The precocious formation of bilirubinate gallstones is the most common complication of hereditary spherocytosis (HS), and the prevention of this problem represents a major impetus for splenectomy in many patients with compensated hemolysis. Because Gilbert syndrome has been considered a risk factor for gallstone formation, there are reasons for postulating that the association of this common inherited disorder of hepatic bilirubin metabolism with HS could increase cholelithiasis. To test this hypothesis, 103 children with mild to moderate HS who, from age 1, have undergone a liver and biliary tree ultrasonography every year, were retrospectively examined. The 2-bp (TA) insertion within the promoter of the uridine diphosphate-glucuronosyltransferase gene (UGT1A1), associated with Gilbert syndrome, was screened. The risk of developing gallstones was statistically different among the 3 groups of patients: homozygotes for the normal UGT1A1 allele, heterozygotes, and homozygotes for the allele with the TA insertion. Fitting a Cox regression model, in fact, a statistically significant hazard ratio of 2.19 (95% confidence interval: 1.31 to 3.66) was estimated from one to the next of these genetic classes. The individual proneness to form gallstones from TA insertion in the TATA-box of the UGT1A1 promoter should be considered during the follow-up of patients with HS. Although patients with HS were the only ones studied, extrapolating these data to patients who have different forms of inherited (eg, thalassemia, intraerythrocytic enzymatic deficiency) or acquired (eg, autoimmune hemolytic anemia, hemolysis from mechanical heart valve replacement) chronic hemolysis can be warranted.
HEREDITARY SPHEROCYTOSIS (HS) is a common hemolytic anemia and, taking into account the heterogeneity of expression, it has a prevalence of approximately 1:2,000 in Europe and North America. The underlying primary molecular lesion is heterogeneous; it may affect several membrane proteins including spectrin, ankyrin, band 3, and, more rarely, protein 4.2.1,2 Most patients do have well-compensated hemolysis. It is only when complications occur that they are brought to medical attention. Because the early formation of bilirubinate gallstones is the most common complication occurring in about half of HS patients, the prevention of this problem represents a major impetus for splenectomy in many patients with mild or moderate hemolysis.3 The pathogenesis of gallstones in HS is related to the high biliary concentration of monoconjugated bilirubin which, in turn, may play a pivotal role in gallstone formation by behaving as a source of unconjugated monohydrogenated bilirubin and as a possible coprecipitant with calcium.4
Gilbert syndrome, a benign condition of decreased bilirubin conjugation because of diminished activity of the conjugating enzyme uridine diphosphate-glucuronyl transferase (UGT1A1), has been associated with an increased production of monoconjugated bilirubin.5 A variant promoter for the UGT1A1 gene containing a two-base pair addition (TA) in the TA(6)TAA element has been recently described at the homozygous state in patients with Gilbert syndrome.6 The extra nucleotides have been shown to decrease the expression of the UGT1A1 gene and consequently the bilirubin conjugation.7 Homozygosity for the promoter variant is frequently encountered in European and African populations with a prevalence of 11% to 19%.8 Coinheritance of Gilbert syndrome has been found to be associated with increased bilirubin levels in patients with heterozygous β-thalassemia or glucose-6-phosphate dehydrogenase (G6PD) deficiency and with increased incidence of neonatal icterus in G6PD deficiency.9-11Simultaneous presence of Gilbert syndrome has been described as a possible cause of HS misdiagnosis.12-14 We recently showed that newborns with HS, homozygotes for the UGT1A1 allele with the (TA) insertion in the promoter, have enhanced jaundice, which requires phototherapy.15
The study’s aim was to determine whether Gilbert syndrome and HS, if co-inherited, could act synergistically to increase gallstone formation. Children with HS (103) were subdivided by their UGT1A1 genotype (normal, heterozygotes, and homozygotes for the mutation causing Gilbert syndrome) and retrospectively examined. Regardless of their erythrocyte membrane alteration, patients with HS, who coinherited Gilbert syndrome, had an almost 5-fold greater tendency to form gallstones than normal HS patients.
MATERIALS AND METHODS
Patients.
One hundred three unsplenectomized children, with mild to moderate HS (mean age: 9.8 ± 4 years; range, 2 to 14 years), belonging to 93 families, were clinically examined in a retrospective way. Classification of the disease’s clinical forms (mild and moderate) was performed by following well-established criteria.3Particularly, mild HS is associated with compensated hemolysis without anemia, whereas hemolysis is incompletely compensated in moderate HS. But, in these patients, hemoglobin levels are constantly higher than 80 to 90 g/L and reticulocytes are usually less than 10%.
To detect gallstones early, these patients have undergone a liver and biliary tree ultrasonography every year, starting at age 1. It must be underscored that abdominal ultrasonography represents the most reliable and readily accessible study to detect gallstones and that it has been reported to have a success rate as high as 95%.16
Erythrocyte membrane study.
The pattern of red blood cell membrane proteins was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, by using, with some modifications,17 both the discontinuous buffer system of Laemmli18 with acrylamide linear gradient from 5% to 15% and the continuous buffer system of Fairbanks et al19 with exponential gradient of acrylamide from 3.5% to 17%.
Genetic analysis.
Genomic DNA was extracted from peripheral blood leukocytes by using standard methods. Screening of the TA insertion in the A(TA)6 TATAA motif within the promoter of the UGT1A1 gene was performed by polymerase chain reaction with the primers described by Monaghan et al.7 Analysis of the amplified DNA fragments was performed in a 6%, silver stained, polyacrylamide gel.
Statistical analysis.
Continuous data are presented as mean ± SD. Differences between gaussian variables mean values were assessed by using the Student t-test; the significance is defined as P < .05. Rates of developing gallstones were calculated for each patient category by dividing the number of events (ie, gallstone formation) by the cumulative number of event-free years of observation. Assuming that the hazard rate was probably not constant during the follow-up, the Kaplan-Meier estimates were calculated. The plots of the complement of Kaplan-Meier estimates for each genotype were compared by a logrank test.
Finally, a Cox regression model was used to get an estimate of the hazard ratio (HR) of gallstone formation in function of the time of their development between the 3 classes of patients. A 95% confidence interval (CI) was calculated to measure the statistical precision of the estimate.
RESULTS
We found 41 (40%) of the HS patients homozygous for the UGT1A1 wild-type allele (TA(6)/TA(6)) and 39 (37%) heterozygotes (TA(6)/TA(7)). Twenty-three (22%) patients were homozygotes for the mutated allele (TA(7)/TA(7)). The frequency distribution of the 3 different genotypes was similar to the normal population in Southern Italy.20
The average reticulocyte number was 212 ± 60 (109/L) in the group of homozygous wild-type allele patients, 220 ± 75 (109/L) in heterozygotes, and 195 ± 55 (109/L) in homozygotes for the mutated allele. The nonsignificant differences among the 3 groups suggest a similar degree of hemolysis.
Patients with the UGT1A1 TA(7)/TA(7) genotype showed higher mean serum bilirubin levels than heterozygotes and normal patients (4.8 ± 1.9 mg/dL, 3.2 ± 1.6 mg/dL, and 2.4 ± 1.3 mg/dL, respectively). Differences among the 3 groups were significant (TA(7)/TA(7)v TA(7)/TA(6): P < .01; TA(7)/TA(7)v TA(6)/TA(6): P< .0005 ).
Five of 41 (12%) patients with the UGT1A1 TA(6)/TA(6) genotype developed gallstones, whereas this complication was discovered in 10 of 39 (26%) heterozygotes and in 11 of 23 (48%) homozygous patients for the allele with the TA insertion. Normal, heterozygotes, and homozygotes totaled 428, 384, and 202 for event-free years, and the rates of cholelithiasis formation were 0.012, 0.026, and 0.055, respectively.
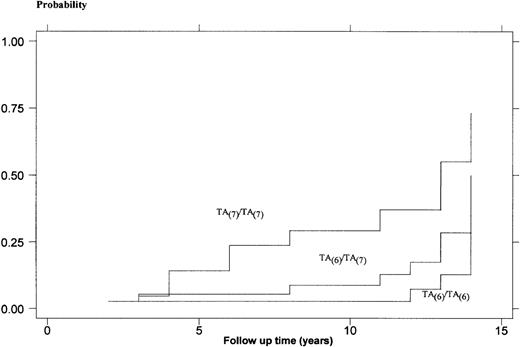
Figure 1 shows the different plots of the complement of Kaplan-Meier estimates among the 3 genotypes. The difference between the 3 plots estimated by the logrank test was statistically significant (P = .003) and represented by every follow-up time, the cumulative rate difference among the groups. The coefficient calculated by Cox regression model (TA(7)/TA(6)vTA(6)/TA(6) and TA(7)/TA(7)vTA(7)/TA(6) ) was 2.19 (95% CI: 1.31-3.66) and represented an estimate of the HR in developing gallstones. The presence of the mutated UGT1A1 allele, nevertheless, does not appear to be a major determinant of the age of gallstone formation. Although gallstone diagnosis occurred at a younger age in patients with the UGT1A1 TA(7)/TA(7)genotype, it was not significantly different compared with the normal and heterozygous children (8.7 ± 4.4 years v 11.2 ± 4.7 years and 10.4 ± 4.6 years, respectively).
Kaplan-Meier probabilities of forming gallstones for 103 patients with HS. Patients are categorized on the basis of their UGT1A1 genotype.
Kaplan-Meier probabilities of forming gallstones for 103 patients with HS. Patients are categorized on the basis of their UGT1A1 genotype.
Combined spectrin and ankyrin reduction was the most frequent biochemical alteration in the 3 categories of patients, followed by band 3 deficiency and isolated spectrin reduction (Table 1). We didn’t find statistically significant differences concerning the tendency to form gallstones when separating HS patients on the basis of their biochemical phenotype. In fact, this complication was found in 6 of 23 (26%) patients with isolated spectrin reduction, in 13 of 46 (28%) patients showing combined spectrin and ankyrin reduction, and in 7 of 27 (26%) patients with band 3 reduction.
Erythrocyte Membrane Protein Deficiencies Found in 103 Patients With HS Subdivided by Their UGT1A1 Genotype
| UGT1A1 Genotypes . | Spectrin (%) . | Ankyrin and Spectrin (%) . | Band 3 (%) . | Unknown (%) . |
|---|---|---|---|---|
| TA(6)/TA(6) | 10 (24) | 21 (51) | 9 (22) | 1 (2) |
| TA(6)/TA(7) | 9 (23) | 16 (41) | 11 (28) | 3 (8) |
| TA(7)/TA(7) | 4 (17) | 9 (39) | 7 (31) | 3 (13) |
| UGT1A1 Genotypes . | Spectrin (%) . | Ankyrin and Spectrin (%) . | Band 3 (%) . | Unknown (%) . |
|---|---|---|---|---|
| TA(6)/TA(6) | 10 (24) | 21 (51) | 9 (22) | 1 (2) |
| TA(6)/TA(7) | 9 (23) | 16 (41) | 11 (28) | 3 (8) |
| TA(7)/TA(7) | 4 (17) | 9 (39) | 7 (31) | 3 (13) |
DISCUSSION
If and when to splenectomize patients with mild to moderate HS has been constantly debated. It must be emphasized that splenectomy carries a surgical risk as well as the risk of postsplenectomy sepsis.21,22 Moreover, it has recently been reported that after age 40 the rate of myocardial infarction or stroke in HS patients without a spleen is 5.6 times the rate in patients with a spleen and, consequently, a conservative approach to splenectomy in people with HS and mild to moderate anemia has been suggested.23 In the decision regarding splenectomy of HS children with normal or only slightly low hemoglobin levels and without growth and activity impairment,24 the likelihood to form gallstones and relative complications may be considered.25-27
We show a statistically significant association between the strongly associated with Gilbert syndrome UGT1A1 TA(7)/TA(7) genotype and the increased rate of gallstones in HS children. Furthermore, the relative risk of gallstones seems to increase in an allele dose-dependent fashion, with the addition of 1 or 2 mutated UGT1A1 alleles.
These results may be explained by the combined effect of hemolysis from HS and Gilbert syndrome in producing high levels of bilirubin monoconjugates. When these interact with bile salts, calcium, or other biliary components, they could initiate pigment and cholesterol gallstone development.28-31
The contribution of coinherited Gilbert syndrome to the risk of forming gallstones should be esteemed during the follow-up of the patient with spherocytosis. It might be considered also in the decision regarding splenectomy, although this treatment is debatable in patients with mild to moderate HS, particularly because new treatments for gallstones lower the risk of this complication.2
The predisposition to gallstones is greatly increased in HS patients with Gilbert syndrome but, rather surprisingly, the age of onset for this complication, although lower compared with the other 2 groups, was not statistically different. Probably, the number of patients analyzed and the extension of the follow-up were not large enough to give statistical importance to this slight difference.
The presence of a wide distribution of membrane protein alterations (ie, isolated or ankyrin-combined spectrin deficiency and band 3 reduction) in the 3 groups of patients with HS reflects considerable heterogeneity of the primary molecular disorders. In fact, alterations of the following genes may, in turn, be involved: SPTB, ANK1, and EPB3. They map to 14q23-q24.2, 8p11-2, and 17q12-q21, respectively.32 This strongly suggests that the phenomenon (ie, greater predisposition to form gallstones in HS patients with Gilbert syndrome) is linked to a common phenotype (ie, chronic hemolysis) rather than to a particular molecular defect in HS. For this reason, although the 103 children investigated belonged to 93 families (ie, there were 10 couples of siblings), we, performing statistical analysis, didn’t cluster families, but chose to treat each patient as independent.
In patients who coinherited HS and Gilbert syndrome, the formation of gallstones represents an interesting example of how different genes (SPTB, ANK1, and EPB3) mutated to produce the same phenotype (HS), in turn interacting with another mutated gene (UGT1A1 with a [TA] insertion in the promoter), can produce a multigenic disease.
The association of an increased bilirubin load from chronic hemolysis and the diminished hepatic conjugation of bilirubin, increases the risk of gallstones. Consequently, although patients with HS were the only ones studied, extrapolating these data to patients who have different forms of inherited (eg, thalassemia, intraerythrocytic enzymatic deficiency) or acquired (eg, autoimmune hemolytic anemia, hemolysis from mechanical heart valve replacement) chronic hemolysis can be warranted.33 34 Although confirmatory studies are still needed, the assessment of the UGT1A1 genotype might be helpful in the management of these categories of patients.
Finally, gastroenterologists who study patients with gallbladder disease, stimulated by these data, might do well to search for the interaction between Gilbert syndrome and, until now unrecognized, slight hemolysis in some of their patients.
Supported by a grant from the “Regione Campania: Ricerca Sanitaria Finalizzata 86/87” and by Telethon Prog. E 645.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Emanuele Miraglia del Giudice, MD, Dipartimento di Pediatria, Seconda Università di Napoli, Via S.Andrea delle Dame N°4, 80138 Napoli, Italy; e-mail:nobili@unina.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal