Abstract
CD34+ hematopoietic stem cells from normal individuals and from patients with chronic myelogenous leukemia can be induced to differentiate into dendritic cells (DC). The aim of the current study was to determine whether acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) cells could be induced to differentiate into DC. CD34+ AML-M2 cells with chromosome 7 monosomy were cultured in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor (TNF), and interleukin-4 (IL-4). After 3 weeks of culture, 35% of the AML-M2 cells showed DC morphology and phenotype. The DC phenotype was defined as upmodulation of the costimulatory molecules CD80 and CD86 and the expression of CD1a or CD83. The leukemic nature of the DC was validated by detection of chromosome 7 monosomy in sorted DC populations by fluorescence in situ hybridization (FISH). CD34+ leukemic cells from 2 B-ALL patients with the Philadelphia chromosome were similarly cultured, but in the presence of CD40-ligand and IL-4. After 4 days of culture, more than 58% of the ALL cells showed DC morphology and phenotype. The leukemic nature of the DC was validated by detection of the bcr-abl fusion gene in sorted DC populations by FISH. In functional studies, the leukemic DC were highly superior to the parental leukemic blasts for inducing allogeneic T-cell responses. Thus, CD34+ AML and ALL cells can be induced to differentiate into leukemic DC with morphologic, phenotypic, and functional similarities to normal DC.
DENDRITIC CELLS (DC) are potent initiators of T-cell–mediated immune responses.1 DC pulsed with tumor antigens can effectively prime T-cell responses in vitro and in vivo and are being developed as cancer vaccines in humans.2,3 Human DC can be generated in vitro from CD34+ stem cells present in bone marrow (BM), umbilical cord blood, and peripheral blood cells.4-8 It has recently been reported that chronic myelogenous leukemia (CML) cells can be induced to differentiate into DC.9-12 DC derived from CML cells retain the bcr-abl genotype. The implication is that CML cells with both a leukemic genotype and a DC phenotype might be potent inducers of T-cell responses to leukemia antigens.
The current study examined whether CD34+ acute leukemic cells can similarly be induced to differentiate into leukemic DC. The expectation of being able to induce differentiation of acute leukemia cells into DC is different than the expectation for CML cells. CML is a disorder of pluripotent hematopoietic stem cells. It is known that CML cells can maturate along normal lines of hematopoietic development into normal functioning myeloid and lymphoid cells carrying the Philadelphia (Ph) chromosome. By contrast, in both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), the malignant hematopoietic cells fail to undergo extensive maturation, resulting in the expansion of large numbers of leukemic blasts blocked at specific phenotypic and functional stages of differentiation.
Leukemia cells present in patients are often commingled with normal hematopoietic cells. When studying mixed populations, determination of whether CD34+ leukemic cells can be induced to differentiate into DC requires a leukemia marker that persists throughout the differentiation process. The presence of normal CD34+ stem cells with leukemic CD34+ blasts can confound the interpretation of results, because some of the normal CD34+ stem cells will differentiate into DC. In addition, the presence of normal monocytes in peripheral blood should also be taken into account. To determine whether acute leukemia cells differentiate into DC, it is necessary to document the leukemic origin of the DC. A leukemia-specific marker is required. Cell surface phenotype markers could be used, but are less satisfactory, because most myeloid leukemic cells express many of the same markers as normal DC.13
In the current study, AML and ALL cells with defined genotypic and/or phenotypic abnormalities were chosen to monitor the differentiation process of the leukemic clone. CD34+ leukemias were chosen for this initial study on the presumption that the behavior of leukemic cells arrested at a more immature stage of differentiation might more closely resemble that of pluripotent stem cells in response to differentiating growth factors and cytokines. The results show that cells with DC morphology and phenotype similar to those described for normal DC can be derived from the malignant clone in AML and ALL patients. The derived leukemic DC are more efficient in the stimulation of allogeneic T lymphocytes than are the original unprocessed leukemic blasts. Thus, leukemia derived DC can now be tested as stimulators of antileukemia T-cell responses in vitro and as leukemia vaccines in vivo.
MATERIALS AND METHODS
Isolation of peripheral blood mononuclear cells (PBMC) from acute leukemia patients and generation of DC.
PBMC or BM from acute leukemia patients were collected at diagnosis from the Division of Hematology, Ospedale S. Giovanni Battista (Torino, Italy) and from the Division of Hematology, Universita’ “La Sapienza” (Rome, Italy). PBMC were isolated after centrifugation over a Ficoll density gradient (Sigma, St Louis, MO) and cryopreserved in medium consisting of 90% fetal bovine serum (FBS; Hyclone Laboratories Inc, Logan, UT) and 10% dimethyl sulfoxide (DMSO; Sigma). Before use, cells were thawed, washed, resuspended in serum-free medium (X Vivo-10; BioWhittaker, Walkersville, MD), and seeded into 25-cm2 flasks at 2 × 105/mL. Cells from AML patients were stimulated with 80 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), 80 ng/mL interleukin-4 (IL-4; a gift of Immunex, Seattle, WA), and 10 ng/mL tumor necrosis factor α (TNFα; Biosource International, Camarillo, CA). Cells from ALL patients were stimulated with 3 μg/mL CD40-Ligand (CD40L; trimer; a gift of Immunex) and 80 ng/mL IL-4. Cytokines and fresh medium were added every 5 to 7 days to the cultures.
Phenotypic analysis.
When a significant proportion of cells in culture started to show DC morphology, ie, being larger in size, associated in clusters, and having typical cytoplasmic motile processes, they were analyzed for phenotype. Fluorescein- or phycoerythrin-conjugated mouse monoclonal antibodies (MoAbs) to the following antigens were used: CD1a, CD7, CD10, CD19, CD33, CD34, CD40, CD86, HLA class I, HLA-DR (Pharmingen, San Diego, CA), CD13 CD14, CD80 (Becton Dickinson, San Jose, CA), and CD83 (Immunotech, Westbrook, ME). Fluorescein- or phycoerythrin-conjugated isotype control mouse MoAbs were purchased from Pharmingen. CD34 is a marker of hematopoietic stem cells. CD13 and CD33 are myeloid antigens. CD19 is expressed by both mature and immature B cells and CD10 is expressed during the early stages of B-cell differentiation. CD80 (also known as BB1/B7-1), CD86 (also known as B70/B7-2), and CD40 are accessory and costimulatory molecules. CD1a and CD83 (also known as HB15) are considered to be DC markers.1,13 14 CD14 is expressed by monocytes. CD7 is a lymphoid marker that can be aberrantly expressed by some myeloid leukemias. Thawed blasts or cultured leukemic cells were incubated with fluorescein- or phycoerythrin-conjugated MoAbs, alone or in combination, for 20 minutes, washed with a phosphate-buffered saline (PBS) buffer containing 1% human serum, and analyzed using a FACSCalibur flow cytometer (Becton Dickinson). To exclude dead cells and debris, the viable cells were gated based on propidium iodide staining (Sigma). DC were defined by the expression of either CD1a or CD83 coupled with the expression of the costimulatory molecules CD80 and CD86. When a significant upmodulation of these molecules was observed, cells were used for fluorescence in situ hybridization (FISH) analysis and allogeneic mixed lymphocyte reactions (allo-MLR; see below).
Magnetic cell sorting.
When a DC phenotype was obtained, the cells were magnetically sorted into CD80+ or CD83+ populations using the MACS system (Miltenyi Biotec, Auburn, CA). Briefly, cells were labeled with MoAbs against CD80 or CD83 for 20 minutes, washed twice, and resuspended in 80 μL of MACS buffer (PBS without Ca2+ and Mg2+, supplemented with 1% bovine serum albumin [BSA] and 5 mmol/L EDTA). Twenty microliters of MACS superparamagnetic beads conjugated with a rat antimouse IgG1 MoAb (for CD80 sorting) or with a mouse antifluorescein MoAb (for CD83 sorting) was added to the cell suspension and incubated for 15 minutes at 6°C to 12°C. After washing twice in MACS buffer, cells were separated on a magnetic stainless steel wool column according to the manufacturer’s recommendations. Negative cells were collected from the column eluate, and positive cells remained attached to the magnetized matrix. To obtain the positive fraction, the column was removed from the magnet and flushed with MACS buffer into another tube. The purity of the positive fraction obtained with this technique was always greater than 95%.
FISH.
FISH was performed on cytospin preparations of original naive leukemic blasts and unsorted and sorted leukemia-derived DC. Cells were cytospun into glass slides and fixed in 4% buffered formalin. Fixed slides were hybridized with commercially obtained probes for a chromosome 7-specific α satellite (Oncor Inc, Gaithersburg, MD) or the bcr-abl gene rearrangement (Vysis Inc, Downers Grove, IL) following standard techniques for FISH. Briefly, slides were aged in 2× SSC at 73°C, denaturated in 70% formamide/2× SSC, and dehydrated in an ethanol series. Probe was denatured according to the manufacturer’s recommendations, applied to slides, and hybridized overnight in a humidified chamber at 37°C. Hybridized slides were washed in 0.4× SSC/0.3% NP-40 at 73°C for 2 minutes and rinsed in 2× SSC/0.1% NP-40. Slides hybridized with the 7 probe were detected with anti–digoxigenin-fluorescein. The bcr-abl probe is directly labeled and required no detection step. Slides were counterstained in Vectashield with the fluorescent dye 4,6-diamino-2 phenyl-indole (DAPI). Cells were analyzed using a Zeiss Axioscope equipped with epifluorescence and appropriate filter combinations for viewing the fluorophores (Carl Zeiss, Thornwood, NY). One hundred cells were analyzed in each sample to quantitate the signal pattern.15
Stimulatory function of the leukemic DC.
Allo-MLR were set up with PBMC from healthy donors as responders and naive leukemic cells or leukemia-derived DC as stimulators. A total of 105 responder cells were seeded in 96-well plates in the presence of varying concentration of stimulator cells, which had been irradiated at 3,000 rads. Cultures were incubated at 37°C in AIM V medium (GIBCO BRL, Grand Island, NY) in 5% CO2 for 5 days. Cells were then pulsed with 1 μCi of tritiated thymidine ([3H]-thymidine; Amersham-Life Science, Boston, MA) 18 hours before harvesting into glass fiber filters. [3H]-thymidine incorporation was measured on a β-counter and expressed as mean counts per minutes (cpm) of triplicates.
RESULTS
In vitro generation of leukemic DC from AML-M2 blasts.
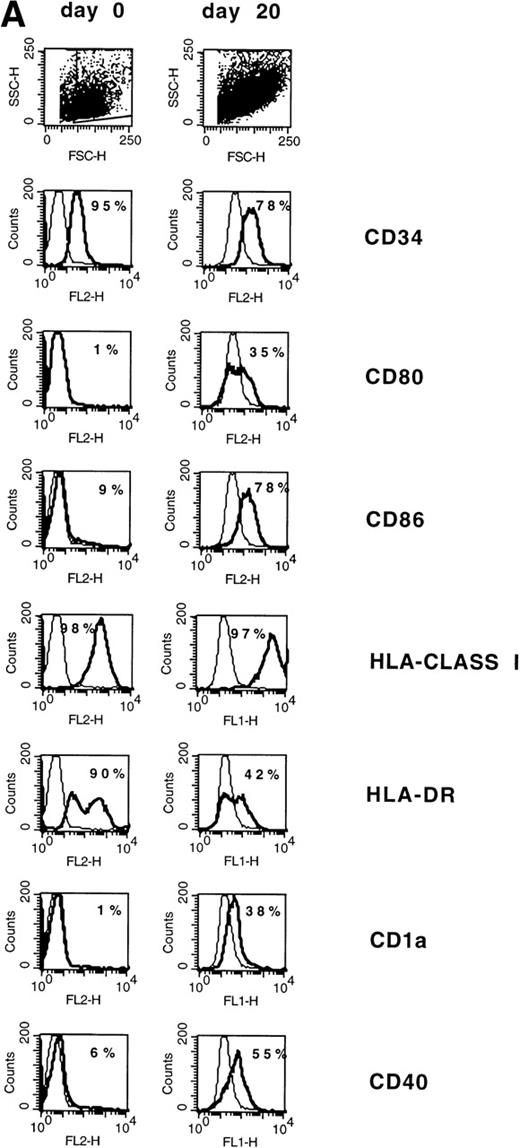
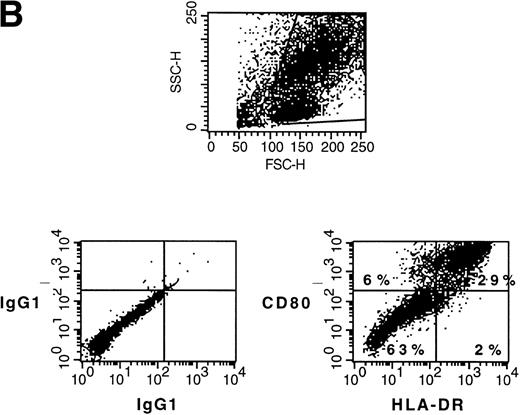
Normal CD34+ BM cells and CD34+ CML BM cells can be induced to differentiate into DC by GM-CSF, TNFα, and IL-4. To assess whether CD34+ AML cells can be similarly induced to differentiate into DC, PBMC containing 95% CD34+ leukemic blasts from a patient with AML-M2 (chromosome 7 monosomy) were cultured in the presence of 80 ng/mL GM-CSF, 10 ng/mL TNFα, and 80 ng/mL IL-4 for 3 weeks. Before culture, the AML blasts were essentially negative for CD80, CD86, CD1a, and CD40 but positive for HLA-DR and HLA class I (Fig1A). After 20 days of culture, approximately 35% of the cells exhibited the typical morphology of DC with thin cytoplasmic processes and increased size. The morphologic DC showed upmodulation of CD80, CD86, CD40 and CD1a (Fig 1A). HLA-class I expression was not modified and CD14 was negative (not shown). In the overall population, HLA-DR expression was decreased (Fig 1A); however, when tested in a subsequent experiment, the majority of CD80+ cells were HLA-DR+ (Fig 1B). Normal CD34+ stem cells lose CD34 expression when induced to differentiate into DC. By marked contrast, CD34 continued to be expressed by the majority of AML cells with DC morphology (Fig 1A).
(A) Phenotype of naive AML blasts and leukemia-derived DC (patient TA). Freshly thawed PBMC from an AML-M2 patient and the same cells after 20 days of culture in the presence of GM-CSF, TNF, and IL-4 were stained with fluorescein-or phycoerythrin-conjugated antibodies and analyzed by flow cytometry. To exclude debris, the viable cells were gated based on propidium iodide staining. Histograms represent the log of fluorescence (horizontal axis) versus the relative cell number (vertical axis). Thin lines represent the isotype-matched indifferent murine MoAb control. The number in each box represents the percent of positive cells. (B) CD80/HLA-DR coexpression on leukemia-derived DC (patient TA). Cells at day 20 of culture were double-stained with phycoerythrin-conjugated anti-CD80 MoAb (vertical axis) and anti-HLA-DR fluorescein-conjugated MoAb (horizontal axis). Fluorochrome-conjugated isotype-matched murine MoAbs (IgG1) were used as a negative control. The number in each quadrant represents the percentage of positive cells.
(A) Phenotype of naive AML blasts and leukemia-derived DC (patient TA). Freshly thawed PBMC from an AML-M2 patient and the same cells after 20 days of culture in the presence of GM-CSF, TNF, and IL-4 were stained with fluorescein-or phycoerythrin-conjugated antibodies and analyzed by flow cytometry. To exclude debris, the viable cells were gated based on propidium iodide staining. Histograms represent the log of fluorescence (horizontal axis) versus the relative cell number (vertical axis). Thin lines represent the isotype-matched indifferent murine MoAb control. The number in each box represents the percent of positive cells. (B) CD80/HLA-DR coexpression on leukemia-derived DC (patient TA). Cells at day 20 of culture were double-stained with phycoerythrin-conjugated anti-CD80 MoAb (vertical axis) and anti-HLA-DR fluorescein-conjugated MoAb (horizontal axis). Fluorochrome-conjugated isotype-matched murine MoAbs (IgG1) were used as a negative control. The number in each quadrant represents the percentage of positive cells.
The initial PBMC population contained 95% leukemia cells. The maintenance of expression of CD34 by the cytokine-induced DC provided substantial evidence that the DC were of leukemic origin rather than from a minor population of normal precursors in the PBMC. To further assess whether the DC were of leukemic origin, the induced DC population was analyzed for chromosome 7 monosomy by FISH. To obtain a more homogeneous DC population for analysis, CD80+ cultured cells were positively selected by immunomagnetic sorting. Before sorting, 35% of cultured cells expressed CD80. All of the original blasts and 94% of sorted CD80+ cells showed chromosome 7 monosomy (Fig 2). Interphase nuclei from normal PBMC, which were used as a control, showed a false-positive background of 5%.
Genotype of CD80-sorted, leukemia-derived DC (patient TA). The figure shows DAPI counterstained nuclei from a CD80-sorted positive culture of leukemia-derived DC hybridized with a chromosome 7-satellite (green signal) probe. Cells with 1 signal indicating monosomy 7 or 2 signals indicating the normal pattern are shown.
Genotype of CD80-sorted, leukemia-derived DC (patient TA). The figure shows DAPI counterstained nuclei from a CD80-sorted positive culture of leukemia-derived DC hybridized with a chromosome 7-satellite (green signal) probe. Cells with 1 signal indicating monosomy 7 or 2 signals indicating the normal pattern are shown.
To investigate the function of the leukemic DC as stimulators of T-cell responses, their ability to stimulate an allo-MLR was compared with that of the original leukemic population. Unfractionated cell populations from 20-day cultures containing DC and thawed blasts from peripheral blood were used to stimulate PBMC from an unrelated normal donor at stimulator:responder ratios ranging from 0.5:1 to 0.06:1. The leukemic DC elicited significantly higher proliferation than did the original leukemic population (Fig 3).
Proliferation of PBMC in response to allogeneic naive AML blasts or leukemia-derived DC (patient TA). Allo-MLR was performed with 105 allogeneic PBMC as responder cells and different numbers of thawed AML blasts or leukemia-derived DC at stimulator:responder ratios ranging from 0.5:1 to 0.06:1. Leukemic DC were derived from 20-day cultures. Proliferation was measured 5 days after stimulation by [3H]-thymidine incorporation.
Proliferation of PBMC in response to allogeneic naive AML blasts or leukemia-derived DC (patient TA). Allo-MLR was performed with 105 allogeneic PBMC as responder cells and different numbers of thawed AML blasts or leukemia-derived DC at stimulator:responder ratios ranging from 0.5:1 to 0.06:1. Leukemic DC were derived from 20-day cultures. Proliferation was measured 5 days after stimulation by [3H]-thymidine incorporation.
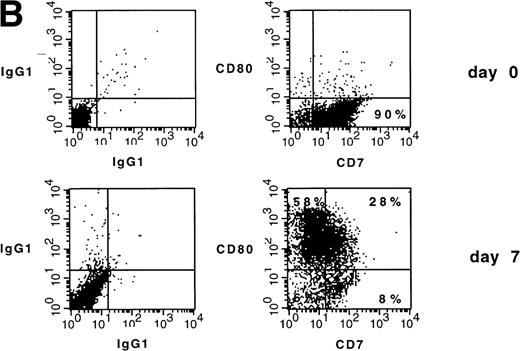
We investigated whether CD34+ AML-M2 cells from a second patient could be similarly induced to differentiate into DC. The leukemic blasts did not have any detectable chromosomal abnormality. Thus, an aberrantly expressed CD7 lymphoid marker on the cell surface was used to identify the leukemic origin of cultured DC. The AML cells from the patient denoted SC were defined phenotypically as myeloid blasts with aberrant CD7. The blasts expressed CD13, CD33, CD34, and CD7—a phenotype that cannot be assigned to normal myeloid cells. After 7 days of culture in the presence of GM-CSF, TNFα, and IL-4, upmodulation of CD80, CD86, and CD1a was obtained (Fig 4A). Thirty-three percent of CD80+ cells were CD7+ (ie, 28% of 86%; Fig 4B). In the peripheral blood of healthy donors, CD7 can normally be expressed by a subset of T lymphocytes and natural killer (NK) cells and also by monocytes very weakly. Given the fact that monocyte (CD14), T-cell (CD3), and NK-cell (CD56) markers were negative in 7-day cultures from this patient (data not shown), CD7 expression is most likely attributable to leukemic cells. Data did not show whether the CD80+/CD7− cells are leukemic cells that have downmodulated CD7 or are normal DC that were induced from normal precursor after cytokine exposure. Allogeneic MLR stimulatory activity of the cultured DC population was also validated for this patient (not shown), but this stimulatory activity might reside either in the CD80+/CD7+ or in the CD80+/CD7− population.
(A) Phenotype of naive AML blasts and leukemia-derived DC (patient SC). Freshly thawed PBMC from an AML-M2 patient and the same cells after 7 days of culture in the presence of GM-CSF, TNF, and IL-4 were stained with fluorescein-or phycoerythrin-conjugated antibodies and analyzed by flow cytometry. To exclude debris, the viable cells were gated based on propidium iodide staining. Histograms represent the log of fluorescence (horizontal axis) versus the relative cell number (vertical axis). Thin lines represent the isotype-matched indifferent murine MoAb control. The number in each box represents the percentage of positive cells. (B) CD7/CD80 coexpression on naive AML blasts and leukemia-derived DC (patient SC). Cells at day 0 and day 7 of culture were double-stained with fluorescein-conjugated anti-CD7 MoAb (horizontal axis) and anti-CD80 phycoerythrin-conjugated MoAb (vertical axis). Fluorochrome-conjugated isotype-matched murine MoAbs (IgG1) were used as a negative control. The number in each quadrant represents the percentage of positive cells.
(A) Phenotype of naive AML blasts and leukemia-derived DC (patient SC). Freshly thawed PBMC from an AML-M2 patient and the same cells after 7 days of culture in the presence of GM-CSF, TNF, and IL-4 were stained with fluorescein-or phycoerythrin-conjugated antibodies and analyzed by flow cytometry. To exclude debris, the viable cells were gated based on propidium iodide staining. Histograms represent the log of fluorescence (horizontal axis) versus the relative cell number (vertical axis). Thin lines represent the isotype-matched indifferent murine MoAb control. The number in each box represents the percentage of positive cells. (B) CD7/CD80 coexpression on naive AML blasts and leukemia-derived DC (patient SC). Cells at day 0 and day 7 of culture were double-stained with fluorescein-conjugated anti-CD7 MoAb (horizontal axis) and anti-CD80 phycoerythrin-conjugated MoAb (vertical axis). Fluorochrome-conjugated isotype-matched murine MoAbs (IgG1) were used as a negative control. The number in each quadrant represents the percentage of positive cells.
In vitro generation of leukemic DC from B-cell ALL blasts.
The data given above shows that CD34+ AML-M2 can be induced to differentiate into DC. Some B-cell acute lymphocytic leukemias are CD34+ and might also be inducible into DC. The growth and differentiation factors for B cells are different than those for myeloid cells. It is unknown whether human B cells can be induced to differentiate into DC. To assess whether CD34+ B-cell ALL cells can be induced to differentiate into DC, we studied the effect of CD40L plus IL-4 on CD34+ ALL cells that contained Ph+ as a cytogenetic marker. Two patients were studied.
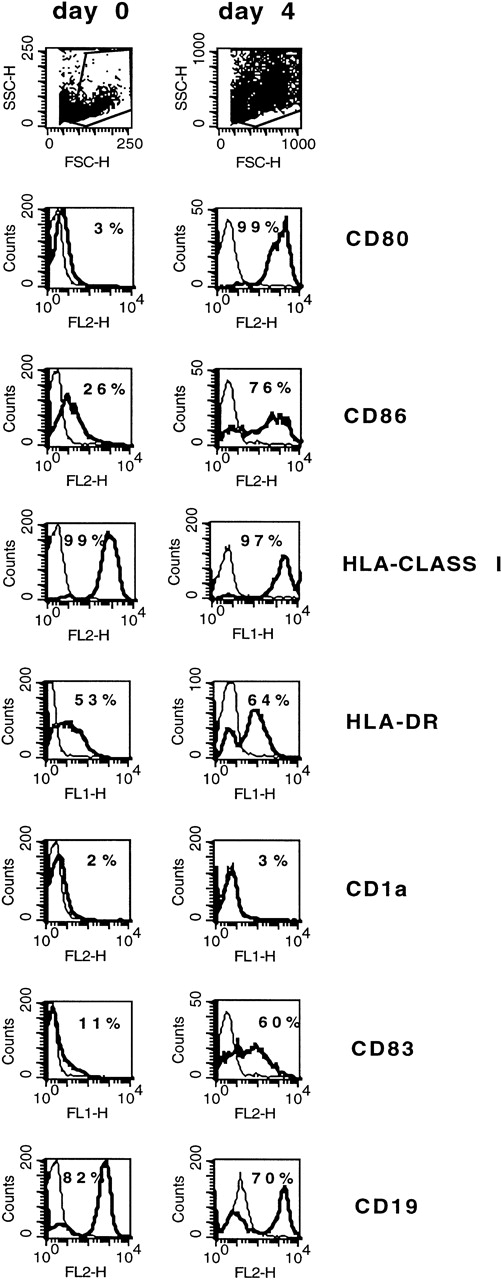
Blast cells from the first ALL patient (patient AB) were positive for the B-cell markers CD10 and CD19 and negative for the myeloid markers CD33 and CD13. Before culture, the leukemic BM contained 92% leukemic blasts. Blasts were essentially 100% CD34+, HLA class I+, and HLA-DR+; 28% CD40+, 11% CD83+, 26% CD86+, and CD1a−and CD80−. After 4 days of culture of BM cells with 3 μg/mL CD40L and 80 ng/mL IL-4, CD80, CD83, and CD86 increased from 3%, 11%, and 26% to 99%, 60%, and 76%, respectively (Fig 5). CD19 decreased from 82% to 70%. CD1a expression remained negative, and HLA class I and DR remained high (Fig 5). Double staining with fluorescein-conjugated anti-CD83 MoAb and phycoerythrin-conjugated MoAb anti-CD80, -CD34, -CD19, or -CD10 showed that 68%, 25%, 97%, and 26% of the CD83+ cells were positive for CD80, CD34, CD19, and CD10, respectively (not shown).
Phenotype of ALL blasts and of leukemia-derived DC (patient AB). Freshly thawed BM cells from a Ph+ ALL patient and the same cells after 4 days of culture in the presence of CD40L and IL-4 were stained with fluorescein- or phycoerythrin-conjugated antibodies and analyzed by flow cytometry. To exclude debris, the viable cells were gated based on propidium iodide staining. Histograms represent the log of fluorescence (horizontal axis) versus the relative cell number (vertical axis). Thin lines represent the isotype-matched indifferent murine MoAb control. The number in each box represents the percentage of positive cells.
Phenotype of ALL blasts and of leukemia-derived DC (patient AB). Freshly thawed BM cells from a Ph+ ALL patient and the same cells after 4 days of culture in the presence of CD40L and IL-4 were stained with fluorescein- or phycoerythrin-conjugated antibodies and analyzed by flow cytometry. To exclude debris, the viable cells were gated based on propidium iodide staining. Histograms represent the log of fluorescence (horizontal axis) versus the relative cell number (vertical axis). Thin lines represent the isotype-matched indifferent murine MoAb control. The number in each box represents the percentage of positive cells.
To examine the leukemic origin of the DC, cells from day 7 of culture were positively selected according to CD83 expression and examined for the presence of the bcr-abl fusion gene by FISH. Before culture, 74% of unfractionated blasts were bcr-abl positive. After culture, 27% of CD83-sorted positive cells were bcr-abl+(Fig 6). Cells from a normal control PBMC culture showed 4 of 100 interphase nuclei analyzed with a fusion signal, giving a background false-positive frequency of 4%. Provided that the bcr-abl− blasts observed may not be leukemic cells, the results show that ALL can be induced to differentiate into cells with a DC phenotype, but imply that normal DC precursors may coexist with the leukemic blasts.
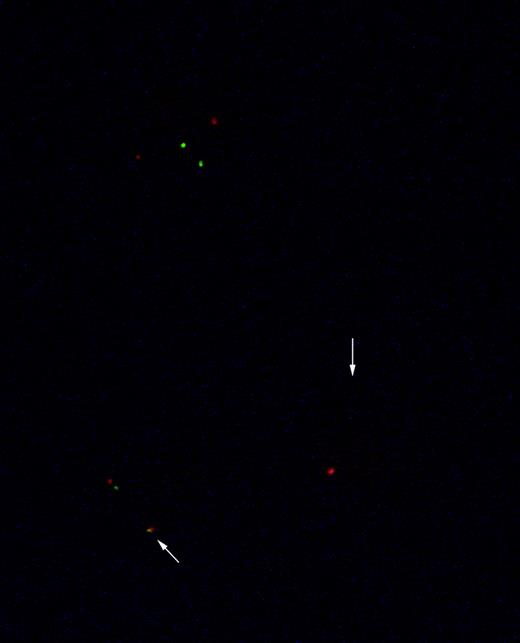
Genotype of CD83-sorted, Ph+leukemia-derived DC (patient AB). The figure shows interphase nuclei from a CD83-sorted positive culture of leukemia-derived DC. A normal interphase nucleus shows 2 orange (bcr) and 2 green (abl) signals. Nuclei with bcr-abl fusion show 1 orange signal (bcr), 1 green signal (abl), and the fusion (bcr-abl) orange and green signal (arrow).
Genotype of CD83-sorted, Ph+leukemia-derived DC (patient AB). The figure shows interphase nuclei from a CD83-sorted positive culture of leukemia-derived DC. A normal interphase nucleus shows 2 orange (bcr) and 2 green (abl) signals. Nuclei with bcr-abl fusion show 1 orange signal (bcr), 1 green signal (abl), and the fusion (bcr-abl) orange and green signal (arrow).
The stimulatory capacity of cultured ALL BM cells was assessed by allo-MLR. PBMC from an unrelated normal donor were stimulated to proliferate in response to cultured ALL cells containing leukemia-derived DC to a greater extent than in response to original leukemic blasts (Fig 7). The difference was more evident when a lower number of stimulator was used. Because many of the cells with DC phenotype were bcr-abl−, it cannot yet be concluded that leukemic DC were responsible for the stimulatory capacity. Unfortunately, it is not possible to sort cells on the basis of bcr-abl expression to determine the stimulatory capacity of ALL DC.
Proliferation of PBMC in response to allogeneic naive ALL blasts or leukemia-derived DC (patient AB). Allo-MLR was performed with 105 allogeneic PBMC from normal unrelated donor as responder cells and different numbers of thawed ALL blasts or leukemia-derived DC at stimulator:responder ratios ranging from 1:1 to 0.037:1. Leukemia DC were derived from 4-day cultures. Proliferation was measured 5 days after stimulation by [3H]-thymidine incorporation.
Proliferation of PBMC in response to allogeneic naive ALL blasts or leukemia-derived DC (patient AB). Allo-MLR was performed with 105 allogeneic PBMC from normal unrelated donor as responder cells and different numbers of thawed ALL blasts or leukemia-derived DC at stimulator:responder ratios ranging from 1:1 to 0.037:1. Leukemia DC were derived from 4-day cultures. Proliferation was measured 5 days after stimulation by [3H]-thymidine incorporation.
Results from the second CD34+ ALL patient (denoted patient NA) were superimposable on the above results (data not shown). At diagnosis, the leukemic BM contained 96% leukemic blasts that were CD10+ and CD19+ and CD13− and CD33−. After 7 days of culture in the presence of CD40L and IL-4, cells with DC morphology and phenotype were generated. CD80, CD83, and CD86 increased from 2%, 13%, and 41%, respectively, to 78%, 58%, and 98%, respectively. HLA class I and HLA-DR were unchanged. Before culture, 85% of unfractionated blasts were bcr-abl+. After culture, 59% of CD83-sorted positive cells were bcr-abl+. In allo-MLR, the superior stimulatory activity of the cultured DC population compared with the original blasts was also validated for this patient.
DISCUSSION
Malignant cells often have low immunogenicity due in part to low expression levels of requisite costimulatory molecules such as CD80 and CD86. Transfection of costimulatory molecules can render nonimmunogenic malignant cells immunogenic.16 An alternative ploy for providing appropriate costimulatory molecules is to fuse malignant cells with B cells or DC.17-19 The present study shows that for some leukemias the same end result can be achieved by inducing differentiation along the DC pathway.
DC are potent inducers of T-cell responses. The cytokine-derived leukemic DC in the current study functioned well as inducers of allogeneic T-cell responses. Although not proven, the assumption is that the leukemic DC will better stimulate antileukemia T-cell responses than normal leukemic cells. Potential applications of leukemic DC for immunotherapy include (1) vaccination in vivo to induce antileukemia T-cell immunity; (2) priming in vitro to induce leukemia-specific T cells for identifying immunogenic leukemia antigens; and (3) activating antileukemia T cells in vitro for use in allogeneic or autologous T-cell therapy regimens.
For using leukemic DC to induce antileukemia T-cell responses, it is crucial to verify that the DC are derived from the malignant clone. DC were generated from 3 leukemia patients with defined genetic abnormalities: 1 AML-M2 with monosomy 7 and 2 ALL with t(9;22). FISH analysis confirmed that the derived DC, sorted on the basis of DC phenotypic markers, had the same genetic abnormalities expressed by freshly isolated leukemic cells. This not only proves that the DC originated from the malignant clone, but also suggests that the leukemic DC continue to express at least some leukemia-related proteins associated with the genetic abnormality. As an example, 27% and 59% of CD83+ sorted DC derived from the 2 ALL studied contained the bcr-abl fusion gene. The cytokine-induced DC are likely to present peptides derived from the bcr-abl protein substantially more efficiently and effectively to T cells than the parental leukemia cells. It is likely that other aberrantly expressed downstream leukemia proteins will be more efficiently presented. Alternatively, important leukemia-associated proteins might be lost during the cytokine-induced DC differentiation. The decreased expression of surface markers related to leukemic phenotype such as CD7 in AML and CD10 in ALL suggests that the pattern of genes expressed by leukemic cells changes during the differentiation process. We have not yet otherwise examined or catalogued the expression by leukemic DC of proteins related to the malignant status.
In initial experiments CD34+ AML-M2 cells were stimulated with GM-CSF, TNFα, and IL-4, because normal CD34+ stem cells and CD34+ CML stem cells can be differentiated into DC by the use of these cytokines.9-12 In contrast, the CD34+ ALL cells were stimulated with CD40L plus IL-4. The Ph+ ALL blasts belonged to the B-lymphoid lineage, being positive for the B-cell markers CD10 and CD19 and negative for the myeloid markers CD33 and CD13. CD40L is known to induce proliferation and upregulation of costimulatory molecules on normal and malignant B cells.20-23 Lymphoid DC derived from normal thymic precursors have been described,24 25 but it was not known that B-cell precursors, either normal or leukemic, could maturate into DC-like cells. Stimulation of the CD34+ ALL cells with CD40L and IL-4 induced morphologic and phenotypic changes, including the expression of the CD83 DC-associated marker along with concurrent persistent expression of the CD19 B-cell marker. Persistent expression of CD19 also validated that the leukemic DC were derived from the B-cell malignancy.
Differentiating agents have been used for leukemia therapy with some success.26 27 A very optimistic view of future biologic therapy of leukemia might include therapy with agents such as those used herein to induce DC differentiation in vivo. Induction of DC differentiation in vivo might both decrease the replication rate of leukemic cells and stimulate an immune response to aberrantly expressed leukemia antigens.
The cytokine-derived leukemic DC possessed morphologic, phenotypic, and functional characteristics of DC. The recognized valuable characteristics of DC for specific immunotherapy are the ability to present antigen and to stimulate specific T-cell responses. Stimulation of T-cell responses requires stimulation by antigen in the context of costimulatory molecules. The crucial role of costimulatory molecules, especially CD80, in the generation of an antileukemic response has been shown in murine leukemia models.28-31 For the current study, the most important goal was to achieve increased expression of the costimulatory molecules CD80 and CD86. For all 4 CD34+leukemias in this study, CD80 was negative in the original blast populations and was increased in cytokine-derived DC cells, with the degree of fluorescence intensity varying from case to case. The same held true for CD86, which was weakly expressed in some of the original blast populations and increased in the induced population. The expression of costimulatory molecules was associated with the expression of CD1a or CD83 on the leukemic DC, as demonstrated by double florescence staining (not all data shown). The presence of these markers helped to define the maturation pattern of the leukemic cells towards the DC lineage.1 13
T cells recognize peptides in the binding cleft of major histocompatibility complex (MHC) molecules. Thus, efficient antigen presentation also requires expression of HLA molecules. Class I MHC and class II MHC were expressed equivalently by the parental leukemic blasts and the derived leukemic DC. Ability to stimulate allogeneic T-cell responses in a standard MLR was used to evaluate T-cell stimulatory capacity of the DC. For the 4 leukemic DC lines generated, the ability to stimulate allogeneic T cells was substantially superior to that of the naive blast population. The presumption is that the ability to present proteins expressed endogenously by the leukemia cells, including leukemia-associated proteins, is also increased. For normal DC, the ability to present exogenous antigens is highly dependent on the degree of maturation of DC. The leukemic DC were not yet evaluated for ability to present endogenous or exogenous proteins.
This report demonstrated that CD34+ leukemic cells from 2 AML-M2 patients and 2 ALL patients could be induced to differentiate into DC. From the point of view of morphology and phenotype, acute leukemia encompasses multiple different diseases. The response to the differentiating activity of growth factors and cytokines is likely to differ substantially for leukemia subtypes according to the stage of maturation of the various subtypes. It is not surprising, therefore, that another group could not detect upmodulation of costimulatory molecules in 1 case of AML after stimulation with GM-CSF, TNFα, and IL-4.31 Our most recent data from experiments not presented are in agreement with this observation. We have studied a total of 8 AML patients, including the 2 patients here. Upmodulation of costimulatory molecules and DC markers was obtained in 5 of 8 cases (in 3 of 4 CD34+ AML and in 2 of 4 CD34−AML). However, a leukemia marker was available in only the 2 cases presented here. Thus, some leukemic cells cannot be induced to differentiate into DC with the cytokine regimens used. Different sets of cytokines and differentiating agents might be explored.
ACKNOWLEDGMENT
The authors thank Immunex Corp for the kind gift of CD40L trimer and Kate Rupert for technical assistance for FISH.
Supported by National Institutes of Health, National Cancer Institute Grants No. 5 R37 CA30558 and CA18029, and by Associazione Italiana per la Ricerca sul Cancro (AIRC), Milano, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to M.A. Cheever, MD, Corixa Corp, 1124 Columbia St, Suite 200, Seattle, WA 98104; e-mail:cheever@corixa.com.


![Fig. 3. Proliferation of PBMC in response to allogeneic naive AML blasts or leukemia-derived DC (patient TA). Allo-MLR was performed with 105 allogeneic PBMC as responder cells and different numbers of thawed AML blasts or leukemia-derived DC at stimulator:responder ratios ranging from 0.5:1 to 0.06:1. Leukemic DC were derived from 20-day cultures. Proliferation was measured 5 days after stimulation by [3H]-thymidine incorporation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/6/10.1182_blood.v94.6.2048/5/m_blod41821003x.jpeg?Expires=1767714921&Signature=FTKwvkIyCV~HFNxiyHMakUgCMBeUlulGNCdhvrXGHa64oJYNb2ARLV824iy94ytNTxnUgiJbAfMUXXGvqIyzmobbvZUDAo3imDegog7Hv77BHGaiOZJeaX2OO6vHRzIE7O-HsnputKqljz6AmzORzRL0aFBD58lQAHyDHaWxbfOap5BGOJNXsd9HrX14rhtRMWzRFtyu86ugX5SRBoUUxPdcQMkT7eJ5I~9mywt3Rso7KLsWGuc9taochflC~JsPRZx7f9fCJxPCkGdg2hYPMI-uDd6i6EmkFwyO7QMNUju8RgQWuQwf3bB8bZJtcLWYcqgwYle4ABUnIkbzwlXUkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 7. Proliferation of PBMC in response to allogeneic naive ALL blasts or leukemia-derived DC (patient AB). Allo-MLR was performed with 105 allogeneic PBMC from normal unrelated donor as responder cells and different numbers of thawed ALL blasts or leukemia-derived DC at stimulator:responder ratios ranging from 1:1 to 0.037:1. Leukemia DC were derived from 4-day cultures. Proliferation was measured 5 days after stimulation by [3H]-thymidine incorporation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/6/10.1182_blood.v94.6.2048/5/m_blod41821007x.jpeg?Expires=1767714922&Signature=vf0pcem-DGmEDpGcx-I8xu0yD5SCwwf1oM51eAkv405Ve5qQJ0AwRxI78VkrIaRSRGyL6F4la-3b8hvMpSHRSpMejZaqkVnN9rz18zCKdFMy9Hn9ZVWyRdIVyjw3rNwKGpvhqstI7lhlrPud3KgnrQlYEQjhUrMhw2Be7Wl6TqoxOxehHA6BXnzDeLfslemWANNgHwDsOwSCmVeDdwjlb6lcYagxl0gD9voqdMRC3ufRmq7zGebzZlc08NLNzCIVwEZ6OXvqhQKmn4pE3gktuuj~iReeYuwLMfMo5fuJLGc9JyTb5NojZBsuHyJwLOSlHQ3KAdDnftVcFCOKIKIazg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal