Abstract
The adhesion molecule von Willebrand factor (vWF) activates platelets upon binding 2 surface receptors, glycoprotein (GP) Ib-V-IX and integrin IIbβ3. We have used 2 approaches to selectively activate GP Ib using either the snake venom lectin alboaggregin-A or mutant recombinant forms of vWF (▵A1-vWF and RGGS-vWF) with selective binding properties to its 2 receptors. We show that activation of GP Ib induces platelet aggregation, secretion of 5-hydroxy tryptamine (5-HT), and an increase in cytosolic calcium. Syk becomes tyrosine phosphorylated and activated downstream of GP Ib, and associates with several tyrosine-phosphorylated proteins including the Fc receptor γ-chain through interaction with Syk SH2 domains. GP Ib physically associates with the γ-chain in GST-Syk-SH2 precipitates from platelets stimulated through GP Ib, and 2 Src family kinases, Lyn and Fyn, also associate with this signaling complex. In addition, GP Ib stimulation couples to tyrosine phosphorylation of phospholipase Cγ2. The Src family-specific inhibitor PP1 dose-dependently inhibits phosphorylation of Syk, its association with tyrosine-phosphorylated γ-chain, phosphorylation of PLCγ2, platelet aggregation, and 5-HT release. The results indicate that, upon activation, GP Ib is physically associated with FcR γ-chain and members of the Src family kinases, leading to phosphorylation of the γ-chain, recruitment, and activation of Syk. Phosphorylation of PLCγ2 also lies downstream of Src kinase activation and may critically couple early signaling events to functional platelet responses.
PLATELET ADHESION to subendothelial structures is an early critical event in hemostasis and thrombosis. von Willebrand factor (vWF) is a major adhesive glycoprotein (GP) required for normal hemostasis in conditions of high shear stress,1-4 such as occur in small arterioles and arterial capillaries. In the presence of shear stress or modulators such as ristocetin, vWF is able to induce signaling in platelets including hydrolysis of phosphoinositides, a transient increase in cytosolic calcium, activation of protein kinase C, cytoskeletal reorganization, and platelet aggregation.5-9
Platelets have 2 receptors for vWF that are sequentially bound upon interaction with vWF: GP Ib in the GP Ib-V-IX complex and the integrin αIIbβ3.10 Previous studies have shown that the tyrosine kinase Syk becomes tyrosine phosphorylated and activated in vWF-stimulated platelets11,12 downstream of the primary receptor GP Ib, and Syk has been shown to play an essential role in signaling through another adhesion molecule, collagen.13-20 Therefore, it was important to establish the mechanism by which Syk is activated downstream of GP Ib in vWF-stimulated platelets. Syk is classically activated by engagement of its tandem Src homology 2 (SH2) domains with doubly phosphorylated tyrosine residues in proteins containing immune-receptor tyrosine-containing activation motifs (ITAMs).21 Neither GP Ib-V-IX nor αIIbβ3 contain ITAM sequences, and only 2 ITAM-containing proteins have been described in platelets; the Fc receptor γ-chain (FcR γ-chain) and the low-affinity receptor for IgG, FcγRIIA.13,22,23 It is possible that either or both of these proteins are required for vWF signaling in platelets and there is evidence to show constitutive association of FcγRIIA with GP Ib-V-IX.24,25 However, the FcR γ-chain has been shown to be critically involved in signaling downstream of collagen19 and its putative signaling receptor GP VI15,16 26 and may, therefore, also be involved in mediating GP Ib signaling to Syk.
We have used 2 approaches to selective activation of GP Ib-V-IX: (1) use of alboaggregin-A, a lectin purified from the venom of the white-lipped tree viper Trimeresurus albolabris, which binds to GP Ib inducing activation, and (2) use of mutant recombinant vWFs that bind selectively to either GP Ib or the integrin αIIbβ3. Recently, a group of viper venom proteins has been reported to interact with GP Ib on platelets resulting in either platelet agglutination and inhibition of ristocetin-induced vWF binding or induction of platelet activation.27-31 One such protein isolated from the venom of T albolabris, the 50-kD C-type lectin alboaggregin-A, has been shown to potently induce platelet activation through binding to GP Ib.28,32 In the present study we have isolated alboaggregin-A to use as a selective tool to induce platelet activation downstream of GP Ib. In addition, we have used 2 recombinant mutant forms of vWF, in combination with the modulator ristocetin, to differentially activate GP Ib or the integrin αIIbβ3. ΔA1-vWF is a deletion mutant of wild-type human vWF in which the major part of the A1 domain, responsible for binding GP Ibα, has been ablated (residues 478-716).33 RGGS-vWF has a point mutation (1746D→G) in the RGD sequence of vWF, disabling its binding to integrin αIIbβ3,34 while allowing normal binding to GP Ib. Using these 2 approaches, we set out to establish a signaling pathway downstream of GP Ib, investigating the role of Src family kinases, FcR γ-chain, Syk, and PLCγ2 in functional responses downstream of this receptor.
MATERIALS AND METHODS
Materials.
Plasma vWF (pvWF) and 2 mutant recombinant forms of vWF (ΔA1-vWF and RGGS-vWF) were kind gifts from Prof J.J. Sixma and Dr T. Vink (Utrecht, The Netherlands), and were prepared as previously described.33 34T albolabris venom was a generous gift from Prof R.D.G. Theakston (Liverpool, UK). The anti-GP Ib mouse monoclonal antibody, MoAb 6D1, was a kind gift from Prof B. Coller (New York, NY). Antiphosphotyrosine MoAb 4G10 and polyclonal anti-FcR γ-chain antibody were from Upstate Biotechnology Inc (TCS Biologicals Ltd, Bucks, UK). Polyclonal anti-Syk, anti-Lyn, anti-GP Ib, anti-Fyn, and anti-PLCγ2 antibodies were from Santa Cruz Biotechnology (Autogen Bioclear, Calne, Wiltshire, UK). GST-fusion protein of Syk tandem SH2 domains (GST-Syk-SH2) was kindly supplied by Dr S. Watson (Oxford, UK). A suspension of type I collagen fibers from equine tendon (Horm collagen) was from Nycomed (Munich, Germany). γ-[32P]-ATP and enhanced chemiluminescence (ECL) reagents were from Amersham Plc (Amersham, UK). Ristocetin, protein A-Sepharose CL 4B, Tween 20, and phenylmethylsulfonyl fluoride (PMSF) were from Sigma (Poole, Dorset, UK). Acrylamide/bisacrylamide solution was from National Diagnostics (Hull, UK). The Src family kinase inhibitor PP1 was from Alexis Corp (Nottingham, UK). All other reagents were of analytical grade.
Preparation and stimulation of human platelets.
Human blood was drawn from drug-free volunteers on the day of the experiment using acid citrate dextrose (ACD: 120 mmol/L sodium citrate, 110 mmol/L glucose, 80 mmol/L citric acid 1:7, vol/vol) as anticoagulant. Platelet-rich plasma (PRP) was prepared by centrifugation (200g, 20 minutes) and platelets were isolated by centrifugation of PRP (1,000g, 10 minutes) in the presence of prostacyclin (0.1 μg/mL). The pellet was resuspended to a density of 4.108 platelets/mL in modified Tyrode’s-HEPES buffer (145 mmol/L NaCl, 2.9 mmol/L KCl, 10 mmol/L HEPES, 1 mmol/L MgCl2, 5 mmol/L glucose, pH 7.3). Indomethacin (10 μmol/L) was added to platelet suspensions throughout subsequent procedures. Stimulation of platelets was performed at 37°C in an aggregometer with continuous stirring at 800 rpm. Concentrations of pvWF (10 μg/mL) and RGGS-vWF (6 μg/mL) were shown to give near-maximal responses, while ΔA1-vWF was used at 3 μg/mL because it had been shown that this concentration allows maximal binding to integrin αIIbβ3.33
Platelet aggregation and release of 5-HT.
Platelet aggregation was measured by optical turbidometry35using a platelet aggregometer (Chronolog Corp, Havertown, PA). For aggregation studies, platelets were suspended in Tyrode’s-HEPES without EGTA, with the exception of those experiments conducted to distinguish fibrinogen-dependent aggregation from adhesion, where platelets were preincubated with EGTA (1 mmol/L) to block fibrinogen binding. The data shown represent the decrease in optical density as a percentage of the maximum possible decrease. For assessment of 5-HT release, platelets were loaded with [3H]-5-HT by incubation with 0.2 μCi/mL PRP for 1 hour at 37°C. Platelets were preincubated with EGTA (1 mmol/L) before stimulation to prevent aggregation, and the reaction was terminated by brief microcentrifugation and [3H]-5-HT release into supernatant was determined by scintillation spectrometry. [3H]-5-HT release was expressed as a percentage of the total tissue content as described previously.19
Measurement of cytosolic [Ca2+].
This was performed as previously described.36 Briefly, PRP was incubated with Fura-2-AM (3 μmol/L) at 30°C for 45 minutes, and platelets prepared as described above. Platelets were stimulated with various concentrations of alboaggregin-A under stirred conditions at room temperature in the absence of EGTA. Fluorescence excitation was made at wavelengths 340 and 380 nm, and emission at 510 nm was measured using a Perkin-Elmer LS50B spectrofluorimeter. Data are presented as the excitation fluorescence ratio (340:380 nm).
Purification of alboaggregin-A.
Alboaggregin-A was prepared from the crude venom of the viperT albolabris, as previously described28-30,32by ion exchange and phenyl sepharose hydrophobic chromatography, and showed physical and functional characteristics identical to that purified by other workers.28-30 32 Briefly, the protein had an apparent molecular weight of 50 kD by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreduced conditions and induced platelet aggregation and 5-HT release in a manner not blocked by the thrombin protease inhibitor PPACK (data not shown). Functional characteristics of alboaggregin-A are described in Results.
Immunoblotting.
Platelets were activated in the presence of EGTA (1 mmol/L) for all blotting and protein precipitation studies. Reactions were stopped by adding an equal volume of Laemmli buffer (2X) and samples were heated for 5 minutes at 95°C. Proteins were separated by either 10% SDS-PAGE or by SDS-PAGE on 10% to 18% gradient slab gels and transferred to polyvinylidene difluoride (PVDF) blotting membranes using a semi-dry transfer system (60 minutes, 15 V). Membranes were incubated for 60 minutes at room temperature with primary followed by secondary antibodies and detected by ECL (Amersham, UK).
Immunoprecipitation and GST-fusion protein precipitation.
Reactions were stopped by lysis with an equal volume of 2X extraction buffer (2% [vol/vol] Triton X-100, 300 mmol/L NaCl, 20 mmol/L Tris, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 10 mmol/L EDTA, 2 mmol/L Na3VO4, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1 μg/mL pepstatin A, pH 7.3), and insoluble material removed by centrifugation (13,000g, 5 minutes, 4°C). Supernatants were then precleared by incubation with protein A-Sepharose (PAS) for immunoprecipitation experiments, or with glutathione-agarose for GST-fusion protein precipitations, for 1 hour at 4°C, followed by centrifugation (13,000g, 5 minutes, 4°C). For immunoprecipitations, supernatants were then incubated with PAS and the appropriate immunoprecipitating antibody for 120 minutes at 4°C, while for GST fusion protein precipitations, supernatants were incubated with glutathione-agarose beads and GST-Syk-SH2 (10 μg/mL) for 120 minutes at 4°C. Beads were then washed once in extraction buffer and twice more in TBS-T before addition of Laemmli sample-treatment buffer. Precipitated proteins were then subjected to SDS-PAGE, transferred to PVDF membrane, and probed with appropriate antibodies as described in Immunoblotting.
In vitro kinase assay.
Immunoprecipitated Syk was suspended in 20 μL kinase assay buffer (5 mmol/L MgCl2, 5 mmol/L MnCl2, 100 mmol/L NaCl, 10 μmol/L adenosine triphosphate (ATP), 20 mmol/L HEPES at pH 7.2) and the reaction started by addition of [γ-32P]-ATP (250 μCi/mL). After incubation for 15 minutes at 25°C, the reaction was terminated by addition of 0.5 mL ice-cold 100 mmol/L EDTA. Samples were subjected to SDS-PAGE and phosphorylated proteins were visualized by autoradiography.
RESULTS
Alboaggregin-A induces platelet aggregation, release of 5-HT, and an increase in cytosolic calcium through activation of GP Ib.
A concentration-response relationship was determined for induction of secretion of 5-HT (Fig 1A) by alboaggregin-A (60 seconds), and the EC50 concentration of 3.5 μg/mL was used throughout the rest of the study to activate platelets, unless otherwise stated. At this concentration, alboaggregin-A induced platelet aggregation, which was substantially inhibited by calcium chelation with EGTA (1 mmol/L) or the monoclonal anti-GP Ib blocking antibody 6D1 (9 μg/mL) (Fig 1B) demonstrating platelet activation through binding GP Ib. In contrast, aggregation in response to collagen (100 μg/mL, 2 minutes) was not inhibited in the presence of 6D1 (10 μg/mL). EGTA (1 mmol/L) partially inhibited platelet aggregation by collagen, the remaining response being caused by calcium-independent adhesion of platelets to collagen fibers. Secretion of 5-HT induced by alboaggregin-A was inhibited by MoAb 6D1 (Fig 1C). In contrast with aggregation, however, secretion was unaffected by EGTA, demonstrating alboaggregin-A to bind GP Ib in the absence of extracellular calcium. However, collagen-induced secretion of 5-HT was unaffected by either EGTA or MoAb 6D1. Alboaggregin-A also induced a dose-dependent rapid increase in cytosolic calcium, as assessed by the change in 340:380-nm fluorescence ratio of Fura-2–loaded platelets (Fig 1D).
Alboaggregin-A induces platelet aggregation, release of 5-HT and an increase in cytosolic calcium upon binding to GP Ib. For 5-HT release, studies platelets were preloaded with [3H]-5-HT and stimulated with various concentrations of alboaggregin-A (A, D) or 3.5 μg/mL alboaggregin-A (B, C) for 1 minute. Collagen (100 μg/mL; 2 minutes) was used for comparison and as a negative control (B, C). Release of 5-HT is presented as a percentage of total 5-HT content, and a dose-response relationship was determined in (A), showing an EC50 of 3.5 μg/mL. Platelet aggregation is presented as the decrease in optical density induced by agonist as a percentage of the maximal possible decrease (B). Platelets were pretreated with MoAb 6D1 (9 μg/mL) for 6 minutes, or EGTA (1 mmol/L) for 10 minutes, before stimulation with agonist (B, C). For assessment of changes in cytosolic calcium (D), platelets were preloaded with the calcium indicator dye Fura-2 and fluorescence measurements were made at emission wavelength 510 nm. Data are presented as the ratio of fluorescence measurements at excitation wavelengths 340 and 380 nm. Alboaggregin-A is added at the time point indicated by the arrow, at concentrations indicated on the right of the graphs. Data presented are means ± SEM for 3 experiments (A, B, C) or are representative of 3 separate experiments (D).
Alboaggregin-A induces platelet aggregation, release of 5-HT and an increase in cytosolic calcium upon binding to GP Ib. For 5-HT release, studies platelets were preloaded with [3H]-5-HT and stimulated with various concentrations of alboaggregin-A (A, D) or 3.5 μg/mL alboaggregin-A (B, C) for 1 minute. Collagen (100 μg/mL; 2 minutes) was used for comparison and as a negative control (B, C). Release of 5-HT is presented as a percentage of total 5-HT content, and a dose-response relationship was determined in (A), showing an EC50 of 3.5 μg/mL. Platelet aggregation is presented as the decrease in optical density induced by agonist as a percentage of the maximal possible decrease (B). Platelets were pretreated with MoAb 6D1 (9 μg/mL) for 6 minutes, or EGTA (1 mmol/L) for 10 minutes, before stimulation with agonist (B, C). For assessment of changes in cytosolic calcium (D), platelets were preloaded with the calcium indicator dye Fura-2 and fluorescence measurements were made at emission wavelength 510 nm. Data are presented as the ratio of fluorescence measurements at excitation wavelengths 340 and 380 nm. Alboaggregin-A is added at the time point indicated by the arrow, at concentrations indicated on the right of the graphs. Data presented are means ± SEM for 3 experiments (A, B, C) or are representative of 3 separate experiments (D).
Alboaggregin-A induces tyrosine phosphorylation of Syk and PLCγ2 and activation of Syk.
Previous reports have shown that a variety of platelet agonists, including collagen, thrombin, and activation of GP Ib, are able to induce the tyrosine phosphorylation and activation of Syk.11-13,19,37 Figure 2A shows that both alboaggregin-A and collagen, which was used for comparison, induced tyrosine phosphorylation of Syk above basal levels. In addition, Syk activity, assayed in vitro as autophosphorylation, was shown to increase markedly upon activation with alboaggregin-A (Fig2B). Tyrosine phosphorylation of PLCγ2 has previously been reported in response to collagen and crosslinking FcγRIIA in platelets,13,14,20,38 39 and here we show that stimulation of platelets with alboaggregin-A caused tyrosine phosphorylation of PLCγ2 (Fig 2C). Collagen-induced phosphorylation of PLCγ2 is shown for comparison. Alboaggregin-A induced greater phosphorylation of Syk than collagen, possibly due to phosphorylation of additional tyrosine residues not phosphorylated upon collagen stimulation.
Alboaggregin-A induces tyrosine phosphorylation of Syk and PLCγ2 and activates Syk kinase. For (A), Syk and for (C), PLCγ2 were immunoprecipitated from lysates of basal platelets (lane 1) or platelets stimulated with either alboaggregin-A (3.5 μg/mL; lane 2) for 1 minute or collagen (100 μg/mL; lane 3) for 2 minutes and Western blotted with either 4G10 [A(i) and C(i)] and anti-Syk [A(ii)] or anti-PLCγ2 [C(ii)]. For (B), a kinase assay was performed in vitro on Syk immunoprecipitates from basal platelets (lane 1) or platelets stimulated with alboaggregin-A (3.5 μg/mL; lane 2) for 1 minute, and presented as an autoradiograph. The results are representative of 3 separate experiments.
Alboaggregin-A induces tyrosine phosphorylation of Syk and PLCγ2 and activates Syk kinase. For (A), Syk and for (C), PLCγ2 were immunoprecipitated from lysates of basal platelets (lane 1) or platelets stimulated with either alboaggregin-A (3.5 μg/mL; lane 2) for 1 minute or collagen (100 μg/mL; lane 3) for 2 minutes and Western blotted with either 4G10 [A(i) and C(i)] and anti-Syk [A(ii)] or anti-PLCγ2 [C(ii)]. For (B), a kinase assay was performed in vitro on Syk immunoprecipitates from basal platelets (lane 1) or platelets stimulated with alboaggregin-A (3.5 μg/mL; lane 2) for 1 minute, and presented as an autoradiograph. The results are representative of 3 separate experiments.
Syk associates with FcR γ-chain, mediated by tandem SH2 domains of Syk.
Figure 3A shows that alboaggregin-A induced a marked, early association between FcR γ-chain and GST-Syk-SH2, which was likely to be caused by a rapid phosphorylation of FcR γ-chain. The association decreased at later time points, probably reflecting a time-dependent dephosphorylation of the γ-chain. In addition, Fig 3B shows that pvWF (10 μg/mL) in the presence of ristocetin (1 mg/mL) stimulated a marked, early association of tyrosine phosphorylated γ-chain with GST-Syk-SH2, which diminished with time. Additional tyrosine-phosphorylated proteins of 44, 56, and 59 kD were found to associate with GST-Syk-SH2 both in alboaggregin-A–stimulated and vWF-stimulated platelets, although these proteins remain unidentified. This finding leaves the possibility that there are routes other than the γ-chain by which Syk may be activated downstream of GP Ib. Precipitates from collagen-stimulated platelets are shown for comparison.
Alboaggregin-A and pvWF induce association of tyrosine phosphorylated FcR γ-chain with GST-Syk-SH2. Platelets were stimulated with either alboaggregin-A (3.5 μg/mL), pvWF (10 μg/mL), and ristocetin (1 mg/mL) or with collagen (100 μg/mL) for the indicated times, and proteins were precipitated from cell lysates using 10 μg of GST-Syk SH2 per lane. Precipitated proteins were separated by SDS-PAGE and immunoblotted with 4G10 [A(i) and B(i)] and anti-FcR γ-chain [A(ii) and B(ii)]. For (A), lane 1 is resting platelets and lanes 2 through 4 were stimulated with alboaggregin-A for the times indicated. For (B), lanes 1 and 7 were resting platelets, lanes 2 through 6 were stimulated with pvWF in the presence of ristocetin for the times indicated, and in lane 8, platelets were stimulated with collagen. Results shown are representative of at least 3 separate experiments.
Alboaggregin-A and pvWF induce association of tyrosine phosphorylated FcR γ-chain with GST-Syk-SH2. Platelets were stimulated with either alboaggregin-A (3.5 μg/mL), pvWF (10 μg/mL), and ristocetin (1 mg/mL) or with collagen (100 μg/mL) for the indicated times, and proteins were precipitated from cell lysates using 10 μg of GST-Syk SH2 per lane. Precipitated proteins were separated by SDS-PAGE and immunoblotted with 4G10 [A(i) and B(i)] and anti-FcR γ-chain [A(ii) and B(ii)]. For (A), lane 1 is resting platelets and lanes 2 through 4 were stimulated with alboaggregin-A for the times indicated. For (B), lanes 1 and 7 were resting platelets, lanes 2 through 6 were stimulated with pvWF in the presence of ristocetin for the times indicated, and in lane 8, platelets were stimulated with collagen. Results shown are representative of at least 3 separate experiments.
vWF induces Syk phosphorylation and association with FcR γ-chain through activation of GP Ib.
To show that vWF-stimulated association was downstream of GP Ib, Syk was immunoprecipitated from platelets challenged with mutant vWFs that differentially bind either GP Ib or the integrin αIIbβ3. Figure4A shows that although pvWF induced Syk phosphorylation and association of Syk with tyrosine-phosphorylated γ-chain, this response was absent in platelets activated by ΔA1-vWF, a mutant unable to bind GP Ib, in the presence of ristocetin (1 mg/mL). On the other hand, RGGS-vWF, a mutant that is unable to bind αIIbβ3 but is able to bind GP Ib, induced both tyrosine phosphorylation of Syk (data not shown) and association with the γ-chain (Fig 4B). Furthermore, as has previously been reported,13,14,16,20 37 both thrombin and collagen were able to induce tyrosine phosphorylation of Syk (data not shown). However, Syk associated with FcR γ-chain only in collagen-stimulated, but not thrombin-stimulated, platelets (Fig 4B). These findings indicate that FcR γ-chain associates with Syk only in cells stimulated through GP Ib or by collagen, but not by thrombin.
Mutant forms of vWF induce differential association between Syk and tyrosine phosphorylated FcR γ-chain.Platelets were stimulated with mutant vWF or pvWF in the presence of ristocetin for 45 seconds, thrombin for 45 seconds, or collagen for 120 seconds. Syk was immunoprecipitated, run on SDS-PAGE, and Western blotted with 4G10 [A(i) and B(i)] or with anti-Syk [A(ii) and B(ii)]. [A(i)]: lane 1, resting platelets; lane 2, pvWF (10 μg/mL) and ristocetin (1 mg/mL); lane 3, ▵A1-vWF (3 μg/mL) and ristocetin (1 mg/mL). [B(i)] Phosphorylation of FcR γ-chain which has been precipitated in association with Syk from basal platelets (lane 1); platelets stimulated with pvWF (10 μg/mL, lane 2), ▵A1-vWF (3 μg/mL, lane 3), RGGS-vWF (6 μg/mL, lane 4) in the presence of ristocetin (1 mg/mL); thrombin (1 U/mL, lane 5) or collagen (100 μg/mL, lane 6). Equal amounts of Syk were present in each lane [B(ii)]. Immunoblots shown are representative of 4 separate experiments.
Mutant forms of vWF induce differential association between Syk and tyrosine phosphorylated FcR γ-chain.Platelets were stimulated with mutant vWF or pvWF in the presence of ristocetin for 45 seconds, thrombin for 45 seconds, or collagen for 120 seconds. Syk was immunoprecipitated, run on SDS-PAGE, and Western blotted with 4G10 [A(i) and B(i)] or with anti-Syk [A(ii) and B(ii)]. [A(i)]: lane 1, resting platelets; lane 2, pvWF (10 μg/mL) and ristocetin (1 mg/mL); lane 3, ▵A1-vWF (3 μg/mL) and ristocetin (1 mg/mL). [B(i)] Phosphorylation of FcR γ-chain which has been precipitated in association with Syk from basal platelets (lane 1); platelets stimulated with pvWF (10 μg/mL, lane 2), ▵A1-vWF (3 μg/mL, lane 3), RGGS-vWF (6 μg/mL, lane 4) in the presence of ristocetin (1 mg/mL); thrombin (1 U/mL, lane 5) or collagen (100 μg/mL, lane 6). Equal amounts of Syk were present in each lane [B(ii)]. Immunoblots shown are representative of 4 separate experiments.
FcR γ-chain associates with Fyn, Lyn, and GP Ib in platelets activated with alboaggregin-A.
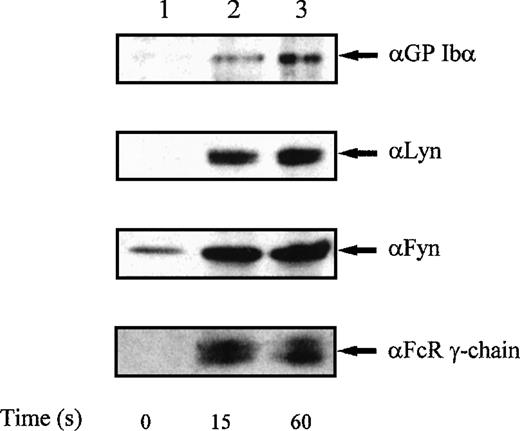
Recent studies have reported a selective role for Fyn and Lyn, but not other Src-family kinase members, in platelets stimulated through the collagen receptor GP VI.14 40 We used GST-Syk-SH2 to precipitate γ-chain from alboaggregin-A–activated platelets, and identified co-associating proteins by Western blotting. Under these conditions, both Fyn and Lyn associated with GST-Syk-SH2 complexed to FcR γ-chain (Fig 5); in addition, GP Ib precipitated with this signaling complex, showing a physical association between these signaling elements under stimulated conditions.
Coprecipitation of FcR γ-chain, GPIb, Lyn, and Fyn with GST-Syk-SH2 from platelets stimulated with alboaggregin-A.Platelets were either unstimulated (lane 1) or were stimulated with alboaggregin-A (3.5 μg/mL) for 15 seconds (lane 2) or 60 seconds (lane 3), and proteins were precipitated with 10 μg of GST-Syk-SH2 per lane. Precipitated proteins were then separated by SDS-PAGE and immunoblotted with GPIb, Lyn, Fyn, or FcR γ-chain, as indicated. Results shown are representative of 3 separate experiments.
Coprecipitation of FcR γ-chain, GPIb, Lyn, and Fyn with GST-Syk-SH2 from platelets stimulated with alboaggregin-A.Platelets were either unstimulated (lane 1) or were stimulated with alboaggregin-A (3.5 μg/mL) for 15 seconds (lane 2) or 60 seconds (lane 3), and proteins were precipitated with 10 μg of GST-Syk-SH2 per lane. Precipitated proteins were then separated by SDS-PAGE and immunoblotted with GPIb, Lyn, Fyn, or FcR γ-chain, as indicated. Results shown are representative of 3 separate experiments.
PP1 inhibits Syk phosphorylation, association with tyrosine-phosphorylated γ-chain, and phosphorylation of PLCγ2.
The Src family kinase inhibitor PP1 was shown to dose-dependently inhibit phosphorylation of multiple proteins induced by alboaggregin-A (data not shown). Figure 6A shows that PP1 inhibited phosphorylation of Syk and its association with tyrosine-phosphorylated γ-chain in a concentration-dependent manner. Interestingly, 5 μmol/L PP1 almost abolished γ-chain phosphorylation and association, but only partially inhibited Syk phosphorylation. This may provide further evidence for the existence of γ-chain–independent pathways to activation of Syk by GP Ib.
PP1 dose-dependently inhibits alboaggregin-A–induced tyrosine phosphorylation of FcR γ-chain, Syk, and PLCγ2.Platelets were preincubated for 3 minutes at 37°C with 0.25% dimethyl sulfoxide (DMSO) or various concentrations of PP1 before stimulation with alboaggregin-A (3.5 μg/mL) for 1 minute. Platelet suspensions were then lysed and either Syk or PLCγ2 immunoprecipitated. Precipitated proteins were then separated by SDS-PAGE and immunoblotted with 4G10 [A(i) and B(i)] and subsequently with either anti-Syk [A(ii)] or anti-PLCγ2 [B(ii)]. Immunoblots shown are representative of 3 separate experiments.
PP1 dose-dependently inhibits alboaggregin-A–induced tyrosine phosphorylation of FcR γ-chain, Syk, and PLCγ2.Platelets were preincubated for 3 minutes at 37°C with 0.25% dimethyl sulfoxide (DMSO) or various concentrations of PP1 before stimulation with alboaggregin-A (3.5 μg/mL) for 1 minute. Platelet suspensions were then lysed and either Syk or PLCγ2 immunoprecipitated. Precipitated proteins were then separated by SDS-PAGE and immunoblotted with 4G10 [A(i) and B(i)] and subsequently with either anti-Syk [A(ii)] or anti-PLCγ2 [B(ii)]. Immunoblots shown are representative of 3 separate experiments.
Figure 6B shows that phosphorylation of PLCγ2 was dose-dependently inhibited by PP1 such that there was partial inhibition at 5 μmol/L PP1 and full inhibition by 20 μmol/L PP1. This result parallels that for Syk phosphorylation and is consistent with PLCγ2 activation being closely coupled to Syk activation.
Inhibition of Src family kinases inhibits GP Ib-mediated platelet aggregation and secretion of 5-HT.
To demonstrate a functional requirement for Src family kinases in platelet activation through GP Ib, we showed that PP1 dose-dependently inhibited both platelet aggregation and release of 5-HT induced by alboaggregin-A (Fig 7). Full inhibition of responses was achieved by 20 μmol/L PP1, in parallel with inhibition of tyrosine phosphorylation of Syk and PLCγ2.
PP1 dose-dependently inhibits alboaggregin-A–induced platelet aggregation and 5-HT secretion. Platelets were preincubated for 3 minutes at 37°C with 0.25% DMSO or various concentrations of PP1 (indicated on the right of the graphs) before stimulation with alboaggregin-A (3.5 μg/mL) for 1 minute. Platelet aggregation was measured as a decrease in optical density of a stirred platelet suspension and 5-HT release was measured after loading cells with [3H]-5-HT. Both platelet aggregation and release of 5-HT are presented as percentages of the responses induced in the absence of PP1. Data presented are means ± SEM for 3 experiments.
PP1 dose-dependently inhibits alboaggregin-A–induced platelet aggregation and 5-HT secretion. Platelets were preincubated for 3 minutes at 37°C with 0.25% DMSO or various concentrations of PP1 (indicated on the right of the graphs) before stimulation with alboaggregin-A (3.5 μg/mL) for 1 minute. Platelet aggregation was measured as a decrease in optical density of a stirred platelet suspension and 5-HT release was measured after loading cells with [3H]-5-HT. Both platelet aggregation and release of 5-HT are presented as percentages of the responses induced in the absence of PP1. Data presented are means ± SEM for 3 experiments.
DISCUSSION
It has become clear that vWF is able to induce platelet activation,5,6,41,42 and it is now emerging that tyrosine phosphorylation events may play a central signaling role in vWF-induced activation.1,43-45 Furthermore, many of these signaling events are directly downstream of the primary vWF receptor GP Ib-V-IX, which has been shown to induce tyrosine phosphorylation of multiple platelet proteins.43-45 Recent evidence suggests that vWF may activate the nonreceptor tyrosine kinase Syk through GP Ib. Antibody-induced cross-linking of GP Ib induces a small aggregation response associated with activation of Syk12 and, in the presence of the modulator botrocetin, vWF induces tyrosine phosphorylation and activation of Syk downstream of GP Ib.11
The aim of this report was to elucidate mechanisms that couple GP Ib to Syk activation, and to determine signaling events downstream of Syk that may regulate functional activities. Although there may be other mechanisms by which Syk may be activated,46,47 it is generally recognized that Syk forms signaling complexes at the plasma membrane by interaction of its tandem Src homology 2 (SH2) domains with proteins containing phosphorylated ITAMs.21,48,49 None of the components of GP Ib-V-IX complex possess ITAM motifs; however, platelets express at least 2 ITAM-containing proteins; the low-affinity receptor for IgG, FcγRIIA, and FcR γ-chain.13,22 23 It is possible that GP Ib-V-IX forms a functional complex with either or both of these proteins, allowing it to couple to Syk upon activation.
The FcR γ-chain forms an integral part of several antibody Fc receptors and has recently been shown to be complexed constitutively to the platelet receptor for the adhesion molecule collagen, GP VI.15,26 It becomes tyrosine phosphorylated and associates with Syk upon activation of platelets with collagen or the GP VI-specific C-type lectin convulxin.15,16,23,26,50 In knock-out studies, both the γ-chain and Syk have been shown to be essential for activation of platelets by collagen19 and, importantly in the absence of FcR γ-chain, collagen is unable to induce Syk activation, showing the FcR γ-chain to be an essential upstream regulator of Syk. We were therefore interested in investigating whether Syk activation downstream of GP Ib might involve an essential receptor complex with the FcR γ-chain.
Several tyrosine-phosphorylated proteins associate with Syk upon activation with vWF or alboaggregin-A, and prominent amongst these is a doublet of 14 kD that was shown to be the FcR γ-chain. The association was through the SH2 domains of Syk and was absent in experiments using the ΔA1-vWF mutant, which is unable to bind GP Ib, but maintained with RGGS-vWF, which is unable to bind the integrin αIIbβ3, thus strongly supporting the hypothesis that the FcR γ-chain couples GP Ib to Syk. Interestingly, although thrombin was also able to induce phosphorylation of Syk, in agreement with other investigators,47,51 there was no association with the γ-chain, indicating a γ-chain-independent mechanism to Syk activation downstream of this receptor. Collagen, on the other hand, induced phosphorylation of Syk that associated with phosphorylated FcR γ-chain, in agreement with previous reports.14,19,23 Therefore, it is clear that FcR γ-chain is likely to represent a major pathway to activation of Syk downstream of GP Ib, but the presence of other tyrosine-phosphorylated proteins precipitated by Syk SH2 domains leaves open the possibility that there are other routes to Syk activation independent of FcR γ-chain, downstream of GP Ib. This would be consistent with phenotypes in knockout mice that we observed previously.19 In the absence of Syk, mice suffer a severe petechial hemorrhaging during fetal development19,52,53 and platelets from these mice do not respond to collagen. In contrast, although platelets from mice lacking FcR γ-chain also lack a response to collagen, they do not manifest a major bleeding disorder, suggesting multiple mechanisms by which Syk may be activated downstream of several receptors. One possible link to Syk in human platelets would be FcγRIIA, which has been shown to be constitutively associated with GP Ib,24,25 although this would not explain the difference in knockout phenotypes because mice do not possess the FcγRIIA gene.54
It is now established that Src family kinases are essential for primary signals to Syk through ITAM motif-containing proteins.21,55,56 Platelets express abundant quantities of several Src family kinases including Src, Fyn, Lyn, Yes, Hck, Fgr, and Lck.57-60 It has previously been shown that Src transiently associates with Syk in vWF-activated platelets,11 and that Src may be activated by vWF, translocating to the cytoskeleton upon activation.61 In the present study we show that both Fyn and Lyn physically associate with GST-Syk-SH2:FcR γ-chain complex upon platelet activation through GP Ib. In addition, GP Ib itself forms a component of this signaling complex. The technique used to precipitate FcR γ-chain in these studies, in which we use GST-Syk-SH2 to precipitate tyrosine-phosphorylated FcR γ-chain, only allows precipitation of γ-chain from activated cells. This limitation does not allow us to conclude whether the association between FcR γ-chain and the other signaling components takes place constitutively or upon platelet activation. However, there is recent evidence that Src-family kinases may associate with nonphosphorylated γ-chain,62and we present evidence that Fyn may bind to GST-Syk-SH2 constitutively (see Fig 5). We therefore speculate that Fyn may form a physical link between the γ-chain and Syk under basal conditions. We also have preliminary evidence that FcR γ-chain is constitutively associated to GP Ib because it is isolated from unstimulated cell lysates precipitated with alboaggregin-A bound to a solid phase (S.F., A.W.P., unpublished observations, September 1998). Taken together, these results may parallel previous data for the platelet collagen receptor, GP VI, in which FcR γ-chain associates with the receptor and Src family members Fyn and Lyn irrespective of activation.14
Therefore, it is concluded that GP Ib, γ-chain, Fyn, Lyn, and Syk form a physical complex upon activation of GP Ib, leading to multiple functional events in platelets including platelet aggregation, 5-HT release, and an increase in cytosolic calcium. We were interested to establish whether the receptor-signaling complex would also signal to activation of PLCγ2, which has previously been shown to be tyrosine phosphorylated in collagen-stimulated platelets.13,38,39PLCγ2 has been shown to be downstream of Syk in hematopoietic cells including platelets,19,63-65 and may functionally couple the proximal GP Ib signaling complex to downstream functional events. In this report we show that PLCγ2 becomes tyrosine phosphorylated downstream of GP Ib. In addition, Src family kinases are essential for its tyrosine phosphorylation because PP1, which has selectivity for Src family kinases over Syk at concentrations up to 100 μmol/L,55,66 dose-dependently inhibits PLCγ2 phosphorylation as well as Syk phosphorylation, platelet aggregation, and secretion of 5-HT. FcR γ-chain phosphorylation is also inhibited by PP1, although at 5 μmol/L the inhibition of γ-chain phosphorylation is greater than that for Syk or PLCγ2. This disproportion between the phosphorylation of these proteins may provide further evidence that Syk is activated by γ-chain–independent pathways, such as that described by Gao et al,46 or there may be sufficient signal amplification at the level of the γ-chain. It is also interesting that 20 μmol/L PP1, which blocks phosphorylation of all signaling proteins studied here, does not fully block 5-HT release. This provides evidence for Src-family kinase-independent pathways to platelet activation by GP Ib. These pathways may include PI 3-kinase, which has been shown to be activated by vWF,61 leading to direct activation of PLCγ267-71 without a requirement for tyrosine phosphorylation. 14-3-3 proteins have also been shown to bind GP Ib-V-IX components72-75 and may form an important Src-family kinase-independent signaling pathway.
Based on the present findings, we put forward a working model in which GP Ib associates with FcR γ-chain, either constitutively or upon activation of the receptor, and that Src family kinases Fyn and Lyn phosphorylate FcR γ-chain leading to binding of Syk through its tandem SH2 domains, activation of Syk, and finally PLCγ2 tyrosine phosphorylation and activation leading to platelet functional responses. However, this model leaves several important questions to be addressed; the possibility of FcR γ-chain–independent mechanisms by which GP Ib can couple to Syk activation warrants further investigation. Furthermore, details of the construction of the receptor-FcR γ-chain-Fyn-Lyn-Syk complex, under basal and stimulated conditions, remain to be elucidated. Recent studies have also established that PLCγ2 can become activated in tyrosine phosphorylation-dependent and -independent manners.67-71 It remains unknown whether PLCγ2 becomes activated downstream of GP Ib and, if so, whether tyrosine phosphorylation of PLCγ2 is the sole mechanism by which PLCγ2 may become activated downstream of this receptor.
ACKNOWLEDGMENT
The authors are grateful to Prof Jan Sixma and Dr Tom Vink for the kind gift of pvWF and mutant recombinant forms of vWF; Prof David Theakston for generously supplying venom from the viper T albolabris; Dr Jon Gibbins for his time, resources, and expertise in assisting preparation of purified alboaggregin-A; Prof Barry Coller for kindly supplying MoAb 6D1; and Dr Steve Watson for the generous donation of GST-Syk-SH2.
Supported by grants from the Biotechnology and Biological Sciences Research Council, UK and the British Heart Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Alastair W. Poole, PhD, Department of Pharmacology, School of Medical Sciences, University Walk, Bristol BS8 1TD, UK; e-mail: A.Poole@bris.ac.uk.

![Fig. 1. Alboaggregin-A induces platelet aggregation, release of 5-HT and an increase in cytosolic calcium upon binding to GP Ib. For 5-HT release, studies platelets were preloaded with [3H]-5-HT and stimulated with various concentrations of alboaggregin-A (A, D) or 3.5 μg/mL alboaggregin-A (B, C) for 1 minute. Collagen (100 μg/mL; 2 minutes) was used for comparison and as a negative control (B, C). Release of 5-HT is presented as a percentage of total 5-HT content, and a dose-response relationship was determined in (A), showing an EC50 of 3.5 μg/mL. Platelet aggregation is presented as the decrease in optical density induced by agonist as a percentage of the maximal possible decrease (B). Platelets were pretreated with MoAb 6D1 (9 μg/mL) for 6 minutes, or EGTA (1 mmol/L) for 10 minutes, before stimulation with agonist (B, C). For assessment of changes in cytosolic calcium (D), platelets were preloaded with the calcium indicator dye Fura-2 and fluorescence measurements were made at emission wavelength 510 nm. Data are presented as the ratio of fluorescence measurements at excitation wavelengths 340 and 380 nm. Alboaggregin-A is added at the time point indicated by the arrow, at concentrations indicated on the right of the graphs. Data presented are means ± SEM for 3 experiments (A, B, C) or are representative of 3 separate experiments (D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1648/5/m_blod41731001x.jpeg?Expires=1767719139&Signature=1gZOyYON2purUCYtmlOL9OEmNio28pIfz4qzeSJ63sMdKeYHOH3O0jDZxlZ~AYrUCYJWzKYoJluFroCexsWH3ji7QM6FLLgYcXAWVk20XoFtYmk7WWJHsGSRcSryj2GUEpbZsWEJI3kqAt8d9opUR63rokbe5OT2JbkPWr6-z0lL5in7AJTgRiyzJ5ZBh5NbOhnVTo3Pph5-cmRHZQXQAPvlIP6hEXMlFtLF3ED9mPbdiMH0-but61Y9hSQJZKsvtekaVecWMX7DK~P0FfAsJlz8TyTFTF6EUjOyEb9aBqd8fQdHeQNqY~f3KqsS~C2AvTOq~d3BDnm6z7ywEOxy7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Alboaggregin-A induces tyrosine phosphorylation of Syk and PLCγ2 and activates Syk kinase. For (A), Syk and for (C), PLCγ2 were immunoprecipitated from lysates of basal platelets (lane 1) or platelets stimulated with either alboaggregin-A (3.5 μg/mL; lane 2) for 1 minute or collagen (100 μg/mL; lane 3) for 2 minutes and Western blotted with either 4G10 [A(i) and C(i)] and anti-Syk [A(ii)] or anti-PLCγ2 [C(ii)]. For (B), a kinase assay was performed in vitro on Syk immunoprecipitates from basal platelets (lane 1) or platelets stimulated with alboaggregin-A (3.5 μg/mL; lane 2) for 1 minute, and presented as an autoradiograph. The results are representative of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1648/5/m_blod41731002w.jpeg?Expires=1767719139&Signature=cynVfjbOtEejAbY8-FzDios-dC1c6k7E-tLqeYa4U4LKSvGqm9DwlTAcpG9w0rmhD7s03xBhemBPt8pauxiOi1EAZfgwWq0grV4CouAHuLbG6TZzNo-cfraiH80EhSTWAcjFxRA2xUhUZMpo~FpSk-Cw0I9RG132~uoGaAOck0c04UlDvYaepDRHEmtHwZCNRTAqonr-0Uk3IgIJ82MsJVDIPS9vObqvOAEPB-wISQFggiygiVl~xmcjNPH1kybIRLOYC6l8ktJO19Js8ePlJ64ViajvlPRud1s1l3x99ZHOGxvijF5eAhZLlWylfBTxT7NpIx9ODte2939Pa5WxOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Alboaggregin-A and pvWF induce association of tyrosine phosphorylated FcR γ-chain with GST-Syk-SH2. Platelets were stimulated with either alboaggregin-A (3.5 μg/mL), pvWF (10 μg/mL), and ristocetin (1 mg/mL) or with collagen (100 μg/mL) for the indicated times, and proteins were precipitated from cell lysates using 10 μg of GST-Syk SH2 per lane. Precipitated proteins were separated by SDS-PAGE and immunoblotted with 4G10 [A(i) and B(i)] and anti-FcR γ-chain [A(ii) and B(ii)]. For (A), lane 1 is resting platelets and lanes 2 through 4 were stimulated with alboaggregin-A for the times indicated. For (B), lanes 1 and 7 were resting platelets, lanes 2 through 6 were stimulated with pvWF in the presence of ristocetin for the times indicated, and in lane 8, platelets were stimulated with collagen. Results shown are representative of at least 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1648/5/m_blod41731003w.jpeg?Expires=1767719139&Signature=uOPJtt-Ic4k9hywvzW7fkpTy0MpqBufeplN1e-VdSRT2oKIiw4yk94eFtN7ZvKUJzxUjZVTWR2vKS7Q-qvJ6Q0n1EDKPLBrOHFPUbbOwEq6V8bBEhf~Z3MpZjqUOo1Yd2BtUApnagdGO0e7ItPRoWWl6tQpw9EQFDCWw2nV2odN-zIyvOjiMShJCAOnzssoW1xu0lhtycaKw52pR-oCi4DHY6IcZwXUUDPOtjyFYu2EeZICaHdPeQYglUN1InC8byoEJ9FcFhysp2-1MXgx431nzqxqOO-WjXTAeUMkU-SBREWr8OkgsRAJsdcDtNR~XWbDhxYLXsZ16cZ9fqR54DA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Mutant forms of vWF induce differential association between Syk and tyrosine phosphorylated FcR γ-chain.Platelets were stimulated with mutant vWF or pvWF in the presence of ristocetin for 45 seconds, thrombin for 45 seconds, or collagen for 120 seconds. Syk was immunoprecipitated, run on SDS-PAGE, and Western blotted with 4G10 [A(i) and B(i)] or with anti-Syk [A(ii) and B(ii)]. [A(i)]: lane 1, resting platelets; lane 2, pvWF (10 μg/mL) and ristocetin (1 mg/mL); lane 3, ▵A1-vWF (3 μg/mL) and ristocetin (1 mg/mL). [B(i)] Phosphorylation of FcR γ-chain which has been precipitated in association with Syk from basal platelets (lane 1); platelets stimulated with pvWF (10 μg/mL, lane 2), ▵A1-vWF (3 μg/mL, lane 3), RGGS-vWF (6 μg/mL, lane 4) in the presence of ristocetin (1 mg/mL); thrombin (1 U/mL, lane 5) or collagen (100 μg/mL, lane 6). Equal amounts of Syk were present in each lane [B(ii)]. Immunoblots shown are representative of 4 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1648/5/m_blod41731004w.jpeg?Expires=1767719139&Signature=QpVcFBR2H81XBwgYMkfvjQJ5ZX4a2q1K~bR6q2ttcvnOBDAznKuWQHZ3ZuPTlGyyiYRQvkD6cIeqidKK~K1DUDrJ4nT2tClM8nfEawAH78-A8K84dihVsVQp5GakKb3PGRS7xiodBAvShkvz7~kYwXFjk5hcUmFBvS4ysH-jn0ovVX5yZC8M8h2eFZqqqbhnY7Yyl7ft8pq2Ag87OKBbP3yE9FN~r2s0a0hMsopu0mHCeNg4UWnW9aW8UarXjUmnpm0S0EeFj0oH5HV~3CfpDkW3bSjEbVnWgUqr99Zi0Sgja55EKoFCxlK0YqoMUgK8twBiXL8FRpvLZDoS~G2TYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. PP1 dose-dependently inhibits alboaggregin-A–induced tyrosine phosphorylation of FcR γ-chain, Syk, and PLCγ2.Platelets were preincubated for 3 minutes at 37°C with 0.25% dimethyl sulfoxide (DMSO) or various concentrations of PP1 before stimulation with alboaggregin-A (3.5 μg/mL) for 1 minute. Platelet suspensions were then lysed and either Syk or PLCγ2 immunoprecipitated. Precipitated proteins were then separated by SDS-PAGE and immunoblotted with 4G10 [A(i) and B(i)] and subsequently with either anti-Syk [A(ii)] or anti-PLCγ2 [B(ii)]. Immunoblots shown are representative of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1648/5/m_blod41731006w.jpeg?Expires=1767719139&Signature=KimJfobKblE4beUPt9~1IhHkMtdH82amgmDKuJ4~S-qH5y5yrXDpNQGEwJ0urPY-HKQdXHrVMLJ0F40e5x~nB-byVx20Bp5nlsSMJ-0WfUw8~k9WZl69qCQjuOeCK~tB3GeNMTKTCzbwZEzR1R91~1aKE9PX~LhtkcN8S9wbDZJFhKZFS1iCcFwwxoCYitPMWNCJ1pwccbBpaYc~dJBUSy3bBdX57iZ5HJEnwhHRQH6gHLZOEFHqpsi3qX4VHt3GaEB7ex1AqFj9D3OcidDdaUkX2eZdfGRFC5SLbIO8l-xsPMdJ0lh3JBQhoUOkMIZEiDCbspeYD0ll3OaZ-TjlcQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. PP1 dose-dependently inhibits alboaggregin-A–induced platelet aggregation and 5-HT secretion. Platelets were preincubated for 3 minutes at 37°C with 0.25% DMSO or various concentrations of PP1 (indicated on the right of the graphs) before stimulation with alboaggregin-A (3.5 μg/mL) for 1 minute. Platelet aggregation was measured as a decrease in optical density of a stirred platelet suspension and 5-HT release was measured after loading cells with [3H]-5-HT. Both platelet aggregation and release of 5-HT are presented as percentages of the responses induced in the absence of PP1. Data presented are means ± SEM for 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/5/10.1182_blood.v94.5.1648/5/m_blod41731007x.jpeg?Expires=1767719139&Signature=YX4vhU4ydtFDRwNQX9G5MUGsXxJLJthNG7y-FOt9QGGSYv81ig41KvdXUKa1sGIdMeDap~lt1f6za-8emT5jrCbrsbkb6eBPtM304GRNyyqfTxmBOH6b2TIlysWKWlWzHBfIg~-urxP~MnVLFkXkKCzZkhauHRuNCeUHdkQRgKgnnbwruQOPROE6La0jvAWLg6azxh~2I9bVsk92bQ6V6QrhkDbaF1iQ2Z7S0jj826aBixBkOAT0SoTSLJ5O8HDEcLDhuWFUdNGpAGS-YYljETiFqY0NFqXd0YZYZa8XRQMwQSeAUbjlHEiXOGmZeOtzPzQw8NupZEHwLGvi5SrgDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal