Eleven patients with relapsed fludarabine-resistant B-cell chronic lymphocytic leukemia (CLL) or leukemic variants of low-grade B-cell non-Hodgkin’s lymphoma (NHL) were treated with the chimeric monoclonal anti-CD20 antibody rituximab (IDEC-C2B8). Peripheral lymphocyte counts at baseline varied from 0.2 to 294.3 × 109/L. During the first rituximab infusion, patients with lymphocyte counts exceeding 50.0 × 109/L experienced a severe cytokine-release syndrome. Ninety minutes after onset of the infusion, serum levels of tumor necrosis factor- (TNF-) and interleukin-6 (IL-6) peaked in all patients. Elevated cytokine levels during treatment were associated with clinical symptoms, including fever, chills, nausea, vomiting, hypotension, and dyspnea. Lymphocyte and platelet counts dropped to 50% to 75% of baseline values within 12 hours after the onset of the infusion. Simultaneously, there was a 5-fold to 10-fold increase of liver enzymes, d-dimers, and lactate dehydrogenase (LDH), as well as a prolongation of the prothrombin time. Frequency and severity of first-dose adverse events were dependent on the number of circulating tumor cells at baseline: patients with lymphocyte counts greater than 50.0 × 109/L experienced significantly more adverse events of National Cancer Institute (NCI) grade III/IV toxicity than patients with less than 50.0 × 109/L peripheral tumor cells (P= .0017). Due to massive side effects in the first patient treated with 375 mg/m2 in 1 day, a fractionated dosing schedule was used in all subsequent patients with application of 50 mg rituximab on day 1, 150 mg on day 2, and the rest of the 375 mg/m2 dose on day 3. While the patient with the leukemic variant of the mantle-cell NHL achieved a complete remission (9 months+) after treatment with 4 × 375 mg/m2 rituximab, efficacy in patients with relapsed fludarabine-resistant B-CLL was poor: 1 partial remission, 7 cases of stable disease, and 1 progressive disease were observed in 9 evaluable patients with CLL. On the basis of these data, different infusion schedules and/or combination regimens with chemotherapeutic drugs to reduce tumor burden before treatment with rituximab will have to be evaluated.
RECENT META-ANALYSES have confirmed that B-cell chronic lymphocytic leukemia (B-CLL) cannot be cured by currently available treatment options. Advanced stage patients have a median survival of 27 months.1 Therefore, therapeutic approaches to CLL are being reassessed with emphasis on laboratory-directed therapy, early enrollment of patients in clinical trials, introduction of fludarabine into combination protocols, and development of new drugs. Advances in the understanding of the biology of CLL cells have identified targets for more selective immunotherapy using monoclonal antibodies (MoAbs). Clinical antitumor efficacy of MoAbs has recently been demonstrated in patients with low-grade follicular NHL2-4 and is of interest in CLL.5-7
Fast, but transient, clearance of circulating tumor cells without major toxicity was reported after administration of the MoAb, Ab89, in a patient with diffuse, poorly differentiated lymphocytic lymphoma and a white blood cell count of 110.0 × 109/L.8Various phase I/II clinical trials were conducted investigating the safety and efficacy of the immunoglobulin G2a (IgG2a) murine MoAb, T101, in patients with advanced B-CLL.9 T101 binds to the CD5 antigen expressed on normal T lymphocytes, malignant T lymphocytes, and in B-CLL. The antibody caused a rapid, but transient, decrease in circulating tumor cells at different dose levels. Severe generalized reactions were observed after intravenous bolus injection, while toxicity was only minimal during a 50-hour infusion. More recently, the humanized monoclonal anti-CD52 antibody Campath-1H binding to malignant cells in more than 95% of B- and T-cell lymphomas produced promising results when used in both untreated10and pretreated CLL.7 The overall response rate was 42%.7 Toxicity was considerable with cellular immunosuppression and subsequent opportunistic infections in most of the heavily pretreated patients. Lower doses of Campath-1H are associated with less pronounced side effects and are currently being investigated for treatment of patients whose disease is still at an early stage.
The chimeric MoAb, IDEC-C2B8 (rituximab), binds to the CD20 antigen expressed on B lymphocytes and more than 95% of all B-cell NHL. The effector mechanism of rituximab includes complement-mediated cell lysis and antibody-dependent cellular cytotoxicity. In addition, rituximab interferes with the calcium channel function of the CD20 antigen and induces apoptosis by triggering the influx of calcium into CD20+ cells.11 Rituximab has exhibited significant antitumor activity in more than 300 patients with low-grade NHL. Intravenous application at doses of 375 mg/m2 given once weekly for 4 weeks induced complete or partial remissions in 50% of patients with relapsed advanced low-grade follicular NHL.2,3 12
Because CD20 is expressed on malignant lymphocytes in CLL in various densities, we initiated a monocenter clinical study investigating 375 mg/m2 rituximab weekly for 4 weeks in 11 patients with heavily pretreated B-CLL or leukemic variants of other low-grade NHL. The study will record that patients with lymphocyte counts exceeding 50.0 × 109/L experience a severe cytokine-release syndrome upon the first infusion of the anti-CD20 MoAb.
MATERIALS AND METHODS
Patients.
Written informed consent was obtained from all patients before study entry. Patients had to have B-CLL according to National Cancer Institute (NCI) criteria of any Rai stage or leukemic variants of other low-grade NHL requiring therapy and had to have received at least 2 prior chemotherapeutic regimens, including anthracycline- and/or fludarabine-containing schedules. Criteria for requiring therapy were as follows: disease-related symptoms, anemia and/or thrombocytopenia, bulky lymphadenopathy, and/or clinically relevant splenomegaly. Patients with prolymphocytic leukemia or Richter’s syndrome and those receiving steroids were excluded. Prior treatment with rituximab was also an exclusion criterion.
Antibody and concomitant medication.
Patients received 375 mg/m2 of the anti-CD20 MoAb rituximab (IDEC-C2B8) once weekly for 4 weeks given as an intravenous infusion in saline solution over a period of 3 to 10 hours. Because the first patient with high peripheral lymphocyte counts experienced severe side effects after attempted administration of 375 mg/m2rituximab in 1 day, all other 10 patients were treated according to a modified schedule with application of 50 mg rituximab on day 1, 150 mg on day 2, and 400 to 500 mg on day 3 of the first infusion cycle. Each dose was dissolved in 1,000 mL saline solution and the infusion was started at a rate of 50 mL per hour. During the first hour, vital signs were monitored every 15 minutes. If there were no adverse events during the first hour of treatment, the infusion rate was increased by 100 mL/h every half hour up to 300 mL/h. In case of adverse events of NCI toxicity grade II-IV, the infusion was interrupted. After the symptoms had disappeared, infusion at half the rate was continued. If adverse events were of grade IV toxicity, treatment was stopped and continued the following day starting with an initial infusion rate of 50 mL/h. During the following 3 cycles, the 375 mg/m2 rituximab dose was given in 1 day. The infusions were started at a rate of 50 mg rituximab per hour and were increased up to 400 mg/h once tolerated well. One thousand milligrams of acetaminophen was administered before the beginning of each infusion. A concomitant infusion of 2,000 mL of saline solution was given to prevent renal damage due to rapid cell elimination. For the same reason, all patients received 300 mg allopurinol once daily during the entire treatment period.
Patient monitoring.
Patients were monitored for safety and clinical antitumor effects using regular medical history, physical examination, and laboratory studies including complete and differential blood count, chemistry panel, quantitative serum IgGs, serum complement, and urinalysis performed at baseline, weeks 1, 2, 3, and 4, as well as 4 and 8 weeks after completion of the last infusion. Toxicity was evaluated according to NCI adult toxicity criteria. Standardized NCI/WG (National Cancer Institute-Sponsored Working Group) criteria were used for assessment of response to rituximab treatment13: complete remission was defined as the absence of lymphadenopathy, hepatomegaly, splenomegaly, or constitutional symptoms for at least 4 weeks after onset of response. In addition, blood counts had to be normal and less than 30% lymphocytes had to be present in a bone marrow biopsy. A partial remission was characterized by a 50% reduction in blood lymphocytes and a 50% reduction in lymphadenopathy and/or 50% reduction in splenomegaly and/or hepatomegaly. Symptoms had to be stable for at least 4 weeks after onset of response. Patients with progressive disease had to present with at least 1 of the following: a greater than 50% increase in the size of at least 2 lymph nodes or new palpable lymph nodes, a ≥50% increase of splenomegaly or hepatomegaly without evidence of transformation to a more aggressive histology, or a greater than 50% increase in the absolute number of circulating lymphocytes.
Flow cytometry.
Flow cytometric phenotyping of mononuclear cells of the peripheral blood was performed after red blood cell lysing of blood samples (Lysing solution; Becton Dickinson, Heidelberg, Germany). The cells were incubated with fluorochrome (fluorescein isothiocyanate [FITC], phycoerythrin [PE], perchlorophyll [PerCP]) conjugated mouse anti-human MoAbs (anti-CD3, anti-CD4, anti-CD5, anti-CD8, anti-CD14, anti-CD16, anti-CD19, anti-CD20, anti-CD25, anti-CD45, anti-CD56, anti-HLA-DR [Becton Dickinson]), and appropriate isotype controls for 20 minutes at 4°C. After 2 washing procedures in phosphate-buffered saline containing 0.1% bovine serum albumin and 0.01% NaN3, samples were measured on a flow cytometer (FACSCalibur; Becton Dickinson) with a minimum of 10,000 mononuclear cells acquired for each staining. Analysis and calculations were performed using CellQuest and Attractors software (Becton Dickinson).
Cytokine analysis.
Cytokine levels in the serum of blood samples obtained before and during rituximab treatment were measured by enzyme-linked immunosorbent assay (ELISA) (interleukin-2 [IL-2], IL-4, IL-10, IL-12, interferon-γ [IFN-γ] [Labserv, Giessen, Germany], IL-1α, IL-6, tumor necrosis factor-α [TNF-α] [DPC Biermann, Bad Nauheim, Germany]) according to the manufacturers’ protocols. Samples and calibrations suitable for the determination of serum cytokine concentrations were run as doublets, and the cytokine concentrations in the patients’ serum samples were calculated from the standard curves as picograms per milliliter.
Statistical analysis.
Patients were retrospectively stratified into 2 groups according to their peripheral lymphocyte counts at baseline. Group A comprised patients with less than 50 × 109/L lymphocytes, while patients with lymphocyte counts of 50 × 109/L and higher were assigned to group B. Differences in pre and/or posttreatment laboratory values between both groups were evaluated for statistical significance using the Student’s t-test. To compare incidence and degree of adverse events in both groups, NCI toxicity grades of side effects were added up for all patients of 1 group; the Student’s t-test was used to determine significance of differences.
RESULTS
Patient characteristics.
Ten patients (4 women, 6 men) with B-CLL and 1 male patient (no. 7) with a leukemic variant of mantle-cell NHL were treated in this study. Patient characteristics are summarized in Table 1. Median age was 58 years (range, 26 to 79 years). All patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 1 or 2 and active disease requiring therapeutic intervention. Four patients had CLL Rai stage IV, 3 patients Rai stage III, 2 patients Rai stage II, and 1 patient Rai stage I. The median time from first diagnosis to enrollment in this study was 6.3 years (range, 2.5 to 10 years). At the time of study entry, all patients were in second or higher relapse having received a median of 3 prior chemotherapeutic regimens (range, 2 to 5). Fludarabine had been applied to all patients at relapse except for patient no. 9, who had received VACOP-B (etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, bleomycin) as first-line therapy followed by autologous bone marrow transplantation. Seven weeks before treatment with rituximab, patient no. 2 had received 473 mg of the humanized anti-CD52 antibody Campath-1H, but progressed after 6 weeks of treatment. Splenectomy had been performed in 2 cases either because of severe autoimmune thrombocytopenia (patient no. 5) or because of a prior history of gastric cancer 5 years before onset of the CLL (patient no. 11). Lymphocyte counts at baseline varied from 0.2 × 109/L to 294.3 × 109/L, with CD20-expression on more than 90% of lymphocytes in all but 3 cases (Table 1). Patients were stratified retrospectively according to their peripheral lymphocyte counts at baseline. Group A consisted of patient nos. 1 to 5 with lymphocyte counts less than 50.0 × 109/L, while patient nos. 6 to 11 with lymphocyte counts of 50.0 × 109/L and higher were comprised in group B. Patient no. 7 had a mantle-cell lymphoma with 68.1 × 109/L peripheral lymphocytes and stage IV disease according to the Ann Arbor system.
Patient Characteristics at Study Entry
| Group . | Patient No. . | Age (yr)/Sex . | Histology (REAL) . | Rai Stage . | Years After First Diagnosis . | No. of Prior Therapies . | Lymphocyte Counts (×109/L) . | CD20+ Lymphocytes (%) . | Thrombocyte Counts (×109/L) . |
|---|---|---|---|---|---|---|---|---|---|
| A | 1 | 75/F | B-CLL | II | 2½ | 2 | 0.2 | 92.2 | 175 |
| 2 | 58/F | B-CLL | III | 10 | 5 | 6.5 | 74.0 | 202 | |
| 3 | 56/M | B-CLL | I | 5 | 5 | 7.7 | 93.6 | 314 | |
| 4 | 71/M | B-CLL | III | 4½ | 2 | 11.5 | 15.8 | 14 | |
| 5 | 69/M | B-CLL | IV | 7½ | 5 | 37.2 | 92.9 | 10 | |
| B | 6 | 66/M | B-CLL | IV | 8½ | 3 | 60.8 | 95.7 | 105 |
| 7 | 79/M | Mantle cell NHL | Ann Arbor IV | 5 mo | 2 | 68.1 | 92.8 | 172 | |
| 8 | 59/M | B-CLL | IV | 6½ | 4 | 71.1 | 97.3 | 49 | |
| 9 | 26/F | B-CLL | III | 2½ | 2 | 89.3 | 90.8 | 137 | |
| 10 | 47/M | B-CLL | II | 6½ | 4 | 124.0 | 91.3 | 155 | |
| 11 | 64/F | B-CLL | IV | 2½ | 4 | 294.3 | 70.9 | 74 |
| Group . | Patient No. . | Age (yr)/Sex . | Histology (REAL) . | Rai Stage . | Years After First Diagnosis . | No. of Prior Therapies . | Lymphocyte Counts (×109/L) . | CD20+ Lymphocytes (%) . | Thrombocyte Counts (×109/L) . |
|---|---|---|---|---|---|---|---|---|---|
| A | 1 | 75/F | B-CLL | II | 2½ | 2 | 0.2 | 92.2 | 175 |
| 2 | 58/F | B-CLL | III | 10 | 5 | 6.5 | 74.0 | 202 | |
| 3 | 56/M | B-CLL | I | 5 | 5 | 7.7 | 93.6 | 314 | |
| 4 | 71/M | B-CLL | III | 4½ | 2 | 11.5 | 15.8 | 14 | |
| 5 | 69/M | B-CLL | IV | 7½ | 5 | 37.2 | 92.9 | 10 | |
| B | 6 | 66/M | B-CLL | IV | 8½ | 3 | 60.8 | 95.7 | 105 |
| 7 | 79/M | Mantle cell NHL | Ann Arbor IV | 5 mo | 2 | 68.1 | 92.8 | 172 | |
| 8 | 59/M | B-CLL | IV | 6½ | 4 | 71.1 | 97.3 | 49 | |
| 9 | 26/F | B-CLL | III | 2½ | 2 | 89.3 | 90.8 | 137 | |
| 10 | 47/M | B-CLL | II | 6½ | 4 | 124.0 | 91.3 | 155 | |
| 11 | 64/F | B-CLL | IV | 2½ | 4 | 294.3 | 70.9 | 74 |
Abbreviation: REAL, Revised European American Lymphoma Classification.
Infusion schedule in patients with lymphocytosis.
Patient no. 9 was the first patient with lymphocytosis who was treated with rituximab at our center. The 375 mg/m2 dose of the anti-CD20 MoAb was administered in 1 day according to the infusion schedule established for patients with advanced relapsed follicular NHL in prior clinical trials. During the 10-hour infusion, a massive cytokine-release syndrome was observed associated with fever, chills, vomiting, NCI grade IV thrombocytopenia, drop of coagulation parameters, massive elevation of lactate dehydrogenase (LDH), as well as an increase in liver enzymes. Symptoms, as well as abnormal serum parameters, persisted for 2 days. As a direct consequence, the infusion regimen was changed to a fractionated schedule for all subsequent patients with more than 10.0 × 109/L peripheral lymphocytes: on day 1, 50 mg rituximab was administered over 5 to 7 hours; on day 2, 150 mg over the same period of time; and on day 3, the remainder of the 375 mg/m2 dose (400 to 500 mg).
Effect of rituximab on peripheral malignant lymphocytes.
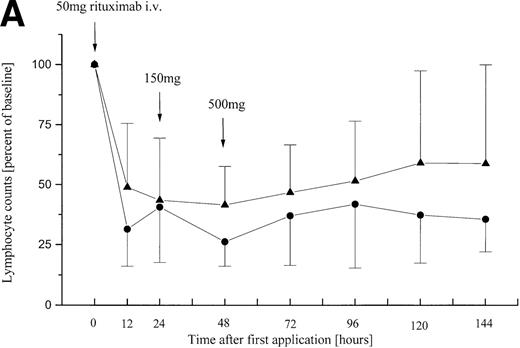
During the first infusion of rituximab, a rapid reduction of the number of peripheral malignant lymphocytes was observed in all 11 patients irrespective of their initial tumor load (Fig 1A). Blood samples taken 90 minutes after onset of the infusion showed a marked depletion of tumor cells. A total reduction of 50% to 75% of peripheral lymphocytes compared with baseline was observed 12 hours after the onset of the rituximab infusion (Fig 1A). Tumor cell counts gradually started to increase again 48 to 96 hours after beginning of the first infusion. Fluorescence-activated cell sorting (FACS) analyses indicated that all CD19/CD20-coexpressing cells were affected. After each of the following treatment cycles with rituximab, lymphocyte counts dropped, but the reduction was not as pronounced as with the first infusion (data not shown). However, tumor cell counts reached baseline values in 7 of 11 patients 4 to 6 weeks after the end of the fourth rituximab infusion.
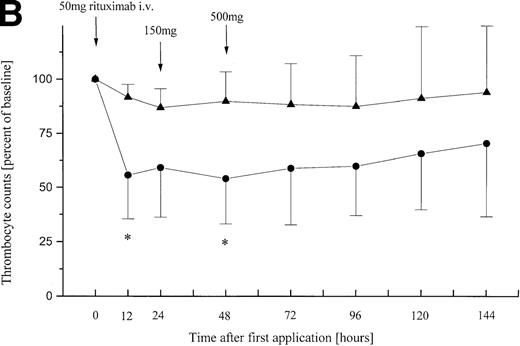
Relative peripheral cell counts in patients (n = 11) with B-CLL or leukemic mantle-cell lymphoma during 144 hours after onset of the first rituximab infusion. Mean values are plotted as percent of mean baseline counts, separately for group A (◂) consisting of 5 patients with lymphocyte counts less than 50 × 109/L and group B (•) of 6 patients with lymphocyte counts greater than 50 × 109/L at baseline. (A) Relative lymphocyte counts. (B) Relative thrombocyte counts (n = 10; patient no. 9 was excluded from analysis because of platelet transfusion 12 hours after onset of infusion). *These differences between posttreatment thrombocyte counts in group A and B are statistically significant (P = .02; P = .03).
Relative peripheral cell counts in patients (n = 11) with B-CLL or leukemic mantle-cell lymphoma during 144 hours after onset of the first rituximab infusion. Mean values are plotted as percent of mean baseline counts, separately for group A (◂) consisting of 5 patients with lymphocyte counts less than 50 × 109/L and group B (•) of 6 patients with lymphocyte counts greater than 50 × 109/L at baseline. (A) Relative lymphocyte counts. (B) Relative thrombocyte counts (n = 10; patient no. 9 was excluded from analysis because of platelet transfusion 12 hours after onset of infusion). *These differences between posttreatment thrombocyte counts in group A and B are statistically significant (P = .02; P = .03).
First-dose side effects.
Despite the fractionated infusion schedule, patients with tumor cell counts exceeding 50.0 × 109/L experienced severe side effects during application of rituximab on day 1, resulting in a temporary interruption of the infusion in 5 of 6 group B patients. Incidence and severity of adverse events during the first antibody infusion were dependent on the patients’ lymphocyte counts at baseline (Table 2). Group A patients experienced significantly less and milder adverse reactions than patients with lymphocyte counts exceeding 50.0 × 109/L (group B) (P = .0017). There were no NCI grade III or IV toxicities upon the first rituximab infusion in any of the group A patients. By contrast, patients in group B experienced 9 grade III adverse events and 1 NCI grade IV thrombocytopenia. The patient with the highest peripheral lymphocyte count (294.3 × 109/L) experienced NCI grade III dyspnea, nausea with vomiting, chills, and a tachycardia with a heart rate up to 120/min. Most importantly, the rituximab infusion had to be terminated to prevent further deterioration of her condition after 5 mg had been applied over a period of 12 hours on day 1. Retreatment with 45 mg on day 2 and 50 mg on day 3 was accompanied by nausea, chills, and fever of NCI toxicity II-III. Side effects persisted during administration of 250 mg rituximab on days 4 and 5, respectively. Due to the severity of the adverse events, this particular treatment was discontinued. Fever and chills were observed in all patients during the first infusion except for the patient with the lowest lymphocyte count (no. 1; 0.2 × 109/L) (Table 2). During the second rituximab cycle, fever and chills of only mild to moderate toxicity occurred again in patients with CD20+ lymphocyte counts exceeding 50.0 × 109/L at the time of the second infusion (data not shown).
Adverse Events During the First Administration of Rituximab According to the NCI Adult Toxicity Criteria
| Patient Lymphocytes (×109/L) . | Group A . | Group B . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. 1 0.2 . | No. 2 6.5 . | No. 3 7.7 . | No. 4 11.5 . | No. 5 37.2 . | No. 6 60.8 . | No. 7 68.1 . | No. 8 71.1 . | No. 9 89.3 . | No. 10 124.0 . | No. 11 294.3 . | |
| Chills | 0 | 0 | I | I | 0 | III | I | 0 | II | 0 | II |
| Fever | 0 | I | II | I | 0 | I | II | 0 | II | I | 0 |
| Dyspnea | 0 | 0 | 0 | 0 | 0 | I | 0 | I | 0 | 0 | III |
| Tachycardia | 0 | 0 | 0 | I | 0 | I | II | 0 | II | 0 | II |
| Nausea | 0 | 0 | 0 | I | 0 | 0 | II | II | III | I | III |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 0 | II | I | I | I |
| Hypokaliemia | 0 | 0 | 0 | 0 | 0 | 0 | I | 0 | I | 0 | I |
| Hypocalcemia | II | 0 | I | I | 0 | 0 | II | II | II | II | I |
| Increase of liver enzymes | 0 | I | 0 | 0 | 0 | II | 0 | I | III | I | I |
| Elevation of LDH | I | I | I | 0 | 0 | III | I | I | III | I | I |
| Hyperuricemia | 0 | I | I | 0 | I | I | I | II | 0 | II | I |
| Hypoproteinemia | I | I | I | I | I | I | I | II | II | I | I |
| Elevation of CRP | I | I | 0 | I | I | II | 0 | I | II | I | 0 |
| Elevation of d-dimers | 0 | I | I | 0 | I | I | 0 | I | III | 0 | I |
| Prolongation of prothrombin time | 0 | 0 | I | 0 | 0 | 0 | 0 | 0 | II | 0 | 0 |
| Anemia | NE | 0 | 0 | I | 0 | I | I | II | I | I | I |
| Thrombocytopenia | 0 | 0 | 0 | 0 | NE | I | III | I | IV | II | II |
| Patient Lymphocytes (×109/L) . | Group A . | Group B . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. 1 0.2 . | No. 2 6.5 . | No. 3 7.7 . | No. 4 11.5 . | No. 5 37.2 . | No. 6 60.8 . | No. 7 68.1 . | No. 8 71.1 . | No. 9 89.3 . | No. 10 124.0 . | No. 11 294.3 . | |
| Chills | 0 | 0 | I | I | 0 | III | I | 0 | II | 0 | II |
| Fever | 0 | I | II | I | 0 | I | II | 0 | II | I | 0 |
| Dyspnea | 0 | 0 | 0 | 0 | 0 | I | 0 | I | 0 | 0 | III |
| Tachycardia | 0 | 0 | 0 | I | 0 | I | II | 0 | II | 0 | II |
| Nausea | 0 | 0 | 0 | I | 0 | 0 | II | II | III | I | III |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 0 | II | I | I | I |
| Hypokaliemia | 0 | 0 | 0 | 0 | 0 | 0 | I | 0 | I | 0 | I |
| Hypocalcemia | II | 0 | I | I | 0 | 0 | II | II | II | II | I |
| Increase of liver enzymes | 0 | I | 0 | 0 | 0 | II | 0 | I | III | I | I |
| Elevation of LDH | I | I | I | 0 | 0 | III | I | I | III | I | I |
| Hyperuricemia | 0 | I | I | 0 | I | I | I | II | 0 | II | I |
| Hypoproteinemia | I | I | I | I | I | I | I | II | II | I | I |
| Elevation of CRP | I | I | 0 | I | I | II | 0 | I | II | I | 0 |
| Elevation of d-dimers | 0 | I | I | 0 | I | I | 0 | I | III | 0 | I |
| Prolongation of prothrombin time | 0 | 0 | I | 0 | 0 | 0 | 0 | 0 | II | 0 | 0 |
| Anemia | NE | 0 | 0 | I | 0 | I | I | II | I | I | I |
| Thrombocytopenia | 0 | 0 | 0 | 0 | NE | I | III | I | IV | II | II |
Taken into account are any abnormalities that occurred during 48 hours after onset of the first antibody infusion.
Abbreviation: NE, not evaluable due to autoimmune thrombocytopenia in patient no. 5 and autoimmune haemolysis in patient no. 1.
Thrombocytopenia of NCI grade I-IV toxicity occurred in all patients during the first infusion except for patients no. 1-4 with the lowest lymphocyte counts at baseline (0.2 × 109/L − 11.5 × 109/L) (Table 2). The extent of the decline in platelet counts induced by the first dose of rituximab was highly dependent on the number of circulating tumor cells at baseline (P = .02) (Fig 1B). Patient no. 5 had a severe disease-related autoimmune thrombocytopenia and was thus not evaluable for rituximab-associated thrombocytopenia. Patient no. 9 experienced an NCI grade IV thrombocytopenia upon the first infusion. This patient had to have a platelet transfusion, but platelet counts started to increase spontaneously 48 hours after onset of the rituximab infusion. A decrease in hemoglobin levels NCI grade I-II was observed in patients no. 3, 6, 9, and 11 without necessity of erythrocyte transfusion in any of them. Patient no. 1 was not evaluable for changes in the red blood cell count due to a disease-associated autoimmune hemolysis.
Changes of several other laboratory parameters were more pronounced in group B compared with group A patients during the first infusion, although the differences were not statistically significant (Table 3). An increase in d-dimer levels observed in 7 patients was associated with a sharp decline of platelets and a prolongation of the prothrombin time. Fibrin monomers were not detected in the serum of any of the patients. Simultaneously, serum concentrations of liver enzymes, including alanine aminotransferase, aspartate aminotransferase, and γ-glutamyl transpeptidase, increased to values exceeding 5 times the normal range, while concentrations of alkaline phosphatase, as well as direct and indirect bilirubin, remained stable throughout antibody treatment. Levels of LDH increased in 9 patients during treatment, with 2 of them (patients no. 6 and 9) peaking at values higher than 2,000 U/mL (Table 2).
Laboratory Parameters During First Rituximab Infusion
| Parameter . | Normal Range . | Group A . | Group B . | 2-Tailed P Value A v B3-150 . | ||||
|---|---|---|---|---|---|---|---|---|
| Median Prior Rituximab . | Median Postrituximab . | Change . | Median Prior Rituximab . | Median Postrituximab . | Change . | |||
| LDH | (120-240 U/L) | 283 | 364 | +81 | 318 | 772 | +454 | .213 |
| AST | (<16 U/L) | 12 | 13 | +1 | 10 | 34 | +24 | .137 |
| ALT | (<20 U/L) | 9 | 7 | −2 | 9 | 28 | +19 | .075 |
| γ-GT | (<19 U/L) | 11 | 12 | +1 | 19 | 48 | +29 | .094 |
| D-dimers | (<0.6 mg/L) | 1.9 | 2.1 | +0.2 | 1.2 | 9.4 | +8.2 | .234 |
| Calcium | (2.2-2.6 mmol/L) | 2.48 | 2.20 | −0.28 | 2.35 | 1.93 | −0.42 | .233 |
| Protein | (66-83 g/L) | 62 | 57 | +5 | 69 | 55 | −14 | .049 |
| CRP | (<8 mg/L) | 13 | 30 | +17 | 8 | 38 | +30 | .474 |
| Uric acid | (<6.1 mg/dL) | 6.4 | 5.7 | −0.7 | 6.1 | 7.4 | +1.3 | .052 |
| Hemoglobin | (12-16 g/dL) | 9.9 | 9.8 | −0.1 | 10.3 | 9.0 | −1.3 | .140 |
| Prothrom time | (70-120%) | 95 | 90 | −5 | 90 | 71 | −19 | .126 |
| Potassium | (3.6-4.8 mmol/L) | 4.0 | 4.3 | +0.3 | 4.0 | 3.8 | −0.2 | .076 |
| C3 complement | (0.75-1.40 g/L) | 0.862 | 0.674 | −0.19 | 0.803 | 0.582 | −0.22 | .249 |
| C4 complement | (0.10-0.34 g/L) | 0.566 | 0.263 | −0.30 | 0.714 | 0.252 | −0.46 | .272 |
| Parameter . | Normal Range . | Group A . | Group B . | 2-Tailed P Value A v B3-150 . | ||||
|---|---|---|---|---|---|---|---|---|
| Median Prior Rituximab . | Median Postrituximab . | Change . | Median Prior Rituximab . | Median Postrituximab . | Change . | |||
| LDH | (120-240 U/L) | 283 | 364 | +81 | 318 | 772 | +454 | .213 |
| AST | (<16 U/L) | 12 | 13 | +1 | 10 | 34 | +24 | .137 |
| ALT | (<20 U/L) | 9 | 7 | −2 | 9 | 28 | +19 | .075 |
| γ-GT | (<19 U/L) | 11 | 12 | +1 | 19 | 48 | +29 | .094 |
| D-dimers | (<0.6 mg/L) | 1.9 | 2.1 | +0.2 | 1.2 | 9.4 | +8.2 | .234 |
| Calcium | (2.2-2.6 mmol/L) | 2.48 | 2.20 | −0.28 | 2.35 | 1.93 | −0.42 | .233 |
| Protein | (66-83 g/L) | 62 | 57 | +5 | 69 | 55 | −14 | .049 |
| CRP | (<8 mg/L) | 13 | 30 | +17 | 8 | 38 | +30 | .474 |
| Uric acid | (<6.1 mg/dL) | 6.4 | 5.7 | −0.7 | 6.1 | 7.4 | +1.3 | .052 |
| Hemoglobin | (12-16 g/dL) | 9.9 | 9.8 | −0.1 | 10.3 | 9.0 | −1.3 | .140 |
| Prothrom time | (70-120%) | 95 | 90 | −5 | 90 | 71 | −19 | .126 |
| Potassium | (3.6-4.8 mmol/L) | 4.0 | 4.3 | +0.3 | 4.0 | 3.8 | −0.2 | .076 |
| C3 complement | (0.75-1.40 g/L) | 0.862 | 0.674 | −0.19 | 0.803 | 0.582 | −0.22 | .249 |
| C4 complement | (0.10-0.34 g/L) | 0.566 | 0.263 | −0.30 | 0.714 | 0.252 | −0.46 | .272 |
Abbreviations: LDH, lactate dehydrogenase; AST, aspartate transaminase; ALT, alanine transaminase; γ-GT, γ-glutamyltranspeptidase; CRP, C-reactive protein.
P values compare the changes of the laboratory parameters during rituximab treatment in group A v group B patients.
NCI grade I-II hypocalcemia was observed in 8 patients during the first rituximab infusion (Table 2). Changes in the serum potassium levels occurred less frequently. Hyperkaliemia, as well as hypokaliemia (NCI grade I-II), were observed after administration of rituximab, but high potassium concentrations were not associated with elevated creatinine levels or high peripheral lymphocyte counts at baseline. Changes in creatinine concentrations were not observed during or after therapy in any patient, including 2 patients (nos. 5 and 7) with impaired renal function and elevated creatinine values at baseline (3.3 and 1.4 mg/dL, respectively). Eight of 11 patients developed NCI grade I or II hyperuricemia despite additional application of allopurinol throughout rituximab treatment.
Serum complement levels (C3, C4) were measured before and 12 hours after onset of the first infusion of rituximab in 9 of 11 patients (Table 3). Levels of C4 decreased in 7 patients during infusion. In all 9 patients C3 levels declined during therapy, but changes were not statistically significant.
Serum cytokine levels.
At baseline, serum levels of TNF-α and IL-6 for all patients were below 20 pg/mL and 25 pg/mL, respectively. Cytokine concentrations were also measured 90 minutes and 7 hours after the onset of the rituximab infusion in most patients (Table 4). Ninety minutes after the start of the infusion, serum cytokine levels peaked. In group B patients, mean peak concentrations were 500 pg/mL for TNF-α and 280 pg/mL for IL-6, respectively. Ninety minutes after the onset of the infusion, group B patients had higher serum TNF-α (P = .049) and IL-6 (P = .08) levels than group A patients (Table 4), suggesting a significant correlation between the number of circulating tumor cells at baseline and the extent of cytokine release upon the first rituximab infusion. After completion of the first rituximab infusion, TNF-α and IL-6 levels returned to baseline values in all patients. Measurable serum levels of IFN-γ were only detected in the patient with the highest peripheral lymphocyte count (no. 11; 294 × 109/L) who had an IFN-γ level of 195 pg/mL 90 minutes after onset of the infusion (baseline, 15 pg/mL).
Cytokine Levels During the First Rituximab Infusion
| IL-6 (normal range, <25 pg/mL) . | |||
|---|---|---|---|
| Patient . | IL-6 Prior Infusion . | IL-6 90 min Postinfusion . | IL-6 7 hrs Postinfusion . |
| Gr. A No. 1 | 25 | 18 | NE |
| Gr. A No. 2 | 9 | 14 | NE |
| Gr. A No. 3 | 20 | 18 | NE |
| Gr. A No. 4 | 16 | 25 | 16 |
| Gr. A No. 5 | 11 | 16 | NE |
| Gr. B No. 6 | 17 | 53 | 15 |
| Gr. B No. 7 | 20 | 62 | 25 |
| Gr. B No. 8 | NE | NE | NE |
| Gr. B No. 9 | 20 | 244 | 40 |
| Gr. B No. 10 | 17 | 319 | 20 |
| Gr. B No. 11 | 18 | 744 | 18 |
| TNF-α (normal range, <20 pg/mL) | |||
| Patient | TNF-α Prior Infusion | TNF-α 90 min Postinfusion | TNF-α 7 h Postinfusion |
| Gr. A No. 1 | 10 | 18 | NE |
| Gr. A No. 2 | 48 | 106 | NE |
| Gr. A No. 3 | 35 | 34 | NE |
| Gr. A No. 4 | 23 | 184 | 8 |
| Gr. A No. 5 | 35 | 78 | NE |
| Gr. B No. 6 | 17 | 71 | 17 |
| Gr. B No. 7 | 20 | 195 | 28 |
| Gr. B No. 8 | NE | NE | NE |
| Gr. B No. 9 | 25 | 351 | 47 |
| Gr. B No. 10 | 12 | 843 | 42 |
| Gr. B No. 11 | 68 | 741 | 56 |
| IL-6 (normal range, <25 pg/mL) . | |||
|---|---|---|---|
| Patient . | IL-6 Prior Infusion . | IL-6 90 min Postinfusion . | IL-6 7 hrs Postinfusion . |
| Gr. A No. 1 | 25 | 18 | NE |
| Gr. A No. 2 | 9 | 14 | NE |
| Gr. A No. 3 | 20 | 18 | NE |
| Gr. A No. 4 | 16 | 25 | 16 |
| Gr. A No. 5 | 11 | 16 | NE |
| Gr. B No. 6 | 17 | 53 | 15 |
| Gr. B No. 7 | 20 | 62 | 25 |
| Gr. B No. 8 | NE | NE | NE |
| Gr. B No. 9 | 20 | 244 | 40 |
| Gr. B No. 10 | 17 | 319 | 20 |
| Gr. B No. 11 | 18 | 744 | 18 |
| TNF-α (normal range, <20 pg/mL) | |||
| Patient | TNF-α Prior Infusion | TNF-α 90 min Postinfusion | TNF-α 7 h Postinfusion |
| Gr. A No. 1 | 10 | 18 | NE |
| Gr. A No. 2 | 48 | 106 | NE |
| Gr. A No. 3 | 35 | 34 | NE |
| Gr. A No. 4 | 23 | 184 | 8 |
| Gr. A No. 5 | 35 | 78 | NE |
| Gr. B No. 6 | 17 | 71 | 17 |
| Gr. B No. 7 | 20 | 195 | 28 |
| Gr. B No. 8 | NE | NE | NE |
| Gr. B No. 9 | 25 | 351 | 47 |
| Gr. B No. 10 | 12 | 843 | 42 |
| Gr. B No. 11 | 68 | 741 | 56 |
Abbreviation: NE, not evaluable.
Clinical response.
Nine of 10 patients with CLL were evaluable for response according to NCI response criteria for CLL. Patient no. 11 was taken off study early because of the severity of adverse events during the first infusion. Seven patients experienced stable disease after treatment with 4 × 375 mg/m2 rituximab. A marked reduction of lymphocyte counts was observed in most patients lasting 4 to 6 weeks after their fourth rituximab infusion. Then, lymphocyte counts increased again requiring salvage treatment in 7 of 10 patients. Five patients experienced a reduction in size of initially enlarged lymph nodes lasting 3 weeks after the end of treatment with rituximab. Patient no. 4 was withdrawn from therapy due to nonresponsive disease after the third cycle of rituximab. In patient no. 8, lymphocyte counts were reduced from 71.1 × 109/L to 12 × 109/L and remained stable for 19+ weeks. According to NCI response criteria, this patient experienced a partial remission, which is still ongoing. Three months after completion of the fourth rituximab infusion, patient no. 5 with the severe autoimmune-thrombocytopenia was retreated with 4 × 375 mg/m2 rituximab. After the first 4 MoAb infusions, his platelet counts had increased. During rituximab retreatment, thrombocyte counts did not normalize, but bleeding stigmata ceased and the necessity of platelet transfusion was decreased.
Patient no. 7 with the leukemic variant of the mantle-cell lymphoma presented 68 × 109/L peripheral lymphocytes, bone marrow infiltration, and splenomegaly (20 × 6.5 cm) at baseline. After treatment with 4 × 375 mg/m2 rituximab, white blood cell counts, as well as thrombocyte counts and hemoglobin values, began to normalize. The size of the spleen had markedly decreased (7 × 5 cm) 3 months after the last MoAb infusion and was totally normalized after 6 months (6 × 4 cm). The patient still is in complete remission 9 months after completion of the rituximab treatment.
DISCUSSION
The following findings emerge from this study: (1) incidence and severity of adverse events during the first infusion of rituximab in patients with peripheral lymphocytosis are dependent on the number of circulating CD20+ tumor cells. Patients with lymphocyte counts exceeding 50 × 109/L experience significantly more side effects of NCI grade III and IV toxicity than patients with less than 50 × 109/L lymphocytes (P = .0017). (2) Application of the first dose of rituximab to patients with high numbers of circulating CD20+ cells results in a cytokine-release syndrome with peaks of TNF-α and IL-6 serum concentrations 90 minutes after the onset of the antibody infusion. Peak levels of these 2 cytokines are significantly higher in patients with lymphocyte counts exceeding 50.0 × 109/L before rituximab therapy than in patients with fewer circulating lymphocytes (P = .049 and P = .08, respectively). (3) Although peripheral lymphocyte counts decrease rapidly after treatment with 375 mg/m2 rituximab once weekly for 4 weeks in patients with relapsed fludarabine- or anthracycline-resistant B-CLL, overall remission rates are modest within this therapeutic schedule.
In patients with relapsed follicular low-grade NHL and normal lymphocyte counts, treatment with 4 × 375 mg/m2 of the monoclonal anti-CD20 antibody rituximab is a safe and well-tolerated therapeutic regimen.2 3 Adverse events typically occur early during the first antibody infusion and comprise fever, chills, rash, and nausea. They usually are transient and of NCI grade I or II toxicity. Thus far, patients with lymphocyte counts exceeding 10.0 × 109/L have been excluded from therapy with rituximab in clinical trials.
The present study concerns itself with antibody treatment of patients with high numbers of circulating CD20+ cells. Incidence and severity of side effects during the application of the first dose of rituximab are significantly dependent on the lymphocyte counts at baseline (P = .0017) (Table 2). While group A patients with less than 50 × 109/L peripheral tumor cells usually experience few side effects of mild to moderate (NCI grade I/II) toxicity during the first infusion, several adverse events of NCI grade III and IV toxicity occurred in group B patients with lymphocyte counts exceeding 50 × 109/L. These patients experienced a rapid increase in body temperature and massive chills 30 to 90 minutes after the onset of the rituximab infusion. Dyspnea and tachycardia were observed in 5 of 6 group B patients. Blood samples taken shortly after the onset of side effects showed a sharp decline of peripheral CD20+ lymphocytes in all patients with B-CLL (Fig 1A), while hemoglobin usually remained stable. Thrombocyte counts of group B patients decreased to significantly lower nadirs than those of group A patients (P = .02), suggesting that the degree of thrombocytopenia is dependent on peripheral lymphocyte counts at baseline. These findings support earlier observations in a patient with small lymphocytic histology and extensive bone marrow infiltration who was treated with 375 mg/m2 rituximab in the first multiple-dose trial.2 Within the first 24 hours, this patient experienced grade IV thrombocytopenia with platelet counts decreasing from a baseline value of 93,000/μL to a nadir of 19,000/μL, although there were no signs of disseminated intravascular coagulation. Seven additional patients with extended bone marrow infiltration and circulating CD20+ cells2,14also experienced transient thrombocytopenia after the first infusion of IDEC-C2B8. Therefore, Maloney et al2 have suggested that the decrease of thrombocyte counts after rituximab therapy is correlated to the number of circulating B cells. Platelet removal was considered to be dependent on platelet activation due to the binding of thrombocytic Fc receptors to circulating immune complexes. The CD20 antigen is not expressed on thrombocytes and IDEC-C2B8 has not been found to react, in vitro or in vivo, with platelets or their predecessors.2 12
In our study, other infusion-related side effects observed during the first application included fever, chills, nausea, dyspnea, and hypotension. The marked decline of the peripheral lymphocytes was also associated with an elevation of LDH (in 9 patients), uric acid (in all patients), and liver transaminases (in 6 patients), prolongation of the prothrombin time (in 2 patients) and an increase in d-dimers (in 7 patients) without detection of fibrin monomers in any of the patients. Byrd et al14 describe the same characteristic infusion-related syndrome in 5 patients with CLL, prolymphocytic leukemia, or diffuse large B-cell lymphoma and elevated lymphocyte counts at baseline (73.0 × 109/L to 132.0 × 109/L). The severity of side effects was most pronounced after a total of 25 to 50 mg rituximab had been infused. These symptoms were different from those observed during a tumor lysis syndrome in patients with aggressive lymphoma. It is more likely that a rapid release of various cytokines during the first rituximab infusion caused this characteristic syndrome in patients with high circulating lymphocyte counts (Table 4). Serum levels of IL-6 and TNF-α peaked 90 minutes after the onset of the infusion, and peak levels were always accompanied by maximum clinical side effects. Cytokine concentrations returned to baseline values as soon as the infusion was completed and also remained stable during treatment with rituximab on days 2 and 3, respectively. During the second rituximab cycle 1 week later, cytokine concentrations increased again in patients with lymphocyte counts exceeding 50.0 × 109/L, but peak values were lower when compared with the first infusion (data not shown). Serum levels of IFN-γ were not elevated in any patient apart from the 1 with the highest peripheral tumor load (294.3 × 109/L lymphocytes). A similar cytokine profile was observed after the intravenous application of bacteria-derived endotoxins15 or after the infusion of antimicrobial drugs to patients with infections caused by endotoxin-releasing bacteria. This so-called Jarisch-Herxheimer reaction typically occurs 60 to 90 minutes after the beginning of an antibiotic treatment.16 Before the onset of symptoms such as fever, rigor, hypotension, or a sharp decrease in peripheral white blood cells, there is a substantial increase in circulating levels of TNF-α, IL-6, and IL-8,17 while IFN-γ levels remain stable. Peak serum TNF-α levels of 240 ± 70 pg/mL in patients with Jarisch-Herxheimer reaction15 are comparable to peak TNF-α levels (440 pg/mL) measured in group B patients 90 minutes after the start of the rituximab infusion (Table4).
Cytokine-release syndromes clinically characterized by fever, chills, rash, and nausea have been observed after initial treatment with various other MoAbs.9,18,19 Usually, side effects and changes in laboratory parameters were more pronounced when the majority of target cells was located in the blood stream or when the therapeutic antibody dose was rapidly injected rather than infused over several hours.7,9,10 In contrast to the pattern of cytokine release reported after rituximab treatment in this study, maximum release of IFN-γ, as well as IL-2, IL-6, and TNF-α, was observed after intravenous application of MoAbs targeting T-cell antigens. The cytokine release after treatment with the murine anti–T-cell (anti-CD3) MoAb OKT318 is thought to be caused by T-cell opsonization and subsequent lympholysis of OKT3-coated cells, as well as by OKT3-induced T-cell activation. Intravenous administration of the humanized MoAb, Campath-1H (anti-CD52), is also associated with a first-dose cytokine-release syndrome involving TNF-α, IFN-γ, and IL-6.19 In vitro studies have shown that binding of Campath-1H to the low-affinity Fc-receptor for IgG (FcγR) on natural killer cells was responsible for the rapid elevation of cytokine levels after treatment of CLL patients.19 A cytokine-release syndrome with elevated levels of TNF-α and IFN-γ has also been reported after infusion of the bispecific antibody OC/TR F(ab′)2 for ligation of T lymphocytes to ovarian cancer cells.20 In patients with peripheral lymphocytosis, the source of the cytokines upon initial treatment with rituximab is unclear: one reason for early release of cytokines might be the agglutination of small lymphocytes in the lung, liver, and spleen. This assumption is supported by results of the autopsy of a patient with Richter’s syndrome who died about 12 hours after initiation of a rituximab infusion. After infusion of 50 mg rituximab, the patient’s general state deteriorated and he died of catecholamine-resistant hypotension and respiratory failure. An autopsy was performed (T. Ruediger, C. Mueller-Hermelink; personal communication, May 1999) and leukostasis was detected in all vessels, prominently in the lung, the brain, and the heart. Leukostasis has been previously described to be associated with an activation of the complement system21 and changes in the endothelial adhesivity induced by elevated cytokine levels. Pulmonary leukostasis may contribute to dyspnea and bronchospasm often encountered in patients with high peripheral lymphocyte counts after treatment with rituximab.5 14 Apoptosis of CD20+ CLL cells might be another reason for rapid cytokine release after intravenous application of rituximab. Nevertheless, only a few apoptotic cells were detected in the patient with Richter’s syndrome who died after rituximab treatment.
In the present study, response rates were poor after administration of 375 mg/m2 rituximab once weekly for 4 weeks in patients with fludarabine-resistant B-CLL in second or higher relapse. One of 10 patients attained partial remission according to NCI response criteria for CLL, which is ongoing at 3 months, while 7 patients had stable disease 6 weeks after the last rituximab infusion had been applied (Table 5). One reason for the poor overall response in this small group of heavily pretreated patients might be the less pronounced expression of CD20 on the surface of CLL cells compared with a much higher density of CD20 in follicular or mantle-cell NHL. Tefferi et al22 classify the CD20 intensity as weak in 62% of 93 previously untreated patients with B-CLL, moderate in 12%, and strong in 26%. In our study, the CD20 antigen was expressed on more than 90% of lymphocytes in 7 of 11 patients as assessed by FACS analysis. Because Taji et al23have not found any correlation between the density of the CD20 expression on the cell surface and the degree of growth inhibition of lymphoma cell lines incubated with rituximab in vitro, patient no. 4 with CD20 expression on only 15% of his tumor cells was treated with rituximab. Interestingly, he showed progressive disease during treatment. Nguyen et al4 have also observed a correlation between response rates and CD20 antigen density in some NHL entities in vivo. The patient with the leukemic variant of the mantle-cell NHL who was treated with 4 × 700 mg rituximab experienced a complete remission, which is currently ongoing (9+ months).
Maximal Response and Time to Progression After Administration of 375 mg/m2 Rituximab Once Weekly for Four Weeks
| Patient . | Lymphocyte Counts (×109/L) at Baseline . | Response . | Time to Progression After Last Infusion (wk) . |
|---|---|---|---|
| Gr. A No. 1 | 0.2 | SD | >19 (ongoing) |
| Gr. A No. 2 | 6.5 | SD | 3 |
| Gr. A No. 3 | 7.7 | SD | 8 |
| Gr. A No. 4 | 11.5 | PD | 0 |
| Gr. A No. 5 | 37.2 | SD | 3 |
| Gr. B No. 6 | 60.8 | SD | 8 |
| Gr. B No. 7 | 68.1 | CR | >36 (ongoing) |
| Gr. B No. 8 | 71.1 | PR | 19 |
| Gr. B No. 9 | 89.3 | SD | 3½ |
| Gr. B No. 10 | 124.0 | SD | 9 |
| Gr. B No. 11 | 294.3 | NE | NE |
| Patient . | Lymphocyte Counts (×109/L) at Baseline . | Response . | Time to Progression After Last Infusion (wk) . |
|---|---|---|---|
| Gr. A No. 1 | 0.2 | SD | >19 (ongoing) |
| Gr. A No. 2 | 6.5 | SD | 3 |
| Gr. A No. 3 | 7.7 | SD | 8 |
| Gr. A No. 4 | 11.5 | PD | 0 |
| Gr. A No. 5 | 37.2 | SD | 3 |
| Gr. B No. 6 | 60.8 | SD | 8 |
| Gr. B No. 7 | 68.1 | CR | >36 (ongoing) |
| Gr. B No. 8 | 71.1 | PR | 19 |
| Gr. B No. 9 | 89.3 | SD | 3½ |
| Gr. B No. 10 | 124.0 | SD | 9 |
| Gr. B No. 11 | 294.3 | NE | NE |
Abbreviations: NE, not evaluable for response because only 1 dose rituximab (375 mg/m2) was administered. SD, stable disease; PD, progressive disease; CR, complete remission; PR, partial remission.
More promising remission rates in pretreated B-CLL patients have been reported after treatment with the anti-CD52 MoAb Campath-1H.7 Lower concentrations (3 to 30 mg) of Campath-1H were injected 3 times a week over a period of 4 to 8 weeks. Thus, a different dosing schedule with prolonged application of smaller doses may improve the efficacy of rituximab, thereby reducing toxicity in patients with blood-borne lymphoma. In addition, the amount of antibody administered according to the standard dose rituximab schedule may be too low for binding to the high numbers of circulating CD20+ tumor cells in CLL. Therefore, O’Brien et al6 started a phase I/II study investigating dose escalation of rituximab in patients with fludarabine-resistant B-CLL. After using 375 mg/m2 rituximab for the first infusion, treatment was continued with once-weekly doses of 500 mg/m2, 650 mg/m2, and 825 mg/m2 for 3 weeks. A reduction in blood lymphocytosis has been observed in all patients treated so far, with 1 partial remission in the 500 mg/m2 dosing group. The therapeutic effect on lymph nodes has been poor, but dose escalation is continuing.
In patients with B-CLL and substantial numbers of peripheral lymphocytes, side effects during the first rituximab infusion seem to be dependent on the total amount of antibody administered over a certain period of time. Therefore, we decided to use a fractionated dosing schedule for the first rituximab application in all patients with B-CLL or leukemic variants of other low-grade NHL, consisting of 50 mg rituximab on day 1, 150 mg on day 2, and the remainder of the 375 mg/m2 dose on day 3. However, treatment in patients with more than 50.0 × 109/L lymphocytes should be initiated only after very careful consideration, because severe first-dose infusion-related adverse events have also been observed with this modified infusion schedule in patients with very high lymphocyte counts. Therefore, reducing the numbers of circulating tumor cells to counts below 50.0 × 109/L by using chemotherapeutic regimens seems reasonable before treatment with rituximab.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to A. Engert, MD, PhD, Department I of Internal Medicine, University of Cologne, Joseph-Stelzmann-Str. 9, D-50924 Cologne, Germany; e-mail: sabine.kluge@medizin.uni-koeln.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal