Abstract
Macrophage-derived chemokine (MDC) is a recently identified CC chemokine that is a potent chemoattractant for dendritic cells, natural killer (NK) cells, and the Th2 subset of peripheral blood T cells. In normal tissues, MDC mRNA is expressed principally in the thymus. Immunohistochemical analysis performed on 5 human postnatal thymuses showed high MDC immunoreactivity, which was selectively localized to epithelial cells within the medulla. To examine the effects of MDC on immature T cells, we have identified cDNA clones for mouse and rat MDC. Expression of MDC in murine tissues is also highly restricted, with significant levels of mRNA found only in the thymus. Thymocytes express high-affinity binding sites for MDC (kd = 0.7 nmol/L), and, in vitro, MDC is a chemoattractant for these cells. MDC-responsive murine thymocytes express mRNA for CCR4, a recently identified receptor for MDC. Phenotypic analysis of MDC-responsive cells shows that they are enriched for a subset of double-positive cells that express high levels of CD3 and CD4 and that have reduced levels of CD8. This subset of MDC-responsive cells is consistent with the observed expression of MDC within the medulla, because more mature cells are found there. MDC may therefore play a role in the migration of T-cell subsets during development within the thymus.
DURING T-CELL DEVELOPMENT, a complex differentiation program takes place within the thymus leading to the generation of mature T lymphocytes.1 These events include the expression and rearrangement of the T-cell receptor genes and commitment to the CD4 or CD8 lineage.2 In addition, cells that recognize foreign antigen in the context of self major histocompatibility complex (MHC) are expanded (positive selection), whereas those that respond vigorously to self antigens are deleted by activation of the apoptotic pathway (negative selection).3
The mechanisms regulating positive and negative selection have yet to be fully elucidated. Early studies suggested that cortical epithelial cells were solely responsible for mediating positive selection,1,3 whereas thymic dendritic cells were responsible for negative selection.3 It was subsequently demonstrated that both epithelial and dendritic cells were capable of mediating negative selection.3 This led to the suggestion that anatomical constraints within the thymus may place restrictions on the cell-cell interactions that occur during T-cell development.4 For example, immature, double-positive T cells will encounter predominantly epithelial cells in the thymic cortex. As these cells mature and migrate into the medulla, they will encounter dendritic cells and thymic macrophages in addition to medullary epithelial cells.4 The factors that regulate such selective migration of immature T-cell subsets have not been extensively characterized.
The migration of mature T cells in the periphery has been more extensively studied and is regulated, in large part, by chemokines. Chemokines are a family of secreted proteins that are characterized by the spacing of 4 conserved cysteines.5-7 In the CC branch of the chemokine family, the first 2 cysteines are juxtaposed, whereas in the CXC branch they are separated by a nonconserved residue. CC chemokines are chemoattractants for monocytes, T cells, eosinophils, and mast cells,8 whereas the CXC chemokines act principally on neutrophils.5
Many chemokines are constitutively expressed in the thymus, including TARC,9 TECK,10 PARC11(also known as DC-CK-112), IP-10,13I-309,14 and macrophage-derived chemokine (MDC),15,16 suggesting that they may play a role in thymocyte migration and ultimately thymic development. In addition, several chemokine receptors are expressed predominantly in the thymus, including CCR417 and CCR8.14
We identified MDC during the sequencing of randomly selected cDNA clones from a human macrophage library.15,18 MDC is a potent chemoattractant for dendritic cells, activated natural killer (NK) cells, and some T cells.15,16,19 We have recently identified CCR4 as a receptor for MDC.19 CCR4 is expressed by the Th2 subset of mature CD4 T cells20 21; therefore, MDC may play a role in the selective migration of these cells in conditions such as allergic inflammation that are mediated by Th2 cells.
In normal human tissues, MDC and its receptor CCR4 are expressed almost exclusively in the thymus, potentially playing a role in the migration of immature T cells. To further investigate the role of MDC in T-cell development, we have examined the localization of MDC in the postnatal human thymus. Furthermore, we have identified cDNA clones for both mouse and rat MDC to examine the phenotype of murine thymocytes that respond to MDC.
MATERIALS AND METHODS
Mice.
Single-cell suspensions of thymocytes from female BALB/c mice (3 to 6 weeks old) were prepared by grinding freshly isolated thymuses between frosted glass slides. Fibrous tissue was removed by filtration through a 100-μm sterile cell strainer (VWR Scientific Products, Brisbane, CA). Cells were pelleted by centrifugation at 1,200 rpm for 5 minutes and resuspended in buffer (0.15 mol/L NH4Cl, 10 mmol/L KHCO3, and 0.1 mmol/L EDTA, pH 7.2) for red blood cell lysis. Cell viability was determined by trypan blue exclusion and was always greater than 95%. Cell suspensions from at least 4 animals were combined for each experiment.
Human thymuses.
Normal postnatal thymus specimens were obtained from 5 children during corrective cardiac surgery at the Apuano Pediatric Hospital of Massa Carrara, Italy. The 5 children were 5 days, 7 days, 5 months, 3 years, and 8 years of age, respectively. The procedures followed in the study were in accordance with the ethical standards of the responsible regional committee on human experimentation.
Production of antibodies to MDC.
Monoclonal antibodies 252Y, 252Z, and 272D were generated by immunizing mice with synthetic human MDC according to standard protocols.22 Each hybridoma was cloned twice by limiting dilution and isotyped using the Isostrip system (Boehringer Mannheim, Indianapolis, IN). Based on the capacity of these monoclonals to compete with one another for binding to MDC, 252Y and 252Z recognize common or overlapping epitopes, whereas 272D recognizes an epitope that is distinct from that recognized by 252Y and 252Z (M. Fahning, unpublished observations). Polyclonal antibodies to mouse MDC were generated by immunizing rabbits with synthetic mouse MDC coupled to KLH.
Immunohistochemical analysis.
Immunohistochemical staining was performed on 10-μm cryostat sections fixed in 4% paraformaldehyde for 20 minutes or in acetone for 10 minutes. Sections were subsequently exposed to 0.3% hydrogen peroxide-methanol solution to quench endogenous peroxidase activity. After 30 minutes of preincubation with normal horse serum (Vectastain ABC kit; Vector Laboratories, DBA, Milan, Italy), sections were incubated for 30 minutes with anti-MDC monoclonal antibodies (252Y, 252Z, and 272D; 5 μg/mL), followed by biotinylated antimouse IgG antibody and the avidin-biotin-peroxidase complex as described.23 3-Amino-9-ethylcarbazole (AEC) was used as a peroxidase substrate. Finally sections were counterstained with Gill’s hemotoxylin and mounted with Kaiser’s glycerol-gelatin. All incubations were performed at room temperature. As a negative control, primary antibody was replaced with an isotype matched antibody with irrelevant specificity or mouse ascites fluid.
Double immunostaining was performed with anti-MDC (252Z; 5 μg/mL) and anti–pan-T (anti-CD3; 5 μg/mL; Ancell, Bayport, MN) or macrophage-associated antigen mannose receptor (anti–PAM-124; 5 μg/mL) or anti-pan cytokeratin (C-11; 0.5 μg/mL; Sigma Immunochemical, Milan, Italy) antibody, using the avidin-biotin-peroxidase system with 2 different substrates, as previously described.23 To identify expression of CD3 and MDC or PAM-1 and MDC on the same section, the AEC (red) and the Vector SG (blue-gray) substrates were used, respectively. No counterstain was applied.
Isolation of cDNA clones for mouse and rat MDC.
cDNA libraries from mouse and rat thymus were purchased from Stratagene (La Jolla, CA). Libraries were screened using a gel-purified fragment encompassing the complete coding region of human MDC. The fragment was labeled by random priming using the Random Prime cDNA Labeling Kit (Boehringer Mannheim) according to the manufacturer’s instructions. One million clones from each library were hybridized in 5× SSC, 5× Denhardt’s solution, 1% sodium dodecyl sulfate (SDS), and 30% formamide at 42°C. After hybridization overnight, filters were washed in 2× SSC and 0.1% SDS at 50°C. Hybridizing plaques were identified by autoradiography and plaque-purified. In vivo excision of the Bluescript phagemid was performed according to the manufacturer’s instructions (Stratagene).
Computer analysis.
Protein comparisons (see Fig 2) were performed with the Geneworks program (Intelligenetics, Mountain View, CA).
Preparation of chemokines.
The mature form of murine MDC was chemically synthesized by Gryphon Sciences (South San Francisco, CA) using t-butyl-oxycarbonyl chemistry on a peptide synthesizer (model 430A; Applied Biosystems, Foster City, CA). Lyophilized powder was dissolved at 10 mg/mL in 4 mmol/L HCl and immediately diluted to 0.1 mg/mL in phosphate-buffered saline (PBS) plus 0.1% bovine serum albumen (BSA) for storage at −80°C. Additional chemokines were purchased from Peprotech (Rocky Hill, NJ). Recombinant murine MDC was expressed as a fusion protein with secreted placental alkaline phosphatase (SEAP) as previously described for human MDC.19 The MDC-SEAP expression plasmid was transfected into COS cells using diethyl aminoethyl (DEAE)-Dextran followed by 1 minute of treatment with 10% dimethyl sulfoxide (DMSO).22 Transfected cells were cultured overnight in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cells were then washed once with calcium- and magnesium-free PBS and placed in fresh DMEM medium containing 1% FBS. After an additional 3 days of culture, supernatants were collected, filtered through a 0.45-μm membrane, and stored at 4°C. The concentration of MDC-SEAP was determined based on the specific activity of SEAP.25
Binding assays.
For saturation binding experiments, 1 × 106thymocytes were incubated for 1 hour at 4°C in the presence of increasing concentrations of MDC-SEAP. Assays were performed in a total volume of 0.2 mL of binding buffer (RPMI 1640 medium containing 25 mmol/L HEPES, pH 7.4, 1% BSA, and 0.02% sodium azide). After incubation, cells were washed 4 times in binding buffer and lysed in 50 mL of 10 mmol/L Tris-HCl, pH 8.0, and 1% Triton X-100. Samples were heated to 65°C for 15 minutes to inactivate endogenous cellular phosphatases, centrifuged to remove cellular debris, and stored at −20°C until assay. Alkaline phosphatase activity in each sample was determined using the Great Escape Detection kit (Clontech, Palo Alto, CA) according to the manufacturer’s instructions.
Chemotaxis assays.
Approximately 106 freshly isolated thymocytes were resuspended in 0.1 mL of RPMI 1640 containing 0.5% BSA and loaded into the upper well of a transwell chamber (3-μm pore size; Costar, Corning, NY). Chemokine was added in the same buffer to the lower well in a volume of 0.6 mL. After 4 hours at 37°C, cells in the lower chamber were collected and counted by fluorescence-activated cell sorting (FACS). Data are expressed as the mean number of cells that migrate through the filter. Each experiment was performed in triplicate at least 3 times, unless otherwise indicated. Cells that migrated in the absence of MDC served as a negative control.
FACS analysis.
Phycoerythrin (PE)-conjugated antibodies to mouse CD3 and CD8 and fluorescein isothiocyanate (FITC)-conjugated antibodies to CD4 were purchased from Pharmingen (San Diego, CA). For analysis by flow cytometry, 106 thymocytes were washed once in PBS and resuspended in 0.1 mL of FACS buffer (RPMI 1640, 1% BSA, and 0.02% sodium azide). Cells were incubated with 1 μg of anti-CD4-FITC and 1 μg of anti-CD8-PE or 1 μg of anti-CD4-FITC and 1 μg of anti-CD3-PE for 1 hour on ice. Cells were washed twice in PBS and resuspended in 0.2 mL of 1% paraformaldehyde in PBS. Analysis was performed on Becton Dickinson FACscan using Lysis II software (Becton Dickinson, Mountain View, CA).
Northern analysis.
The expression of murine MDC mRNA was determined by Northern blotting. Tissue was isolated from normal rats and snap-frozen in liquid nitrogen. Total RNA was isolated using RNA Stat-60 (Tel-Test “B” Inc, Friendswood, TX). Total RNA was fractionated on a 0.8% agarose formaldehyde gel (20 μg per lane) and transferred to nitrocellulose as described.15 The blot was hybridized with the coding region of rat MDC. The β-actin cDNA was from Clontech. Probes were labeled by random-priming as described for cDNA library screening. Hybridization (5× SCC, 5× Denhardt’s solution, 1% SDS, and 50% formamide at 42°C) and washing (0.2× SSC and 0.1% SDS at 50°C) were performed at high stringency.
Reverse transcription-polymerase chain reaction (RT-PCR).
CCR4 expression in MDC responsive cells was determined by RT-PCR. Total RNA was isolated from cells that had migrated in response to MDC (cells were prepared as described for FACS analysis) using RNA Stat-60 (Tel Test “B” Inc). cDNA was synthesized using the 1st Strand cDNA Synthesis Kit (Boehringer Mannheim), according to the manufacturer’s instructions, in a total volume of 50 μL. Reactions were performed in the presence or absence of reverse transcriptase. One tenth of the resulting cDNA was used as a template in the PCR reaction, using oligonucleotide primers specific for murine CCR4. The forward primer was ATGAATGCCACAGAGGTCACAGAC and the reverse primer was CAAAGCGTCACGGAAGTCTACTAG. Ten microliters of the PCR reaction was separated on a 1.5% agarose gel. The identity of the amplified products was confirmed by dideoxy sequencing.
RESULTS
Immunolocalization of MDC.
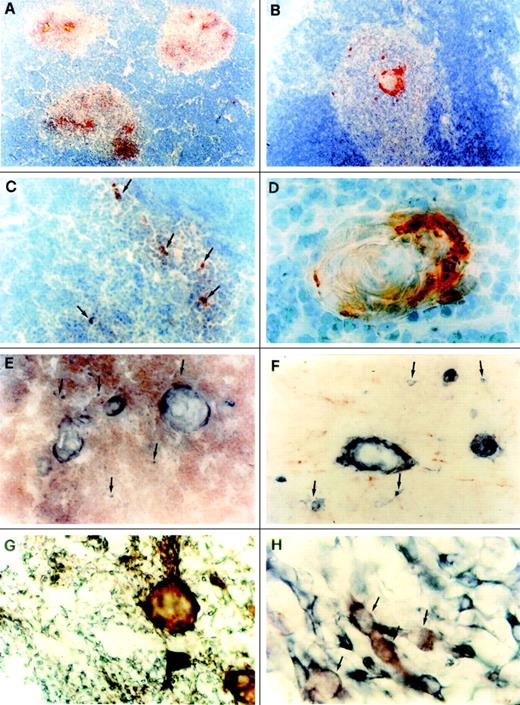
To more precisely localize MDC expression within the thymus, 5 postnatal human thymuses were examined using immunohistochemistry. Using monoclonal antibodies specific for human MDC (252Y and 252Z), MDC reactivity appeared to be selectively localized in cells scattered in the medullary areas and in the outer walls of Hassal’s corpuscles, whereas no MDC-positive cells were found in the cortex (Fig1A through D). This pattern of MDC immunoreactivity was confirmed using a monoclonal antibody (272D) that recognizes a different epitope on MDC to that seen by 252Y and 252Z. No staining of medullary thymic epithelial cells or Hassal’s corpuscles was found by using an isotype-matched control antibody (data not shown). The nature of MDC-expressing cells in the human thymus was investigated by double immunostaining CD3 (T cells) or PAM-1 (macrophages and dendritic cells) or cytokeratin (epithelial cells). Obvious separation was seen between staining for MDC and staining for CD3 (Fig 1E) or PAM-1 (Fig 1F). In contrast all MDC-reactive cells also stained positive for cytokeratin (Fig 1G and H), suggesting that MDC expression within the thymus is restricted to medullary epithelial cells.
Distribution of MDC in the human thymus. (A) Selective MDC expression in the medullary areas. The section was immunostained with anti-MDC monoclonal antibody using the avidin-biotin-peroxidase method and the AEC substrate (red color; original magnification × 40). (B) MDC immunostaining in the medulla is clearly visible in cells of the outer layer of a Hassal’s corpuscle and in the other cells scattered throughout the medulla (red color; original magnification × 100). (C) High power magnification of cells scattered in the medulla (shown by arrows) showing MDC immunostaining (red color; original magnification × 250). (D) High power magnification of a Hassal’s corpuscle showing strong MDC immunoreactivity (original magnification × 1,000). (E) Double immunostaining for MDC (blue-gray) and CD3 (red); individual cells staining for MDC but not CD3 are shown by arrows (original magnification × 250). (F) Double immunostaining for MDC (blue-gray) and PAM-1 (red) showing clear cut separation between MDC-positive Hassal’s corpuscles or single cells (arrows) and macrophage dendritic cells (original magnification × 250). (G) Double immunostaining for MDC (red) and cytokeratin (blue-gray). Hassal’s corpuscles and some cells staining for both MDC and cytokeratin (purple-brown), as well as many cells staining for cytokeratin alone (blue-gray) are visible (original magnification × 250). (H) Higher magnification of medullary cells showing double immunostaining for MDC and cytokeratin (purple-brown; indicated by arrows) or cytokeratin alone (blue-gray; original magnification × 1,000). (A) through (D) were counterstained with Gill’s hematoxylin, whereas no counterstain was applied in (E) through (H).
Distribution of MDC in the human thymus. (A) Selective MDC expression in the medullary areas. The section was immunostained with anti-MDC monoclonal antibody using the avidin-biotin-peroxidase method and the AEC substrate (red color; original magnification × 40). (B) MDC immunostaining in the medulla is clearly visible in cells of the outer layer of a Hassal’s corpuscle and in the other cells scattered throughout the medulla (red color; original magnification × 100). (C) High power magnification of cells scattered in the medulla (shown by arrows) showing MDC immunostaining (red color; original magnification × 250). (D) High power magnification of a Hassal’s corpuscle showing strong MDC immunoreactivity (original magnification × 1,000). (E) Double immunostaining for MDC (blue-gray) and CD3 (red); individual cells staining for MDC but not CD3 are shown by arrows (original magnification × 250). (F) Double immunostaining for MDC (blue-gray) and PAM-1 (red) showing clear cut separation between MDC-positive Hassal’s corpuscles or single cells (arrows) and macrophage dendritic cells (original magnification × 250). (G) Double immunostaining for MDC (red) and cytokeratin (blue-gray). Hassal’s corpuscles and some cells staining for both MDC and cytokeratin (purple-brown), as well as many cells staining for cytokeratin alone (blue-gray) are visible (original magnification × 250). (H) Higher magnification of medullary cells showing double immunostaining for MDC and cytokeratin (purple-brown; indicated by arrows) or cytokeratin alone (blue-gray; original magnification × 1,000). (A) through (D) were counterstained with Gill’s hematoxylin, whereas no counterstain was applied in (E) through (H).
Cloning of murine MDC.
Mouse and rat thymus cDNA libraries were hybridized with a human MDC probe at low stringency. Three cDNA clones were obtained for mouse MDC and 2 clones were obtained for rat MDC. In addition, a single sequence with identity to our mouse MDC clones has been deposited in the expressed sequence tag (EST) database (Genbank no. AA175762). An alignment of the predicted mature protein sequence of mouse, rat, and human MDC is shown in Fig 2. The start of the mature sequence for rodent MDC is based on comparison with the human sequence. Mouse, rat, and human MDC are highly conserved around the predicted signal peptide cleavage site; 11 of the first 13 residues of the mature protein are completely identical. The start of the mature protein is also in accord with rules governing signal sequence cleavage.26
Comparison of the mature forms of mouse, rat, and human MDC. (A) Residues that are shared with the murine sequences are shown by (.). (B) Dendrogram analysis illustrating similarity of CC chemokines. The sequences reported here for mouse and rat MDC have been deposited with Genbank under the accession numbers AF163477 andAF163476.
Comparison of the mature forms of mouse, rat, and human MDC. (A) Residues that are shared with the murine sequences are shown by (.). (B) Dendrogram analysis illustrating similarity of CC chemokines. The sequences reported here for mouse and rat MDC have been deposited with Genbank under the accession numbers AF163477 andAF163476.
Over the entire mature protein sequence, mouse and rat MDC are 85% identical and share 65% identity with human MDC. Many of the amino acid differences between human and rodent MDC are conservative substitutions (eg, Ile to Leu or Lys to Arg). Dendrogram analysis shows that these genes are more closely related to each other than to any other CC chemokine family members (Fig 2B).
Tissue expression of murine MDC mRNA.
The expression pattern of MDC was examined in normal rat tissues by Northern hybridization. As presented in Fig 3, MDC mRNA could be readily detected in thymus but not in the other tissues examined (lymph node, brain, and heart). On prolonged exposure of the autoradiograph (12 days) expression of MDC could also be detected in lymph node (data not shown). To confirm the presence and integrity of the RNA samples, the blot was probed for β-actin, which was readily detected in all tissues.
Northern blot analysis of MDC expression in rat tissues. Total RNA was isolated from various rat tissues, fractionated on a formaldehyde agarose gel, and blotted onto nitrocellulose. The blot was hybridized sequentially with cDNA probes for MDC and β-actin. Probe removal was confirmed between each round of hybridization by autoradiography. Exposure times were 2 days for MDC and 8 hours for β-actin. The more rapidly migrating species present in heart represents hybridization of the β-actin probe to γ-actin, which is the major species of actin present in this tissue.27
Northern blot analysis of MDC expression in rat tissues. Total RNA was isolated from various rat tissues, fractionated on a formaldehyde agarose gel, and blotted onto nitrocellulose. The blot was hybridized sequentially with cDNA probes for MDC and β-actin. Probe removal was confirmed between each round of hybridization by autoradiography. Exposure times were 2 days for MDC and 8 hours for β-actin. The more rapidly migrating species present in heart represents hybridization of the β-actin probe to γ-actin, which is the major species of actin present in this tissue.27
Characterization of the MDC receptor on murine thymocytes.
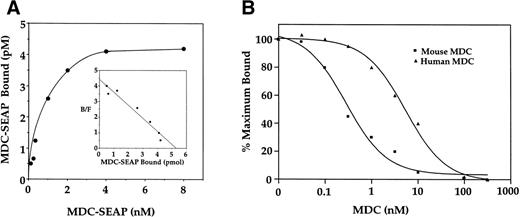
To investigate the effects of MDC on immature T cells, we initially examined the specific binding of MDC to mouse thymocytes using a mouse MDC-SEAP fusion protein. When binding experiments were performed using increasing concentrations of MDC-SEAP, saturable binding to thymocytes was observed (Fig 4A). Scatchard analysis showed that the binding was to a single high-affinity site with a calculated kd of 0.7 nmol/L (shown inset). Binding of MDC-SEAP to mouse thymocytes could be competed with unlabeled mouse or human MDC. The IC50 for mouse MDC was approximately 0.5 nmol/L, whereas that for human MDC was approximately 3.0 nmol/L (Fig 4B).
Binding characteristics of mouse MDC:SEAP to murine thymocytes. (A) Mouse thymocytes (1 × 106 cells) were incubated with increasing concentrations of mouse MDC:SEAP. Nonspecific binding was determined in the presence of 500-fold molar excess of unlabeled murine MDC. (Inset) Scatchard analyis of the binding data. (B) Competition binding of mouse or human MDC with mouse MDC:SEAP to mouse thymocytes.
Binding characteristics of mouse MDC:SEAP to murine thymocytes. (A) Mouse thymocytes (1 × 106 cells) were incubated with increasing concentrations of mouse MDC:SEAP. Nonspecific binding was determined in the presence of 500-fold molar excess of unlabeled murine MDC. (Inset) Scatchard analyis of the binding data. (B) Competition binding of mouse or human MDC with mouse MDC:SEAP to mouse thymocytes.
Induction of thymocyte chemotaxis by chemokines.
To determine if thymocytes functionally respond to murine MDC, chemotaxis experiments were performed in which the chemotactic response of thymocytes to MDC and a panel of 8 additional chemokines was examined. MDC induced a dose-dependent chemotaxis of freshly isolated thymocytes, as shown in Fig 5G. The bell-shaped curve is a chemotactic response that is characteristic of chemokines. Chemotaxis was detectable at concentrations of MDC as low as 10 nmol/L (P < .05), although maximal chemotaxis was observed at 100 nmol/L of MDC (P < .01; Fig5G). The number of thymocytes that migrated in response to MDC was relatively small, representing approximately 0.2% of the starting population. A similar induction of thymocyte chemotaxis was seen in response to the chemokines ELC and SLC (Fig 5H and I), whereas MCP-4, RANTES, Lymphotactin, and MIP1δ failed to stimulate significant chemotaxis (Fig 5A, B, C, and F).
Chemotaxis of murine thymocytes to chemokines. Freshly isolated thymocytes from 3- to 6-week-old BALB/c mice were assayed for chemotaxis in response to a panel of chemokines. The number of cells migrating was determined by FACS analysis. All assays were performed on samples in triplicate; data shown are representative of three to 5 independent experiments. Significant chemotaxis above background is indicated by *P < .05 or **P < .01.
Chemotaxis of murine thymocytes to chemokines. Freshly isolated thymocytes from 3- to 6-week-old BALB/c mice were assayed for chemotaxis in response to a panel of chemokines. The number of cells migrating was determined by FACS analysis. All assays were performed on samples in triplicate; data shown are representative of three to 5 independent experiments. Significant chemotaxis above background is indicated by *P < .05 or **P < .01.
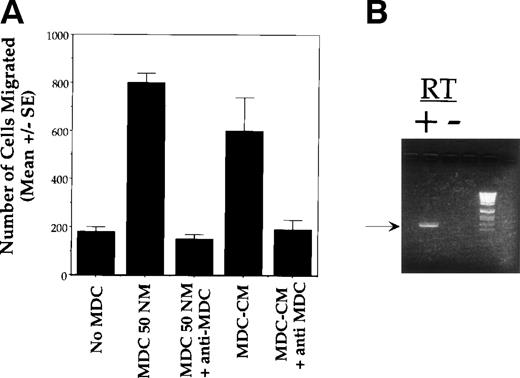
To determine whether the observed chemotaxis was due to MDC or secondary to the induction of other factors, we performed the following experiment. Murine thymocytes were cultured for 4 hours in the presence of 50 nmol/L MDC. Thymocytes were removed by centrifugation, and this (MDC-conditioned) media was used to stimulate the chemotaxis of fresh thymocytes. As shown in Fig 6A, the chemotactic activityof this media was inhibited by antibodies to MDC.
MDC acts directly on CCR4 expressing thymocytes. (A) The migration of thymocytes is MDC-dependent. Freshly isolated thymocytes from 3- to 6-week-old BALB/c mice were assayed for chemotaxis in response to murine MDC as follows. Thymocytes were cultured for 4 hours in the presence of 50 nmol/L MDC, and cells were removed by centrifugation. The resulting MDC-conditioned media was then used to stimulate the chemotaxis of fresh thymocytes in the presence or absence of a neutralizing antibody to MDC. The number of cells migrating was determined by FACS analysis. All assays were performed on samples in triplicate; data shown are the mean ± SEM and are representative of 3 independent experiments. (B) Expression of CCR4 by cells that had migrated in response to MDC was determined by RT-PCR.
MDC acts directly on CCR4 expressing thymocytes. (A) The migration of thymocytes is MDC-dependent. Freshly isolated thymocytes from 3- to 6-week-old BALB/c mice were assayed for chemotaxis in response to murine MDC as follows. Thymocytes were cultured for 4 hours in the presence of 50 nmol/L MDC, and cells were removed by centrifugation. The resulting MDC-conditioned media was then used to stimulate the chemotaxis of fresh thymocytes in the presence or absence of a neutralizing antibody to MDC. The number of cells migrating was determined by FACS analysis. All assays were performed on samples in triplicate; data shown are the mean ± SEM and are representative of 3 independent experiments. (B) Expression of CCR4 by cells that had migrated in response to MDC was determined by RT-PCR.
We have previously identified CCR4 as a receptor for human MDC,19 and both human and murine CCR4 are expressed at high levels in the thymus.17 RT-PCR analysis was performed to determine whether thymocytes that had migrated in response to MDC expressed CCR4. As shown in Fig 6B, CCR4 mRNA was found to be expressed by cells that had migrated in response to MDC.
Phenotypic characterization of MDC and ELC-responsive thymocytes.
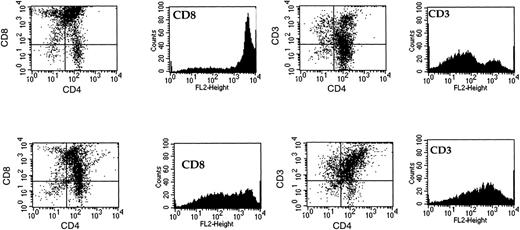
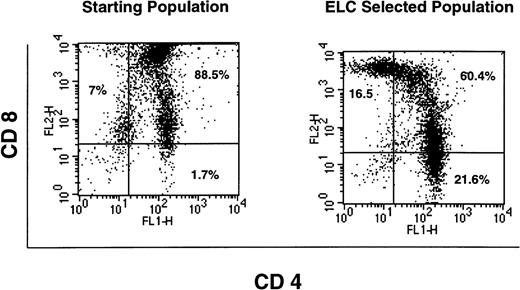
Thymocytes were isolated after chemotaxis to MDC or ELC and analyzed by flow cytometry for the expression of CD4, CD8, and CD3. The majority of the cells in the thymus (80% to 90%) are CD4+CD8+.4 The remainder are composed of mature single-positive cells and the double-negative population, representing cells at the earliest stages of T-cell development. We observed similar results for the total thymocyte population, as presented in Fig 7(upper panels). Thymocytes that had migrated in response to an optimal concentration of MDC (100 nmol/L) were also collected and analyzed. These cells were highly enriched for double-positive thymocytes that have reduced levels of expression of CD8. Although these cells were still CD8+, the mean number of CD8 molecules expressed per cell is reduced, and this is reflected in the reduction in the median flourescence for the CD8+ marker (from 3,429 fluorescence units to 311; Fig 7). In addition, the expression of the T-cell receptor complex (CD3) was augmented in the MDC-responsive population, because the median fluorescence was increased 8.3-fold (Fig 7, lower panels). The levels of CD4 were not significantly different between the total thymocyte population and those cells that had migrated in response to MDC. Thus, MDC preferentially attracts lineage-committed thymocytes that are CD3+CD4+CD8low. In contrast, ELC acts as a chemoattractant for T cells that are at a more advanced stage of development, causing the selective accumulation of both single-positive CD4 and CD8 thymocytes in addition to double-positive cells ( Fig 8).
Phentotypic analysis of MDC-responsive thymocytes. FACS analysis was performed on thymocytes after chemotaxis towards an optimal concentration of MDC (100 nmol/L). The upper panels show staining of freshly isolated thymocytes with CD4 and CD8 or CD3. The lower panels show staining of thymocytes that had migrated in response to MDC for the same markers. Data shown are representative of 3 separate experiments.
Phentotypic analysis of MDC-responsive thymocytes. FACS analysis was performed on thymocytes after chemotaxis towards an optimal concentration of MDC (100 nmol/L). The upper panels show staining of freshly isolated thymocytes with CD4 and CD8 or CD3. The lower panels show staining of thymocytes that had migrated in response to MDC for the same markers. Data shown are representative of 3 separate experiments.
Phentotypic analysis of ELC-responsive thymocytes. FACS analysis was performed on thymocytes after chemotaxis towards an optimal concentration of ELC (500 nmol/L). The left-hand panel shows staining of freshly isolated thymocytes with CD4 and CD8, whereas the right-hand panel shows staining of those thymocytes that had migrated in response to ELC. Data shown are representative of 3 separate experiments.
Phentotypic analysis of ELC-responsive thymocytes. FACS analysis was performed on thymocytes after chemotaxis towards an optimal concentration of ELC (500 nmol/L). The left-hand panel shows staining of freshly isolated thymocytes with CD4 and CD8, whereas the right-hand panel shows staining of those thymocytes that had migrated in response to ELC. Data shown are representative of 3 separate experiments.
DISCUSSION
In an effort to characterize the role of MDC in the thymus, we have examined its expression by immunocytochemistry. Using different monoclonal antibodies that recognize nonoverlapping epitopes on MDC, MDC immunoreactivity was localized to epithelial cells in the medullary areas of 5 human thymuses (ages ranged from 5 days to 8 years). Epithelial expression of MDC was unexpected, because we previously observed MDC expression in macrophages and dendritic cells.15 18 We cannot exclude the possibility that this finding may reflect the local accumulation of MDC protein perhaps associated with epithelial glycosaminoglycans. However, we were unable to detect MDC immunoreactivity associated with dendritic cells, macrophages, or T cells in the medulla. The absence of detectable MDC expression in macrophages and dendritic cells within the thymus may reflect phenotypic and functional differences between cells derived in vitro from peripheral blood and those resident within the thymic microenvironment. MDC may therefore act to attract developing T lymphocytes towards medullary epithelial cells.
To examine the effects of MDC on the chemotaxis of thymocytes in vitro, murine thymocytes were studied. cDNA clones for murine MDC were isolated. Rat and mouse MDC are 85% identical, and they share approximately 65% identity with the human molecule. Dendrogram analysis confirms that the rat and mouse sequences described here are more closely related to human MDC than to other CC chemokines. The murine sequences have an identical pattern of in vivo gene expression to that reported for human MDC and interact with CCR4, providing further support that they are murine homologues of MDC.
Binding studies showed interaction of MDC with a single class of high-affinity receptors present on thymocytes (kd = 0.7 nmol/L). Competition assays demonstrated that the binding of MDC-SEAP to these cells could be inhibited by mouse and human MDC. The receptor responsible for thymic MDC effects is likely to be CCR4. CCR4 mRNA is expressed constitutively in the thymus,17 and thymocytes that had migrated in response to MDC expressed CCR4 mRNA. CCR4 is the only receptor thus far identified for MDC, and murine MDC is chemotactic for L1.2 cells stably transfected with human CCR4 (data not shown). Alternatively, other receptors for MDC may yet be characterized. Consistent with this, macrophages and dendritic cells express little or no CCR4 and yet respond to MDC by chemotaxis.15
MDC was shown to be chemotactic for thymocytes, although maximum chemotaxis was observed at relatively high concentrations (100 nmol/L). These results are similar to those observed for monocytes,15 but dendritic cells and T cells respond at 1 to 10 nmol/L.15,19 Although it is conceivable that the primary role of MDC in the thymus is distinct from chemotaxis,28 we believe this to be unlikely and may reflect limitations in our in vitro chemotaxis assay. Within the thymus, MDC may be more efficiently presented to immature T cells, perhaps in the context of cell surface proteoglycans. This type of presentation has been described for interleukin-829 and macrophage inflammatory protein-1β (MIP-1β).27 It is noteworthy that the chemokines ELC and SLC, which also stimulated significant thymocyte chemotaxis, did so at high concentrations greater than 100 nmol/L. Alternatively, under physiological conditions, the microenvironment of the thymus may be able to generate high local concentrations of MDC. This is quite possible, because MDC was first isolated as a highly abundant cDNA from cultured human macrophages.15 18
Phenotypic analysis of thymocytes that migrated in response to MDC showed that they were enriched for double-positive cells that had reduced the levels of cell surface expression of CD8. In addition, these cells showed an increase in the density of their T-cell receptor, CD3. At this latter stage of development, thymocytes, which have been positively selected, migrate from the cortex into the medulla. Although we cannot formally exclude species differences in the biological role of MDC between humans and mice, these findings, together with our immunolocalization studies on human thymuses, suggests that MDC may act to attract thymocytes from the cortex into the medulla.1-3The role of the medulla in T-cell development is poorly understood,3,4 but it may play an important role in negative selection.3,4 Cells undergoing apoptosis have been identified in situ within the thymic medulla,30 and tolerance to human C-reactive protein by CD4+ T cells is mediated by medullary thymic epithelium.31 In previous work, we have shown that medullary epithelial cells from human thymus express high levels of the ligand for CD30 (CD30L).23 CD30 is a member of the tumor necrosis factor (TNF) receptor superfamily and activation of this receptor by its ligand can lead to activation of the apoptotic pathway.32 Studies in the mouse have suggested a role for CD30 in the negative selection of autoreactive T cells within the thymus.33
The demonstration that MDC acts as a chemoattractant for thymocytes and is localized to cells in the medulla that express the ligand for CD30 (a potential mediator of apoptosis) suggests that MDC may target potentially autoreactive T lymphocytes for elimination by activation of the apoptotic pathway. These findings are consistent with the hypothesis that medullary epithelial cells play an important role in the negative selection. MDC is also chemotactic for cells of the monocyte/macrophage lineage.15,18 Macrophages play an important role in the phagocytosis and removal of apoptotic cells within the thymus.34 MDC may also attract macrophages towards areas where there are apoptotic cells.
However, CD30 expression is restricted to a small number of cells within the thymus,23,33 and its expression is not significantly increased in those cells that migrate in response to MDC (data not shown). This may be due to the transient expression of CD30 during T-cell development.23 33 Alternatively, the effects of MDC as a chemoattractant for immature T cells may not be restricted to those cells that are destined to undergo negative selection. Rather, MDC may act more broadly to attract thymocytes from the cortex to the medulla.
A number of chemokines are expressed at high levels in the thymus, including PARC11 (also known as DC-CK112), TECK,9 I-309,14 and TARC.10 One possible explanation for the diversity of chemokine expression may be to control the diversity of cell types within the thymus and to allow for the complexity of cell movements that occur during T-cell development. For example, immature T-cell progenitors that originate in the bone marrow enter the subcapsular region of the thymus; from there, they migrate into the thymic cortex and then into the medulla before exiting to the periphery as mature differentiated T cells.1,3 It is tempting to speculate that the selective expression of chemokines and their receptors regulate the migration (and perhaps other properties) of developing T-cell subsets. Consistent with this, we and others have found that the chemokine ELC acts as a chemoattractant for T cells that are at a more advanced stage of development than those that migrate in response to MDC (Fig 8; see also Ngo et al35). Formal testing of this hypothesis will require the detailed characterization of the temporal and spatial pattern of chemokine expression during T-cell development within the thymus, along with the selective inactivation of these chemokines in the germline by homologous recombination.
ACKNOWLEDGMENT
The authors thank Dina Levitan and Marsalina Quiggle for oligonucleotide synthesis and DNA sequencing, Rick Jasman for FACS analysis, Reyna Simon and Michael Siani (Gryphon Sciences) for chemical synthesis of murine MDC, and Mitch Fahning for monoclonal antibody preparation and characterization.
P.R. and S.R. are supported by AIRC and Italian Ministry of Health (AIDS Project).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Patrick W. Gray, PhD, ICOS Corp, 22021 20th Ave SE, Bothell, WA 98021; e-mail: pgray@icos.com.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal