Basophils (Ba) and mast cells (MC) are important effector cells of inflammatory reactions. Both cell types derive from CD34+hematopoietic progenitors. However, little is known about the cell subsets that become committed to and give rise to Ba and/or MC. We have generated a monoclonal antibody (MoAb), 97A6, that specifically detects human Ba, MC (lung, skin), and their CD34+ progenitors. Other mature hematopoietic cells (neutrophils, eosinophils, monocytes, lymphocytes, platelets) did not react with MoAb 97A6, and sorting of 97A6+ peripheral blood (PB) and bone marrow (BM) cells resulted in an almost pure population (>98%) of Ba. Approximately 1% of CD34+ BM and PB cells was found to be 97A6+. Culture of sorted CD34+97A6+ BM cells in semisolid medium containing phytohemagglutinin-stimulated leukocyte supernatant for 16 days (multilineage assay) resulted in the formation of pure Ba colonies (10 of 40), Ba-eosinophil colonies (7 of 40), Ba-macrophage colonies (3 of 40), and multilineage Ba-eosinophil-macrophage and/or neutrophil colonies (12 of 40). In contrast, no Ba could be cultured from CD34+97A6− cells. Liquid culture of CD34+ PB cells in the presence of 100 ng/mL interleukin (IL)-3 (Ba progenitor assay) resulted in an increase of 97A6+ cells, starting from 1% of day-0 cells to almost 70% (basophils) after day 7. Culture of sorted BM CD34+97A6+ cells in the presence of 100 ng/mL stem cell factor (SCF) for 35 days (mast cell progenitor assay) resulted in the growth of MC (>30% on day 35). Anti-IgE–induced IgE receptor cross-linking on Ba for 15 minutes resulted in a 4-fold to 5-fold upregulation of 97A6 antigen expression. These data show that the 97A6-reactive antigen plays a role in basophil activation and is expressed on multipotent CD34+ progenitors, MC progenitors, Ba progenitors, as well as on mature Ba and tissue MC. The lineage-specificity of MoAb 97A6 suggests that this novel marker may be a useful tool to isolate and analyze Ba/MC and their progenitors.
BASOPHILS and mast cells are multifunctional hematopoietic effector cells involved in allergic and inflammatory reactions.1-4 These cells produce various biologically active mediators which, after cell stimulation, can be released into the extracellular space.4-7 Despite being of similar morphology and similar biochemical and functional properties, basophils and mast cells represent distinct cell lineages within the hematopoietic system. In fact, basophils differ from mast cells in their antigenic properties, their response to diverse agonists, and their mediator content.8-10 With regard to cytokine receptors, mature basophils express significant amounts of interleukin (IL)-3Rα (CD123), but not c-kit (CD117), whereas tissue mast cells express high levels of CD117, but not CD123.8,11-13 In contrast with eosinophils, basophils and mast cells express huge amounts of the high affinity IgE receptor FcεRI.14 In addition, basophils can be distinguished from eosinophils and mast cells by their unique expression of dipeptidylpeptidase IV (CD26) and lactosylceramide (CDw17).14
Basophils and mast cells originate from CD34+ progenitor cells.15-18 An important differentiation factor for human basophils (and eosinophils) appears to be IL-3.19 In particular, when normal bone marrow–derived progenitor cells are cultured in the presence of IL-3 for 14 or 16 days, a substantial number of cultured cells are basophils and eosinophils.18-20 Other cytokines, like granulocyte-macrophage colony-stimulating factor (GM-CSF) or IL-5 can also promote basophil and eosinophil differentiation.21Interestingly, neither IL-3, IL-5, or GM-CSF induce the differentiation of human mast cells in vitro.15-19 However, the differentiation of human mast cells is inducible by stem cell factor (SCF), the ligand of the c-kit gene product.16,18,22-25 In addition, several codifferentiation factors (IL-6, IL-4) reportedly influence the SCF-induced in vitro differentiation of human mast cells.18 26
Although it is generally accepted that basophils, eosinophils, and mast cells arise from CD34+ cells, little is known about the phenotype and receptors that define the subsets of their precursor cells. The distribution of cytokine receptors suggests that c-kit is expressed early in mast cell differentiation together with other cytokine receptors.12,25 However, the other cytokine receptors are lost during progenitor cell maturation, whereas c-kit expression is maintained.12 In the case of basophils, c-kit expression apparently decreases during maturation, whereas the receptor for IL-3 is expressed throughout the maturation process.27
So far, only a few markers that show specificity for either human basophils or mast cells have been generated.28-33 One of these, Bsp-1, was found to react with basophils, but not with tissue mast cells.28,29 Another marker, 2D7, specifically recognizes a cytoplasmic antigen of basophils, but does not react with eosinophils, neutrophils, or mast cells.30 The antibody YB5B8 was initially described as a mast cell–specific marker.31 In fact, this antibody did not react with other mature myeloid cells. Later, it was found that the YB5B8-reactive antigen is the human equivalent of c-kit and is expressed on mast cells as well as on hematopoietic progenitors.32 33
In this report, we describe a monoclonal antibody, 97A6, which specifically recognizes mature basophils and mast cells, but no other mature hematopoietic and nonhematopoietic cell types. Moreover, we provide evidence that antibody 97A6 also reacts with CD34+basophil and mast cell progenitor cells and that the detected antigen plays a role in basophil activation.
MATERIALS AND METHODS
Immunization and Hybridoma Production
Antibody 97A6 was raised by immunization of a 4- to 8-week-old female Balb/c mouse with the erythro-megakaryoblastic cell line UT-7,34 which was grown in RPMI 1640 medium supplemented with 10% fetal calf serum in the presence of 10 μg/mL IL-3 (Behring Werke, Marburg, Germany). The mice were injected 5 times intraperitoneally with 107 cells in 2-week intervals, and 10 days after the last injection, an intrasplenic boost of 2 × 105 cells was applied. The spleens were removed 4 days later for fusion with the SP2/0 myeloma cell line. The resulting hybridomas were grown in RPMI 1640 (GIBCO-BRL, Eggenstein, Germany) containing 10% fetal calf serum and hypoxanthine-aminopterine-thymidine (HAT; Sigma Chemicals, München, Germany). Culture supernatants that were positive on UT-7 cells were screened on peripheral blood (PB) and bone marrow (BM) cells. Hybridoma cells secreting antibodies specific for only a small subset of mononuclear PB and/or BM cells were selected and cloned twice by limiting dilution. Clone 97A6 fulfilled these criteria and was selected. The IgG1 isotype of the resulting monoclonal antibody (MoAb) 97A6 was determined by enzyme-linked immunosorbent assay (ELISA) (Boehringer Mannheim, Mannheim, Germany). For biotinylation, MoAb 97A6 was purified on a Protein-G Sepharose column (Pharmacia Biotech, Freiburg, Germany), dialyzed against 0.1 mol/L sodium borate buffer, pH 9.3, concentrated to 1 mg/mL by ultrafiltration (Ultrafree PF-filter, 10.000 NMWL; Millipore, Eschborn, Germany), and incubated for 2.5 hours at room temperature with a freshly prepared solution of 1 mg/mL ε-amino caproic acid N-hydroxyl succinimide biotin (Biotin-X-NHS; Calbiochem, Heidelberg, Germany) at a molar ratio of 30:1.35 The reaction was stopped by thorough dialysis against Hanks’ balanced salt solution (HBSS). Conjugation of MoAb 97A6 with R-Phycoerythrin (PE) via a hetero-bireactive cross-linker was performed according to the method described by Oi et al.36
Isolation of Primary Hematopoietic Cells
BM samples from healthy donors (n = 5) and PB cells from patients with chronic myeloid leukemia (CML; n = 15) were obtained after informed consent was given and were separated on a Ficoll-Hypaque density gradient (1.077 g/cm3) by collecting the interphase cells. Eight of fifteen patients suffered from CML in chronic phase (0.5% to 3% blasts, 0.5% to 1.5% basophils), 2 of 15 patients had CML in accelerated phase (15% to 20% blasts, 7% to 8% basophils), and 5 of 15 patients suffered from CML in myeloid blast crisis (98% to 99% blasts, basophils not counted). Buffy coat PB cells from normal volunteers (n = 3) were obtained from the Blood Transfusion Department (Institute for Transfusion Medicine, Tübingen, Germany) according to institutional guidelines. Before analysis, mononuclear cells were Ficoll-separated. For additional analysis of neutrophils and eosinophils, buffy coat PB cells were treated with ammonium chloride to remove erythrocytes.
Isolation of Primary Tissue Mast Cells
Lung mast cells were enriched from surgical specimens of 3 patients with bronchiogenic carcinoma (lobectomy). Informed consent was obtained before surgery. Foreskin was obtained from 3 children undergoing circumcision. Informed consent was obtained from parents in each case. Uterine tissue was obtained from 3 patients with uterine myomata after informed consent was given. Tissue mast cells were isolated following published techniques.37-40 In brief, tissue specimens were cut into small pieces and washed in Tyrode’s buffer (200 mg/L KCl, 50 mg/L NaH2PO4.H2O, 8 g/L NaCl, 1 g/L glucose; Mg/Ca-free). Tissue fragments were then incubated in 2 mg/mL collagenase type II (Sebak, Suben, Austria) for 90 to 180 minutes at 37°C. After digestion, dispersed cells were recovered, washed, and examined for the presence of mast cells by toluidine blue (Sigma, München, Germany) staining. Isolated cells were cultured in RPMI 1640 plus 10% fetal bovine serum (FBS) at 37°C for at least 12 hours before experiments were conducted.
Cell Lines
The human mast cell line HMC-1, established from a patient suffering from mast cell leukemia,41 was kindly provided by JH Butterfield, Mayo Clinic, Rochester, MN. HMC-1 cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% FBS, glutamine, and antibiotics at 37°C and 5% CO2. The basophil precursor cell line KU-812, raised from a patient suffering from CML,42 was a kind gift from K Kishi, Niigata University, Niigata, Japan. The eosinophilic cell line EOL-1, established from a patient suffering from acute eosinophilic leukemia,43 was purchased from the German Collection of Microorganisms and Cell Cultures (DSM; Braunschweig, Germany). KU-812 and EOL-1 cells were cultured in RPMI 1640 medium with 10% FBS, glutamine, and antibiotics (37°C, 5% CO2). The erythro-megakaryoblastic cell line UT-734 was a kind gift from Dr Komatsu (Togichi, Japan), and was grown in RPMI 1640, supplemented with 10% FBS and 10 ng/mL recombinant human (rh) IL-3 (Behring Werke). In addition, the following cell lines were used for screening with MoAb 97A6: the tumor cell lines DU.4475, MCF-7, and T-47D (breast carcinoma); NCI-H128 and NCI-69 (small cell lung carcinoma); SK-MES, SK-LU1, Calu 1, Calu 3, and Calu 6 (lung carcinoma); HELA (cervix carcinoma); 5637 (urinary bladder carcinoma); A431 (epidermal carcinoma); IMR-32 (neuroblastoma); TE-671 (medulloblastoma); A172 and U138 (glioblastoma); and the leukemic cell lines K562 and HEL (erythroleukemia); KG-1, KG-1a, HL-60, and U937 (myeloid leukemia); HSB-2, CCRF-CEM, and Molt-4 (T-lymphoid leukemia); Daudi (Epstein-Barr virus [EBV]-transformed B-cell line); and Reh (B-lymphoid leukemia) were obtained from the American Type Culture Collection (ATCC, Rockville, MD). The cell lines MO7-e (megakaryoblastic leukemia); Nalm-1, BV-173, 207, 380, and 697 (B-lymphoid leukemia); CML-T1 (T-lymphoid leukemia); EM-2 (myeloid leukemia); and U-266 (myeloma) were purchased from the German Collection of Microorganisms and Cell Cultures. The following additional cell lines were kind gifts: MEG-O1 (megakaryoblastic leukemia) from Dr H. Saito (Nagoia, Japan),44 TF-1 (erythroleukemic leukemia) from Dr T. Kitamura (Tokyo, Japan),45 MOLM-1 (megakaryoblastic leukemia) from Dr J. Minowada (Okayama, Japan),46 and OCI/AML-4 (myeloid leukemia) from Dr E.A. McCulloch (Toronto, Canada).47
Purification of PB and BM CD34+ Cells
Selection of normal donor BM CD34+ cells (n = 5) from 5-10 × 107 or normal donor PB CD34+ cells (n = 3) from 1 × 109 Ficoll-Hypaque–isolated cells was performed on a magnetic activated cell sorting (MACS) column according to the instructions of the manufacturer (Miltenyi, Bergisch Gladbach, Germany). Purity of selected CD34+ cells was 96% to 99%, and the recovery was greater than 65%.
Selection of Peripheral Blood Basophils
Ficoll-Hypaque–selected buffy coat PB cells from healthy donors (n = 3) or Ficoll-Hypaque–separated cells from patients in chronic or accelerated phase of CML (n = 2) were enriched for basophils on a magnet using the StemSep basophil enrichment antibody cocktail (CellSystems, Remagen, Germany). Negative selection of basophils was achieved by magnetic depletion of undesired cells and was performed according to the manufacturer’s guidelines (CellSystems). The purity of the selected basophils was determined by morphology. The values ranged between 89% to 98%. Alternatively, PB cells (n = 3) were labeled with MoAb 97A6-biotin, washed 3 times, and magnetically stained with anti-IgG-MACS beads (Miltenyi). The selection by MACS was conducted as described by the manufacturer. To monitor positivity for MoAb 97A6, cells were stained with streptavidin-phycoerythrin (SA-PE) plus MoAb 97A6-PE and analyzed on a FACSCalibur (Becton Dickinson, Heidelberg, Germany) flow cytometer. Approximately 99% (99% ± 0.8%) of the selected cells were 97A6+ basophils, as determined by flow cytometry and morphology.
Basophil Progenitor Cell Assays
Liquid suspension culture.
5 × 105 CD34+ PB cells (n = 3) were incubated at 37°C in serum-free IMDM medium48supplemented with 1% bovine serum albumin (BSA), 700 μg/mL holo-transferrin, 40 μg/mL human low density proteins, and 10 μg/mL insulin (Sigma, München, Germany). For the generation of basophils, cells were stimulated in the presence of 100 ng/mL IL-3 (Behring Werke) for 14 days. Half-medium changes were performed twice weekly.
Colony assay (multilineage assay).
Duplicate cultures containing either 103 MACS-selected CD34+ BM cells, 103 fluorescence-activated cell sorting (FACS)–sorted CD34+97A6+cells, or 103 CD34+97A6−cells (n = 2) were plated in 1-mL dishes (Greiner, Nürtingen, Germany). The cells were added to a ready-made medium containing 0.9% methylcellulose and a combination of growth factors (3 U/mL rh erythropoietin and 5% phytohemagglutinin-stimulated human leukocyte–conditioned medium as a source of IL-3 and other cytokines; CellSystems). After 16 days of incubation at 37°C in a humidified atmosphere (5% CO2), the colonies were scored. Cells derived from colonies of the CD34+97A6+cultures were picked with an elongated Pasteur pipette, transferred onto slides, and air-dispersed. For morphological analysis, cells were stained with May-Grünwald-Giemsa solution.
Mast Cell Progenitor Assay
Duplicate cultures of 103 MACS-selected CD34+, 103 FACS-sorted CD34+97A6+, and 103 CD34+97A6− BM cells were incubated in 24-well plates (Greiner) in serum-free IMDM medium48 supplemented with 1% BSA, 700 μg/mL holo-transferrin, 40 μg/mL human low density proteins, and 10 μg/mL insulin, 100 ng/mL SCF (PreproTech, Frankfurt, Germany), and 10 ng/mL IL-6 (PreproTech), for 35 days at 37°C in a humidified atmosphere (5% CO2). After 14 and 28 days of culture, the medium was replaced by serum-free IMDM medium supplemented with 100 ng/mL SCF. After 35 days of culture, the resulting cells were analyzed for morphology and tryptase content. For morphological analysis, cytocentrifuge preparations were stained with May-Grünwald-Giemsa. Tryptase content was determined by a fluoro-immuno-enzyme (FIA; Pharmacia, Uppsala, Sweden) assay as described.49,50 The detection limit of this assay was found to be 1 ng/mL; no cross-reactivity with histamine, heparin, or other known growth factors was found.49 50
Histamine Release Experiments
Histamine release experiments were performed as described51using primary basophils (3 healthy donors) and primary tissue mast cells (lung, foreskin, and uterus, each 3 donors). Cell suspensions were adjusted to final concentrations of 0.5 to 1.5 × 106/mL. Basophils (immediately after isolation) and mast cells (after at least 12 hours in culture) were incubated with varying concentrations (0.01, 0.1, 1.0, and 10 μg/mL) of MoAb 97A6 or anti-IgE MoAb E-124-2-8/Dε2 (Immunotech, Marseille, France) in histamine release buffer (HRB, Immunotech; 20 mmol/L 1,4-piperazinediethanesulfonic acid [PIPES], 1 g/L glucose, 1 mmol/L CaCl2) or with buffer (HRB) alone for 45 minutes at 37°C. Mast cells were incubated with myeloma IgE (derived from the cell line U266) for 3 hours at 4°C before the challenge with anti-IgE. After incubation with agonists, cells were centrifuged at 4°C, and cell-free supernatants were recovered by aspiration. Liberated histamine in supernatants was measured by radioimmunoassay (Immunotech). Total histamine in cell suspensions was quantified after cell lysis in distilled water and freeze thawing. Histamine release was expressed as percentage of total histamine.
Immunofluorescence Analysis
Indirect staining of cell lines.
Cells from growing cell lines were washed in FACS buffer (Hanks supplemented with 1% BSA and 0.01% NaN3) before incubation with 20% human AB serum for 10 minutes at 4°C to prevent unspecific binding of mouse antibodies. Cells were then incubated with 10 μg/mL of the primary antibody (MoAb 97A6) for 30 minutes on ice. After washing 3 times, cells were stained with the F(ab)′2 fragment of a fluorescein isothiocyanate (FITC)–conjugated goat-antimouse antiserum (Dunn, Asbach, Germany) for 30 minutes at 4°C. After washing, cells were suspended in FACS buffer and analyzed on a FACSCalibur flow cytometer. In selected experiments, 97A6 antigen expression was analyzed on HMC-1 and KU-812 cells incubated for 4 or 12 hours with the following cytokines before staining with MoAb 97A6 (100 ng/mL): recombinant human IL-2, IL-4, IL-5, IL-7 (Genzyme, Cambridge, MA); IL-9, IL-10, IL-13, SCF (PreproTech, Rocky Hill, NJ); IL-3, IL-8 (Sandoz, Vienna, Austria); and IL-1α, IL-1β, IL-6, IL-11, IL-12, IL-15, IL-16, IL-17, and IL-18 (R&D, Minneapolis, MN).
Two-color staining of BM and PB cells.
Unseparated BM and PB cells or MACS-selected PB CD34+ cells cultured for 14 days in the presence of IL-3 and collected after defined time intervals were labeled with 97A6-PE and FITC-conjugated antibodies against CD10 (W8E7), CD26 (L272), CD34 (8G12), CD44 (L178), CD61 (RUU-PL7F12), CD71 (L01.1), HLA-DR (L243) (Becton Dickinson), CD13 (SJ1D1), CD16 (3G8), CD33 (D3HL60.251), CD38 (T16), CD41 (P2) (Immunotech, Krefeld, Germany), CD117 (9B9) (Hölzel Diagnostics, Cologne, Germany), IgE (polyclonal) (BioSource, Ratingen, Germany), CD55 (1A10) and CD59 (p282(H19)) (PharMingen, Hamburg, Germany) for 30 minutes on ice and washed twice. Alternatively, BM cells were labeled with anti-CD123-PE (7G3; PharMingen) and MoAb 97A6-biotin, washed, and stained with streptavidin-FITC (SA-FITC; DAKO, Krefeld, Germany) for 15 minutes on ice. In other approaches, BM cells were labeled with 97A6-PE (IgG1) and the CD164-specific antibody 103B2/9E10 (IgG3),52 53 the CD81-specific antibody JS-64 (IgG2a; Immunotech), or the CDw17-specific antibody MEM74 (IgM; BioSource), and stained with the F(ab)′2 fragments of FITC-conjugated isotype-specific goat antimouse antisera (Medac, Hamburg, Germany). After washing twice, cells were analyzed on a flow cytometer.
Three-color staining.
Unseparated BM and PB cells were stained with anti-CD34-PerCP (HPCA-2-PerCP; Becton Dickinson), 97A6-PE, and FITC-conjugated antibodies or nonconjugated antibodies stained with anti–isotype-specific secondary antibodies as described for 2-color staining of BM and PB cells. To determine FcεR1 coexpression, cells were incubated for 30 minutes with 50 μL undiluted culture supernatant derived from the human myeloma cell line U266 (as a source of clonal IgE antibody) before staining with anti-IgE-FITC. After washing twice, cells were analyzed on a flow cytometer. For data acquisition, 25,000 to 100,000 events were analyzed on a FACSCalibur flow cytometer, and the resulting data files were stored and evaluated on a Macintosh computer using the Cellquest software.
Three-color staining was also applied to analyze the expression of 97A6 antigen on peripheral blood basophils (CD123++HLD-DR−) of healthy volunteers (n = 2). In these experiments, cells were first incubated with MoAb 97A6 (or isotype-matched control antibody) for 30 minutes at 4°C, washed 2 times, and then incubated with the F(ab)′2 fragment of an FITC-conjugated goat-antimouse antiserum. To prevent unspecific binding of the secondary-step reagent with antibody conjugates used in the next step, cells were incubated with excess mouse IgG for 15 minutes at 4°C. In the next step, cells were stained with anti-CD123-PE (clone 9F5; PharMingen) and anti-HLA-DR-PerCP (clone L243; Becton Dickinson) for 30 minutes at 4°C. After washing, cells were analyzed on a FACSCalibur flow cytometer. Basophils were identified as CD123++HLA-DR−.
Double staining of basophils and mast cells with toluidine blue and immunofluorescence.
Expression of cell surface antigens on basophils (n = 3) and tissue mast cells (lung, n = 3; foreskin, n = 3; uterus, n = 3) was analyzed by combined toluidine blue/immunofluorescence staining as described.38,54-56 In brief, cells were preincubated in AB serum for 30 minutes, washed, incubated with MoAb for 30 minutes (4°C), washed, and then exposed to second-step fluorescein-labeled goat F(ab′)2 IgG antimouse antibody for 30 minutes (4°C). Cells were then fixed in 0.025% glutaraldehyde for 1 minute, washed, and incubated with toluidine blue (0.0125%) for 8 to 12 minutes. After washing, cells were examined under bright-field and fluorescence-light (fluorescence microscope; Olympus, Vienna, Austria). At least 20 cells were analyzed in each sample. In selected experiments, expression of 97A6 on c-kit+ tissue mast cells was analyzed by 2-color immunostaining using a PE-conjugated anti–c-kit MoAb.32
Cell Sorting
Cell sorting was performed on a FACSVantage cytometer (Becton Dickinson) equipped with an air-cooled argon laser. FITC and PE fluorochromes were excited at 488 nm, and emission of FITC was detected through a 530-nm band pass filter, and PE through a 570-nm band pass filter. Instrument alignment and compensation of FITC versus PE signals were accomplished using a mixture of Calibrite beads (Becton Dickinson). The following fractions were sorted into tubes containing 50 μL of phosphate-buffered saline (PBS) supplemented with 40% BSA: the IgE+97A6+ fraction of unseparated BM cells stained with anti-IgE and 97A6-PE, the CD34+97A6+ and CD34+97A6− fractions of MACS-selected CD34+ BM stained with anti-CD34-FITC and 97A6-PE, and the 97A6+ fractions of cultured CD34+ cells stimulated with IL-3 and stained with anti-CD34-FITC and 97A6-PE. For morphological analysis, sorted cells were cytocentrifuged onto glass slides and stained with May-Grünwald-Giemsa. For liquid culture experiments or cultures of hematopoietic progenitors in methylcellulose, defined numbers of sorted cells were put into dishes or plates.
MoAb 97A6–Mediated Modulation of 97A6 Reactive Antigen Expression
UT-7 cells were incubated in PBS for 2 hours at 37°C, either with a control antibody or with 5 μg/mL of MoAb 97A6. After washing with ice-cold PBS in the presence of NaN3, cells were labeled either with a control antibody or with MoAb 97A6, and stained with a PE-conjugated secondary antibody. Modulation of antigen expression (downregulation or upregulation) was determined as described.57
Activation of Basophils by Acarids and IgE Receptor Cross-Linking
One hundred microliters of heparinized blood from normal donors (n = 3) or from donors with allergy against acarids (n = 2) was incubated with serial dilutions of either anti-IgE antibody (clone E124-2-8/Dε2, mouse IgG1; Immunotech) or acarids allergen extract (Dermatophagoı̈des farinae + D pteronissynus; reactivity index 10; Stallergenes, Anthony, France) for 15 minutes at 37°C. To stop the Ca2+-dependent reaction, the cells were washed with PBS/20 mmol/L EDTA and resuspended in 100 μL PBS/BSA. The cells were then incubated with 20 μL MoAb 97A6-PE (25 μg/mL) for 15 minutes at room temperature. After washing and red blood cell lysis with IOTest lysing reagent (Immunotech #0486), the cells were resuspended in PBS/BSA and analyzed on a FACSCalibur flow cytometer. Modulation of 97A6 antigen was evaluated as change in the mean fluorescence intensity of cells gated on the 97A6+population.
Immunoprecipitation of the Cell Surface Molecule(s) Detected by MoAb 97A6
Immunoprecipitates and gel electrophoresis were performed as described.58 In brief, KU-812 cells were surface-labeled with 125I-iodine by the lactoperoxidase method and lysed with NP-40. Immunoprecipitation was performed with preformed complexes of 97A6 antibody immobilized on Sepharose beads conjugated with a secondary antibody. As a control, immunoprecipitation was performed with beads in the absence of the 97A6 antibody. Apparent molecular weights were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)59 after boiling the samples in SDS under reducing conditions in the presence of 2-mercaptoethanol and under nonreducing conditions, followed by separation on 5% to 15% polyacrylamide gels using 14C-methylated marker proteins.
RESULTS
Antibody 97A6 Specifically Recognizes PB and BM Basophils
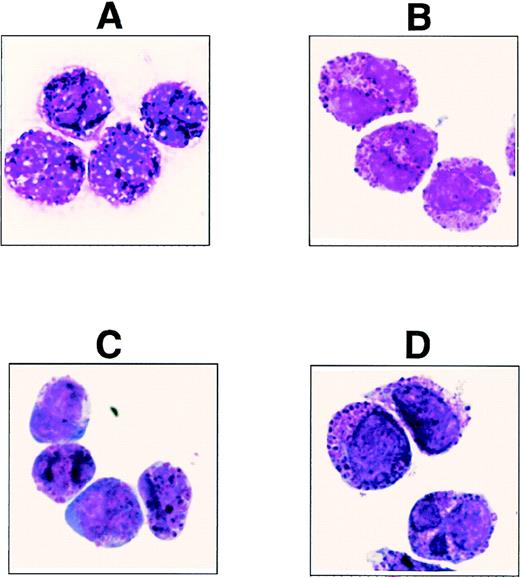
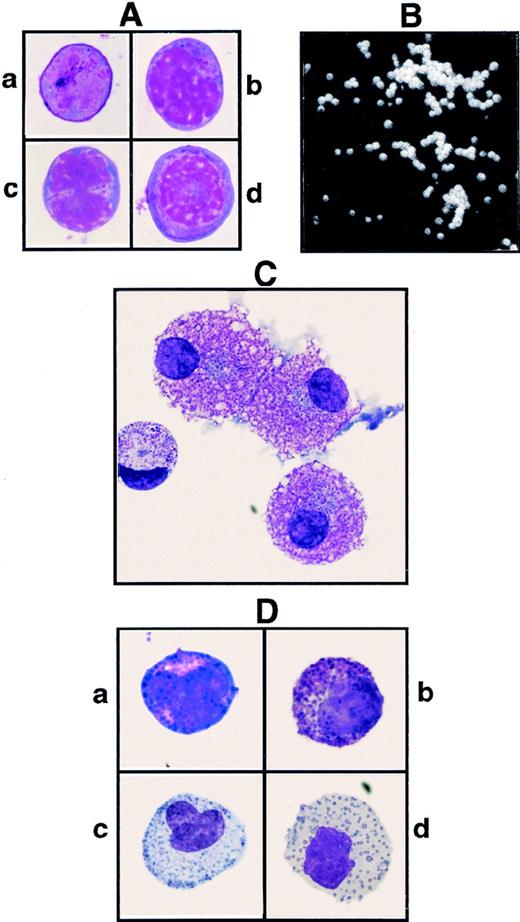
In an attempt to generate antibodies specific for rare hematopoietic cells, we produced the antibody (MoAb) 97A6 and found that the detected surface antigen is expressed on PB basophils, but not on other blood cells. To confirm the specific reaction of MoAb 97A6 with basophils, MACS-separated 97A6+ PB cells (99% pure) were stained with May-Grünwald-Giemsa for morphological analysis. Figure 1A shows the presence of a pure basophil population as identified by their typical metachromatic granules. Using a commercially available kit for basophil enrichment (StemSep; Cell Systems, Remagen, Germany), the isolated cells were found to display a very similar morphology (Fig 1B).
May-Grünwald-Giemsa–stained cells derived from (A) MACS-selected 97A6+ PB cells (positive enrichment), (B) StemSep (basophil kit)–selected PB cells (negative enrichment), (C) StemSep (basophil kit)–selected PB cells from a patient with accelerated phase CML, and (D) FACS-sorted 97A6+IgE+ BM cells.
May-Grünwald-Giemsa–stained cells derived from (A) MACS-selected 97A6+ PB cells (positive enrichment), (B) StemSep (basophil kit)–selected PB cells (negative enrichment), (C) StemSep (basophil kit)–selected PB cells from a patient with accelerated phase CML, and (D) FACS-sorted 97A6+IgE+ BM cells.
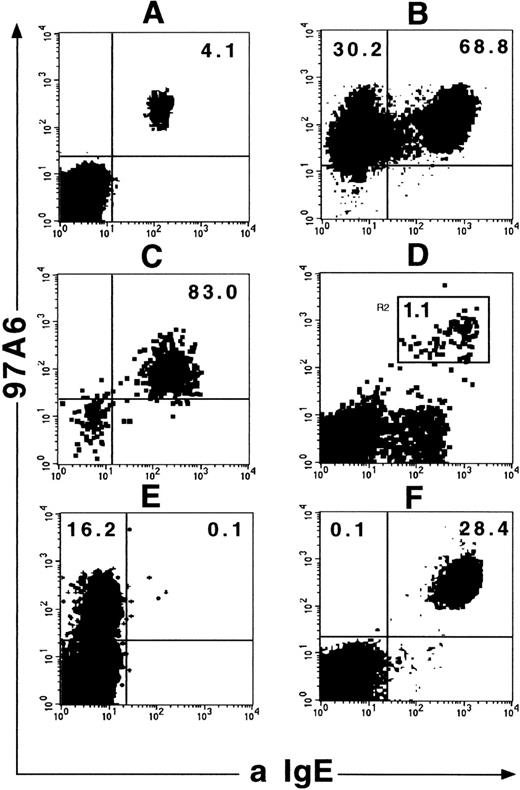
In the next step, the coexpression of IgE binding sites on these cells was analyzed. Figure 2A shows that unseparated 97A6+ PB cells express surface-bound IgE (n = 3). Surprisingly, an additional 97A6+IgE−population (30%) was found in MACS-selected cells (n = 3; Fig 2B). However, FACS separation of 97A6+ cells showed basophils with identical morphology in both the IgE+ and IgE− fractions (not shown). In contrast, StemSep-selected basophils were exclusively 97A6+IgE+ (n = 3; Fig 2C). Similar to PB cells, staining of BM cells with MoAb 97A6 and anti-IgE results in the appearance of a 97A6+IgE+ population (n = 3; Fig 2D), which also contains pure basophils (>98% pure) as determined by FACS sorting and May-Grünwald-Giemsa staining (Fig1D). The specificity of MoAb 97A6 for PB basophils was also confirmed by combined toluidine blue/immunofluorescence staining analysis. In fact, MoAb 97A6 exclusively reacted with metachromatic PB basophils (>90% of basophils reactive) in all donors analyzed (n = 5). Platelets and other PB cells, including lymphocytes, monocytes, CD10+ neutrophils, and CD81+eosinophils,60 were 97A6− (not shown). In addition, no 97A6 antigen expression was found on the eosinophilic leukemia cell line EOL and all the tested myeloid (EM2, KG-1, KG-1a, U937, HL60, OCI/AML-4), B-lymphoid (Reh, BV-173, Nalm-1, 207, 380, 697, U266, Daudi), T-lymphoid (CML-T1, HSB-2, CCRF-CEM, Molt-4), erythroid (HEL, TF-1, K562), and nonhematopoietic cell lines (DU.4475, T-47D, MCF-7, NCI-H128, NCI-H69, SK-MES, Calu 1, Calu 3, Calu 6, SK-LU1, HELA, 5637, IMR-32, TE-671, A172, U138, A431). In contrast, the megakaryocytic cell lines MEG-O1 and UT-7 (but not Molm-1, MO7-e), as well as the basophil cell line KU-812 and the mast cell line HMC-1 (subpopulation of 10% to 50%), were 97A6+ (not shown).
Coexpression of 97A6 antigen and IgE on PB and BM cells. Ficoll-Hypaque PB or BM cells were stained with MoAb 97A6-PE and anti-IgE-FITC and analyzed on a FACSCalibur flow cytometer. (A) Expression on Ficoll-Hypaque PB cells gated on “lymphocytes” (low side scatter cells). (B) Expression on MACS-selected 97A6+ PB cells. (C) Expression on PB cells selected with the StemSep basophil kit. (D) Expression on Ficoll-Hypaque BM cells. The gate in this plot indicates the sort window for morphological analysis of cells shown in Fig 1D. Plot (E) shows the display of a selected PB sample from a patient with CML in accelerated phase and plot (F) from a patient with CML in chronic phase. Note that the 97A6+IgE− sample in E contains immature basophils (morphology shown in Fig 1C).
Coexpression of 97A6 antigen and IgE on PB and BM cells. Ficoll-Hypaque PB or BM cells were stained with MoAb 97A6-PE and anti-IgE-FITC and analyzed on a FACSCalibur flow cytometer. (A) Expression on Ficoll-Hypaque PB cells gated on “lymphocytes” (low side scatter cells). (B) Expression on MACS-selected 97A6+ PB cells. (C) Expression on PB cells selected with the StemSep basophil kit. (D) Expression on Ficoll-Hypaque BM cells. The gate in this plot indicates the sort window for morphological analysis of cells shown in Fig 1D. Plot (E) shows the display of a selected PB sample from a patient with CML in accelerated phase and plot (F) from a patient with CML in chronic phase. Note that the 97A6+IgE− sample in E contains immature basophils (morphology shown in Fig 1C).
It is a well-documented fact that the number of basophils in the PB from patients with CML increases during transition from the chronic to the accelerated phase.61 To test whether MoAb 97A6 also detects basophils in this disease, the PB cells from 8 patients with CML in chronic, 2 with accelerated, and 5 with blast phase (myeloid blast crisis) were analyzed for the coexpression of bound IgE and 97A6 reactive antigen. Figure 2 shows 1 of 2 samples of accelerated phase CML with a 97A6+IgE−(E) phenotype and a representative sample (1 of 8) of chronic phase CML with a 97A6+IgE+ phenotype (F). Blast crisis CML samples appeared to be 97A6−, probably because of the high content of leukemic blasts (98% to 99%; not shown). Figure 1C shows the immature appearance of a StemSep-selected accelerated phase CML sample of the 97A6+IgE− phenotype. The low amounts of basophil granules were found in both analyzed samples. Notably, morphological counting of these samples revealed only 60% to 80% basophils, although more than 99% of the isolated cells were 97A6+ (not shown). The remaining cells were characterized as very immature blast-like cells, suggesting that some CML samples contain, in addition to mature basophils, immature 97A6+basophil precursors, which are not readily identified by morphology or conventional phenotypic analysis.
MoAb 97A6 Recognizes Tissue Mast Cells
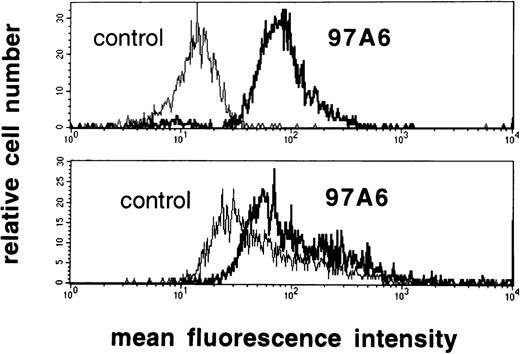
The reactivity of MoAb 97A6 with tissue mast cells from lung (n = 3), uterus (n = 3), and skin (n = 3) was analyzed. Mast cells from all sources were 97A6+, as assessed by combined immunofluorescence/toluidine blue staining. Other tissue cells were 97A6−. Expression of 97A6 antigen on mast cells was also confirmed by 2-color immunofluorescence staining experiments using a PE-labeled anti–c-kit antibody. Figure 3shows that c-kit+ lung mast cells express lower levels of 97A6 antigen when compared with CD123++HLA-DR− PB basophils. Hence, MoAb 97A6 also stains human mast cells, although at lower intensity than basophils.
97A6 antigen expression on lung mast cells and PB basophils. To quantify the intensity of 97A6 antigen expression on basophils and mast cells, normal PB basophils (top) and lung mast cells (bottom) were analyzed by multicolor flow cytometry on a FACSCalibur. PB cells (source of basophils) were stained with MoAb 97A6 plus goat antimouse IgG-FITC, as well as with anti-CD123-PE and anti–HLA-DR-PerCP. The upper histogram results from cells gated on the CD123++HLA-DR− population. Lung mast cells were stained with MoAb 97A6 plus goat antimouse IgG-FITC and anti–c-kit-PE. The lower histogram results from cells gated on the c-kit+ population. Control histograms represent cells stained with IgG1 control antibodies instead of MoAb 97A6.
97A6 antigen expression on lung mast cells and PB basophils. To quantify the intensity of 97A6 antigen expression on basophils and mast cells, normal PB basophils (top) and lung mast cells (bottom) were analyzed by multicolor flow cytometry on a FACSCalibur. PB cells (source of basophils) were stained with MoAb 97A6 plus goat antimouse IgG-FITC, as well as with anti-CD123-PE and anti–HLA-DR-PerCP. The upper histogram results from cells gated on the CD123++HLA-DR− population. Lung mast cells were stained with MoAb 97A6 plus goat antimouse IgG-FITC and anti–c-kit-PE. The lower histogram results from cells gated on the c-kit+ population. Control histograms represent cells stained with IgG1 control antibodies instead of MoAb 97A6.
Functional Studies With MoAb 97A6 and Modulation of 97A6 Reactive Antigen
To test whether MoAb 97A6 can induce cross-linking of surface molecules on basophils and mast cells in a similar fashion as anti-IgE antibodies, histamine release experiments were conducted. Incubation of cells with 1 μg/mL of anti-IgE MoAb E-124-2-8 at 37°C (cross-linking of FCεRI) resulted in histamine release in both basophils (16.0% ± 22.3% v 1.4% ± 0.4% control) and mast cells from skin (21.3% ± 3.4% v 2.5% ± 0.4%), uterus (10.2% ± 7.7% v 2.7% ± 0.7%), and lung (11.7% ± 5.2% v 2.6% ± 1.2%). In contrast, incubation of cells with MoAb 97A6 was not followed by a significant histamine secretion above control values (not shown). To determine a potential modulating activity of MoAb 97A6 on the expression of the cognate antigen, UT-7 cells were incubated with MoAb 97A6 for 2 hours at 37°C and stained with MoAb 97A6-PE. However, FACS analysis revealed no change in cell surface expression of the 97A6 reactive antigen. To study the regulation of 97A6 antigen expression on basophil progenitor cells, KU-812 cells were incubated for 4 and 12 hours with various cytokines and analyzed for reactivity with MoAb 97A6. However, no effect on 97A6 antigen expression was observed when cells were stimulated with the tested cytokines (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12, IL-13, IL-15, IL-16, IL-17, IL-18, SCF [not shown]).
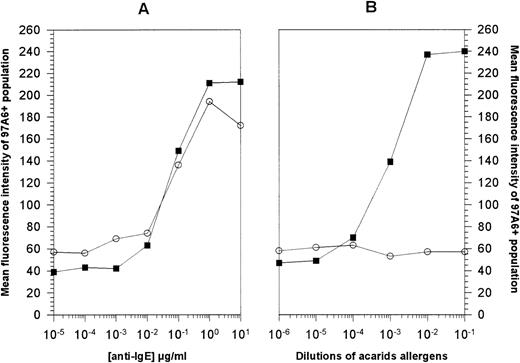
In another set of experiments, the effect of acarids allergen on basophil activation and of IgE receptor cross-linking by anti-IgE antibody was analyzed. Figure 4 shows that stimulation of PB cells with anti-IgE antibody for 15 minutes results in a dose-dependent upregulation of 97A6 antigen expression on basophils from normal donors (n = 3), as well as from donors allergic to acarids (n = 2). A concentration of 1 μg/mL of anti-IgE antibody resulted in the optimal upregulation of 97A6 antigen expression on basophils from normal (364% ± 28%) and allergic (543% and 328%) donors (Fig 4A). When the same cells were stimulated with acarids allergens, a dose-dependent increase of 97A6 surface antigen expression (510% and 307%) was only detected on the basophils from the allergic, but not from the normal donors (Fig 4B). This suggests that the 97A6 antigen plays an important role and is functionally involved in the IgE-dependent activation of basophils.
Activation of basophils with (A) anti-IgE antibody and (B) acarids. PB cells from normal volunteers (○) or from donors with allergy against acarids (▪) were stimulated with serial dilutions of either anti-IgE antibody or acarids allergen extracts for 15 minutes at 37°C. After washing, the cells were stained with MoAb 97A6-PE and analyzed on a FACSCalibur flow cytometer. The mean fluorescence intensities of cells gated on the 97A6+ population are plotted against the concentrations of the stimulating agents.
Activation of basophils with (A) anti-IgE antibody and (B) acarids. PB cells from normal volunteers (○) or from donors with allergy against acarids (▪) were stimulated with serial dilutions of either anti-IgE antibody or acarids allergen extracts for 15 minutes at 37°C. After washing, the cells were stained with MoAb 97A6-PE and analyzed on a FACSCalibur flow cytometer. The mean fluorescence intensities of cells gated on the 97A6+ population are plotted against the concentrations of the stimulating agents.
In Vitro Culture of CD34+ PB Cells in the Presence of IL-3
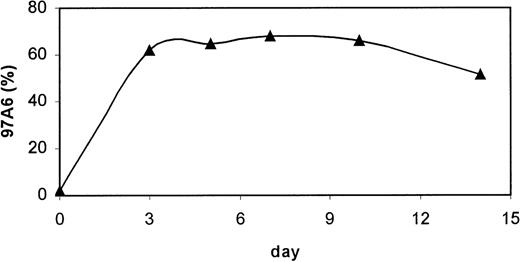
IL-3 is a major stimulating agent for growth and differentiation of basophils.15 19 To test the influence of this cytokine on the generation of 97A6+ basophils, MACS-selected CD34+ PB cells were stimulated in serum-free medium with 100 ng/mL IL-3 for 14 days (n = 3), and 97A6 antigen expression was monitored at defined time intervals. Figure5 shows a significant increase from 1% ± 0.5% 97A6+cells of the day 0 population to >60% 97A6+ cells on day 3 of culture. The amount of 97A6+ cells slightly increased after day 7 of culture to almost 70% and dropped to 51% ± 10.2% on day 14. Mainly basophils could be identified in the 97A6+ population, as assessed by May-Grünwald-Giemsa staining. The remaining cells were eosinophils, some macrophages, lymphocytes, and immature myeloid progenitor cells.
Developmental expression of 97A6 antigen of CD34+ PB cells in the presence of IL-3. MACS-selected CD34+ PB cells from normal PB were cultured in serum-free medium in the presence of 100 ng/mL IL-3 (n = 3) and analyzed for 97A6 antigen expression at different stages of culture. Percentages of 97A6-positive cells are plotted against days of culture. 10,000 cells of each sample were analyzed on a FACSCalibur flow cytometer, and percentage positive cells was calculated with the Cellquest software.
Developmental expression of 97A6 antigen of CD34+ PB cells in the presence of IL-3. MACS-selected CD34+ PB cells from normal PB were cultured in serum-free medium in the presence of 100 ng/mL IL-3 (n = 3) and analyzed for 97A6 antigen expression at different stages of culture. Percentages of 97A6-positive cells are plotted against days of culture. 10,000 cells of each sample were analyzed on a FACSCalibur flow cytometer, and percentage positive cells was calculated with the Cellquest software.
In Vitro Culture of CD34+ Cells in the Presence of SCF
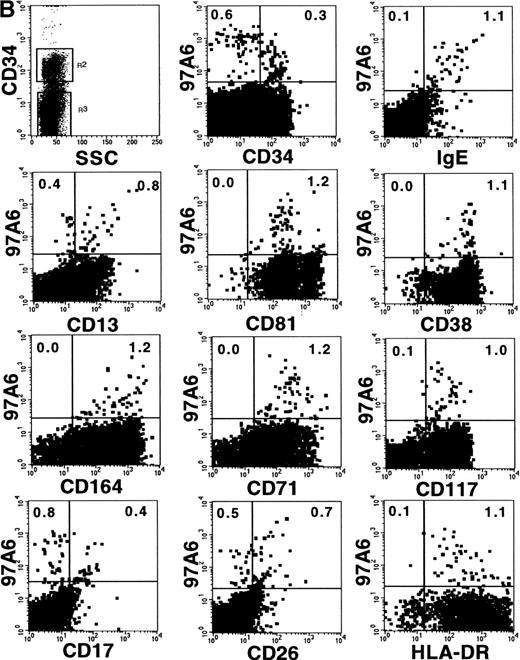
SCF is a major stimulator for mast cell growth and differentiation.12 To determine whether mast cells are derived from a 97A6+ progenitor, MACS-selected CD34+ BM cells, stained with anti-CD34-FITC and MoAb 97A6-PE, were fractionated by FACS into CD34+97A6+ (about 1% of CD34+cells) and CD34+97A6− cells. One thousand cells of each fraction (CD34+, CD34+97A6+, and CD34+97A6−) were cultured in serum-free medium containing 100 ng/mL SCF and 10 ng/mL IL-6. After 14 and 28 days of culture, the medium was replaced with serum-free medium containing 100 ng/mL SCF. After 35 days of culture, the cells were counted and analyzed for morphology and tryptase content. Morphological examination showed comparable distributions of mast cells in all 3 fractions (36% of CD34+97A6+, 32% of CD34+97A6−, and 30% of CD34+cultures). Figure 6C (see page 2346) shows representative mast cells from CD34+97A6+ cultures. The identity of mast cells was confirmed by measuring the cellular tryptase content. Table 1 shows significant amounts of this mast cell–specific enzyme in all 3 fractions (1.2, 1.4, and 1.5 pg/mast cell in the CD34+97A6+, CD34+97A6−, and CD34+fractions, respectively), suggesting that mast cell progenitors are found in both the CD34+97A6+ and CD34+97A6− fractions.
Determination of Mast Cell and Tryptase Content in CD34+ BM Cells Stimulated With 100 ng/mL SCF in Liquid Culture for 35 Days
| . | CD34+ . | CD34+97A6+ . | CD34+97A6− . |
|---|---|---|---|
| Cell count | 20 × 104 | 3.5 × 104 | 8.5 × 104 |
| % mast cells | 30% | 36% | 32% |
| Total number of mast cells | 6 × 104 | 1.26 × 104 | 2.72 × 104 |
| Tryptase/104 mast cells | 15.16 ng | 11.19 ng | 14.19 ng |
| . | CD34+ . | CD34+97A6+ . | CD34+97A6− . |
|---|---|---|---|
| Cell count | 20 × 104 | 3.5 × 104 | 8.5 × 104 |
| % mast cells | 30% | 36% | 32% |
| Total number of mast cells | 6 × 104 | 1.26 × 104 | 2.72 × 104 |
| Tryptase/104 mast cells | 15.16 ng | 11.19 ng | 14.19 ng |
One thousand cells of each BM fraction (CD34+, CD34+97A6+, and CD34+97A6−) were cultured in the presence of 100 ng/mL SCF and 10 ng/mL for 35 days. After days 14 and 28 of culture, the medium was replaced with serum-free medium containing 100 ng/mL SCF. After culture, cells were counted and mast cells were identified by morphology. The tryptase content was determined as described in Materials and Methods.
Colony Assays for Hematopoietic Progenitor Cells
We have shown that about 1% of CD34+ PB and BM cells coexpress the 97A6 antigen. Figure 6A shows that the CD34+97A6+ BM population consists of heterogeneous blasts without granules (Fig 6A). Although the cell in Fig 6A(a) does not contain distinguishable cytoplasmic regions, the cells in Fig 6A(b-d) show more pronounced nuclear structures and cytoplasmic regions. To determine whether basophil and/or mixed basophil/eosinophil progenitor cells reside in the CD34+97A6+ population, MACS-selected CD34+ BM cells stained with anti-CD34 and MoAb 97A6 were fractionated by FACS, and 1,000 cells of each fraction (duplicate cultures, n = 2) were plated in an IL-3–containing semisolid medium. After 16 days of culture, the colonies were enumerated and—in the case of CD34+97A6+ cultures—single colonies were transferred to slides for morphological staining with May-Grünwald-Giemsa. Table 2 shows that virtually all the BFU-E, as well as the majority of the myeloid progenitors, are found in the CD34+97A6−fraction, whereas the CD34+97A6+ population almost exclusively contained myeloid colonies. 85% ± 5% of the CD34+97A6+ colonies contained cells with very regular, round shapes (Fig 6B), compared with the irregular cell shapes found in the CD34+97A6− fraction. Morphological examination of these cells showed that 10 of 40 colonies gave rise to pure basophils, 7 of 40 to mixed eosinophil-basophils, 3 of 40 to mixed basophil-macrophage cells, and 12 of 40 to multipotent basophil-eosinophil-macrophage and/or neutrophil colonies, suggesting that the 97A6 reactive antigen is not only expressed on unipotent basophil progenitors, but also on bipotent and multipotent progenitors. Only 6 of 40 of the examined colonies did not contain basophils, but rather immature blasts. Figure 6D shows an example of cells obtained from a basophil/eosinophil colony. Representative cells consist of basophils (a and b) and eosinophils (c and d) of different developmental stages that are characterized by the abundance of basophil or eosinophil granules, respectively. In summary, MoAb 97A6 recognizes a CD34+ basophil progenitor, as well as a common progenitor for basophils and eosinophils, and a multipotent progenitor for myeloid cells of various lineages including basophils.
(A) May-Grünwald-Giemsa–stained CD34+97A6+ BM cells derived from MACS-selected CD34+ cells after staining with anti–CD34-FITC and 97A6-PE, and fractionation by FACS sorting; the cells in a-d show heterogeneity. (B) Colony from CD34+97A6+ BM cells after day 16 of culture in semisolid methylcellulose-containing medium. (C) Mast cells from CD34+97A6+ BM cells after 35 days of culture in serum-free medium in the presence of 100 ng/mL SCF. (D) May-Grünwald-Giemsa–stained cells derived from a bipotent basophil/eosinophil colony type shown in (B). Figure 6D (a, b) and (c, d) show representative basophils (basophilic granules) and eosinophils (eosinophilic granules) of different developmental stages from this colony.
(A) May-Grünwald-Giemsa–stained CD34+97A6+ BM cells derived from MACS-selected CD34+ cells after staining with anti–CD34-FITC and 97A6-PE, and fractionation by FACS sorting; the cells in a-d show heterogeneity. (B) Colony from CD34+97A6+ BM cells after day 16 of culture in semisolid methylcellulose-containing medium. (C) Mast cells from CD34+97A6+ BM cells after 35 days of culture in serum-free medium in the presence of 100 ng/mL SCF. (D) May-Grünwald-Giemsa–stained cells derived from a bipotent basophil/eosinophil colony type shown in (B). Figure 6D (a, b) and (c, d) show representative basophils (basophilic granules) and eosinophils (eosinophilic granules) of different developmental stages from this colony.
Colony Numbers of Sorted BM CD34+97A6+ and CD34+97A6− Cells
| Fraction . | BFU-E . | CFU-GM . | CFU-Baso* . |
|---|---|---|---|
| CD34+ | 33.4 ± 7.4 | 47.5 ± 8.4 | nd |
| CD34+97A6+ | 0.5 ± 0.5 | 12.5 ± 3.5 | 85 ± 5% |
| CD34+97A6− | 46.2 ± 6.8 | 37.3 ± 5.2 | nd |
| Fraction . | BFU-E . | CFU-GM . | CFU-Baso* . |
|---|---|---|---|
| CD34+ | 33.4 ± 7.4 | 47.5 ± 8.4 | nd |
| CD34+97A6+ | 0.5 ± 0.5 | 12.5 ± 3.5 | 85 ± 5% |
| CD34+97A6− | 46.2 ± 6.8 | 37.3 ± 5.2 | nd |
Colony-forming capacity of MACS-selected CD34+ BM cells stained with anti-CD34-FITC and 97A6-PE and FACS-sorted into CD34+97A6+ and CD34+97A6− fractions. Fractionated cells were plated in semisolid methylcellulose cultures as described in Materials and Methods. Cultures were scored for colony growth after 16 days of incubation at 37°C and 5% CO2. Results represent the mean colony numbers ± standard deviation (SD) based on 1 × 103 plated cells from 2 independent experiments with duplicate determinations. For determination of basophil colonies in the CD34+97A6+ fraction, single colonies were transferred with a Pasteur pipette on glass slides, air-dried, and stained with May-Grünwald-Giemsa for morphological analysis.
Abbreviations: BFU-E, burst-forming units-erythrocyte; CFU-GM, colony-forming units-granulocyte/macrophage; CFU-Baso, colony-forming units-basophils; nd, not determined.
CFU-Baso or mixed colonies in which basophils were detected.
Phenotypic Characterization of CD34−97A6+ and CD34+97A6+ PB and BM Basophils
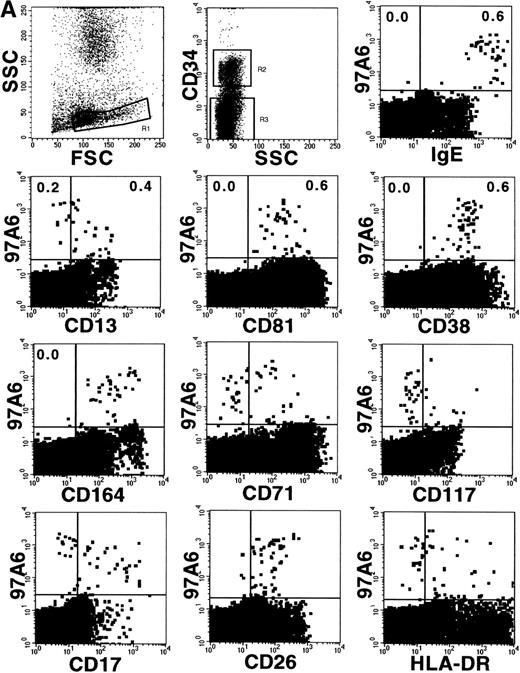
The profile of surface antigen expression on mature PB basophils is well documented.8,14 29 This study extends the analysis of previous reports and describes coexpression of selected markers and 97A6 antigen on CD34+ and CD34−basophils/basophil precursors. The plots in Fig 7A show coexpression of 97A6 antigen and high levels of surface-bound IgE; intermediate levels of CD81, CD38, and CD164; and low levels of CD26 on CD34− BM cells, whereas expression of CD13, CD71, CD17, and HLA-DR is heterogeneous, and CD117 expression is almost absent on this population (gate R3). In contrast, 97A6+ BM cells gated on the CD34+ population (Fig 7B; gate R2) coexpress low levels of receptor-bound IgE, CD17, and CD26, but are positive for CD117 and CD71.
Coexpression of 97A6 antigen and defined CD antigens on CD34+ and CD34− BM cells. BM cells were stained with anti-CD34-PerCP, 97A6-PE, and FITC-conjugated antibodies with the indicated specificities. When antibodies with specificities for CD81 (IgG2a), CD164 (IgG3), and CD17 (IgM) were used, the cells were labeled with the indicated antibodies and stained after washing with F(ab′)2 fragments of FITC-conjugated isotype-specific secondary reagents. Expression on CD34− cells (A) and CD34+cells (B) was analyzed after gating on the corresponding populations. 250,000 cells of each sample were analyzed on a FACSCalibur flow cytometer.
Coexpression of 97A6 antigen and defined CD antigens on CD34+ and CD34− BM cells. BM cells were stained with anti-CD34-PerCP, 97A6-PE, and FITC-conjugated antibodies with the indicated specificities. When antibodies with specificities for CD81 (IgG2a), CD164 (IgG3), and CD17 (IgM) were used, the cells were labeled with the indicated antibodies and stained after washing with F(ab′)2 fragments of FITC-conjugated isotype-specific secondary reagents. Expression on CD34− cells (A) and CD34+cells (B) was analyzed after gating on the corresponding populations. 250,000 cells of each sample were analyzed on a FACSCalibur flow cytometer.
Table 3 summarizes the expression of CD markers on primary BM and PB basophils, as well as on accelerated phase CML and cultured 97A6+ PB cells. In line with previous reports, CD34− PB basophils are negative for CD117, a marker for mast cells, and CD71, a marker for proliferating cells, but express CD17 and high levels of the IL-3 receptor alpha-chain (CD123), a marker expressed on basophils, eosinophils, and monocytes. CD34− BM cells appear to express CD13, CD17, CD71, and HLA-DR more heterogeneously than PB basophils. A similar expression is found on PB basophils from patients in accelerated phase CML. In contrast with the BM counterpart, all CD34+97A6+ PB basophil precursors are negative for CD13, CD17, and CD26 (Table 3), indicating that the PB precursors are slightly more immature. Both precursors are, however, positive for CD117 and CD71, markers which are absent on mature basophils. Unlike mature PB basophils, cultured 97A6+ basophils obtained from CD34+ PB cells stimulated with IL-3 expressed only low levels or no CD38, but high levels of HLA-DR. Taken together, the expression of CD antigens on basophils of different maturational stages and from different sources varies significantly, whereas the 97A6 antigen seems to be the continuously expressed on all types of basophils, suggesting a pivotal role of the 97A6 antigen for this cell type.
Coexpression of CD Antigens on 97A6+Cells
| . | PB CD34− . | PB CD34+ . | BM CD34− . | BM CD34+ . | PB CML . | d3 PB (+IL-3) . | d14 PB (+IL-3) . |
|---|---|---|---|---|---|---|---|
| CD10 | − | nd | − | − | − | nd | − |
| CD13 | + | − | ± | ± | ± | − | ± |
| CD16 | − | nd | nd | nd | ± | nd | − |
| CD17 | + | − | ± | ± | ± | ± | − |
| CD26 | + | − | + | ± | + | ± | ± |
| CD33 | ± | + | + | + | + | + | + |
| CD38 | + | + | + | + | + | + | ± |
| CD44 | + | + | + | + | + | + | ± |
| CD55 | + | + | + | + | + | + | + |
| CD59 | + | + | + | + | + | + | + |
| CD71 | − | + | ± | + | − | + | ± |
| CD81 | ± | nd | + | + | + | + | + |
| CD117 | − | + | − | + | − | + | nd |
| CD123 | + | + | + | + | + | ± | nd |
| CD164 | + | + | + | + | + | − | ± |
| IgE | + | ± | + | + | − | − | ± |
| HLA-DR | − | ± | ± | + | − | + | ± |
| . | PB CD34− . | PB CD34+ . | BM CD34− . | BM CD34+ . | PB CML . | d3 PB (+IL-3) . | d14 PB (+IL-3) . |
|---|---|---|---|---|---|---|---|
| CD10 | − | nd | − | − | − | nd | − |
| CD13 | + | − | ± | ± | ± | − | ± |
| CD16 | − | nd | nd | nd | ± | nd | − |
| CD17 | + | − | ± | ± | ± | ± | − |
| CD26 | + | − | + | ± | + | ± | ± |
| CD33 | ± | + | + | + | + | + | + |
| CD38 | + | + | + | + | + | + | ± |
| CD44 | + | + | + | + | + | + | ± |
| CD55 | + | + | + | + | + | + | + |
| CD59 | + | + | + | + | + | + | + |
| CD71 | − | + | ± | + | − | + | ± |
| CD81 | ± | nd | + | + | + | + | + |
| CD117 | − | + | − | + | − | + | nd |
| CD123 | + | + | + | + | + | ± | nd |
| CD164 | + | + | + | + | + | − | ± |
| IgE | + | ± | + | + | − | − | ± |
| HLA-DR | − | ± | ± | + | − | + | ± |
BM and PB cells were labeled with anti-CD34-PerCP, 97A6-PE, and FITC-conjugated antibodies of the indicated specificities and analyzed on a FACSCalibur flow cytometer. CD34− and CD34+ cells were evaluated after gating on the respective populations using the Cellquest software. CML PB cells and CD34+ PB cells stimulated with 100 ng/mL IL-3 and harvested at days 3 and 14, respectively, were stained with 97A6-PE and FITC-conjugated antibodies of the indicated specificities. ± indicates positive subpopulation.
Abbreviation: nd, not determined.
Immunochemical Characterization of Cell Surface Proteins Detected by MoAb 97A6
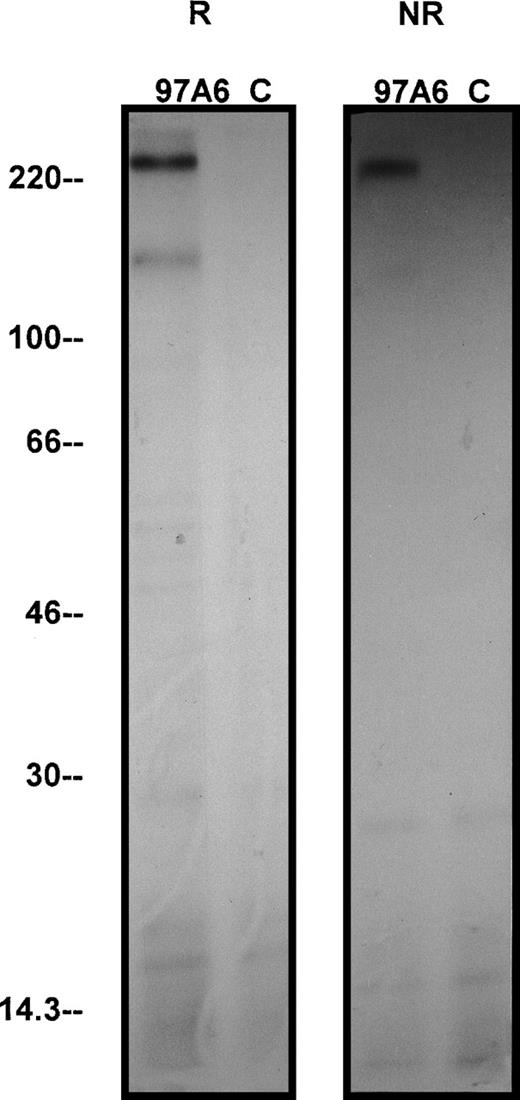
To determine the molecular mass of the protein(s) recognized by MoAb 97A6, KU-812 cells were used for immunoprecipitation. Figure 8 shows that MoAb 97A6 precipitates 2 proteins of 270 kD and 150 kD at reducing conditions and1protein of 270 kD at nonreducing conditions, suggesting that the 97A6 antigen is a disulfide-linked dimer structure of 2 150-kD cell surface proteins. The fact that 2 bands were precipitated under reducing conditions suggests that the reducing and denaturing conditions were not stringent enough to completely dissociate all dimer chains.
Immunoprecipitation of the 97A6 reactive antigen. 107 KU-812 cells were surface-iodinated with125I-iodine by the lactoperoxidase method and immunoprecipitated under reduced and nonreduced conditions with 97A6 antibody-coated beads or control beads, respectively. The precipitates were then separated by 5% to 15% SDS-PAGE and visualized by radiographic photography. “R” indicates reduced conditions; “NR” indicates nonreduced conditions. Molecular weight standards are indicated on the left.
Immunoprecipitation of the 97A6 reactive antigen. 107 KU-812 cells were surface-iodinated with125I-iodine by the lactoperoxidase method and immunoprecipitated under reduced and nonreduced conditions with 97A6 antibody-coated beads or control beads, respectively. The precipitates were then separated by 5% to 15% SDS-PAGE and visualized by radiographic photography. “R” indicates reduced conditions; “NR” indicates nonreduced conditions. Molecular weight standards are indicated on the left.
DISCUSSION
In the present study, the generation of a monoclonal antibody, 97A6, recognizing a cell surface structure on basophils, mast cells, and their progenitors, is described. This novel marker seems to be specific for basophils and mast cells in that (a) no other mature hematopoietic cells (eosinophils, neutrophils, monocytes) are recognized by the reagent; (b) only the basophil cell line KU-812, the mast cell line HMC-1, and a few megakaryocytic cell lines, but not other cell lines, appear to express the 97A6 reactive antigen; and (c) isolated CD34+97A6+ cells give rise to basophils and mast cells in appropriate cultures.
Mast cells and basophils share certain morphological and biochemical properties, as well as expression of the high affinity IgE receptor.8 However, these 2 cell types differ from each other in terms of mediator production, surface receptor expression, and response to distinct stimuli, and can be regarded as 2 separate cell lineages within the hematopoietic system.8 In this regard, the expression of the 97A6 reactive surface antigen on both mast cells and basophils is remarkable. However, although both types of cells are recognized by the 97A6 antibody, a significant difference between the 2 cell types was observed when the staining patterns were compared. In particular, MoAb 97A6 stained primary basophils of all healthy donors and also basophil KU-812 cells very brightly, whereas tissue mast cells and the HMC-1 mast cell line were only weakly stained, and a subpopulation of lung mast cells and HMC-1 appeared to remain in the 97A6-negative fraction. Whether this difference is caused by a different antigen production rate, diverse degradation rate, or other mechanisms (different in mast cells versus basophils) remains unknown. Also, little is known about the regulation of expression of the 97A6 antigen. In CD34+ progenitor cells, IL-3 seems to promote 97A6 antigen expression. On the other hand, neither IL-3 nor the other cytokines tested (IL-1 through IL-18) were found to upregulate or downregulate 97A6 antigen expression in KU-812 cells. In mature basophils, however, we found that IgE receptor cross-linking by anti-IgE leads to an upregulation of 97A6 antigen expression within a short time (15 minutes). Interestingly, the acarids allergen induced a specific 97A6 antigen upregulation on the basophils from donors allergic for this allergen, but not on the basophils from normal donors. This observation points to a distinct role of 97A6 antigen in the mechanism of IgE-dependent basophil activation. The rapid increase of 97A6 antigen expression suggests that this IgE-dependent effect is caused by a rapid redistribution from intracellular stores.
Another interesting observation in this study was that MoAb 97A6 not only recognizes mature basophils and mast cells, but also (a subset of) immature CD34+ PB and BM cells, including clonogenic progenitors (CFUs). CD34+97A6+ cells cultured in a semisolid medium in the presence of various growth factors (including IL-3) gave rise to colonies consisting of basophils only, or of a mixture of various cell types including basophils. These results suggest that the 97A6 antigen is expressed on multipotent, bipotent, and on lineage-restricted basophil precursor cells. Whether these basophil-committed progenitors would also give rise to mast cells in response to SCF was not addressed in this study. However, we were able to show that the 97A6 antigen is indeed expressed on mast cell progenitor cells. In fact, when CD34+97A6+ BM cells were cultured in liquid suspension culture in the presence of the mast cell growth factor SCF together with IL-6, a significant differentiation of mast cells was seen. Interestingly, the CD34+97A6− progenitor subset gave rise to mast cells as well, but did not give rise to basophils in the colony assay. These results may suggest that the 97A6 antigen is not expressed on primitive mast cell progenitor cells, but is acquired on later phases of mast cell development. Alternatively, 2 pathways of mast cell differentiation (97A6+v 97A6−) may exist, giving rise to diverse subsets of mature mast cells. Because a number of markers that indicate mast cell heterogeneity have been described in the past, it might be of interest to establish whether the 2 different progenitor subsets are producing 2 different types of (cultured) mast cells. Another reason for the failure of MoAb 97A6 to detect all mast cell progenitor cells could be low level expression of the reactive antigen on these cells, which would be in line with the weak expression of 97A6 antigen on mature mast cells. In contrast with the mast cell lineage, 97A6 antigen expression is clearly detectable on all stages of basophil differentiation. This assumption is based not only on the colony data, but also on time-course experiments, indicating that IL-3–induced in vitro differentiation of basophils from CD34+ blasts is associated with a rapid and continuous expression of the 97A6 antigen. Moreover, MoAb 97A6 not only recognizes basophil progenitors from normal donors, but also morphologically unidentifiable immature basophil precursors in patients with CML in accelerated phase, suggesting that this antibody is also a valuable tool for a precise determination of the basophil lineage in these patients. In fact, an exact quantitative evaluation of the basophil compartment in CML is of importance because it is known that disease acceleration is often accompanied or preceded by pronounced basophilia. Studies are underway to determine the clinical significance of 97A6+ cells and their increase in CML.
Although the marker profiles for mature basophils and mast cells have been established in the past, little is known so far about the phenotype of CD34+ basophil- and mast cell–committed progenitors. The results of our study suggest that the 97A6 antigen may be associated with basophil/mast cell commitment in CD34+cell populations. In the present study, we also compared the phenotype of CD34+97A6+ basophil/mast cell progenitor cells with that of mature CD34−97A6+blood basophils. Interestingly, the immature CD34+97A6+ progenitors were found to express the transferrin receptor CD71. This observation corresponds well with the proliferative capacity of these cells and is in line with other data on CD34+ cells.9 Another interesting finding was that almost all CD34+97A6+ cells express the SCF receptor c-kit (CD117). With regard to mast cell differentiation, this observation is in line with the assumption that c-kit is expressed at a continuously high level throughout mastopoiesis.8 12 In case of the basophil lineage, it appears that c-kit is expressed at a detectable level only on CD34+97A6+ progenitors, but is lost during cell maturation.
Little is known about the subcellular distribution, function, and molecular structure of the 97A6 antigen. Preliminary experiments performed with KU-812 cells suggest that the antigen is expressed both in the cytoplasm and on the cell surface of basophils. As assessed by immunoprecipitation, the 97A6 antibody appears to detect proteins of 270 kD and 150 kD under reducing conditions and 1 protein of 270 kD under nonreducing conditions. Most likely, the 97A6 antigen consists of a homodimer complex, because no separate bands (apart from the not completely dissociated 270-kD protein) are detected after immunoprecipitation under reducing conditions. To determine the precise role of the 97A6 antigen in basophil development and function, attempts to clone the gene for 97A6 antigen are in progress. Although the molecular structure of the 97A6 antigen is unknown at present, a role of this antigen in basophil activation could be determined: cross-linking of normal donor PB cells with anti-IgE or stimulation of PB cells from donors allergic for a defined allergen results in a dramatic upregulation of the 97A6 antigen. This indicates that an upregulated expression of the 97A6 antigen is associated with basophil activation. However, a ligand that directly interacts with this antigen has not been identified. Cross-linking of the surface determinant by incubating basophils and mast cells with MoAb 97A6 did not result in histamine secretion, and incubation of cells with MoAb 97A6 was not followed by a change in 97A6 antigen expression.
So far, a number of different antibodies specific for either mast cells or basophils have been generated. One of them, the YB5.B8 antibody, was found to react with both mast cells and their uncommitted as well as committed progenitors. Later, it was found that MoAb YB5.B8 recognizes the receptor for SCF, one of the major regulators of mast cell growth and differentiation. In the case of the basophil lineage, however, no marker has been described so far that defines mature as well as immature cells. We now have generated the novel MoAb 97A6 that binds with considerable specificity to basophils and their progenitor cells. The MoAb should therefore be most useful to isolate basophils, to determine their activation, and to analyze their development from multilineage progenitors, and possibly from a progenitor common for both mast cells and basophils. Finally, it might be a valuable tool for a precise enumeration of basophil numbers in CML.
ACKNOWLEDGMENT
The authors thank Heike Letzkus for her excellent help in the generation of antibody 97A6 and for FACS analysis, Minoo Ghannadan and Gerit-Holger Schernthaner for excellent technical assistance, Anke Marxer for MACS and FACS sorting and cell preparation, and Doris Schweigert for photography.
Supported by the Deutsche Forschungsgemeinschaft (SFB 510, project A1), by a grant from the Federal Ministry of Education and Research and the Interdisciplinary Clinical Research Center (project II A1), and the Fonds zur Förderung der Wissenschaftlichen Forschung inÖsterreich, FWF, grant # P12517.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Hans-Jörg Bühring, PhD, Klinik für Innere Medizin, Abteilung II, FACS-Labor, Otfried-Müller-Str.10, 72076 Tübingen, Germany; e-mail:hjbuehri@med.uni-tuebingen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal