein described arise from this earlier precursor. Most available data point to the expression of CD13 on immature rather than mature human mast cells. If CD13 is expressed during mast cell, monocyte, and myeloid cell differentiation in the bone marrow,18 when progenitors retain or lose their c-kit positivity, it is possible to speculate that the mast cell/monocyte progenitor would retain the c-kit+/CD13+ phenotype, whereas other myeloid (ie, basophil) and certain monocyte progenitor cells would lose c-kit expression but express CD13 as these cells differentiate from earlier c-kit+/CD13− cells.
Recently, it has been published that human mast cell progenitors are both CD34+ and CD38+, and often lack HLA-DR.36 When 1,200 single CD34+CD38+ cells were cultured in rhSCF, rhIL-6, and rhIL-3 for 7 days, followed by 3 weeks in rhIL-3 or 7 weeks in rhSCF and rhIL-6, they obtained 13 mast cell colonies, 1 colony with mast cells and another type, and 42 colonies of other cell types, including basophils, macrophages, and eosinophils. They also reported mast cells in CD34+,CD38−, and HLA-DR+sorts. The authors concluded that mast cells originate from progenitors different from myeloid progenitors. Although we agree with this conclusion, by using a sorting strategy based on 2 known mast cell progenitor markers (CD34, c-kit) plus CD13, we were able to obtain all mast cell precursors, with only one other cell type (monocyte) arising from this progenitor. Furthermore, no matter what growth factors were added, 40% to 50% of all colonies were pure mast colonies, and most of the other colonies were mixed mast cell/monocyte colonies. It should be noted that our CD34+/c-kit+/CD13+progenitor cells are HLA-DR+ and express CD38. Thus, the combination of CD34, c-kit, and CD13 more clearly defines the human mast cell progenitor population.
Taken together, these results show that the CD34+/c-kit+/CD13+ cell population contains the precursors for all human mast cells, and a subset of monocytes. Within the CD34+/c-kit+/CD13+ cell population are mast cell, monocyte, and mast cell/monocyte (bipotential) precursors. The proportion of mast cells to monocytes arising from CD34+/c-kit+/CD13+ cells depends on the growth factors added, but in no combination of cytokines added were any other cell types noted.
ACKNOWLEDGMENT
The authors thank Stefania Pittaluga, Hemopathology Section, National Cancer Institute (Bethesda, MD), for her excellent technical assistance, and Dr Calman Prussin, Laboratory of Allergic Diseases, NIAID (Bethesda, MD), for his advice.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Arnold S. Kirshenbaum, MD, NIH/NIAID/Laboratory of Allergic Diseases, Building 10, Room 11C208, 10 Center Dr MSC 1881, Bethesda, MD 20892-1881; e-mail:Akirshenba@atlas.niaid.nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal