A competitive repopulation assay in the dog was used to develop improved gene transfer protocols for hematopoietic stem cell gene therapy. Using this assay, we previously showed improved gene transfer into canine hematopoietic repopulating cells when CD34-enriched marrow cells were cocultivated on gibbon ape leukemia virus (GALV)–based retrovirus vector-producing cells. In the present study, we have investigated the use of fibronectin fragment CH-296 and 2 growth factor combinations to further improve gene transfer efficiency. CD34-enriched marrow cells from each dog were prestimulated for 24 hours and then divided into 3 equal fractions. Two fractions were placed into flasks coated with either CH-296 or bovine serum albumin (BSA) and virus-containing medium supplemented with growth factors, and protamine sulfate was replaced 4 times over a 48-hour period. One fraction was cocultivated on irradiated PG13 (GALV-pseudotype) packaging cells for 48 hours. In 2 animals, cells of the different fractions were transduced in the presence of human FLT-3 ligand (FLT3L), canine stem cell factor (cSCF), and human megakaryocyte growth and development factor (MGDF), and in 2 other dogs, transduction was performed in the presence of FLT3L, cSCF, and canine granulocyte-colony stimulating factor (cG-CSF). The vectors used contained small sequence differences, allowing differentiation of cells genetically marked by the different vectors. After transduction, nonadherent and adherent cells from all 3 fractions were pooled and infused into lethally irradiated dogs. Polymerase chain reaction and Southern blot analysis were used to determine the persistence of the transferred vectors in the peripheral blood and marrow cells after transplantation. The highest levels of gene transfer were obtained when cells were transduced in the presence of FLT3L, cSCF, and cG-CSF (gene transfer levels of more than 10% for more than 8 months so far). Compared with the 2 animals that received cells transduced with FLT3L, cSCF, and MGDF, gene transfer levels were significantly higher when dogs received cells that were transduced in the presence of cG-CSF. Transduction on CH-296 resulted in gene transfer levels that were at least as high as transduction by cocultivation. In summary, the overall levels of gene transfer obtained with these conditions should be sufficiently high to allow stem cell gene therapy studies aimed at correcting genetic diseases in dogs as a model for human gene therapy.
A NUMBER of genetic diseases in the dog, for example, pyruvate kinase deficiency or storage diseases such as iduronidase deficiency,1,2 resemble human diseases and provide unique opportunities to investigate the efficacy of gene therapy for treating these disorders. Unfortunately, gene transfer efficiencies in the dog have lagged behind the promising results reported for monkeys.3-5 Because relevant disease models are available in dogs and not in nonhuman primates, we have continued to study methods to improve gene transfer rates in the canine model. We have used a competitive repopulation assay to evaluate new protocols. In recent studies, we have shown improved gene transfer into dog marrow repopulating cells using CD34-enriched marrow cells and cocultivation on PG13-based packaging cells, a method which is not approved for human gene therapy trials because of concerns over infusion of murine packaging cells. To develop efficient transduction conditions that would not require cocultivation and to further improve gene transfer rates in the dog model, we have investigated the use of fibronectin fragment CH-296 and different growth factor combinations for their ability to improve canine stem cell transduction.
Fibronectin has been shown to facilitate transduction of hematopoietic progenitor cells and repopulating cells in various animal models.5-7 Prestimulation and transduction in the presence of growth factors active on hematopoietic stem/progenitor cells have also been shown to increase gene transfer rates.5,8-10Unfortunately, many of the human growth factors active on hematopoietic stem/progenitor cells are not, or are only partially, active on canine cells. More recently, recombinant canine stem cell factor (cSCF), granulocyte colony-stimulating factor (cG-CSF), and granulocyte-macrophage colony-stimulating factor (cGM-CSF) have become available. All 3 recombinant canine growth factors have been shown to be active on canine hematopoietic progenitor/stem cells.11-15 G-CSF or GM-CSF, however, have usually not been included for the transduction of hematopoietic stem cells in human and nonhuman primates, to avoid potential differentiation and, therefore, loss of hematopoietic repopulating cells.3,5,16 Both human megakaryocyte growth and development factor (MGDF) and human FLT-3 ligand (FLT3L) have been shown to have at least partial activity on canine hematopoietic progenitor cells,17 and inclusion of these growth factors has been shown to enhance transduction and maintenance of hematopoietic stem cells in nonhuman primates.5 9 Based on these observations, we have examined the use of CH-296 and the effect of 2 different growth factor combinations, FLT3L, cSCF, cG-CSF (FSG) and FLT3L, cSCF, MGDF (FSM), on transduction and maintenance of canine hematopoietic repopulating cells. Our results show that transduction of canine CD34-enriched marrow cells on fibronectin fragment CH-296 in the presence of a G-CSF–containing growth factor combination (FSG) results in high-level gene transfer into marrow repopulating cells in dogs.
MATERIALS AND METHODS
Animals.
Dogs were raised and housed at the Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA) under conditions approved by the American Association for Accreditation of Laboratory Animal Care. All animals were provided with commercial chow and chlorinated tap water ad libitum. Marrow draws were performed after animals had been anesthetized with a combination of ketamine-HCl. Animals received broad-spectrum antibiotics and recombinant cG-CSF after transplant until absolute neutrophil count (ANC) was greater than 1,000/μL. As preparation for transplantation, all animals received a single myeloablative dose of 920 cGy total body irradiation (TBI) administered from 2 opposing 60Co sources at 7 cGy/min.
CD34 enrichment of bone marrow cells.
The method has been described recently.18,19 Briefly, bone marrow buffy coat (in dog E585) or Ficoll-separated cells (dogs E617, E649, and E680) were labeled with either biotinylated (E585, E649, E680) or nonbiotinylated (E617) antibody 1H6, (IgG-1 anticanine CD34)18 at 4°C for 30 minutes. The cells were washed twice and then incubated with streptavidin-conjugated microbeads (E585, E649, E680) or rat-monoclonal antimouse IgG1 microbeads (E617) for 30 minutes at 4°C, washed, and then separated using an immunomagnetic column technique (Miltenyi Biotech, Auburn, CA) according to manufacturer’s instructions. After CD34 enrichment, an aliquot of cells was relabeled with 1H6 for 20 minutes at 4°C. The cells were then washed and incubated with goat-antimouse fluorescein isothiocyanate (FITC)–conjugated IgG (CALTAG, Burlingame, CA) for 20 minutes and then analyzed on a FACSCalibur (Becton Dickinson, San Jose, CA).
Retrovirus vectors and cell lines.
The LN, LNX, and LNY vectors carry the bacterial neomycin phosphotransferase (neo) gene conveying G418 resistance and are identical with the exception of different length sequences between theneo gene and the vector 3′ long terminal repeat (LTR). LN and LNX have been described before.4 5 LNY was constructed by inserting 2 copies of a PacI linker into the HindIII site of the LNX vector, resulting in an additional 20 base pairs (5′ CTT TAA TTA ATT AAT TAA AG 3′). These length differences allowed us to use a single pair of primers to amplify different length sequences by polymerase chain reaction (PCR) that could distinguish cells genetically marked by the different vectors. Packaging cell lines were generated using PG13 (GALV-pseudotype) cells, and individual producer clones with equal titers were selected for LN, LNX, and LNY comparisons. Vector titers were about 5 × 105 colony-forming units (CFU)/mL on canine D17 cells.
Transduction of enriched bone marrow cells.
CD34-enriched cells were prestimulated for 24 hours in Iscove’s modified Dulbecco’s medium supplemented with 12.5% horse serum (GIBCO-BRL, Gaithersburg, MD), 12.5% fetal bovine serum (FBS; GIBCO-BRL), 10−6 mol/L hydrocortisone (SIGMA Chemical Co, St Louis, MO), 10−4 mol/L 2-mercaptoethanol (SIGMA), 1% glutamine, 1% penicillin/streptomycin (GIBCO-BRL) in the presence of 50 ng/mL of FLT3L, cSCF, MGDF (dogs E585 and E617) or FLT3L, cSCF, and cG-CSF (dogs E649 and E680). After prestimulation, equal numbers of CD34-enriched cells were placed in 75 cm2 canted-neck flasks (Corning, Corning, NY) either coated with bovine serum albumin (BSA) or CH-296 (RetroNectin; Takara Shuzo, Otsu, Japan) at a concentration of 2 μg/cm2 and preloaded with 20 mL of virus-containing medium as described.7 The third fraction of cells was added to flasks containing irradiated packaging cells.19 For cells transduced on CH-296 and on BSA, fresh retrovirus-containing medium was added 4 times over a period of 48 hours, growth factors were added at a concentration of 50 ng/mL, and protamine sulfate was added at 8 μg/mL. After transduction, nonadherent and adherent cells including packaging cells were counted, pooled, and infused intravenously into the animal within 24 hours of TBI.
Analysis of neo gene expression by colony-forming unit-culture (CFU-C) assay.
CD34-enriched cells were cultured in a double layer agar culture system as previously described.4 Briefly, isolated cells were cultured in alpha minimal essential medium supplemented with FBS (Hyclone, Logan, UT), BSA (fraction V; Sigma), and 1% (wt/vol) agar (Difco, Detroit, MI), overlaid on medium with 0.6% agar (wt/vol) containing 100 ng/mL each of canine SCF, G-CSF, GM-CSF, and 4 U/mL human erythropoietin (all growth factors were kindly provided by Amgen, Thousand Oaks, CA). Cultures were incubated at 37°C in 5% CO2 and 95% air in a humidified incubator. CD34 cells were plated at a density of 10,000 cells per plate, and all cultures were performed in triplicate. Colonies grown with and without G418 (0.5 to 0.7 mg/mL) were enumerated at day 14 of culture using an inverted microscope. Untransduced control cells were plated at the same time with the same G418 concentrations. Only G418 concentrations that did not allow growth of colonies in the untransduced control samples were used to enumerate the transduced G418-resistant colonies.
PCR analysis.
Genomic DNA was prepared using DNAzol (MRC Inc, Cincinnati, OH). Amplification conditions for LN and LNX vectors have been described and were not different for LNY. Briefly, 300 to 500 ng of genomic DNA was amplified with LN(SN) 2257 and LN(ASN) 3210 primers using 2.5 U Taq polymerase (Perkin Elmer Cetus, Norwalk, CT) as previously described.5 Conditions were optimized to ensure linear amplification in the range of intensity of the positive PCR samples: denaturation at 95°C, followed by 27 cycles of 62°C annealing (1 minute) and 95°C denaturation (1 minute) with a final extension at 72°C for 7 minutes. For the detection of LN, LNX, and LNY, PCR was run in the presence of 10 μCi/mL 32P deoxycytidine triphosphate, and PCR products were separated on a 6% polyacrylamide gel. To standardize DNA concentrations between samples, PCR amplification of the β-actin gene was performed using 100 ng of genomic DNA and the following primers: actin-1, 5′ TCC TGT GGC ATC CAC GAA ACT 3′, and actin-2, 5′ GAA GCA TTT GCG GTG GAC GAT 3′. PCR conditions for the amplification of β-actin were the same as for the LN vectors, except that only 16 cycles were run.
Southern blot analysis.
Restriction digests of genomic DNA were performed with XbaI, which allowed the detection of full-length integrated retroviral vector DNA only.
Detection of helper virus.
Helper virus production was analyzed as described before.5Briefly, DNA from peripheral blood mononuclear cells was assayed for helper virus genomes by PCR. Positive control dilutions of DNA from PG13/LN packaging cells into normal dog DNA were run concurrently.
RESULTS
Engraftment in dogs transplanted with transduced CD34-enriched marrow cells.
Four dogs were transplanted with transduced CD34-enriched marrow cells. Cell numbers before and after prestimulation and transduction, purity of CD34+ cells, transduction conditions, and engraftment are shown in Table 1. A median of 3.5 × 106 CD34-enriched marrow cells/kg was obtained after CD34 separation. The median CD34 purity and recovery were 72% (range, 17% to 89%) and 48% (range, 13% to 88%), respectively. Two of the 4 animals received cells transduced in the presence of FLT3L, cSCF, and cG-CSF (FSG), and 2 animals were transplanted with cells transduced in the presence of FLT3L, cSCF, and MGDF (FSM). In 3 of the 4 animals, prestimulation with growth factors for 24 hours resulted in a reduction in cell numbers, which correlated with the degree of CD34 purity. After a 48-hour transduction period, cell numbers were further decreased when FSM was used for transduction (dogs E585 and E617), in contrast with a slight increase in cell numbers in the presence of FSG in dogs E649 and E680 (Table 1). The median cell number infused into the animals was 2.0 × 106 cells/kg; dogs in the FSM group received fewer cells (0.6 and 1.2 × 106) than the 2 other animals in the FSG group (2.8 and 6 × 106). There was, however, no obvious difference between the 2 growth factor groups in the recovery of CFU-C after transduction. In dogs E585 and E617 (FSM), 29% and 125% of CFU-C were recovered, as compared with 60% and 18% in dogs E649 and E680 (FSG).
Number of CD34-Enriched Cells Before and After Prestimulation and Transduction, CD34 Purity, Transduction Conditions, Hematopoietic Recovery and Survival in Dogs Transplanted With Transduced CD34-Enriched Cells
| Dog No. . | No. of CD34- enriched Cells/kg ×106 Before Culture (% CD34+) . | Growth Factor Combination* . | No. of CD34- Enriched cells/kg ×106After Prestimulation . | Transduction Conditions for Indicated Vector . | No. of Cells Infused/kg ×106 (LN/LNX/LNY) (total) . | ANC† >500/μL . | Survival in Months (cause of death) . | ||
|---|---|---|---|---|---|---|---|---|---|
| LN . | LNX . | LNY . | |||||||
| E585 | 4.5 (17) | FSM | 2.0 | Cocultivation | BSA | CH-296 | 0.6/0.3/0.3 (1.2) | 25 | >10 |
| E617 | 1.1 (80) | FSM | 1.1 | Cocultivation | BSA | CH-296 | 0.1/0.2/0.3 (0.6) | 35 | >9 |
| E649 | 6.8 (63) | FSG | 5.1 | Cocultivation | BSA | CH-296 | 1.8/2.4/1.8 (6.0) | 10 | >8 |
| E680 | 2.4 (89) | FSG | 2.2 | CH-296 | No BSA control | Cocultivation | 1.3/1.5 (2.8) | 18 | 5 (infection) |
| Dog No. . | No. of CD34- enriched Cells/kg ×106 Before Culture (% CD34+) . | Growth Factor Combination* . | No. of CD34- Enriched cells/kg ×106After Prestimulation . | Transduction Conditions for Indicated Vector . | No. of Cells Infused/kg ×106 (LN/LNX/LNY) (total) . | ANC† >500/μL . | Survival in Months (cause of death) . | ||
|---|---|---|---|---|---|---|---|---|---|
| LN . | LNX . | LNY . | |||||||
| E585 | 4.5 (17) | FSM | 2.0 | Cocultivation | BSA | CH-296 | 0.6/0.3/0.3 (1.2) | 25 | >10 |
| E617 | 1.1 (80) | FSM | 1.1 | Cocultivation | BSA | CH-296 | 0.1/0.2/0.3 (0.6) | 35 | >9 |
| E649 | 6.8 (63) | FSG | 5.1 | Cocultivation | BSA | CH-296 | 1.8/2.4/1.8 (6.0) | 10 | >8 |
| E680 | 2.4 (89) | FSG | 2.2 | CH-296 | No BSA control | Cocultivation | 1.3/1.5 (2.8) | 18 | 5 (infection) |
Abbreviations: ANC, absolute neutrophil count; BSA, bovine serum albumin.
Growth factor combinations used for prestimulation (24 hours) and transduction (48 hours): FLT3L, cSCF, MGDF (FSM) and FLT3L, cSCF, cG-CSF (FSG).
Days to reach a sustained neutrophil count greater than 500/μL.
All 4 animals engrafted after a median of 22 days (Table 1). Dogs transplanted with cells that were prestimulated and transduced in the presence of cG-CSF (FSG) showed a more rapid granulocyte engraftment (10 and 18 days) compared with the dogs in the FSM group (25 and 35 days), P = .13. There was no difference in platelet engraftment between the 2 groups (data not shown). One dog died 5 months posttransplant as a result of infectious complications. PCR analysis of peripheral blood samples for the presence of GALV envelope sequences was negative in all animals, indicating that there was no helper virus infection present.
Comparison between cocultivation and transduction on CH-296.
In our previous study in the dog, we used cocultivation of CD34-enriched marrow cells on vector-producing cells.19 At the end of the transduction, both packaging cells and hematopoietic cells were infused into lethally irradiated animals. Because infusion of packaging cells has not been approved for the clinical setting, we have explored whether recombinant human fibronectin fragment CH-296 would facilitate transduction into repopulating cells in the dog, similar to our studies in nonhuman primates.5 We have compared transduction on CH-296 with transduction by cocultivation in 4 animals. In 3 dogs, transduction by cocultivation with PG13/LN was compared with transduction on CH-296 with LNY(PG13). We used LNX(PG13) to transduce cells on BSA as a control for the transduction on CH-296. In 1 animal, E680, we switched vectors and used LNY(PG13) for cocultivation and LN(PG13) for the transduction on CH-296 to ensure that differences observed between transduction by cocultivation and transduction on CH-296 were caused by the transduction conditions and not the retroviral vectors.
In all 4 animals, gene transfer efficiencies into CFU-C before transplantation were higher when cells were transduced on CH-296 as compared with cocultivation, regardless of the vector used (Fig 1). After transplantation, animals were observed for the presence of vector-containing cells in peripheral blood and marrow using PCR and Southern blot analysis. Highest gene transfer levels (more than 10%) were obtained when transduction on CH-296 was combined with the growth factor combination FSG (dogs E649 and E680). The relatively high gene transfer levels were confirmed by Southern blot analysis (Fig 2). We have also sorted DM5+ granulocytes, CD3+lymphocytes, and CD14+ monocytes in dog E649 at 8 months after transplant, and PCR analysis of DNA from these different hematopoietic populations showed similar levels of gene transfer (Fig 3). Figure4 summarizes the ratios of gene transfer levels between transduction on CH-296 and cocultivation. In 3 animals (including the animal in which vectors were switched), transduction on CH-296 resulted in higher gene transfer levels than cocultivation (Fig 4). In 1 animal, E585, cocultivation resulted in a higher percentage of vector-containing cells than transduction on CH-296 (Fig 4). The difference of gene transfer levels in peripheral blood cells between cells transduced on CH-296 and cells transduced by cocultivation was more pronounced in the early posttransplant period, suggesting a greater impact of CH-296 on committed hematopoietic progenitor cells than on long-term repopulating cells.
G418-resistant CFU-C after transduction of canine CD34-enriched marrow cells and before transplantation. The bars represent the different transduction conditions: transduction on CH-296 or BSA, or transduction by cocultivation on vector-producing cells. The numbers are means from triplicate analyses.
G418-resistant CFU-C after transduction of canine CD34-enriched marrow cells and before transplantation. The bars represent the different transduction conditions: transduction on CH-296 or BSA, or transduction by cocultivation on vector-producing cells. The numbers are means from triplicate analyses.
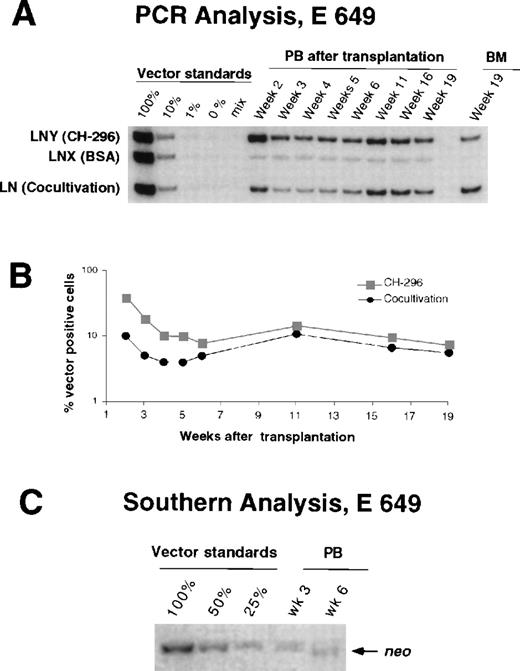
Detection of vector sequences in peripheral blood and marrow from dog E649 transplanted with CD34-enriched marrow cells transduced by 72 hours on CH-296 (LNY) or BSA (LNX), or by cocultivation (LN). (A) PCR analysis of peripheral blood cells and marrow cells. PB, peripheral blood; BM, bone marrow. (B) Percentage of vector-positive DNA as measured by phosphor image analysis of signal intensities for LN, LNX, and LNY corrected for the amount of DNA as determined by PCR for the β-actin gene. (C) Southern blot analysis.
Detection of vector sequences in peripheral blood and marrow from dog E649 transplanted with CD34-enriched marrow cells transduced by 72 hours on CH-296 (LNY) or BSA (LNX), or by cocultivation (LN). (A) PCR analysis of peripheral blood cells and marrow cells. PB, peripheral blood; BM, bone marrow. (B) Percentage of vector-positive DNA as measured by phosphor image analysis of signal intensities for LN, LNX, and LNY corrected for the amount of DNA as determined by PCR for the β-actin gene. (C) Southern blot analysis.
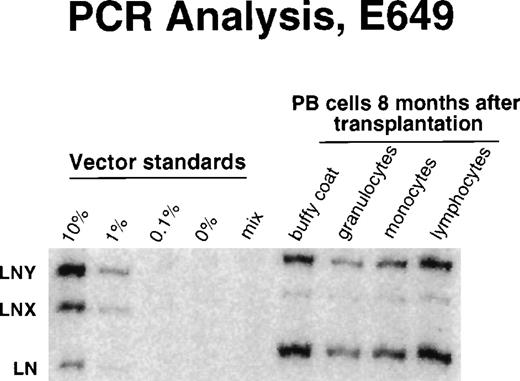
Detection of vector sequences in different hematopoietic lineages in peripheral blood from dog E649 8 months after transplantation with transduced CD34-enriched marrow cells.
Detection of vector sequences in different hematopoietic lineages in peripheral blood from dog E649 8 months after transplantation with transduced CD34-enriched marrow cells.
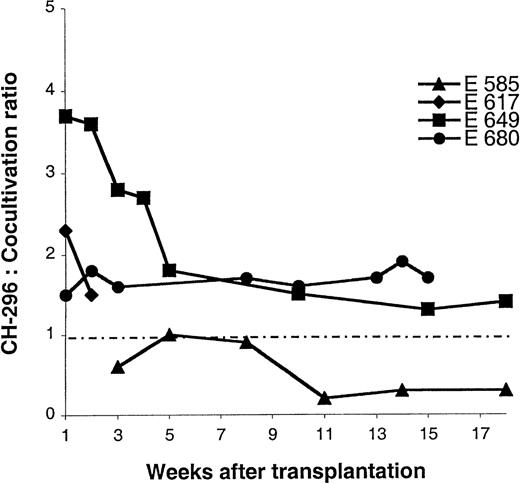
Comparison between transduction on CH-296 and cocultivation. Ratios of gene transfer levels between transduction on CH-296 and cocultivation are plotted over time. Only 2 time points are evaluable for dog E617, because both subsequent gene transfer levels were below the detection threshold.
Comparison between transduction on CH-296 and cocultivation. Ratios of gene transfer levels between transduction on CH-296 and cocultivation are plotted over time. Only 2 time points are evaluable for dog E617, because both subsequent gene transfer levels were below the detection threshold.
Comparison of transduction efficiencies between FSG and FSM.
We used recombinant canine SCF and G-CSF in combination with cocultivation to transduce canine CD34-enriched marrow cells in our previous study.19 This protocol resulted in the persistence of gene-modified cells for more than 18 months at levels up to 10%. In the current study, we wished to investigate whether the inclusion of G-CSF would also promote transduction and maintenance of hematopoietic stem cells when cells were transduced on CH-296. Most gene transfer protocols in humans or nonhuman primates have avoided G-CSF because of concerns that G-CSF might lead to differentiation and loss of hematopoietic repopulating cells. Thus, we have transplanted 2 of the 4 dogs (E585 and E617) with CD34-enriched cells transduced in the presence of FSM and 2 dogs (E649 and E680) with cells transduced with FSG. Even though there was no obvious difference in maintenance of and gene transfer efficiencies into CFU-C before transplantation between the 2 groups (Fig 1), both animals that received cells transduced with the FSM combination had lower gene transfer levels in peripheral blood and marrow than animals that received cells transduced with FSG (Fig 5); this difference was statistically significant, P = .02 (Student’s t-test). These results show that the addition of G-CSF to the transduction protocol may lead to improved transduction/maintenance of hematopoietic repopulating cells.
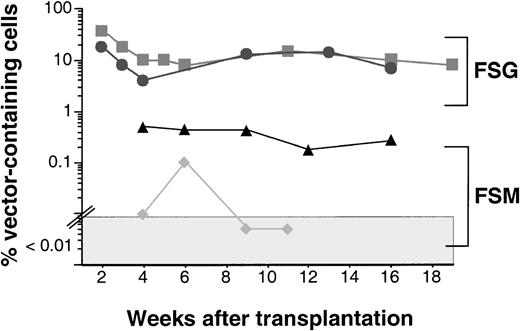
Summary of gene transfer levels in dogs transplanted with gene-modified CD34-enriched marrow cells. Dogs E585 (▴) and E617 (⧫) received gene-modified cells transduced in the presence of FLT3L, cSCF, and MGDF (FSM), and dogs E649 (▪) and E680 (•) received cells transduced in the presence of FLT3L, cSCF, and cG-CSF (FSG). PCR-negative samples (<0.01%) are plotted in the shaded area. The difference between the 2 groups is statistically significant, P= .02 (Student’s t-test).
Summary of gene transfer levels in dogs transplanted with gene-modified CD34-enriched marrow cells. Dogs E585 (▴) and E617 (⧫) received gene-modified cells transduced in the presence of FLT3L, cSCF, and MGDF (FSM), and dogs E649 (▪) and E680 (•) received cells transduced in the presence of FLT3L, cSCF, and cG-CSF (FSG). PCR-negative samples (<0.01%) are plotted in the shaded area. The difference between the 2 groups is statistically significant, P= .02 (Student’s t-test).
DISCUSSION
Most of the previously reported gene transfer studies in the dog resulted in relatively low gene transfer efficiencies.20-22Extended exposure to retroviral vectors in long-term marrow culture systems or pretreatment of donor animals with SCF and G-CSF has been shown to improve gene transfer efficiencies in vitro and in vivo.8,23-26 We have recently shown that cocultivation of CD34-enriched marrow cells on vector-producing packaging cells based on GALV resulted in improved hematopoietic stem cell gene transfer in baboons and dogs.4 19 In this study, we have shown that transduction of marrow CD34-enriched cells on CH-296 in combination with a G-CSF–containing growth factor cocktail (FSG) resulted in high-level gene transfer into hematopoietic repopulating cells in dogs without the need for cocultivation.
Recombinant human fibronectin fragment has been shown by a number of investigators to facilitate gene transfer into hematopoietic stem/progenitor cells.5-7 27 In an attempt to further improve transduction of canine hematopoietic repopulating cells, we have used our competitive repopulation system to investigate whether CH-296 would also facilitate gene transfer into hematopoietic stem cells in dogs. In all 4 dogs, transduction of CFU-C before transplantation was higher when cells were transduced on CH-296 as compared with a BSA control or cocultivation. After transplantation, gene transfer levels remained higher in CH-296–transduced cells in 3 out of 4 animals. In 1 animal, cocultivation resulted in superior gene transfer efficiency; however, overall gene transfer efficiency was low in this animal. Interestingly, the difference in gene transfer efficiency between CH-296 and cocultivation was more pronounced early after transplant, suggesting a greater effect of CH-296 on the transduction of progenitor cells. These results show that CH-296 facilitates gene transfer into short- and long-term repopulating cells in dogs.
The majority of protocols for the transduction of human or nonhuman primate hematopoietic stem/progenitor cells has included multiple cytokines such as interleukin (IL)-3, IL-6, SCF, MGDF, and FLT3L. Most of these cytokines have been shown to have activity on early hematopoietic stem/progenitor cells.5,10,11,14,28,29 However, G-CSF or GM-CSF has not been used for the transduction of hematopoietic stem cells in clinical trials, because of concerns that these growth factors might cause differentiation and, therefore, loss of repopulating cells. We had included cG-CSF during the transduction on vector-producing packaging cells in our previous study, which resulted in improved overall gene transfer into canine marrow repopulating cells. However, transduction in those studies was performed by cocultivation on vector-producing cells, a method that is currently not approved for human application. In addition, packaging cells used in these studies were based on NIH 3T3 cells, which are known to support the maintenance of repopulating cells without the addition of growth factors,30 and they could have prevented the differentiation and loss of stem cells in the presence of cG-CSF. In the present study, we have therefore investigated whether the addition of cG-CSF would also improve gene transfer into hematopoietic repopulating cells on CH-296, a transduction method that requires longer in vitro culture than cocultivation (3 days v 2 days), but has been approved for clinical application. We have compared transduction and engraftment in animals that received CD34-enriched cells transduced in the presence of cG-CSF (FSG) with animals that received cells transduced with an MGDF-containing growth factor combination (FSM). We have chosen FSM based on our encouraging results in monkeys,5 and based on published reports and our own observations that human MGDF has activity on canine progenitor cells.17 In both animals that received CD34-enriched cells transduced in the presence of cG-CSF (FSG), gene transfer levels in peripheral blood and marrow samples after transplantation were higher than in animals that received CD34-enriched cells transduced with FSM. This was in contrast with the similar transduction rates between the 2 growth factor combinations in CFU-C before infusion. The gene transfer rates obtained with the FSM combination resulted in lower gene transfer levels than in our baboon studies in which we used a similar growth factor combination for transduction of growth factor–primed CD34-enriched marrow cells. These results suggest that even though there is some activity of human MGDF on dog cells, as shown by our CFU-C data and by published reports,17 there may be a differential activity on progenitor cells and on stem cells, and it may not be sufficient to adequately support transduction and maintenance of hematopoietic repopulating cells in dogs.
In conclusion, we have developed culture conditions that allow high-level gene transfer into canine hematopoietic repopulating cells in a clinically applicable protocol. Our results suggest that the use of G-CSF during transduction of CD34-enriched cells on CH-296 may further improve retroviral gene transfer into hematopoietic stem cells.
ACKNOWLEDGMENT
The authors thank Eric Bell, Alix Smith, the technicians of the Canine Shared Resource, the hematology and pathology laboratories for their technical assistance, and Drs Beverly Torok-Storb and Shelly Heimfeld for reviewing the manuscript.
Supported in part by Grants HL36444, HL03701, DK 42716, and DK 47754 awarded by the National Institutes of Health. M.G. is supported by the German Krebshilfe. H.-P.K. is a Markey Molecular Medicine Investigator.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Hans-Peter Kiem, MD, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: hkiem@fhcrc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal