Tumor necrosis factor- (TNF-) is released from the cell surface by cleavage of pro–TNF- by metalloproteinases (MPs). In cell cultures, inhibition of MPs has been found not only to reduce the release of TNF-, but also to enhance the surface expression of TNF- and TNF- receptors, which might lead to a proinflammatory effect. To determine the effect of MP inhibition during inflammation in humans, 7 healthy subjects were studied after intravenous injection of lipopolysaccharide (LPS; 4 ng/kg) preceded (−20 minutes) by an oral dose of the MP inhibitor GI5402 (100 mg) or matching placebo. GI5402 strongly reduced LPS-induced TNF- release (P < .001), but did not influence the increase in monocyte-bound TNF-. In addition, GI5402 attenuated the rise in plasma-soluble TNF- receptors types I and II after LPS injection (both P < .001), but did not change the LPS-induced decreases in granulocyte and monocyte TNF- receptor expression. These data suggest that MP inhibitors may be useful as a treatment modality in diseases in which excessive production of TNF- is considered to play an important role. Furthermore, unlike in vitro, no evidence has been found in vivo with MP inhibition for a potential proinflammatory effect due to increases in membrane-bound TNF- and TNF- receptor number.
TUMOR NECROSIS FACTOR-α (TNF-α) has been implicated as an important mediator in the pathogenesis of a variety of inflammatory diseases, including septic shock, rheumatoid arthritis, and Crohn′s disease.1 Two forms of TNF-α have been identified, a membrane-bound precursor protein of 26 kD, and a 17-kD mature secreted form. Both forms can assume a homotrimeric configuration and are biologically active.2,3 The cleavage of cell-associated TNF-α is mediated by metalloproteinases (MPs), including TNF-α-converting enzyme (TACE).4,5 MP inhibitors have been found to prevent TNF-α release in mononuclear cell cultures in vitro and in mice in vivo, and to protect mice from a lethal dose of lipopolysaccharide (LPS).6-14 MPs are also involved in the shedding of other surface molecules, including TNF-α receptors types I and II.9,10,13,15 16
In theory, inhibition of MPs can result in the accumulation of biologically active pro–TNF-α at the cell surface. Indeed, TACE-deficient mouse T cells showed an almost complete abrogation of TNF-α release upon activation, with a simultaneous increase in surface TNF-α expression compared with normal wild-type cells,4 and treatment of mononuclear cells with different MP inhibitors similarly enhanced cell-associated TNF-α expression.4,6,8 Of considerable interest, a recent report has suggested that inhibition of MPs may also render cells more sensitive to TNF-α effects by preventing the shedding of TNF-α receptors from the cell surface, thereby increasing the number of transmembrane TNF-α receptors available for signal transduction.17 In accordance, TACE-deficient cells failed to shed the type II TNF-α receptor upon stimulation with various stimuli, including LPS.15 Hence, the therapeutic potential of MP inhibitors in inflammatory diseases may be hampered by increased expression of membrane-bound TNF-α and TNF-α receptors and thus a potential proinflammatory effect.
Knowledge of the effects of MP inhibitors on the processing of TNF-α and TNF-α receptors through in vivo models of inflammation is limited. In the present study, we sought to determine the effect of a newly developed MP inhibitor on soluble and cell-bound TNF-α and TNF-α receptor levels in healthy humans injected with a single dose of LPS.
MATERIALS AND METHODS
Matrix MP and TACE inhibitory activity of GI5402.
Ki (inhibitory constant) values for GI5402 (Glaxo Wellcome, Greenford, UK) were determined for human 19-kD truncated collagenase (matrix MP [MMP]-1), 20-kD truncated collagenase-3 (MMP-13), stromelysin-1 (MMP-3), and 50-kD truncated gelatinase B (MMP-9) using the fluorogenic substrate, Dnp-Pro-Cha-Gly-Cys(Me)-His-Ala-Lys (N-methylanthranilic acid)-NH2.18 Assays were conducted in a total volume of 0.180 mL assay buffer (200 mmol/L NaCl, 50 mmol/L Tris, 5 mmol/L CaCl2, 10 μmol/L ZnSO4, 0.005% Brij 35, pH 7.6) in each well of a black 96-well microtiter plate. 19-kD collagenase-1, 20-kD collagenase-3, stromelysin-1, and 50-kD gelatinase B concentrations were adjusted to 500 pmol/L, 30 pmol/L, 5 nmol/L, and 100 pmol/L, respectively. A concentration-effect curve was generated for GI5402 using an 11-point 3-fold serial dilution with initial starting concentrations of 100, 10, or 1 μmol/L. Inhibitor and enzyme were incubated for 30 minutes at room temperature and then the reaction initiated with addition of 10 μmol/L fluorogenic substrate (above). The product formation was measured at EX343/EM450 nm (excitation/emission) after 45 to 180 minutes (time dependent on MP studied and hence rate of substrate cleavage) using a Fluostar SLT fluorescence analyzer (TecanUS Inc, Research Triangle Park, NC). Under the above conditions, the substrate concentrations are << than Kmand the Ki can be determined directly by plotting the percentage inhibition versus the log of the inhibitor concentration.
Assays to determine inhibition constants for TACE were run in a 96-well plate using a biotinylated and tritiated peptide substrate based on the cleavage sequence surrounding precursor TNF-α. A microsomal preparation of TACE5 was diluted 1/25 into 10 mmol/L HEPES, pH 7.5, and incubated with a biotinylated and tritiated version of the TACE substrate SPLAQAVRSSSRTPS-NH2. After 2 hours at room temperature, the substrate turnover was determined in the presence or absence of inhibitor using avidin-conjugated scintillation proximity beads. Percentage inhibition versus log (inhibitor) was plotted to determine the IC50 (inhibitory concentration, 50%), where Ki = IC50/(1 + [S]/Km). Depletion of substrate was followed using a scintillation proximity assay (SPA).5
Whole-blood stimulation.
Whole-blood stimulation was performed as described previously.19 Briefly, on the first study occasion, and before dosing, blood was collected aseptically from subjects using a sterile collecting system consisting of a butterfly needle connected to a syringe (Becton Dickinson, Rutherford, NJ). Anticoagulation was obtained using sterile heparin (LEO Pharmaceutical, Weesp, the Netherlands) (final concentration, 10 U/mL blood). Whole blood, diluted 1:1 in sterile RPMI-1640 (GIBCO-BRL, Life Technologies, Grand Island, NY), was stimulated for 24 hours at 37°C with LPS (final concentration, 10 ng/mL) with or without increasing concentrations of GI5402 (30 to 10,000 ng/mL) in sterile polypropylene tubes (Becton Dickinson). A 24-hour incubation period was chosen, since TNF levels have reached a plateau by this time.20 For these experiments, polypropylene tubes were prefilled with 0.75 mL RPMI containing the appropriate concentrations of LPS and GI5402, after which 0.75 mL heparinized blood was added. Tubes were then gently mixed and placed in the incubator. After the incubation plasma was prepared by centrifugation and stored at −20°C until TNF-α assays were performed.
Human endotoxemia.
The study was approved by the Research and Ethics Committees of the Academic Medical Center, Amsterdam, the Netherlands, and written informed consent was obtained from all volunteers before study entry. The study was designed as a double-blind, randomized, cross-over, placebo-controlled study in which 7 male volunteers (mean age, 22 years; range, 20 to 25 years; weight range, 74 to 90 kg) were treated with LPS on 2 occasions, with an interval of 6 weeks between. All study subjects had an unremarkable medical history, physical examination, and routine laboratory examination before study entry. During the 2 study periods, they were admitted to the hospital Clinical Research Unit. On 1 study occasion, fasted subjects were given an oral dose of GI5402, as a 100-mg tablet, which was followed 20 minutes later by an intravenous injection of LPS. On the other occasion, matching placebo preceded the LPS injection. Placebo tablets were visually and chemically identical with the exception of the presence of GI5402. Escherichia coliLPS, lot G (UPS, Rockville, MD) was administered over 1 minute into an antecubital vein at a dose of 4 ng/kg body weight.
Heart rate, blood pressure (by Dinamap, Critikon, Tampa, FL), and oral temperature were measured before GI5402 administration, 20 minutes postadministration (ie, just before LPS injection), and at intervals thereafter up to 24 hours. Clinical symptoms were recorded throughout the study periods using a scoring list for separate signs and symptoms, such as headache, shivers, nausea, vomiting, tiredness, and malaise (0 = absent; 1 = weak; 2 = moderate; 3 = severe).
Sampling and assays.
Blood was obtained from a cannulated forearm vein 0.5 hours before LPS injection (ie, directly before administration of GI5402 or placebo), directly before LPS administration (time = 0 hours) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, and 24 hours thereafter. Blood for FACScan analyses was collected in heparin-containing vacutainer tubes; all other samples were drawn in K3-EDTA–containing tubes. Leukocyte counts and differentials were assessed by a Stekker analyzer (counter STKS; Coulter counter, Bedfordshire, UK). All blood samples (except samples for flow cytometry) were centri-fuged at 2,000gfor 20 minutes at 4°C and plasma was stored at −20°C until assays were performed. The following enzyme-linked immunosorbent assays (ELISAs) were used according to the instructions of the manufacturer and/or were described in detail previously (with detection limits): TNF-α (Medgenix Diagnostics, Brussels, Belgium; 21 pg/mL) and soluble TNF-α receptors type I and II (Hoffman La Roche, Basel, Switzerland; both 70 pg/mL).21 The TNF-α ELISA measures total TNF-α (free and complexed with its receptors)22; the soluble TNF-α receptor assays detect unoccupied TNF-α receptors.21 Blood obtained for FACScan analysis was immediately put on ice. Erythrocytes were lysed with ice-cold isotonic NH4Cl solution (155 mmol/L NH4Cl, 10 mmol/L KHCO3, 0.1 mmol/L EDTA, pH 7.4) for 10 minutes. Cells were centrifuged at 600g for 5 minutes at 4°C. The remaining cells were brought to a concentration of 4 × 106cells/mL in FACS buffer (phosphate-buffered saline [PBS] supplemented with 0.5% bovine serum albumin [BSA], 0.01% NaN3, and 100 mmol/L EDTA). Expression of cell-surface TNF-α was determined using a fluorescein isothiocyanate (FITC)-labeled anti-human TNF-α monoclonal antibody (MoAb) (clone 6401; R&D Systems, Abingdon, UK). This MoAb recognizes both the 26-kD membrane-associated form of TNF and TNF bound to its cell surface receptor.23 24 Saturation binding of TNF-α by white blood cells was determined using phycoerythrin (PE)-labeled human TNF-α (Fluorokine; R&D Systems). Expression of type I and type II TNF-α receptors was determined using biotinylated MoAbs directed against either the type I (p55; clone MR1-2) or type II (p75; clone MR2-1) TNF-α receptor (Monosan, Uden, the Netherlands). These MoAbs are nonneutralizing, ie, they bind to receptor epitopes not involved in TNF-α binding (information supplied by the manufacturer). All FACS reagents were used in concentrations as recommended by the manufacturers, and all analyses were also conducted with the appropriate control antibodies (murine IgG1 or IgG2 [Becton Dickinson]). For each test, 105cells were counted. Mean cell fluorescence (MCF) at greater than 570 nm of forward and side angle scatter-gated granulocytes, monocytes, and lymphocytes was assessed. Data are presented as the difference (linear units) between MCF intensities of specifically and nonspecifically stained cells.
Blood samples (1 mL) for parent drug assay (times relative to LPS administration), were taken pre-GI5402, −10, 0, 10, 25, 40 minutes, and 1, 1.5, 2, 3, 4, 5, 6, 8, 12, and 24 hours post-LPS dose. Samples were drawn into clotting tubes, allowed to clot at room temperature for approximately 30 minutes, centrifuged within 15 minutes at 1,500g for 10 minutes at 4°C, and serum separated and stored at −20°C until assay. GI5402 was measured by liquid chromatography-mass spectrometry following solid-phase extraction with a lower limit of quantification of 1 ng/mL. The maximum observed serum concentration (Cmax) and the time at which the maximum serum concentration was observed (tmax) were calculated using PEARS, an in-house PC-based pharmacokinetic software package.
Statistical analysis.
Values are given either as arithmetic mean ± SEM or geometric mean with 95% confidence intervals (CIs). Differences between GI5402 and placebo treatment periods were tested by analysis of variance (ANOVA) for repeated measures. Changes of variables over time were analyzed using 1-way ANOVA. Differences in maximum symptom scores between the 2 study periods were analyzed with the paired t-test. The correlation between in vitro IC50 values and percentage inhibition of TNF-α in vivo was calculated using the Pearson correlation test. The area under the curve for plasma concentration of TNF-α was calculated from time point −0.5 hours pre-LPS up to the point at which the second derivative of the curve changes sign (5 hours post-LPS in all subjects). All tests were done using SPSS for Windows (Microsoft, Redmond, WA). A 2-tailed P value less than .05 was considered significant.
RESULTS
Inhibitory activity of GI5402 on isolated MMPs and TACE.
GI5402 is a potent inhibitor in vitro of a range of human isolated MMPs, including collagenase-1 (MMP-1), collagenase-3 (MMP-13), stromelysin-1 (MMP-3), and gelatinase B (MMP-9) with IC50values (Ki) in the low nanomolar range (Table1). The compound was relatively nonselective across this range of MMPs. It was also a potent inhibitor of isolated TACE, with an IC50 of 6.6 nmol/L (equivalent to 2.6 ng/mL).
In Vitro Inhibitory Profile of GI5402 Against a Range of Isolated MMPs and TACE
| . | Isolated Enzymes (Ki, nmol/L) . | ||||
|---|---|---|---|---|---|
| MMP-1 . | MMP-3 . | MMP-9 . | MMP-13 . | TACE . | |
| Geometric mean | 2.2 | 26.6 | 4.2 | 1.0 | 6.6 |
| 95% CI | 2.0-2.5 | 19.3-36.7 | 3.7-4.8 | 0.9-1.1 | 4.3-10.2 |
| n | 10 | 7 | 11 | 8 | 5 |
| . | Isolated Enzymes (Ki, nmol/L) . | ||||
|---|---|---|---|---|---|
| MMP-1 . | MMP-3 . | MMP-9 . | MMP-13 . | TACE . | |
| Geometric mean | 2.2 | 26.6 | 4.2 | 1.0 | 6.6 |
| 95% CI | 2.0-2.5 | 19.3-36.7 | 3.7-4.8 | 0.9-1.1 | 4.3-10.2 |
| n | 10 | 7 | 11 | 8 | 5 |
Effect of GI5402 in whole blood in vitro.
In 6 of 7 healthy volunteers, the effect of GI5402 on TNF-α release in LPS-stimulated whole blood was investigated before their participation in the in vivo phase of the study. GI5402 caused a concentration-dependent inhibition of LPS-induced TNF-α release in vitro in all subjects with, on average, greater than 95% inhibition achieved at 10,000 ng/mL GI5402. The inhibitory potency was similar in all subjects, with a mean (n = 6) IC50 of 515 ng/mL (95% CI, 405 to 654 ng/mL) (Table 2). This potency is substantially lower (≈200-fold) than that observed on the isolated TACE enzyme. Apart from differences in methodologies in determining these IC50 values, the difference in potency between whole cell and isolated enzyme may suggest a reduced ability of GI5402 to access the cellularly located enzyme.
Comparison of the In Vitro IC50 to Inhibit TNF Release in LPS-Stimulated Whole Blood With the Magnitude of Inhibition of TNF In Vivo for Each Subject
| Subject No. . | IC50 (ng/mL) In Vitro . | Inhibition of TNFα Release In Vivo (%) . | Cmax(ng/mL) in Serum . | Tmax (h) . |
|---|---|---|---|---|
| 1 | 675.2 | 14 | 954 | 1.0 |
| 2 | 493.4 | 95 | 360 | 1.33 |
| 3 | 470.3 | 74 | 1,032 | 0.75 |
| 4 | 605.3 | 63 | 944 | 1.0 |
| 5 | 352.3 | 72 | 605 | 0.75 |
| 6 | 557.1 | 94 | 1,065 | 0.50 |
| Subject No. . | IC50 (ng/mL) In Vitro . | Inhibition of TNFα Release In Vivo (%) . | Cmax(ng/mL) in Serum . | Tmax (h) . |
|---|---|---|---|---|
| 1 | 675.2 | 14 | 954 | 1.0 |
| 2 | 493.4 | 95 | 360 | 1.33 |
| 3 | 470.3 | 74 | 1,032 | 0.75 |
| 4 | 605.3 | 63 | 944 | 1.0 |
| 5 | 352.3 | 72 | 605 | 0.75 |
| 6 | 557.1 | 94 | 1,065 | 0.50 |
The peak serum concentrations of GI5402 (Cmax) and time to peak concentration (tmax) are also shown. The in vitro IC50 was determined in whole blood from each subject stimulated with LPS (10 ng/mL) for 24 hours at 37°C in the presence of increasing concentrations of GI5402. Percentage inhibition of in vivo TNFα release by GI5402 is expressed relative to LPS-induced TNFα release in the placebo period (area under the curve). The area was calculated from timepoint −0.5 hours up to the point at which the second derivative of the curve changes sign (5 hours in all subjects). Pearson’s correlation test calculated r2 = .36 and P = .2. For definitions of Cmax and tmax, see Materials and Methods.
Clinical signs and circulating leukocyte counts.
Injection of LPS induced a febrile response, peaking after 3.5 hours (38.8 ± 0.3°C), together with transient flu-like symptoms, including headache, nausea, retching, malaise, and chills. In addition, LPS administration resulted in a biphasic change in neutrophil counts, characterized by an initial neutropenia (from 3.4±0.4 to 1.1 ± 0.2 × 109/L after 1 hour) followed by neutrophilia (12 hours: 14.8 ± 1.0 × 109/L), monocytopenia (from 0.57 ± 0.04 to 0.02 ± 0.003 × 109/L after 3 hours), and lymphocytopenia (from 1.7 ± 0.2 to 0.3 ± 0.02 × 109/L after 6 hours). None of these changes were influenced by GI5402 (data not shown).
Soluble and cell-bound TNF-α.
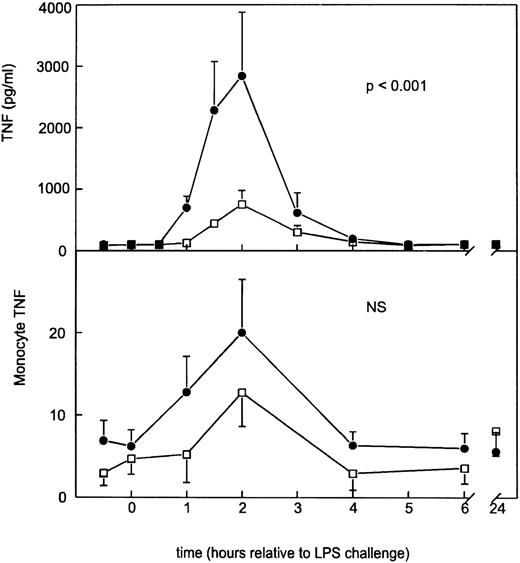
Following placebo, LPS elicited a monophasic increase in plasma soluble TNF-α concentrations peaking after 2 hours (2,839 ± 1,041 pg/mL) (Fig 1). This LPS-induced rise in soluble TNF-α levels was associated with an increase in monocyte-bound TNF-α immunoreactivity from 6.2 ± 2.0 to 20.0 ± 6.5 U (Fig1). GI5402 produced a 74% reduction in the peak LPS-induced increase in plasma-soluble TNF-α levels (2 hours: 750 ± 226 pg/mL;P < .001 v placebo), while it did not significantly alter the enhanced expression of monocyte TNF-α (Fig 1). Granulocyte- and lymphocyte-associated TNF-α immunoreactivities were low and remained unchanged in both study periods (data not shown).
Mean (±SE) plasma concentrations of soluble TNF- (pg/mL) and monocyte membrane-anchored TNF- after intravenous injection of LPS in subjects either receiving GI5402 (□) or placebo (•) given 20 minutes before LPS challenge (lot G, 4 ng/kg). Pvalue indicates the difference between treatment groups. NS, nonsignificant.
Mean (±SE) plasma concentrations of soluble TNF- (pg/mL) and monocyte membrane-anchored TNF- after intravenous injection of LPS in subjects either receiving GI5402 (□) or placebo (•) given 20 minutes before LPS challenge (lot G, 4 ng/kg). Pvalue indicates the difference between treatment groups. NS, nonsignificant.
The GI5402 IC50 against LPS-induced TNF-α release in vitro obtained in 6 of 7 volunteers did not correlate significantly with the relative potency of GI5402 to inhibit LPS-induced TNF-α release in vivo in these same subjects (r2 = .36,P = .2) (Table 2). GI5402 Cmax values in individual subjects and tmax are also shown in Table 2. Cmax values ranged from 360 to 1,065 ng/mL and were greater than the IC50 values determined in vitro for 5 of 6 subjects. Time to peak serum concentration of GI5402 was similar in most subjects (0.75 to 1.0 hours), which was approximately 1 hour before the peak in soluble TNF-α; mean plasma half-life was 4.8 hours (range, 2.8 to 7.2). An examination of the relationship between percent inhibition of soluble TNF-α in vivo and serum concentration of drug was not possible due to the relative lack of pharmacokinetic data for each subject around the time of the peak in soluble TNF-α.
Soluble and cell-bound TNF-α receptors.
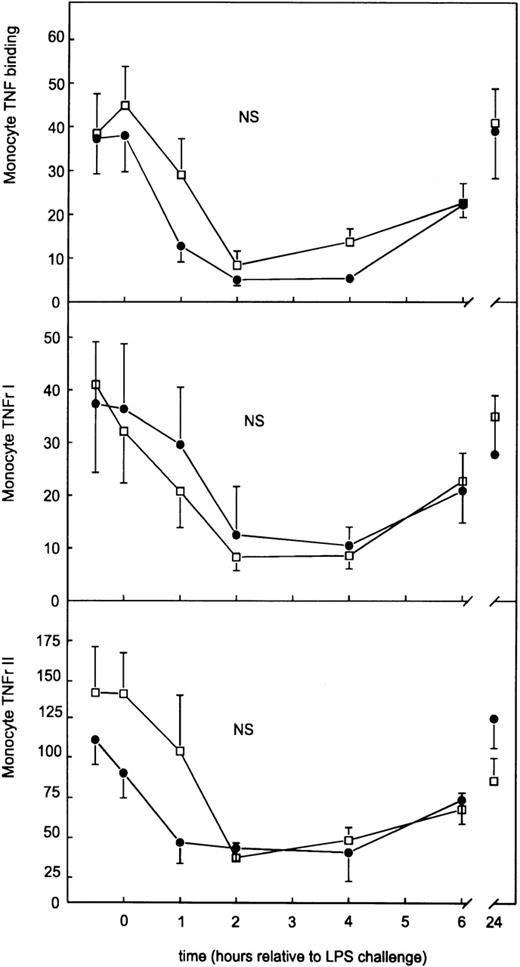
Because MP inhibitors have been found to influence the cell-surface expression of TNF-α receptors,10,11,14 16 we determined the effect of GI5402 on the shedding and surface expression of type I and type II TNF-α receptors. LPS injection induced increases in the plasma concentrations of soluble TNF-α receptor type I and type II, peaking after 2 hours (7.08 ± 0.38 ng/mL and 15.32 ± 0.85 ng/mL, respectively). GI5402 inhibited the release of both soluble TNF-α receptor species (Fig 2); peak concentrations of the type I receptor were 3.75 ± 0.38 ng/mL, and of the type II receptor 8.52 ± 0.89 ng/mL (both P < .001v placebo). LPS also induced a transient decrease in the binding of PE-labeled TNF-α by circulating monocytes and granulocytes, reaching its nadir at 2 to 4 hours (Figs3 and 4). This decreased monocyte and granulocyte PE-labeled TNF-α binding was associated with a reduced expression of both type I and type II TNF-α receptors at the surface of these cell types, also reaching minimum values at 2 to 4 hours. In contrast to its effect on soluble TNF-α receptors, GI5402 did not influence LPS-induced changes in monocyte and granulocyte TNF-α receptor expression (Figs 3 and 4). Lymphocyte TNF-α receptor expression was low throughout both study periods (data not shown).
Mean (±SE) plasma concentrations of soluble TNF- receptor I and II after intravenous injection of LPS in subjects receiving either GI5402 (□) or placebo (•) 20 minutes before LPS challenge (lot G, 4 ng/kg). P value indicates the difference between treatment groups.
Mean (±SE) plasma concentrations of soluble TNF- receptor I and II after intravenous injection of LPS in subjects receiving either GI5402 (□) or placebo (•) 20 minutes before LPS challenge (lot G, 4 ng/kg). P value indicates the difference between treatment groups.
Monocyte surface TNF- receptor and monocyte PE-labeled TNF- binding after intravenous injection of LPS in subjects receiving either GI5402 (□) or placebo (•) given 20 minutes before LPS challenge (lot G, 4 ng/kg). Results are expressed as the difference between specific mean channel fluorescence (MCF) and nonspecific MCF (mean ± SE).
Monocyte surface TNF- receptor and monocyte PE-labeled TNF- binding after intravenous injection of LPS in subjects receiving either GI5402 (□) or placebo (•) given 20 minutes before LPS challenge (lot G, 4 ng/kg). Results are expressed as the difference between specific mean channel fluorescence (MCF) and nonspecific MCF (mean ± SE).
Granulocyte surface TNF- receptor and granulocyte PE-labeled TNF- binding after intravenous injection of LPS in subjects receiving either GI5402 (□) or placebo (•) given 20 minutes before LPS challenge (lot G, 4 ng/kg). Results are expressed as the difference between specific MCF and nonspecific MCF (mean ± SE).
Granulocyte surface TNF- receptor and granulocyte PE-labeled TNF- binding after intravenous injection of LPS in subjects receiving either GI5402 (□) or placebo (•) given 20 minutes before LPS challenge (lot G, 4 ng/kg). Results are expressed as the difference between specific MCF and nonspecific MCF (mean ± SE).
DISCUSSION
Although MP inhibitors have been advocated as therapeutic agents for a number of inflammatory diseases, knowledge of their effects in humans in vivo is limited. Here, we have shown that the orally administered MP inhibitor GI5402 markedly reduced the appearance of soluble TNF-α in the circulation after a bolus intravenous injection of LPS into healthy humans. GI5402 did not influence the LPS-induced increase in monocyte-bound TNF-α immunoreactivity or the decreases in monocyte and granulocyte TNF-α receptor expression, but did attenuate the rise in the plasma concentrations of soluble TNF-α receptors. These results demonstrate for the first time the in vivo effects of a MP inhibitor on TNF-α and TNF-α receptor processing in humans.
Soluble mature TNF-α is released from cells by proteolytic cleavage of a 26-kD biologically active transmembrane precursor between Ala-76 and Val-77. Several years ago, it was recognized that MP inhibitors were able to reduce TNF-α release by stimulated cells, indicating that one or more MP-like enzymes were involved in this process.6-8 Furthermore, it was reported that MP inhibitors could inhibit TNF-α release in rodents in vivo and prevent LPS-induced lethality inD-galactosamine sensitized mice.6-8,11,12,14 however, recent investigations have raised concerns about possible unwanted proinflammatory actions of MP inhibitors secondary to their inhibiting effects on not only TNF-α release, but also on TNF-α receptor processing. Indeed, inhibition of MPs has been associated with enhanced expression of cell-surface TNF-α and TNF-α receptors.4,6,8,17 Therefore, we sought to determine the effects of GI5402, a broad spectrum inhibitor of MPs, on changes in cell-associated and soluble forms of both TNF-α and its receptors in a well-characterized human model of inflammation in which rises in the plasma concentrations of soluble TNF-α and soluble TNF-α receptors have been found to be accompanied by decreased expression of monocyte and granulocyte TNF-α receptors.25-27
In previous studies in healthy male volunteers, single oral doses of GI5402 (10 to 200 mg) were rapidly absorbed, and peak concentrations increased proportionally with dose. In one study in 12 healthy fasted subjects, pharmacokinetic parameters following a 100-mg tablet of GI5402 were Cmax 1,091 (984 to 1,210) ng/mL (geometric mean with 95% CI), tmax 45 (30 to 90) minutes, and half-life 3.9 (3.7 to 4.2) hours (both median and range) (unpublished data, May, 1997). These values compare closely with those obtained in the present study. The concentrations of GI5402 required to inhibit soluble TNF-α production in vivo and in whole blood in vitro also appeared to be similar. The peak plasma concentrations achieved in vivo for the majority of subjects were slightly greater than the IC50 values determined in vitro for inhibition of soluble TNF-α release. The resultant mean inhibition of soluble TNF-α in vivo of approximately 70% is therefore consistent with these slightly greater plasma concentrations of GI5402. Given the Cmax and IC50 concentration data obtained in the present study, this would suggest that measurable inhibition of TNF-α in the LPS model with 100 mg oral GI5402 might be expected to persist for between 4 to 8 hours (ie, 1 to 2 half-lives).
Our finding that GI5402 not only reduced the release of soluble TNF-α, but also that of soluble TNF-α receptors is in accordance with earlier in vitro studies,9,10,13,17 and indicates that the shedding of TNF-α receptors is regulated at least in part by MPs in humans in vivo. The fact that GI5402 did not alter LPS-induced changes in cell-surface TNF-α receptor expression contrasts with in vitro findings with the broad-spectrum MP inhibitor BB2275. This agent reversed the downmodulation of TNF-α receptors on several cell lines stimulated with phorbol myristate acetate, and enhanced the sensitivity of rhabdomyosarcoma cells to TNF-α-induced cytotoxicity.17 Possible explanations for this discrepancy include differences in experimental setting (cell lines in vitrov humans in vivo) and/or differences in the modes or spectrum of action of the different MP inhibitors used. Our finding that during human endotoxemia the release of soluble TNF-α receptors and the downregulation of blood cell-associated TNF-α receptors are not directly linked phenomena is supported by previous observations in this model of human endotoxemia. Indeed, in earlier studies, no correlation existed between the extent of down-modulation of cellular TNF-α receptors and peak plasma levels of soluble TNF-α receptors,25 and neutralization of endogenous TNF-α activity strongly reduced soluble TNF-α receptor release with no or only a modest effect on cell-surface TNF-α receptor expression.27 Possible explanations for this lack of correlation are that (1) soluble TNF-α receptors in plasma are, at least in part, derived from surface TNF-α receptors of cells that are not present in blood (eg, endothelial cells, tissue macrophages); (2) the soluble receptors are derived from an intracellular pool, not from the cell surface; (3) the MP inhibitor may not only reduce the shedding of the receptors, but also their expression; and (4) the downmodulation of peripheral blood monocyte and granulocyte TNF-α receptors is at least partly caused by internalization of their surface receptors, rather than by shedding. In accordance with the latter possibility is the in vitro finding that exposure of macrophages or monocytes to endotoxin results in a rapid and complete loss of cell-surface TNF-α binding sites secondary to internalization of TNF-α receptors.28
Our study also demonstrated a transient rise in monocyte TNF-α immunoreactivity after injection of LPS into normal humans. Although the anti-TNF-α MoAb used cross-reacts with both the 26-kD membrane form of TNF-α and TNF-α bound to its cell-surface receptor,23,24 we consider it likely that TNF-α immunoreactivity detected in the present study predominantly represented the membrane-anchored cytokine. Indeed, at the time point monocyte-bound TNF-α peaked, after 2 hours, the expression of (occupied and unoccupied) TNF-α receptors was strongly reduced, as determined by TNF-α receptor MoAbs binding to epitopes of TNF-α receptors that are not involved in TNF-α binding. These data therefore suggest that monocytes within the circulation contribute to soluble TNF-α release during endotoxemia. Membrane-anchored TNF-α remained low on circulating granulocytes and lymphocytes during endotoxemia, suggesting that these cell types are not important sources for the appearance of soluble TNF-α in the circulation. GI5402 did not cause an accumulation of pro–TNF-α on the surface of circulating cells, indicating that the previously reported rise in cell-associated TNF-α after treatment of cells with MP inhibitors in vitro may not be of great importance when using an MP inhibitor in vivo.4,6,8 The fact that membrane-anchored TNF-α (ie, pro–TNF-α) on monocytes from subjects treated with LPS plus GI5402 never exceeded the levels on monocytes from the same subjects treated with LPS only, suggests that unprocessed TNF-α is transient and has a relatively short half-life on the cell surface. This agrees with previous pulse-chase studies showing that unprocessed pro–TNF-α is rapidly degraded.8,29 Furthermore, our data are in accordance with the finding that MP inhibition did not influence the concentration of membrane-associated TNF-α in livers of mice with Concanavalin A–induced hepatitis.11
Treatment with GI5402 did not reduce clinical signs and symptoms induced by LPS. In this respect, it should be noted that in previous studies neither complete neutralization of endogenous TNF by infusion of a recombinant TNF receptor fusion protein,27,30 nor treatment with the antiinflammatory cytokine interleukin-10,31 influenced LPS-induced clinical symptoms, despite the fact that cytokine release and other inflammatory responses were strongly inhibited. Therefore, it seems likely that LPS at least in part induces symptoms directly. In spite of the lack of an effect on LPS-induced clinical signs, we consider it likely that the overall effect of MP inhibition during endotoxemia is antiinflammatory. GI5402, at oral doses in the range of 9 to 27 mg/kg, produced 90% to 100% inhibition of plasma soluble TNF-α generation in an LPS challenge model in mice (unpublished data, May 1997). By administering galactosamine in conjunction with LPS in this model, LPS induces lethality in the mouse. While GI5402 itself was not tested in the lethality model, a close analog, GW9471 with similar TACE inhibitory potency, did prevent lethality when greater than 90% inhibition of plasma soluble TNF-α generation was achieved.32 Other groups have also demonstrated a protective effect of a TACE inhibitor against LPS lethality.6 Thus, it would appear that there is good evidence for the mechanism of MP inhibition preventing the pathophysiologic effects of shedding of soluble TNF-α.
MP inhibitors have gained much attention in the past few years as potentially new antiinflammatory agents, due to their inhibiting effect on TNF-α processing in vitro and animals in vivo, and the fact that they can be administered orally. Considering that MP inhibitors are likely to be used in the treatment of patients with a number of inflammatory disorders in the near future, it is important to obtain more insight in the effects of these compounds on inflammation in humans in vivo. We found that the MP inhibitor GI5402 strongly reduced the release of soluble TNF-α in the circulation after a bolus intravenous injection of LPS in healthy humans. Undesired proinflammatory effects such as enhanced expression of surface TNF-α or surface TNF-α receptors were not observed. Our data therefore indicate that MP inhibitors may be of use as a treatment modality in patients with diseases in which excessive production of TNF-α is considered to play a central role.
ACKNOWLEDGMENT
We thank Mieke Sala for excellent technical assistance; Jeff Stafford, Mark Bickett, and Tony Leesnitzer for measurement of the isolated MP IC50 values; Alison Mackie and Bob Biddlecombe for pharmacokinetic analysis of GI5402; Emma Seaber for preparation of the protocol; and Dr Richard P. Koopmans for calculation of the IC50 values.
Supported by Glaxo Wellcome, Middlesex, United Kingdom and a grant from the Royal Netherlands Academy of Arts and Science to T.v.d.P.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Pascale E.P. Dekkers, MD, Laboratory of Experimental Internal Medicine, Room G2-105, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands; e-mail: P.E.Dekkers@AMC.UVA.NL.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal