Abstract
Cytogenetic abnormalities of chromosome arm 9p occur frequently in children with acute lymphoblastic leukemia (ALL). We analyzed 201 such cases (11%) in 1,839 children with newly diagnosed ALL treated between 1989 and 1995 on risk-adjusted protocols of the Children’s Cancer Group (CCG). The majority of patients (131; 65%) with a 9p abnormality were classified as higher risk. Nearly all patients had complex karyotypes; most cases had deletions of 9p, add/der(9p), a dicentric involving chromosome arm 9p, and/or balanced translocations and inversions involving 9p. Event-free survival (EFS) estimates at 6 years for patients with and without a 9p aberration were 61% (standard deviation [SD] = 5%) and 76% (SD = 2%;P < .0001). In addition, patients with a 9p abnormality had an increased cumulative incidence of both marrow (P = .04) and central nervous system (P = .0001) relapses. Overall survival also was significantly worse for patients with an abnormal 9p (P < .0001). These effects were most pronounced in standard-risk patients (age 1 to 9 years with white blood cell count <50,000/μL): 6-year EFS of 61% (SD = 9%) versus 80% (SD = 2%; P < .0001). Also, a 9p aberration was an adverse risk factor for B-lineage, but not T-lineage patients. The effect of 9p status on EFS was attenuated, but maintained in a multivariate analysis of EFS after adjustment for Philadelphia chromosome status, age, white blood cell (WBC) count, sex, race, and ploidy group (P= .01). Thus, abnormalities of chromosome arm 9p identify a subgroup of standard-risk patients with increased risk of treatment failure.
CYTOGENETICALLY DETECTABLE deletions and other nonrandom abnormalities of the short arm of chromosome 9, most frequently involving 9p21-22, occur in approximately 10% of children with acute lymphoblastic leukemia (ALL).1-5 These abnormalities initially were associated with the lymphomatous syndrome in a small group of patients1 and subsequently were correlated with multiple higher-risk features including older age, high white blood cell (WBC) count at diagnosis, and lymphomatous characteristics in a larger cohort of children with ALL.5Others reported that this aberration was found in ALL patients with pre-B, common, null, or T-lineage features.2,4Abnormalities in chromosome arm 9p may be associated with increased relapse rates in childhood ALL5,6 or with an excess of extramedullary relapse,5 although an earlier report2 suggested no adverse effect of these aberrations. Conversely, occurrence of a dicentric (9;12) has been associated with favorable outcome in childhood ALL.7 8
The associations observed previously between deletions and other abnormalities of chromosome arm 9p and poor outcome prompted the search for oncogenes or tumor suppressor genes at the 9p21-22 locus. The genes for methylthioadenosine phosphorylase (MTAP),9interferon-α, and interferon-β10,11 were mapped to this locus, and all three genes were found to be deleted in leukemic cell lines and in a high percentage of primary leukemic cells from patients with ALL, some of whom had cytogenetically detectable deletions of 9p.10,11 More recently, the putative tumor suppressor genes MTS1/CDK4I/p16INK4A (CDKN2) and MTS2/p15INK4B (CDKN2B) also were mapped to 9p21-22.12-14 Inactivation of one or both of these genes has been found in primary leukemic cells from both adults and children with ALL, particularly T-lineage ALL, but the prognostic significance of these abnormalities remains controversial.15-20
The observations described above prompted us to examine the prognostic significance of abnormalities in the short arm of chromosome 9 in a very large cohort (N = 1,839) of children with ALL treated on contemporary intensive protocols of the Children’s Cancer Group (CCG). Our data indicate that abnormalities of 9p were correlated with poorer outcome in the overall group of patients. Notably, we found that the effect was largely confined to the subset of patients with B-lineage or standard-risk (age 1 to 9 years and WBC count <50,000/μL) ALL.
MATERIALS AND METHODS
Patients.
Diagnosis of ALL was based on morphological, biochemical, and immunological features of the leukemic cells, including lymphoblast morphology on Wright-Giemsa–stained bone marrow smears, negative staining for myeloperoxidase, and cell-surface expression of 2 or more lymphoid differentiation antigens.21 Leukemic cell expression data for the T-lineage–associated antigen CD7 and the B-lineage–associated antigen CD19 were available for a subset of patients. Within this subset, patients were classified as B lineage if ≥30% of their leukemic cells were positive for CD19 and <30% were positive for CD7; patients were classified as T lineage if ≥30% of their leukemic cells were positive for CD7 and <30% were positive for CD19.21
The current study involved 4,986 children with newly diagnosed ALL enrolled on CCG risk-adjusted protocols between 1988 and 1995. Children 2 to 9 years of age with WBC < 10,000/μL (low-risk ALL) were enrolled on CCG-1881; children 2 to 9 years of age and WBC 10,000 to 49,999/μL or age 12 to 23 months and WBC <50,000/μL (intermediate-risk ALL) were enrolled on CCG-1891. After completion of these studies, patients with low- or intermediate-risk ALL were enrolled on a single protocol, CCG-1922, for standard-risk ALL (age 1 to 9 years and WBC count <50,000/μL) based on National Cancer Institute [NCI] criteria.22 Children age 1 to 9 years with WBC ≥50,000/μL or age ≥10 years (NCI poor-risk group)22 were assigned to CCG-1882. In addition, children with multiple unfavorable features (lymphomatous syndrome ALL)23 were enrolled on the CCG-1901 protocol. Infants less than 12 months of age were excluded from this analysis because they represent a group of patients with distinct cytogenetic features and a very poor outcome.24-27 All protocols were approved by the NCI and the Institutional Review Boards of the participating CCG-affiliated institutions. Informed consent was obtained from parents, patients, or both, according to the guidelines of the Department of Health and Human Services.
Cytogenetic analysis.
Diagnostic karyotyping of leukemic cells was performed by institutional laboratories before initiation of therapy. The recommended procedure called for preparation of banded chromosomes from unstimulated peripheral blood or direct and 24-hour–cultured preparations of fresh bone marrow, as described previously.28 Chromosome abnormalities were designated using the International System for Human Cytogenetic Nomenclature (ISCN; 1995).29 Abbreviations include: add, additional chromosomal material of unknown origin; del, deletion; der, derivative; dic, dicentric; i, isochromosome; and t, translocation. Abnormal clones were defined as 2 or more metaphase cells with identical structural abnormalities or extra chromosomes, or 3 or more metaphase cells with identical missing chromosomes. Diagnosis of a normal karyotype required complete analysis of a minimum of 20 banded metaphases from bone marrow only. A minimum of 2 original karyotypes of each abnormal clone or, in the case of normal cytogenetics, normal cells, were reviewed by at least 2 members of the CCG Cytogenetics Committee. Ploidy group was based on the karyotype of the simplest clone. Among all enrolled patients, 1,839 cases had centrally reviewed and accepted cytogenetic data: 201 cases (11%) had abnormalities of the short arm of chromosome 9; 1,638 cases (89%) lacked 9p abnormalities.
Statistical methods.
Data analysis used patient information current to April 1, 1998. Patients with or without abnormalities of chromosome arm 9p were compared with respect to various clinical, demographic, and laboratory features using χ2 tests for homogeneity of proportions. Most of the outcome analyses used life table methods and associated statistics. The primary endpoint examined was event-free survival (EFS) from entry on study. EFS events included induction failure (nonresponse to therapy or death during induction), leukemic relapse at any site, death during remission, or second malignant neoplasm, whichever occurred first. Patients not experiencing an event at the time of EFS analysis were censored at the time of their last contact. Life table estimates were calculated by the Kaplan-Meier (KM) procedure,30 and the standard deviation (SD) of the life table estimate was obtained using the method of Peto.31 To indicate precision, the KM estimate of EFS and its SD are provided at selected time points. An approximate 95% confidence interval can be obtained from the life table estimate ± 1.96 SDs. Life table comparisons of EFS outcome pattern for patient groups generally used the log rank statistic.31,32P values for life table comparisons are based on the pattern of outcome across the entire period of patient follow-up, but also may be given at specific time points for comparative purposes. P values ≤.05 are referred to as significant. Estimates of the life table relative risk for a particular event were calculated by the O/E method for log-rank analyses.33
The multivariate prognostic effect of 9p status was examined with the Cox regression model,34 using known important prognostic factors for outcome in ALL together with characteristics that were distributed in a significantly different manner for the groups with and without 9p abnormalities. Significance levels were based on the likelihood ratio test. The multivariate analysis relative risk for 9p status was estimated by using the exponentiated maximum likelihood coefficient from the regression model results. Life table analysis of site-specific relapse was performed using a cumulative incidence function.35,36 Cumulative incidence estimates were compared using Gray’s test.37
Concurrently enrolled patients with (N = 1,839) and without (N = 3,147) accepted cytogenetic data were compared with respect to presenting features and treatment outcome. Although percentages of patients in the 2 groups were not markedly different, comparisons of 7 of the 23 characteristics tested did reach statistical significance, which could be due to the large numbers of patients studied. For example, patients with accepted data were more likely to have a mediastinal mass, WBC counts ≥50,000/μL, nonwhite, nonblack race, enlarged lymph nodes, platelet counts ≥150 × 103/μL, T-lineage ALL, and NCI poor-risk status. The overall cohort comprised 1,839 pediatric ALL patients with accepted cytogenetic data. Notably, EFS at 6 years was 74% (SD = 2%) for patients with accepted cytogenetic data and 76% (SD = 2%), for patients who did not have accepted data (P = .26).
RESULTS
Presenting features of pediatric ALL patients with abnormalities of chromosome arm 9p.
Compared with patients lacking a 9p abnormality (N = 1,638), those with a 9p abnormality (N = 201) were more likely to have higher-risk features at diagnosis including older age (P < .0001), higher WBC count (P = .0002), splenomegaly (P = .002), and high hemoglobin levels (P = .01; Table 1 ). More than half (65%) of the patients with a 9p abnormality were classified as NCI poor risk (age ≥10 years or WBC counts ≥50,000/μL), compared with only 35% of patients without a 9p abnormality.
Presenting Features of Children With ALL and Abnormalities of Chromosome Arm 9p
| Variable . | Category . | Patients With Abnormal 9p (N = 201) . | Patients With Normal 9p (N = 1,638) . | P Value* . | ||
|---|---|---|---|---|---|---|
| No. . | (%) . | No. . | (%) . | |||
| Age (yr) | 1-9 | 112 | (56) | 1,248 | (76) | <.0001 |
| ≥10 | 89 | (44) | 390 | (24) | ||
| WBC | <20 | 99 | (49) | 1,043 | (64) | .0002 |
| (×109/L) | 20-49 | 36 | (18) | 240 | (15) | |
| ≥50 | 66 | (33) | 355 | (22) | ||
| Sex | Male | 108 | (54) | 911 | (56) | .61 |
| Female | 93 | (46) | 727 | (44) | ||
| Race | White | 167 | (83) | 1,325 | (81) | .22 |
| Black | 15 | (8) | 95 | (6) | ||
| Other | 19 | (10) | 218 | (13) | ||
| Down | Yes | 3 | (1) | 33 | (2) | .61 |
| syndrome | No | 198 | (99) | 1,605 | (98) | |
| Liver | Normal | 98 | (48) | 811 | (50) | .85 |
| Mod enlarged† | 97 | (48) | 761 | (47) | ||
| Markedly enlarged | 6 | (3) | 59 | (4) | ||
| Spleen | Normal | 73 | (36) | 807 | (49) | .002 |
| Mod enlarged | 112 | (56) | 732 | (45) | ||
| Markedly enlarged | 16 | (8) | 98 | (6) | ||
| Lymph nodes | Normal | 97 | (48) | 834 | (51) | .69 |
| Mod enlarged | 88 | (44) | 694 | (42) | ||
| Markedly enlarged | 16 | (8) | 110 | (7) | ||
| Mediastinal | Absent | 183 | (91) | 1,487 | (91) | .15 |
| mass | Small | 5 | (3) | 79 | (5) | |
| Large | 13 | (7) | 72 | (4) | ||
| Hemoglobin | 1-7.9 | 97 | (48) | 869 | (53) | .01 |
| (g/dL) | 8.0-10.9 | 63 | (31) | 559 | (34) | |
| ≥11.0 | 41 | (20) | 210 | (13) | ||
| Platelets | 1-49 | 100 | (50) | 757 | (46) | .39 |
| (×109/L) | 50-149 | 62 | (31) | 494 | (30) | |
| ≥150 | 39 | (19) | 387 | (24) | ||
| CNS disease | Yes | 8 | (4) | 44 | (3) | .30 |
| at diagnosis | No | 192 | (96) | 1,577 | (97) | |
| Immuno | B-lineage | 102 | (83) | 762 | (81) | .65 |
| phenotype | T-lineage | 21 | (17) | 176 | (19) | |
| NCI risk group | Standard | 71 | (35) | 1,003 | (61) | <.0001 |
| Poor | 130 | (65) | 635 | (39) | ||
| Ploidy group‡ | Normal | 0 | (0) | 564 | (34) | <.0001 |
| Hypodiploid | 55 | (27) | 52 | (3) | ||
| Pseudodiploid | 104 | (52) | 383 | (23) | ||
| Hyperdiploid (47-50) | 29 | (14) | 168 | (10) | ||
| Hyperdiploid (>50) | 13 | (6) | 471 | (29) | ||
| Variable . | Category . | Patients With Abnormal 9p (N = 201) . | Patients With Normal 9p (N = 1,638) . | P Value* . | ||
|---|---|---|---|---|---|---|
| No. . | (%) . | No. . | (%) . | |||
| Age (yr) | 1-9 | 112 | (56) | 1,248 | (76) | <.0001 |
| ≥10 | 89 | (44) | 390 | (24) | ||
| WBC | <20 | 99 | (49) | 1,043 | (64) | .0002 |
| (×109/L) | 20-49 | 36 | (18) | 240 | (15) | |
| ≥50 | 66 | (33) | 355 | (22) | ||
| Sex | Male | 108 | (54) | 911 | (56) | .61 |
| Female | 93 | (46) | 727 | (44) | ||
| Race | White | 167 | (83) | 1,325 | (81) | .22 |
| Black | 15 | (8) | 95 | (6) | ||
| Other | 19 | (10) | 218 | (13) | ||
| Down | Yes | 3 | (1) | 33 | (2) | .61 |
| syndrome | No | 198 | (99) | 1,605 | (98) | |
| Liver | Normal | 98 | (48) | 811 | (50) | .85 |
| Mod enlarged† | 97 | (48) | 761 | (47) | ||
| Markedly enlarged | 6 | (3) | 59 | (4) | ||
| Spleen | Normal | 73 | (36) | 807 | (49) | .002 |
| Mod enlarged | 112 | (56) | 732 | (45) | ||
| Markedly enlarged | 16 | (8) | 98 | (6) | ||
| Lymph nodes | Normal | 97 | (48) | 834 | (51) | .69 |
| Mod enlarged | 88 | (44) | 694 | (42) | ||
| Markedly enlarged | 16 | (8) | 110 | (7) | ||
| Mediastinal | Absent | 183 | (91) | 1,487 | (91) | .15 |
| mass | Small | 5 | (3) | 79 | (5) | |
| Large | 13 | (7) | 72 | (4) | ||
| Hemoglobin | 1-7.9 | 97 | (48) | 869 | (53) | .01 |
| (g/dL) | 8.0-10.9 | 63 | (31) | 559 | (34) | |
| ≥11.0 | 41 | (20) | 210 | (13) | ||
| Platelets | 1-49 | 100 | (50) | 757 | (46) | .39 |
| (×109/L) | 50-149 | 62 | (31) | 494 | (30) | |
| ≥150 | 39 | (19) | 387 | (24) | ||
| CNS disease | Yes | 8 | (4) | 44 | (3) | .30 |
| at diagnosis | No | 192 | (96) | 1,577 | (97) | |
| Immuno | B-lineage | 102 | (83) | 762 | (81) | .65 |
| phenotype | T-lineage | 21 | (17) | 176 | (19) | |
| NCI risk group | Standard | 71 | (35) | 1,003 | (61) | <.0001 |
| Poor | 130 | (65) | 635 | (39) | ||
| Ploidy group‡ | Normal | 0 | (0) | 564 | (34) | <.0001 |
| Hypodiploid | 55 | (27) | 52 | (3) | ||
| Pseudodiploid | 104 | (52) | 383 | (23) | ||
| Hyperdiploid (47-50) | 29 | (14) | 168 | (10) | ||
| Hyperdiploid (>50) | 13 | (6) | 471 | (29) | ||
Global χ2 test for homogeneity.
Mod, moderately. Degree of organomegaly and size of mediastinal mass were determined as described by Steinherz et al.23
Based on the simplest clone.
Based on the stemline (ie, the simplest abnormal clone), 104 patients (52%) with a 9p aberration were classified as pseudodiploid, compared with only 23% of patients lacking an abnormal 9p. Hypodiploidy also was more common among patients with an abnormal 9p, although within the hypodiploid subgroup, similar percentages of those with or without a 9p abnormality (76% and 81%, respectively) had 45 chromosomes (data not shown). Only 6% of patients with a 9p abnormality were high hyperdiploid (>50 chromosomes), compared with 29% of patients without an abnormal 9p.
Karyotypes of patients with an abnormality of chromosome arm 9p.
The chromosome arm 9p abnormalities were classified according to type of rearrangement (Table 2 ). In 87 patients (43%), including 21 cases with monosomy 9, the 9p abnormalities were classified as deletions. Among this group, 34 patients had breakpoints in 9p22, and the remaining 53 cases were missing 9p21 and 9p22 as a result of the particular deletion. An add/der(9p) was found in 41 patients (20%); 40 of these cases resulted in loss of the distal portion of 9p, including 9p22→9pter. Nineteen patients (10%) had an i(9)(q10). A dicentric between chromosome 9 and another chromosome was found in 39 (19%) patients: 18 cases had a dic(9;20) and 12 cases had a dic(9;12). There were 26 cases (13%) with balanced translocations and inversions; these were the only abnormalities that did not result in partial or complete loss of 9p. Seven of these cases had breakpoints in 9p13 and 9 had breakpoints in 9p22.
Karyotypic Features in Patients With an Abnormality of Chromosome Arm 9p
| Category . | Percentage of Patients* . | Total N = 201 . | ||||
|---|---|---|---|---|---|---|
| NCI Standard Risk† N = 77 . | NCI Poor Risk† N = 124 . | |||||
| N . | (%) . | N . | (%) . | N . | (%) . | |
| 9p Abnormality | ||||||
| Balanced translocation | 8 | (10) | 18 | (15) | 26 | (13) |
| Dicentric chromosome 9 | 22 | (29) | 17 | (14) | 39 | (19) |
| dic(9;20)‡ | 12 | (16) | 6 | (5) | 18 | (9) |
| dic(9;12) | 5 | (8) | 7 | (6) | 12 | (6) |
| dic(7;9) | 2 | (3) | 2 | (2) | 4 | (3) |
| other | 3 | (4) | 2 | (2) | 5 | (2) |
| i(9)(q10) | 5 | (6) | 14 | (11) | 19 | (10) |
| del(9p) | 27 | (35) | 60 | (48) | 87 | (43) |
| add/der(9p) | 17 | (22) | 24 | (19) | 41 | (20) |
| Ploidy group2-153 | ||||||
| Hypodiploid (<46) | 20 | (26) | 35 | (28) | 55 | (27) |
| Pseudodiploid | 33 | (43) | 71 | (57) | 104 | (52) |
| Hyperdiploid (47-50) | 16 | (21) | 13 | (10) | 29 | (14) |
| Hyperdiploid (>50) | 8 | (10) | 5 | (4) | 13 | (7) |
| Additional abnormality | ||||||
| t(1;19)(q23;p13) or der(19)t(1;19)(q23; p13)2-155 | 3 | (4) | 10 | (8) | 13 | (6.5) |
| del(6q) | 7 | (9) | 15 | (12) | 22 | (11.0) |
| Abnormal 12p | 13 | (17) | 20 | (26) | 33 | (16.4) |
| t(9;22)(q34;q11) | 3 | (4) | 15 | (12) | 18 | (8.5) |
| Category . | Percentage of Patients* . | Total N = 201 . | ||||
|---|---|---|---|---|---|---|
| NCI Standard Risk† N = 77 . | NCI Poor Risk† N = 124 . | |||||
| N . | (%) . | N . | (%) . | N . | (%) . | |
| 9p Abnormality | ||||||
| Balanced translocation | 8 | (10) | 18 | (15) | 26 | (13) |
| Dicentric chromosome 9 | 22 | (29) | 17 | (14) | 39 | (19) |
| dic(9;20)‡ | 12 | (16) | 6 | (5) | 18 | (9) |
| dic(9;12) | 5 | (8) | 7 | (6) | 12 | (6) |
| dic(7;9) | 2 | (3) | 2 | (2) | 4 | (3) |
| other | 3 | (4) | 2 | (2) | 5 | (2) |
| i(9)(q10) | 5 | (6) | 14 | (11) | 19 | (10) |
| del(9p) | 27 | (35) | 60 | (48) | 87 | (43) |
| add/der(9p) | 17 | (22) | 24 | (19) | 41 | (20) |
| Ploidy group2-153 | ||||||
| Hypodiploid (<46) | 20 | (26) | 35 | (28) | 55 | (27) |
| Pseudodiploid | 33 | (43) | 71 | (57) | 104 | (52) |
| Hyperdiploid (47-50) | 16 | (21) | 13 | (10) | 29 | (14) |
| Hyperdiploid (>50) | 8 | (10) | 5 | (4) | 13 | (7) |
| Additional abnormality | ||||||
| t(1;19)(q23;p13) or der(19)t(1;19)(q23; p13)2-155 | 3 | (4) | 10 | (8) | 13 | (6.5) |
| del(6q) | 7 | (9) | 15 | (12) | 22 | (11.0) |
| Abnormal 12p | 13 | (17) | 20 | (26) | 33 | (16.4) |
| t(9;22)(q34;q11) | 3 | (4) | 15 | (12) | 18 | (8.5) |
Percentage of patients within each risk group; most patients had multiple abnormalities and some patients had more than 1 abnormality of chromosome arm 9p.
NCI standard risk = 1 to 9 years with WBC <50,000/μL; NCI poor risk = age ≥10 years or WBC ≥50,000/μL.6
Abbreviations: del, deletion; add, additional chromosomal material of unknown origin; der, derivative; dic, dicentric; i, isochromosome; t, translocation.
Based on simplest clone.
Standard risk, all 3 cases had an unbalanced translocation; poor risk, 7 cases had an unbalanced, and 3 cases had a balanced translocation.
The 9p abnormalities included 2 cases with a der(9)t(9;13)(p13;q12). Two other patients had translocations involving 9p and chromosome band 15q13: 1 patient had a der(9)t(9;15)(p13;q13) and the other had a t(9;15)(p21;q13). The latter translocations have not been reported before in ALL. There were 3 cases with a t(9;9), 1 balanced and 2 unbalanced. Two of these cases had identical breakpoints at 9p13 and 9q13. The breakpoints in the remaining t(9;9) and in the 3 cases with inv(9) all differed.
Abnormalities of chromosome arm 9p were found in association with a complex karyotype involving multiple aberrations in nearly all of the 201 cases: only 17 cases had a del(9p), an i(9)(q10), or an add(9p) as the sole abnormality; 7 additional cases had only a balanced translocation involving 9p and 1 or more other chromosomes; and 11 cases had a dicentric as the sole abnormality. Eighteen cases also had a Philadelphia chromosome, ie, a t(9;22)(q34;q11); 15 of the 18 patients were classified as poor risk. Thirteen patients with a 9p aberration also had a t(1;19)(q23;p13) (10 unbalanced, 3 balanced). Twenty-two patients had a del(6q) and 33 patients had an abnormality of the short arm of chromosome 12 in addition to their 9p aberrations.
The various chromosome arm 9p abnormalities and additional aberrations described above occurred with a similar hierarchy and frequency in both standard- and poor-risk patients, although dicentrics, particularly dic(9;20), were more common among standard-risk patients, whereas a balanced translocation, a del(9p), and an i(9)(q10) were more common among poor-risk patients. As could be expected, poor-risk patients also were less likely to have hyperdiploidy and more likely to be pseudodiploid. Standard-risk patients were less likely than poor-risk patients to have a t(1;19), an abnormal chromosome arm 12p, or a t(9;22).
Treatment outcome.
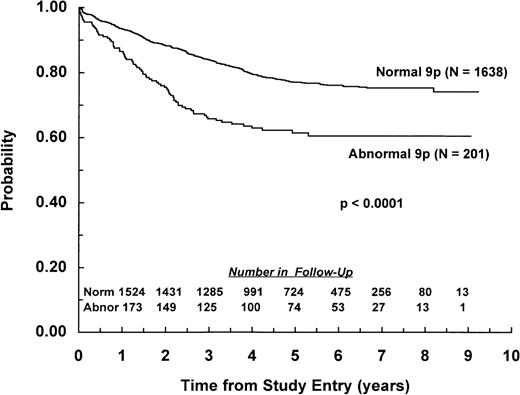
Nearly all (99%) of the 1,839 patients with or without a 9p abnormality achieved remission by day 28 of induction chemotherapy. EFS from study entry was worse for patients with a 9p abnormality compared with those lacking this abnormality, with 6-year EFS of 61% (SD = 4%) and 76% (SD = 2%), respectively (P < .0001; Fig 1). Overall survival also was significantly different between the groups, with 6-year estimates of 70% (SD = 5%) and 85% (SD = 1%), respectively (P < .0001).
EFS for children with ALL and abnormalities of chromosome arm 9p. Probability of EFS for 201 patients with a 9p abnormality and 1,638 patients lacking a 9p abnormality. Numbers of patients remaining in follow-up are shown in the inset.
EFS for children with ALL and abnormalities of chromosome arm 9p. Probability of EFS for 201 patients with a 9p abnormality and 1,638 patients lacking a 9p abnormality. Numbers of patients remaining in follow-up are shown in the inset.
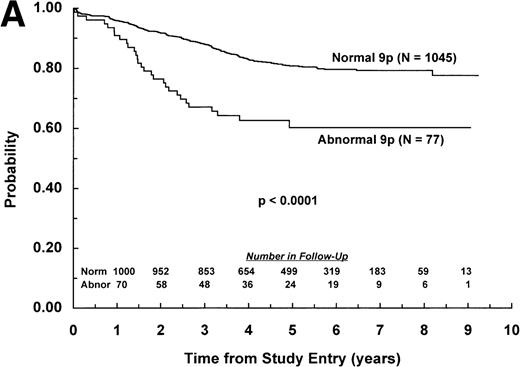
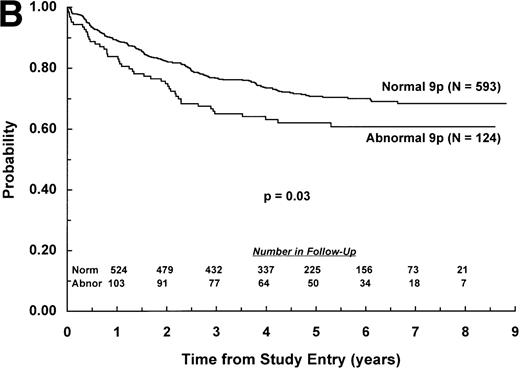
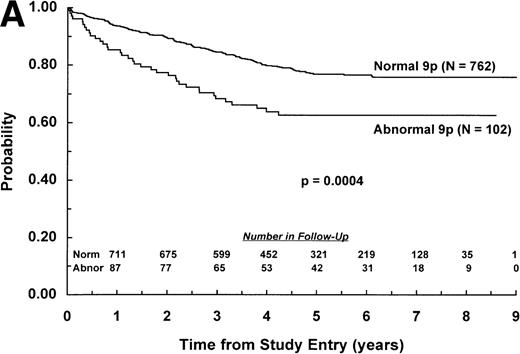
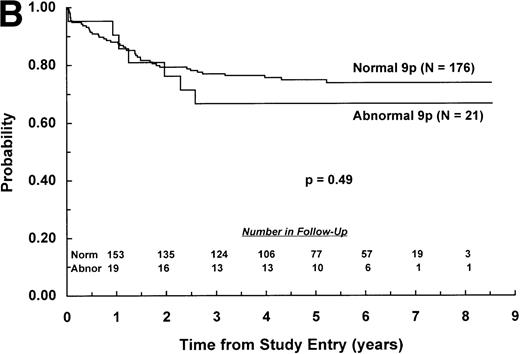
The adverse effect of a 9p abnormality was most pronounced within the subset of patients classified as NCI standard risk, with 6-year EFS of 61% (SD =9%) and 80% (SD = 2%) for patients with and without a 9p abnormality, respectively (P < .0001; Fig 2A). Patients with a 9p abnormality and NCI poor risk also had worse outcome than their normal 9p counterparts, with 6-year EFS of 60% (SD = 7%) and 70% (SD = 3%), respectively (P = .03; Fig 2B). In addition, 9p abnormalities conferred increased risk of treatment failure only for patients with B-lineage ALL. EFS estimates at 6 years among patients with B-lineage ALL were 63% (SD = 7%) and 77% (SD = 3%) for those with and without a 9p abnormality, respectively (P = .0004; Fig 3A ). In contrast, 6-year EFS estimates for patients with T-lineage ALL with and without a 9p abnormality were 67% (SD = 16%) and 74% (SD = 5%), respectively (P = .49; Fig 3B).
EFS for children with ALL according to NCI risk classification. Probability of EFS for (A) NCI standard-risk patients with (N = 77) and without (N = 1,045) a 9p abnormality; or (B) NCI poor-risk patients with (N = 124) or without (N = 593) a 9p abnormality. Numbers of patients remaining in follow-up are shown in the insets.
EFS for children with ALL according to NCI risk classification. Probability of EFS for (A) NCI standard-risk patients with (N = 77) and without (N = 1,045) a 9p abnormality; or (B) NCI poor-risk patients with (N = 124) or without (N = 593) a 9p abnormality. Numbers of patients remaining in follow-up are shown in the insets.
EFS for children with ALL according to immunophenotype. Probability of EFS for (A) B-lineage ALL patients with (N = 102) and without (N = 762) a 9p abnormality; or (B) T-lineage ALL patients with (N = 21) or without (N = 176) a 9p abnormality. Numbers of patients remaining in follow-up are shown in the insets.
EFS for children with ALL according to immunophenotype. Probability of EFS for (A) B-lineage ALL patients with (N = 102) and without (N = 762) a 9p abnormality; or (B) T-lineage ALL patients with (N = 21) or without (N = 176) a 9p abnormality. Numbers of patients remaining in follow-up are shown in the insets.
Patients in this cohort were treated by CCG risk-adjusted treatment programs and each protocol included 2 or more randomized regimens. Similar numbers of patients with or without a 9p abnormality were treated on each regimen of a particular protocol; thus, no associations between treatment regimen and 9p status were clearly evident. Nor were specific 9p abnormalities associated with poorer outcome. We did note a high frequency of dicentric chromosomes, particularly dic(9;12) and dic(9;20), although outcome for either of these groups was not significantly different from that of patients with other 9p abnormalities: dic(9;12) versus other 9p aberration, P = .17 (relative risk = 0.39); dic (9;20) versus other 9p aberration,P = .24 (relative risk = 0.58). There were 2 events among the 12 patients with a dic(9;12) and 5 events among the 18 patients with a dic(9;20).
Overall, there were 76 events among patients with a 9p abnormality and 361 events among patients lacking a 9p abnormality (Table 3). Patients with a 9p abnormality appeared to have an increased frequency of both isolated marrow (33 events in 201 patients) and isolated central nervous system (CNS) relapses (18 events in 201 patients) compared with the frequencies in patients lacking this aberration. Precise estimates for these specific failures, calculated using a cumulative incidence analysis, indicated a significantly increased rate of isolated CNS relapse (9%, SD = 2% v 4%, SD = 0.5%; P = .0001) for patients with or without a 9p aberration, respectively. Cumulative incidence of an isolated marrow relapse also was increased (18%, SD = 3% v 12%, SD = 1%; P = .04).
Frequency and Type of First Events for Patients According to Chromosome Arm 9p Status
| Event Type . | Patients With Abnormal 9p . | Patients With Normal 9p . | ||
|---|---|---|---|---|
| No. . | (%)3-150 . | No. . | (%) . | |
| Marrow relapse, isolated | 33 | (16) | 186 | (11) |
| Marrow relapse + CNS relapse | 2 | (1) | 15 | (1) |
| Marrow relapse + testicular relapse | 0 | (0) | 8 | (1) |
| Marrow relapse + other event3-151 | 0 | (0) | 1 | (0) |
| CNS relapse, isolated | 18 | (9) | 55 | (3) |
| CNS relapse + nonmarrow relapse or other event | 0 | (0) | 1 | (0) |
| Testicular relapse, isolated | 3 | (2) | 15 | (1) |
| Other relapse | 3 | (2) | 8 | (1) |
| Second malignancy | 0 | (0) | 7 | (0) |
| Induction failure or death in induction | 8 | (4) | 26 | (2) |
| Death in remission | 9 | (5) | 39 | (2) |
| Total events | 76 | 361 | ||
| Event Type . | Patients With Abnormal 9p . | Patients With Normal 9p . | ||
|---|---|---|---|---|
| No. . | (%)3-150 . | No. . | (%) . | |
| Marrow relapse, isolated | 33 | (16) | 186 | (11) |
| Marrow relapse + CNS relapse | 2 | (1) | 15 | (1) |
| Marrow relapse + testicular relapse | 0 | (0) | 8 | (1) |
| Marrow relapse + other event3-151 | 0 | (0) | 1 | (0) |
| CNS relapse, isolated | 18 | (9) | 55 | (3) |
| CNS relapse + nonmarrow relapse or other event | 0 | (0) | 1 | (0) |
| Testicular relapse, isolated | 3 | (2) | 15 | (1) |
| Other relapse | 3 | (2) | 8 | (1) |
| Second malignancy | 0 | (0) | 7 | (0) |
| Induction failure or death in induction | 8 | (4) | 26 | (2) |
| Death in remission | 9 | (5) | 39 | (2) |
| Total events | 76 | 361 | ||
Percentage of all patients for given group.
Events other than hematological relapses in the marrow, CNS, or testicles.
Prognostic factors.
Eighteen patients with a 9p abnormality and 26 patients without a 9p abnormality had a cytogenetically detectable Philadelphia chromosome. The prognostic significance of a 9p abnormality was maintained in an analysis of EFS after exclusion of the patients with a Philadelphia chromosome (P = .0001). Thirteen patients with a 9p abnormality and 52 patients without a 9p abnormality had a t(1;19). The effect of a 9p abnormality on EFS and CNS relapse rate was maintained after exclusion of t(1;19)-positive patients (P < .0001 for both analyses). Data on early response to induction therapy, an important prognostic factor for childhood ALL,38 was available for 1,468 patients. Within this subset, approximately 75% of patients with or without a 9p abnormality achieved M1 or M2 marrow status and 25% achieved M3 marrow status by day 7 of induction (P = .60).
The independent significance of a 9p aberration was assessed using a multivariate analysis model that included age, WBC count, sex, race, ploidy group (hypodiploid with <45 chromosomes v high hyperdiploid v all others), and Philadelphia chromosome status. In this model, the effect of 9p status on outcome was attenuated, but remained significant (P = .01; relative risk = 1.4). A stepwise regression analysis indicated that Philadelphia chromosome status, ploidy group (as indicated above), and age had the strongest attenuating influence on the univariate effect of 9p status on EFS.
DISCUSSION
We have analyzed a large cohort of children with newly diagnosed ALL to determine the prognostic significance of cytogenetically detectable aberrations in the short arm of chromosome 9. Among this group, 201 had a 9p aberration. Compared with concurrently enrolled patients who lacked a 9p abnormality, these patients were more likely to have higher WBC counts, older age, splenomegaly, high hemoglobin levels, and hypodiploidy at diagnosis. The majority of patients with a 9p aberration were designated as poor-risk ALL according to NCI criteria.22
The most common 9p abnormalities were deletions, dicentric chromosomes, or derivative chromosomes, and the majority of patients with a 9p aberration were classified as pseudodiploid or hypodiploid; only a small percentage were high hyperdiploid (>50 chromosomes). We also noted a balanced t(9;15)(p21;q13) and an unbalanced der(9)t(9;15)(p13;q13) that have not been reported before in ALL. Most chromosome arm 9p abnormalities were found in association with a complex karyotype involving multiple abnormalities; the most common of these included del(6q), abnormal chromosome arm 12p, t(9;22)(q34;q11), and t(1;19)(q23;p13).
In the overall cohort, both EFS and overall survival were significantly worse for patients with a 9p aberration than for patients without a 9p aberration. The effect of 9p status was attenuated, but remained significant in a multivariate analysis including important prognostic factors such as age, WBC count, ploidy group, sex, race, and presence of a Philadelphia chromosome. The effect of 9p status on outcome was most significant for patients classified as NCI standard risk. In addition, a 9p aberration was an adverse risk factor for B-lineage, but not T-lineage patients. Specific karyotypic features, including dic(9;20) and dic(9;12), the latter of which has been associated with favorable outcome,7 8 did not appear to modulate the overall adverse effect on outcome conferred by the presence of an abnormal chromosome arm 9p.
Standard-risk patients with or without a 9p abnormality were treated on either CCG-1881, CCG-1891,39 or CCG-1922.40Patients on CCG-1881 received standard induction, consolidation, and interim maintenance and were randomized to receive either standard maintenance or delayed intensification and maintenance. Patients on CCG-1891 received therapy including 1 course of delayed intensification and were randomized to receive either (1) standard maintenance; (2) an additional course of interim maintenance and delayed intensification followed by standard maintenance; or (3) maintenance with increased doses of vincristine and prednisone.39 Patients on CCG-1922 received 1 course of delayed intensification in the context of standard therapy, with a double randomization for oral versus intravenous 6-mercaptopurine and for prednisone versus dexamethasone during induction and maintenance.40 Overall outcome on these studies was very favorable, and similar numbers of patients with a 9p abnormality were randomized to each of the regimens. No particular therapeutic regimen appeared to be associated with better or worse outcome, but small numbers of patients precluded a statistical analysis of outcome by regimen.
Previous studies of childhood ALL correlated 9p aberrations with higher risk or lymphomatous features1,5 and reported increased risk of treatment failure for patients with this abnormality,5 even in the case of patients treated on an intensive rotational regimen.6 Other studies with small numbers of patients failed to show a correlation between presence of a 9p aberration and specific subgroups of ALL patients.2 Our results are consistent with the former studies in showing a high frequency of 9p abnormalities in patients with high-risk or lymphomatous features and an overall poorer outcome for patients with a 9p abnormality compared with those lacking this cytogenetic feature. We also noted an increase in the cumulative incidence of CNS relapses among patients with an abnormality in chromosome arm 9p, consistent with results of Murphy et al.5 Importantly, our data indicate that 9p abnormalities identify a subgroup of NCI standard-risk patients with increased risk of treatment failure.
The genes for methylthioadenosine phosphorylase (MTAP),9the interferons-α and -β,10,11 and MTS1/CDK4I/p16INK4A and MTS2/p15INK4B were mapped to 9p21-22 and found to be deleted in primary leukemic cell lines and in primary leukemic cells from patients with ALL.12-14 In the case of hemizygous deletions, however, point mutations were not found in the remaining allele, suggesting that another gene might be involved in leukemogenesis. Our cytogenetic data suggest that deletions, as well as other abnormalities in chromosome arm 9p, are significant prognostic factors in childhood ALL and identify a subgroup of standard-risk patients with increased risk of treatment failure. The mechanisms by which these abnormalities exert their effects and their relationship to the putative leukemogenic genes described above await further study.
Contributing Cytogeneticists
| Investigator . | Institution . |
|---|---|
| A. A. Al Saadi | William Beaumont Hospital, Royal Oak, MI |
| D. C. Arthur | University of Minnesota, Minneapolis, MN |
| H. Aviv | University of Medicine and Dentistry, Newark, NJ |
| B. L. Barnoski | University Medical Center, Camden, NJ |
| P. Benn | University of Connecticut Health Center, Farmingham, CT |
| R. Best | Richland Memorial Hospital, Columbia, SC |
| D. S. Borgaonkar | Medical Center of Delaware, Wilmington, DE |
| C. Bradley | Children’s Hospital and Medical Center, Seattle, WA |
| A. Brothman | University of Utah, Salt Lake City, UT |
| M. G. Butler | Vanderbilt University, Nashville, TN |
| Z. Chen | Genzyme Genetics, Scottsdale, AZ |
| H. Chen | Louisiana State University Medical Center, Shreveport, LA |
| P. D. Cotter | Children’s Hospital, Oakland, CA |
| A. J. Dawson | Cytogenetics/HSC, Winnepeg, MB, Canada |
| G. Dewald | Mayo Clinic, Rochester, MN |
| Y.-S. Fan | London Health Science Centre, London, ON, Canada |
| P. A. Farber | Geisinger Medical Center, Danville, PA |
| A. B. Glassman | M.D. Anderson Cancer Center, Houston, TX |
| T. W. Glover | University of Michigan, Ann Arbor, MI |
| W. Golden | Rainbow Babies & Children’s Hospital, Cleveland, OH |
| S. M. Golin | University of Pittsburgh, Pittsburgh, PA |
| D. Harris | Children’s Mercy Hospital, Kansas City, MO |
| N. A. Heerema | Indiana University, Indianapolis, IN |
| R. Higgins | Children’s Mercy Hospital, Kansas City, MO |
| J. V. Higgins | Butterworth Hospital, Grand Rapids, MI |
| B. Hirsch | University of Minnesota, Minneapolis, MN |
| D. K. Kalousek | British Columbia Children’s Hospital, Vancouver, BC, Canada |
| M. M. LeBeau | University of Chicago, Chicago, IL |
| C. Lee | Izaak Walton Killam Children’s Hospital, Halifax, NS, Canada |
| K. Leppig | University of Washington, Seattle, WA |
| S. Lewis | Scottish Rite Children’s Hospital, Atlanta, GA |
| W. D. Loughman | Children’s Hospital, Oakland, CA |
| C. B. Lozzio | University of Tennessee Medical Center, Knoxville, TN |
| R. E. Magenis | Oregon Health Sciences Center, Portland, OR |
| L. McGavran | Children’s Hospital of Denver, Denver, CO |
| L. E. McMorrow | University of Medicine & Dentistry, Newark, NJ |
| A. Milatovitch | Children’s Hospital and Medical Center, Cincinnati, OH |
| T. K. Mohandas | Harbor/University of California Los Angeles Medical Center, Torrance, CA |
| B. Mouron | Children’s Mercy Hospital, Kansas City, MO |
| A. Murch | King Edward Memorial Hospital, Perth, Australia |
| P. Nowell | Children’s Hospital of Philadelphia, Philadelphia, PA |
| K. Opheim | Children’s Hospital and Medical Center, Seattle, WA |
| L. Pasztor | Children’s Mercy Hospital, Kansas City, MO |
| S. Patil | University of Iowa, Iowa City, IA |
| C. Pehl | Loma Linda University, Redlands, CA |
| M. A. Perle | New York University Medical Center, New York, NY |
| A. Pettigrew | University of Kentucky, Lexington, KY |
| C. Phillips | Emory University Hospital, Atlanta, GA |
| K. Rao | University of North Carolina, Chapel Hill, NC |
| K. E. Richkind | Genzyme Genetics, Santa Fe, NM |
| K. N. Rosenbaum | Children’s Hospital National Medical Center, Washington, DC |
| D. Roulston | University of Chicago, Chicago, IL |
| C. Sandlin | Memorial Genetics Center, Long Beach, CA |
| W. G. Sanger | University of Nebraska, Omaha, NB |
| K. L. Satya-Prakash | Medical College of Georgia, Augusta, GA |
| S. Schwartz | Rainbow Babies & Children’s Hospital, Cleveland, OH |
| G. S. Sekhon | University of Wisconsin, Madison, WI |
| S. Sheldon | University of Michigan, Ann Arbor, MI |
| S. Soukup | Children’s Hospital and Medical Center, Cincinnati, OH |
| R. Sparkes | University of California, Los Angeles, CA |
| R. Stallard | Rainbow Babies & Children’s Hospital, Cleveland, OH |
| W. S. Stanley | Genetics and IVF Institute, Fairfax, VA and Children’s Hospital National Medical Center, Washington, DC |
| J. Stone | Genetrix, Inc, Scottsdale, AZ |
| P. Storto | Michigan State University, Lansing, MI |
| K. S. Theil | Children’s Hospital of Columbus, Columbus, OH |
| B. Torchia | Western Reserve Healthcare, Youngstown, OH |
| D. Van Dyke | Henry Ford Health System, Detroit, MI |
| N. V. Vigfusson | Sacred Heart Medical Center, Spokane, WA |
| D. Warburton | Columbia Presbyterian College of Physicians & Surgeons, New York, NY |
| J. R. Waterson | Children’s Hospital Medical Center, Akron, OH |
| S. L. Wenger | University of Pittsburgh, Pittsburgh, PA |
| G. Williams | Manitoba Cancer Foundation, Winnepeg, MB, Canada |
| K.-L. Yin | Children’s Hospital of Los Angeles, Los Angeles, CA |
| C. W. Yu | Valley Children’s Hospital, Fresno, Fresno, CA |
| T. Zadeh | Genetics Center, Orange, CA |
| M. C. Zapata | Children’s Medical Center, Dayton, OH |
| S. Zneimer | Kaiser-Permanente, San Jose, CA |
| Investigator . | Institution . |
|---|---|
| A. A. Al Saadi | William Beaumont Hospital, Royal Oak, MI |
| D. C. Arthur | University of Minnesota, Minneapolis, MN |
| H. Aviv | University of Medicine and Dentistry, Newark, NJ |
| B. L. Barnoski | University Medical Center, Camden, NJ |
| P. Benn | University of Connecticut Health Center, Farmingham, CT |
| R. Best | Richland Memorial Hospital, Columbia, SC |
| D. S. Borgaonkar | Medical Center of Delaware, Wilmington, DE |
| C. Bradley | Children’s Hospital and Medical Center, Seattle, WA |
| A. Brothman | University of Utah, Salt Lake City, UT |
| M. G. Butler | Vanderbilt University, Nashville, TN |
| Z. Chen | Genzyme Genetics, Scottsdale, AZ |
| H. Chen | Louisiana State University Medical Center, Shreveport, LA |
| P. D. Cotter | Children’s Hospital, Oakland, CA |
| A. J. Dawson | Cytogenetics/HSC, Winnepeg, MB, Canada |
| G. Dewald | Mayo Clinic, Rochester, MN |
| Y.-S. Fan | London Health Science Centre, London, ON, Canada |
| P. A. Farber | Geisinger Medical Center, Danville, PA |
| A. B. Glassman | M.D. Anderson Cancer Center, Houston, TX |
| T. W. Glover | University of Michigan, Ann Arbor, MI |
| W. Golden | Rainbow Babies & Children’s Hospital, Cleveland, OH |
| S. M. Golin | University of Pittsburgh, Pittsburgh, PA |
| D. Harris | Children’s Mercy Hospital, Kansas City, MO |
| N. A. Heerema | Indiana University, Indianapolis, IN |
| R. Higgins | Children’s Mercy Hospital, Kansas City, MO |
| J. V. Higgins | Butterworth Hospital, Grand Rapids, MI |
| B. Hirsch | University of Minnesota, Minneapolis, MN |
| D. K. Kalousek | British Columbia Children’s Hospital, Vancouver, BC, Canada |
| M. M. LeBeau | University of Chicago, Chicago, IL |
| C. Lee | Izaak Walton Killam Children’s Hospital, Halifax, NS, Canada |
| K. Leppig | University of Washington, Seattle, WA |
| S. Lewis | Scottish Rite Children’s Hospital, Atlanta, GA |
| W. D. Loughman | Children’s Hospital, Oakland, CA |
| C. B. Lozzio | University of Tennessee Medical Center, Knoxville, TN |
| R. E. Magenis | Oregon Health Sciences Center, Portland, OR |
| L. McGavran | Children’s Hospital of Denver, Denver, CO |
| L. E. McMorrow | University of Medicine & Dentistry, Newark, NJ |
| A. Milatovitch | Children’s Hospital and Medical Center, Cincinnati, OH |
| T. K. Mohandas | Harbor/University of California Los Angeles Medical Center, Torrance, CA |
| B. Mouron | Children’s Mercy Hospital, Kansas City, MO |
| A. Murch | King Edward Memorial Hospital, Perth, Australia |
| P. Nowell | Children’s Hospital of Philadelphia, Philadelphia, PA |
| K. Opheim | Children’s Hospital and Medical Center, Seattle, WA |
| L. Pasztor | Children’s Mercy Hospital, Kansas City, MO |
| S. Patil | University of Iowa, Iowa City, IA |
| C. Pehl | Loma Linda University, Redlands, CA |
| M. A. Perle | New York University Medical Center, New York, NY |
| A. Pettigrew | University of Kentucky, Lexington, KY |
| C. Phillips | Emory University Hospital, Atlanta, GA |
| K. Rao | University of North Carolina, Chapel Hill, NC |
| K. E. Richkind | Genzyme Genetics, Santa Fe, NM |
| K. N. Rosenbaum | Children’s Hospital National Medical Center, Washington, DC |
| D. Roulston | University of Chicago, Chicago, IL |
| C. Sandlin | Memorial Genetics Center, Long Beach, CA |
| W. G. Sanger | University of Nebraska, Omaha, NB |
| K. L. Satya-Prakash | Medical College of Georgia, Augusta, GA |
| S. Schwartz | Rainbow Babies & Children’s Hospital, Cleveland, OH |
| G. S. Sekhon | University of Wisconsin, Madison, WI |
| S. Sheldon | University of Michigan, Ann Arbor, MI |
| S. Soukup | Children’s Hospital and Medical Center, Cincinnati, OH |
| R. Sparkes | University of California, Los Angeles, CA |
| R. Stallard | Rainbow Babies & Children’s Hospital, Cleveland, OH |
| W. S. Stanley | Genetics and IVF Institute, Fairfax, VA and Children’s Hospital National Medical Center, Washington, DC |
| J. Stone | Genetrix, Inc, Scottsdale, AZ |
| P. Storto | Michigan State University, Lansing, MI |
| K. S. Theil | Children’s Hospital of Columbus, Columbus, OH |
| B. Torchia | Western Reserve Healthcare, Youngstown, OH |
| D. Van Dyke | Henry Ford Health System, Detroit, MI |
| N. V. Vigfusson | Sacred Heart Medical Center, Spokane, WA |
| D. Warburton | Columbia Presbyterian College of Physicians & Surgeons, New York, NY |
| J. R. Waterson | Children’s Hospital Medical Center, Akron, OH |
| S. L. Wenger | University of Pittsburgh, Pittsburgh, PA |
| G. Williams | Manitoba Cancer Foundation, Winnepeg, MB, Canada |
| K.-L. Yin | Children’s Hospital of Los Angeles, Los Angeles, CA |
| C. W. Yu | Valley Children’s Hospital, Fresno, Fresno, CA |
| T. Zadeh | Genetics Center, Orange, CA |
| M. C. Zapata | Children’s Medical Center, Dayton, OH |
| S. Zneimer | Kaiser-Permanente, San Jose, CA |
Supported in part by research grants including CCG Chairman’s Grant No. CA-13539 and CA-60437 from the National Cancer Institute, National Institutes of Health. Contributing CCG cytogeneticists are given in the.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Nyla A. Heerema, PhD, Children’s Cancer Group, Attn. Lucia Noll, PO Box 60012, Arcadia, CA 91066-6012.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal