Abstract
We evaluated demographic characteristics and graft composition as risk factors for acute graft-versus-host disease (GVHD) in 160 adult recipients of HLA-identical allogeneic blood stem cell transplants. The patients received a median nucleated cell dose of 7.9 × 108/kg and median C34+ cell dose of 5.6 × 106/kg. GVHD prophylaxis consisted of cyclosporine (CSA) and steroids, tacrolimus (FK506) and steroids, or FK506 and methotrexate. Grades 2 to 4 GVHD occurred in 31% (95% CI, 23% to 39%), and grades 3 to 4 GVHD in 14% (95% CI, 8% to 20%). In univariate analyses, GVHD prophylaxis with CSA and high CD34+ cell doses were significant risk factors for grades 2 to 4 GVHD, but diagnosis, age, use of total body irradiation, donor sex, female donor for male recipient, donor parity, donor alloimmunization, viral serology, nucleated cell dose, CD3+ cell dose, and CD56+ cell dose did not alter the incidence of GVHD significantly. With a CD34+cell dose less than 8 × 106 CD34+ cells/kg, the risk of grades 2 to 4 GVHD was significantly higher for those who received CSA (39%, 95% CI, 21% to 47%) in comparison with those on FK506 (18%, 95% CI, 10% to 26%) (P = .03), but GVHD prophylaxis regimen had less impact with a higher CD34+cell dose (overall grades 2 to 4 GVHD rate 52%, 95% CI, 37% to 67%). GVHD prophylaxis and CD34+ cell dose are independent risk factors for acute GVHD after allogeneic blood stem cell transplantation.
WHETHER THE HIGH number of lymphocytes in blood stem cell grafts would increase the risk of acute graft-versus-host disease (GVHD) after primary allogeneic transplantation was initially a topic of intense debate. Concern diminished when the first reports by Russell et al1 and Sasaki et al2 showed that engraftment of allogeneic blood stem cells was successful without fatal GVHD, and the feasibility of this approach was further supported by several case series.3-5 Comparisons to historical control marrow recipients6-11 and one randomized Phase II study12 further suggested that the risk of acute GVHD was no greater with blood stem cell grafts than with marrow grafts from HLA-identical donors, and randomized Phase III studies to address this issue are currently ongoing. Interestingly, the rates of grades 2 to 4 GVHD reported have ranged from 14% to 70% for allogeneic blood stem cell transplant recipients with HLA-identical donors,6-18and the reason for this wide range is unclear.
Although histoincompatibility remains the strongest risk factor for acute GVHD, within the subset of patients receiving non–T-cell–depleted marrow transplants from HLA-matched related donors, a number of other variables have been associated with an increased incidence of acute GVHD. These include diagnosis, recipient-donor sex mismatch, recipient age, female donor-male recipient pair, alloimmunized and/or parous donor, increased dose of total body irradiation (TBI), lower intensity of GVHD prophylaxis, and viral seropositivity of recipient or donor.19-24 Herein, we have investigated whether such variables are also risk factors for GVHD after allogeneic blood stem cell transplantation.
PATIENTS AND METHODS
Patients.
Over a consecutive 44-month period, 160 adults received a myeloablative preparative regimen and an unmanipulated blood stem cell graft from an HLA-matched related donor. Patient characteristics are shown in Table 1. All patients had hematologic malignancies or solid tumors. Details of the peritransplant care and administration of the preparative regimens have been described previously.4,9 25-28 “Minitransplant” recipients and recipients of T-cell–depleted blood stem cell transplants were not included. Written informed consent was obtained from each patient, and all protocols were approved by the Institutional Review Board of the M.D. Anderson Cancer Center (Houston, TX).
Characteristics of Allogeneic Blood Stem Cell Recipients
| Number | 160 |
| Median age (range) | 40 yrs (17-61) |
| Sex (Male/Female) | 91/69 |
| Diagnosis | |
| Acute lymphoblastic leukemia | 12 |
| Acute myelogenous leukemia | 36 |
| Chronic lymphocytic leukemia | 7 |
| Chronic myelogenous leukemia | 23 |
| Chronic myeloproliferative disorder | 1 |
| Hodgkin’s disease | 4 |
| Malignant lymphoma | 54 |
| Multiple myeloma | 3 |
| Myelodysplastic syndrome | 7 |
| Solid tumor | 13 |
| Disease status | |
| First remission or chronic phase | 12 |
| Second remission or accelerated phase | 31 |
| Relapse or blast crisis | 117 |
| Preparative regimen | |
| Total body irradiation-based | 33 |
| Busulfan-based | 91 |
| Other | 36 |
| Graft-versus-host disease prophylaxis | |
| CSA/methylprednisolone | 50 |
| FK506/methylprednisolone | 55 |
| FK506/methotrexate | 55 |
| Number | 160 |
| Median age (range) | 40 yrs (17-61) |
| Sex (Male/Female) | 91/69 |
| Diagnosis | |
| Acute lymphoblastic leukemia | 12 |
| Acute myelogenous leukemia | 36 |
| Chronic lymphocytic leukemia | 7 |
| Chronic myelogenous leukemia | 23 |
| Chronic myeloproliferative disorder | 1 |
| Hodgkin’s disease | 4 |
| Malignant lymphoma | 54 |
| Multiple myeloma | 3 |
| Myelodysplastic syndrome | 7 |
| Solid tumor | 13 |
| Disease status | |
| First remission or chronic phase | 12 |
| Second remission or accelerated phase | 31 |
| Relapse or blast crisis | 117 |
| Preparative regimen | |
| Total body irradiation-based | 33 |
| Busulfan-based | 91 |
| Other | 36 |
| Graft-versus-host disease prophylaxis | |
| CSA/methylprednisolone | 50 |
| FK506/methylprednisolone | 55 |
| FK506/methotrexate | 55 |
Donors and blood stem cell collection.
Donor characteristics are shown in Table 2. Details of filgrastim administration and blood stem cell collection have been published.29 The target CD34+ cell dose was 4 × 106/kg, but the actual cell dose infused depended on the result of apheresis and processing. Blood stem cell collections were assessed for CD34+, CD3+, and CD56+ cells by flow cytometry on the day of collection before cryopreservation.29 The absolute number of a particular subset in the apheresis component was calculated by multiplying the ungated percentage of that subset of cells times the total number of cells in the component. On the day of transplantation, the component was thawed and, in some cases, washed before infusion. An aliquot of the infusion was used to determine the number of cells infused. The absolute numbers of CD34+, CD3+, and CD56+ cells infused were calculated by multiplying the percentage of cells recovered after thawing and processing times the absolute number of the subset in the cryopreserved component. The median recovery of total nucleated cells was 82% (range, 45% to 100%). Characteristics of the blood stem cell grafts are summarized in Table 2. There was a weak association between CD34+ and CD3+ or total nucleated cell dose (Spearman correlation coefficient 0.26 and 0.39, respectively).
Characteristics of Allogeneic Blood Stem Cell Donors and Grafts
| Number | 160 |
| Median age (range) | 49 (8-68) |
| Sex | |
| Male | 81 |
| Female | 79 |
| HLA-matched | |
| Sibling | 158 |
| Parent or child | 2 |
| Parous | 57 |
| Transfused | 19 |
| Alloimmunized | 62 |
| Sex-mismatched | 76 |
| Female donor to male recipient | 43 |
| Pair CMV-seronegative | 19 |
| Total nucleated cells infused (range) | 7.9 × 108/kg (1.1-26.4) |
| CD34+ cells infused (range) | 5.6 × 106/kg (1.9-24.9) |
| CD3+ cells infused (range) | 2.3 × 108/kg (0.4-11.4) |
| CD56+ cells infused (range) | 2.8 × 107/kg (0.2-17.3) |
| Number | 160 |
| Median age (range) | 49 (8-68) |
| Sex | |
| Male | 81 |
| Female | 79 |
| HLA-matched | |
| Sibling | 158 |
| Parent or child | 2 |
| Parous | 57 |
| Transfused | 19 |
| Alloimmunized | 62 |
| Sex-mismatched | 76 |
| Female donor to male recipient | 43 |
| Pair CMV-seronegative | 19 |
| Total nucleated cells infused (range) | 7.9 × 108/kg (1.1-26.4) |
| CD34+ cells infused (range) | 5.6 × 106/kg (1.9-24.9) |
| CD3+ cells infused (range) | 2.3 × 108/kg (0.4-11.4) |
| CD56+ cells infused (range) | 2.8 × 107/kg (0.2-17.3) |
GVHD prophylaxis, monitoring, and grading.
Patients received one of three GVHD prophylaxis regimens depending on the standard at the time of transplantation or the requirement of the transplant protocol. Prophylaxis consisted of cyclosporine (CSA) and methylprednisolone (MP), tacrolimus (FK506) and MP, or FK506 and micromethotrexate (MTX). CSA was administered at 3 mg/kg/day IV continuous infusion from day −2, and doses were adjusted to maintain whole blood steady state or trough levels at 250 ng/mL to 350 ng/mL by radioimmunoassay (RIA) for parent drug. FK506 was administered at 0.03 mg/kg/day IV by continuous infusion from day −2, and doses were adjusted to maintain whole blood steady state or trough levels at 5 to 15 ng/mL by IMx assay. After engraftment when the patient was able to eat, CSA or FK506 was converted to oral, continued through day 180, and tapered off thereafter. MTX was administered at 5 mg/m2 IV on days 1, 3, and 6. MP was administered at 1.0 mg/kg/day from days 5 to 28 with a taper thereafter. Patients were observed prospectively for development of acute GVHD. The diagnosis of GVHD was based on clinical evidence with histologic confirmation,30 and GVHD was graded according to the consensus criteria.31
Statistical considerations.
Minimum follow-up at the time of analysis was 180 days after transplantation. Estimates of the incidence of GVHD and treatment-related mortality were calculated by the method of Kaplan and Meier.32 Cause-specific failure rates for GVHD were also computed,33 and these were nearly identical to Kaplan-Meier estimates. Potential prognostic factors are listed in Tables3 and 4. In considering the association of GVHD rates with continuously measured covariates, patients were grouped approximately into quartiles based on covariate values (age, log cell dose). Comparisons of categorical data were by chi-square test, and associations of factors with GVHD rates were analyzed using log-rank tests and proportional hazards regression modeling.34 In the case of CD34+ cell dose, a graphical method based on residuals from a proportional hazards model35 was used to further evaluate the association.
Univariate Analysis of Demographic Risk Factors for Acute Graft-Versus-Host Disease
| Factor . | No. . | Gr 2-4 GVHD % (SE) . | P . | Gr 3-4 GVHD % (SE) . | P . |
|---|---|---|---|---|---|
| Diagnosis | .21 | .68 | |||
| Leukemia/myelodysplasia | 85 | 27 (5) | 11 (3) | ||
| Hodgkin’s/lymphoma/myeloma | 62 | 33 (6) | 17 (5) | ||
| Solid tumor | 13 | 49 (14) | 16 (10) | ||
| Patient age | .57 | .61 | |||
| 17-32 yrs | 39 | 28 (7) | 10 (5) | ||
| 33-39 yrs | 39 | 23 (7) | 10 (5) | ||
| 40-46 yrs | 43 | 34 (7) | 14 (5) | ||
| 47-61 yrs | 39 | 41 (8) | 20 (7) | ||
| Preparative regimen | .91 | .17 | |||
| Total body irradiation | 33 | 30 (4) | 6 (4) | ||
| No total body irradiation | 127 | 34 (8) | 16 (3) | ||
| GVHD prophylaxis regimen | .03 | .15 | |||
| CSA/methylprednisolone | 50 | 45 (7) | 22 (6) | ||
| FK506/methylprednisolone | 55 | 30 (6) | 13 (5) | ||
| FK506/methotrexate | 55 | 21 (6) | 7 (4) | ||
| Donor sex | .80 | .21 | |||
| Male | 81 | 32 (5) | 10 (3) | ||
| Female | 79 | 30 (5) | 17 (4) | ||
| Female donor—male recipient | .71 | .24 | |||
| Yes | 43 | 28 (7) | 19 (6) | ||
| No | 117 | 32 (4) | 12 (3) | ||
| Parous donor | .61 | .79 | |||
| Yes | 57 | 32 (6) | 13 (5) | ||
| No | 103 | 30 (5) | 14 (3) | ||
| Alloimmunized donor | .51 | .56 | |||
| Yes | 62 | 29 (6) | 12 (4) | ||
| No | 98 | 33 (5) | 15 (4) | ||
| Alloimmunized female donor—male recipient | .57 | .92 | |||
| Yes | 30 | 27 (8) | 10 (5) | ||
| No | 130 | 32 (4) | 14 (3) | ||
| Pair CMV-seronegative | .86 | .70 | |||
| Yes | 19 | 33 (11) | 17 (9) | ||
| No | 141 | 31 (4) | 13 (3) |
| Factor . | No. . | Gr 2-4 GVHD % (SE) . | P . | Gr 3-4 GVHD % (SE) . | P . |
|---|---|---|---|---|---|
| Diagnosis | .21 | .68 | |||
| Leukemia/myelodysplasia | 85 | 27 (5) | 11 (3) | ||
| Hodgkin’s/lymphoma/myeloma | 62 | 33 (6) | 17 (5) | ||
| Solid tumor | 13 | 49 (14) | 16 (10) | ||
| Patient age | .57 | .61 | |||
| 17-32 yrs | 39 | 28 (7) | 10 (5) | ||
| 33-39 yrs | 39 | 23 (7) | 10 (5) | ||
| 40-46 yrs | 43 | 34 (7) | 14 (5) | ||
| 47-61 yrs | 39 | 41 (8) | 20 (7) | ||
| Preparative regimen | .91 | .17 | |||
| Total body irradiation | 33 | 30 (4) | 6 (4) | ||
| No total body irradiation | 127 | 34 (8) | 16 (3) | ||
| GVHD prophylaxis regimen | .03 | .15 | |||
| CSA/methylprednisolone | 50 | 45 (7) | 22 (6) | ||
| FK506/methylprednisolone | 55 | 30 (6) | 13 (5) | ||
| FK506/methotrexate | 55 | 21 (6) | 7 (4) | ||
| Donor sex | .80 | .21 | |||
| Male | 81 | 32 (5) | 10 (3) | ||
| Female | 79 | 30 (5) | 17 (4) | ||
| Female donor—male recipient | .71 | .24 | |||
| Yes | 43 | 28 (7) | 19 (6) | ||
| No | 117 | 32 (4) | 12 (3) | ||
| Parous donor | .61 | .79 | |||
| Yes | 57 | 32 (6) | 13 (5) | ||
| No | 103 | 30 (5) | 14 (3) | ||
| Alloimmunized donor | .51 | .56 | |||
| Yes | 62 | 29 (6) | 12 (4) | ||
| No | 98 | 33 (5) | 15 (4) | ||
| Alloimmunized female donor—male recipient | .57 | .92 | |||
| Yes | 30 | 27 (8) | 10 (5) | ||
| No | 130 | 32 (4) | 14 (3) | ||
| Pair CMV-seronegative | .86 | .70 | |||
| Yes | 19 | 33 (11) | 17 (9) | ||
| No | 141 | 31 (4) | 13 (3) |
Univariate Analysis of Graft Characteristics as Risk Factors for Acute Graft-Versus-Host Disease
| Factor . | No. . | Gr 2-4 GVHD % (SE) . | P . | Gr 3-4 GVHD % (SE) . | P . |
|---|---|---|---|---|---|
| Total nucleated cells × 108/kg | .52 | .80 | |||
| 1.1-5.9 | 40 | 26 (7) | 10 (5) | ||
| 6.0-7.8 | 38 | 24 (7) | 13 (6) | ||
| 7.9-10.7 | 42 | 35 (8) | 12 (5) | ||
| 10.8-26.4 | 40 | 39 (8) | 18 (6) | ||
| CD34+cells × 106/kg | .02 | .27 | |||
| 1.9-4.1 | 37 | 26 (8) | 12 (6) | ||
| 4.2-5.4 | 42 | 22 (7) | 12 (5) | ||
| 5.5-8.1 | 40 | 26 (7) | 8 (4) | ||
| 8.2-24.9 | 41 | 50 (8) | 22 (7) | ||
| CD3+ cells × 108/kg | .82 | .99 | |||
| 0.4-1.5 | 35 | 24 (7) | 12 (6) | ||
| 1.6-2.2 | 44 | 31 (7) | 14 (5) | ||
| 2.3-2.9 | 38 | 32 (8) | 13 (5) | ||
| 3.0-11.4 | 43 | 37 (8) | 15 (6) | ||
| CD56+ cells × 107/kg | .46 | .66 | |||
| 0.2-1.7 | 36 | 22 (7) | 8 (5) | ||
| 1.8-2.7 | 43 | 26 (7) | 12 (5) | ||
| 2.8-4.1 | 41 | 38 (8) | 15 (6) | ||
| 4.2-17.3 | 40 | 39 (8) | 19 (7) |
| Factor . | No. . | Gr 2-4 GVHD % (SE) . | P . | Gr 3-4 GVHD % (SE) . | P . |
|---|---|---|---|---|---|
| Total nucleated cells × 108/kg | .52 | .80 | |||
| 1.1-5.9 | 40 | 26 (7) | 10 (5) | ||
| 6.0-7.8 | 38 | 24 (7) | 13 (6) | ||
| 7.9-10.7 | 42 | 35 (8) | 12 (5) | ||
| 10.8-26.4 | 40 | 39 (8) | 18 (6) | ||
| CD34+cells × 106/kg | .02 | .27 | |||
| 1.9-4.1 | 37 | 26 (8) | 12 (6) | ||
| 4.2-5.4 | 42 | 22 (7) | 12 (5) | ||
| 5.5-8.1 | 40 | 26 (7) | 8 (4) | ||
| 8.2-24.9 | 41 | 50 (8) | 22 (7) | ||
| CD3+ cells × 108/kg | .82 | .99 | |||
| 0.4-1.5 | 35 | 24 (7) | 12 (6) | ||
| 1.6-2.2 | 44 | 31 (7) | 14 (5) | ||
| 2.3-2.9 | 38 | 32 (8) | 13 (5) | ||
| 3.0-11.4 | 43 | 37 (8) | 15 (6) | ||
| CD56+ cells × 107/kg | .46 | .66 | |||
| 0.2-1.7 | 36 | 22 (7) | 8 (5) | ||
| 1.8-2.7 | 43 | 26 (7) | 12 (5) | ||
| 2.8-4.1 | 41 | 38 (8) | 15 (6) | ||
| 4.2-17.3 | 40 | 39 (8) | 19 (7) |
RESULTS
Incidence of acute GVHD.
Forty-eight patients developed grades 2 to 4 GVHD for a cumulative incidence of 31% (95% CI, 23% to 39%). Twenty-one patients developed grades 3 to 4 GVHD for a cumulative incidence of 14% (95% CI, 8% to 20%).
Risk factors for acute GVHD.
Patient and donor demographics as well as characteristics of the grafts were evaluated as potential risk factors for grades 2 to 4 and grades 3 to 4 GVHD. Higher rates of grades 2 to 4 GVHD occurred in patients who were older or who had solid tumors, but GVHD prophylaxis regimen was the only demographic factor identified as statistically significant (Table 3). Use of TBI, donor sex, patient-donor sex mismatch, donor parity, donor alloimmunization, and cytomegalovirus (CMV)-seronegativity had no correlation with the risk of grades 2 to 4 GVHD. No demographic factor or graft characteristic correlated significantly with grades 3 to 4 GVHD, although trends generally corresponded to findings for grades 2 to 4 GVHD (Tables 3 and 4).
There was a trend for increasing rates of grades 2 to 4 GVHD with increasing numbers of total nucleated cells, CD34+ cells, CD3+ cells, or CD56+ cells transplanted, but CD34+ cell dose was the only graft characteristic that correlated significantly with grades 2 to 4 GVHD. The rate of GVHD remained fairly stable with increasing numbers of CD34+cells transfused except at the highest quartile (Table 4). The correlation between CD34+ cell dose and GVHD risk was verified in a plot of regression residuals,35 which suggested a sharp increase in risk of GVHD in a range of 6.3 to 10.0 × 106 CD34+ cells/kg with constant risks both above and below that range. A cutpoint of 8 × 106 CD34+ cells/kg was selected for further analysis, because it fell at approximately the midpoint of this range.
A proportional hazards model for grades 2 to 4 acute GHVD was fitted including the two indicator covariates (GVHD prophylaxis and CD34+ cell dose), the interaction factor (GVHD prophylaxis by CD34+ cell dose), age (years), and diagnosis (hematologic disorder or solid tumor). Use of CSA and a CD34+ cell dose greater than 8 × 106CD34+ cells/kg remained independent risk factors (Table 5).
Summary of Regression Model for Grades 2-4 Acute Graft-Versus-Host Disease
| Risk Factor . | Odds Ratio (95% CI) . | P . |
|---|---|---|
| CD34+cell dose | 4.41 (2.00-9.68) | <.01 |
| GVHD prophylaxis | 2.48 (1.14-5.40) | .02 |
| Diagnosis | 2.71 (1.12-6.59) | .03 |
| Age | 1.02 (1.00-1.05) | .11 |
| Prophylaxis × CD34+ | 0.45 (0.14-1.42) | .17 |
| Risk Factor . | Odds Ratio (95% CI) . | P . |
|---|---|---|
| CD34+cell dose | 4.41 (2.00-9.68) | <.01 |
| GVHD prophylaxis | 2.48 (1.14-5.40) | .02 |
| Diagnosis | 2.71 (1.12-6.59) | .03 |
| Age | 1.02 (1.00-1.05) | .11 |
| Prophylaxis × CD34+ | 0.45 (0.14-1.42) | .17 |
Correlation between risk factors and outcomes.
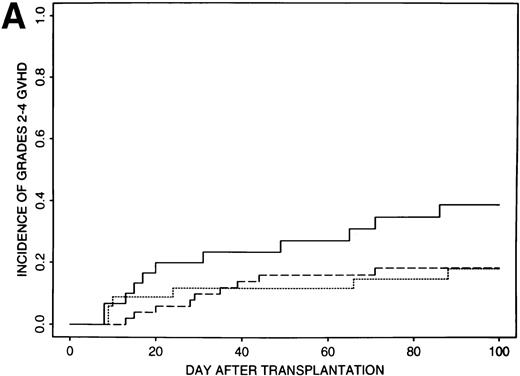
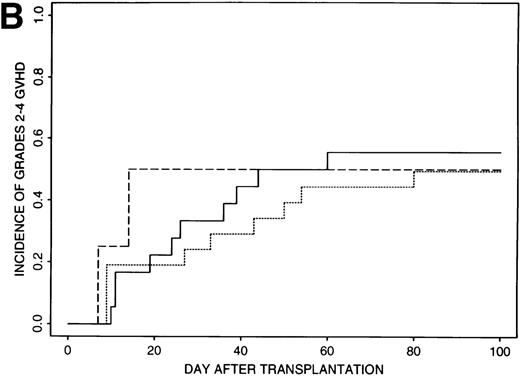
Estimates of GVHD rates suggested that FK506 provided better prophylaxis than CSA for those patients transplanted with less than 8 × 106 CD34+ cell/kg (P = .03); the rate of grades 2 to 4 GVHD was 39% (95% CI, 21% to 57%) for the 32 patients who received CSA and 18% (95% CI, 10% to 26%) for the 85 patients who received FK506 (Fig 1A). Rates of grades 2 to 4 GVHD were higher (52%, 95% CI, 37% to 67%) for patients transplanted with ≥8 × 106CD34+ cell/kg and were similar for all GVHD regimens (49% to 56%) (Fig 1B).
Incidence of grades 2 to 4 GVHD after transplantation of less than 8 × 106 CD34+ cells/kg (A) or greater than 8 × 106 CD34+ cells/kg (B) using CSA/MP (solid line), FK506/MP (dashed line), or FK506/MTX (dotted line) as GVHD prophylaxis.
Incidence of grades 2 to 4 GVHD after transplantation of less than 8 × 106 CD34+ cells/kg (A) or greater than 8 × 106 CD34+ cells/kg (B) using CSA/MP (solid line), FK506/MP (dashed line), or FK506/MTX (dotted line) as GVHD prophylaxis.
Of the 48 patients who developed grades 2 to 4 GVHD, 21 (44%) had skin involvement alone, 37 (77%) had skin with or without visceral GVHD, 9 (19%) had liver involvement, and 26 (54%) had gut involvement. The distribution of organ involvement did not differ significantly by GVHD prophylaxis or CD34+cell dose group (Table 6).
Distribution of Organ Involvement for Grades 2-4 Acute Graft-Versus-Host Disease
| . | CSA Low CD34 . | FK506 Low CD34 . | High CD34 . | All . |
|---|---|---|---|---|
| Number | 11 | 15 | 22 | 48 |
| Skin alone | 6 (55%) | 4 (27%) | 11 (50%) | 21 (44%) |
| Skin | 9 (82%) | 10 (67%) | 18 (82%) | 37 (77%) |
| Liver | 4 (36%) | 2 (13%) | 3 (14%) | 9 (19%) |
| Gut | 5 (45%) | 10 (67%) | 11 (50%) | 26 (54%) |
| . | CSA Low CD34 . | FK506 Low CD34 . | High CD34 . | All . |
|---|---|---|---|---|
| Number | 11 | 15 | 22 | 48 |
| Skin alone | 6 (55%) | 4 (27%) | 11 (50%) | 21 (44%) |
| Skin | 9 (82%) | 10 (67%) | 18 (82%) | 37 (77%) |
| Liver | 4 (36%) | 2 (13%) | 3 (14%) | 9 (19%) |
| Gut | 5 (45%) | 10 (67%) | 11 (50%) | 26 (54%) |
For all patients, treatment-related mortality was 30% (95% CI, 23% to 37%) at day 180 posttransplant. The day-180 treatment-related mortality was 42% (95% CI, 25% to 59%) for patients on CSA who received the low CD34+ cell dose, 24% (95% CI, 15% to 33%) for patients on FK506 who received the low CD34+ cell dose, and 37% (95% CI, 22% to 52%) for those who received the high CD34+ cell dose. The differences in treatment-related mortality were not significant.
DISCUSSION
In our evaluation of risk factors for acute GVHD after allogeneic blood stem cell transplantation, we found that the incidence of GVHD was affected by the immunoprophylaxis regimen and by the CD34+cell dose. The GVHD prophylaxis regimen has long been known to be an important risk factor for GVHD,22,26 and recent studies have shown that FK506 is more potent than CSA for prevention of acute GVHD after allogeneic marrow transplantation,36,37 so our results for GVHD prophylaxis were not unexpected. The correlation between CD34+ cell dose and risk of GVHD has not been noted previously with allogeneic marrow transplantation, but the high CD34+ cell doses as we have collected here from filgrastim-mobilized donors are rarely achieved in a single marrow harvest,4,10 12 so such an association would probably not be identified easily with marrow transplantation.
Of the demographic descriptors evaluated, both we here and Schmitz et al15 noted a trend for increasing risk of acute GVHD with increasing age of patient, but it was not significant in either analysis. None of the other patient-donor variables was found to be significant. This may be due to the fact that donor selection criteria at our center already takes into account known characteristics that increase GVHD risk, potentially biasing the analysis against detection of significance. With improvement in GVHD prophylaxis, however, others have also reported that widely accepted demographic risk factors for acute GVHD may no longer apply for HLA-identical marrow transplant recipients.22 23
In nonrandomized comparisons, the incidence of grades 2 to 4 GVHD in marrow transplant recipients did not vary when using MP or MTX with CSA.38,39 Similarly, in our study of stem cell recipients, when stratified by CD34+ cell dose, patients receiving FK506 had the same risk of GVHD whether used in conjunction with MP or MTX. Bensinger et al7 and Schmitz et al15 also did not find a significant difference in the incidence of acute GVHD comparing MP to MTX used with CSA after allogeneic stem cell transplantation. However, the use of MP in GVHD prophylaxis regimens has been associated with a higher risk of infection and treatment-related mortality after marrow transplantation,17,38 40 so MTX may be the preferred second drug to use in combination regimens.
In large multivariate analyses, total nucleated cell dose has not been identified as an independent risk factor for GVHD after HLA-identical marrow transplantation.19,20,23,24 In one early study, nucleated cell dose was associated with an increased survival, but this was related to a reduction in the incidence of interstitial pneumonitis and early treatment-related complications other than GVHD.41 We also found that nucleated cell dose did not affect acute GVHD incidence in the allogeneic blood stem cell recipients.
For our patients, there was no significant correlation between the incidence of acute GVHD and CD3+ or CD56+ cell dose. The relative lack of severe GVHD despite the high numbers of lymphocytes infused into these patients has been ascribed to a reduction in function of T and NK cells in filgrastim-treated normal donors.42,43 Impaired functions of T cells from blood stem cell donors measured in vitro include a decrease in alloantigen and mitogen proliferative responses, and a lower induction of the CD28 response complex by TCR stimulation.42,44,45 The hyporesponsiveness is thought to be mediated by interleukin-10 (IL-10) produced by monocytes.46 This observation may be an in vitro epiphenomenon, however, because high IL-10 levels in vivo have been associated with increased transplant-related complications,47,48 and sorted CD4+ cells from filgrastim-treated normal donors are not consistently hyporesponsive.42 Others have reported that filgrastim treatment results in a Th2 polarization in vivo,49-51 and in an animal model, this correlated with a reduction in the incidence of acute GVHD,52 but the mechanism by which filgrastim induces the Th2 polarization is unknown.
The association between CD34+ cell dose and GVHD risk has not been reported previously, although CSA-based prophylaxis has been used for most other allogeneic blood stem cell transplant recipients, and even in our study population, the difference in risk of acute GVHD by CD34+ cell dose is minimal for the CSA subgroup alone. The truly striking difference occurs in the FK506 subgroup, in which the risk of GVHD was lower than with CSA only with the low C34+ cell dose. The lack of FK506-responsiveness at high CD34+ cell doses suggests that GVHD in this circumstance may not be mediated totally by lymphocytes. Instead, GVHD at high CD34+ cell doses may be exacerbated by cytokines released by the markedly expanding myeloid population at the time of engraftment. In support of this hypothesis, Hägglund et al23 reported that early engraftment independently predicted development of acute GVHD after allogeneic marrow transplantation. Tumor necrosis factor α (TNFα) has been proposed as the cytokine responsible for this effect,53,54 and high levels of TNFα have been found in peripheral blood monocytes at the time of engraftment.55 Cytokine release from myeloid cells would clearly not be ameliorated by FK506 or CSA.
The ultimate objective of a risk factor analysis is to identify the important variables that may improve outcome when controlled in practice. We have found that the risk of moderate-to-severe acute GVHD after HLA-identical blood stem cell transplantation is increased by high CD34+ cells doses and use of CSA rather than FK506. Although there was a trend for differences in treatment-related mortality when patients were grouped by CD34+ cell dose and GVHD prophylaxis, the differences did not achieve statistical significance, albeit the power of the analysis may have been limited by the small number of patients studied. Nevertheless, given the association of increased risk of acute GVHD with CD34+ cell dose and immunoprophylaxis regimen, these factors should be taken into consideration in any analysis of GVHD after allogeneic blood stem cell transplantation and in the design of new treatment protocols. We caution, however, that the critical cutpoint for CD34+ cell dose may differ between institutions due to the variability in the techniques for measuring CD34+ cell numbers.
Supported in part by The Tony Anderson Fund, Fujisawa USA, and the Cancer Center Core Grant (No. CA-16672) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.