Abstract
We previously reported that treatment of human peripheral blood monocytes or dendritic cells (DC) with calcium ionophore (CI) led to the rapid (18 hour) acquisition of many characteristics of mature DC, including CD83 expression. We therefore investigated whether less-mature myeloid cells were similarly susceptible to rapid CI activation. Although the promyelocytic leukemia line HL-60 was refractory to cytokine differentiation, CI treatment induced near-uniform overnight expression of CD83, CD80 (B7.1), and CD86 (B7.2), as well as additional characteristics of mature DC. Several cytokines that alone had restricted impact on HL-60 could enhance CI-induced differentiation and resultant T-cell sensitizing capacity. In parallel studies, CD34pos cells cultured from normal donor bone marrow developed marked DC-like morphology after overnight treatment with either rhCD40L or CI, but only CI simultaneously induced upregulation of CD83, CD80, and CD86. This contrasted to peripheral blood monocytes, in which such upregulation could be induced with either CI or rhCD40L treatment. We conclude that normal and transformed myeloid cells at many stages of ontogeny possess the capacity to rapidly acquire many properties of mature DC in response to CI treatment. This apparent ability to respond to calcium mobilization, even when putative signal-transducing agents are inoperative, suggests strategies for implementing host antileukemic immune responses.
A NUMBER OF RECENT STUDIES have suggested that human myeloid leukemias express antigens that can enable their recognition by T cells, with attendant immune-mediated modulation of tumor growth.1,2 Because tumors of myeloid and lymphoid lineage share the ontogeny of professional antigen-presenting cells (APC),3 the capacity of such malignant cells to present endogenously expressed tumor-associated antigen (Ag) directly to T cells has been the focus of intense recent interest. Studies of myelogenous leukemias as well as lymphocytic leukemias, lymphomas, and multiple myelomas indicate that native malignant cells typically have heterogenous or absent expression of costimulatory molecules essential for efficient T-cell sensitization.4-9 However, costimulatory molecule expression can frequently be induced by agents or combinations of agents identical to those that enhance APC function in these cells’ nonmalignant counterparts. For example, cytokine combinations such as recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF), tumor necrosis factor-alpha (rTNF-α) and interleukin-4 (rIL-4), when added to cultured bone marrow or peripheral blood mononuclear cells (PBMC) obtained from chronic myelogenous leukemia (CML) patients, also can induce dendritic cell (DC)-like differentiation in the Philadelphia chromosome-expressing (Phpos) malignant cell subpopulation, thereby enhancing APC function.10,11 Similar results have been obtained when peripheral blasts from patients with acute myelogenous leukemia (AML) were cultured with rGM-CSF, rTNF-α, stem cell factor, and IL-6.2 These results indicate that CML and AML cells from at least some individuals remain susceptible to normal signal transductive stimuli that promote elements of DC-like differentiation and enhanced APC function. Similarly, culture of B-cell–derived lymphoma, leukemia, and multiple myeloma cells with CD40 ligand (CD40L) can induce marked expression of costimulatory molecules such as B7.1, B7.2, and ICAM-1 on the tumor cells, similar to the upregulation that occurs after CD40L-stimulation of normal DC and B cells.7-9 12-14
Efforts to enhance APC function in leukemia cells via cytokine or ligand treatments are dependent on the malignant cells’ expression of specific receptors for the stimulating agent(s) as well as a capacity for normal membrane signal transduction. One strategy for bypassing such dependence is to transduce malignant cells with expression constructs that contain the genes encoding costimulatory molecules such as B7.1. Whereas this approach has successfully enhanced tumor cell immunogenicity in several studies,15-17 strategies that target activation of endogenous differentiation pathways may remain clinically advantageous when they can induce a wider panoply of APC functional characteristics.
We recently showed that pharmacologic agents that mobilize intracellular calcium can be used to enhance APC function in both human peripheral blood monocytes and immature DC, apparently bypassing initial receptor/ligand requirements involving cytokines or ligands previously shown to influence such development.18 We found that each of these myeloid subpopulations responded to calcium ionophore (CI) treatment by developing the morphologic characteristics and functions of highly activated, mature DC. Whereas full morphologic differentiation during CI treatment required 72 to 96 hours of culture, many immunophenotypic alterations occurred promptly within the first 20 hours of culture, including decreased expression of the monocyte/macrophage-associated molecule CD14 (LPS binding protein receptor), increased expression of major histocompatibility complex (MHC) molecules, upregulation of B7.2, CD40, and ICAM-1 expression, and de novo expression of both B7.1 and the DC-associated activation marker CD83.19 In addition, enhanced T-cell sensitizing efficiency was evident within the first 20 to 40 hours of treatment. Such rapid activation kinetics contrasted to the much slower activation observed when peripheral blood monocytes are treated with cytokine combinations such as rGM-CSF, rIL-4, and rTNF-α. For example, monocyte CD83 expression appeared within 4 hours and peaked at 20 hours of CI treatment, whereas with combination cytokine treatment CD83 expression is seldom observed before 5 to 7 days of culture. These contrasting kinetics do not preclude the possibility that cytokine treatment and CI treatment induce DC differentiation and/or activation through ultimately convergent molecular mechanisms. However, pharmacologic calcium-mobilizing agents have the potential clinical advantage of bypassing initial surface receptor/signal transduction requirements.
Our previous studies of peripheral blood monocytes and immature DC raised the possibility that calcium-mobilizing agents might also trigger elements of differentiation/activation in relatively undifferentiated myeloid leukemia cells as well as in normal myeloid bone marrow progenitor cells, rendering such treatment a means to induce numerous DC characteristics in these populations. The present report shows that, in addition to peripheral blood monocytes and DC, both transformed and untransformed myeloid cells at earlier stages of maturation possess the ability to respond rapidly to CI treatment. Although the kinetics of morphologic responses vary among the individual studied myeloid populations, a common observation for each studied population is marked upregulation of the activation marker CD8319 as well as the costimulatory molecules B7.1 and B7.2 within 20 hours of initiation of CI treatment, regardless of the myeloid cells’ prior differentiation status.
MATERIALS AND METHODS
Reagents and Ab.
CI A23187 (Sigma, St. Louis, MO) was used as described previously.18 In some experiments, cultures were supplemented with the following cytokines: human interferon-gamma (rIFN-γ) (Biogen, Cambridge MA; specific activity 1.6 × 107 U/mg); rh-c-kit ligand (sp act 2.7 × 105 U/mg), rhTNF-α (sp act 1.1 × 108IU/mg), and rhIL-4 (sp act 2.9 × 107 IU/mg) (all R&D Systems, Minneapolis, MN); and rhGM-CSF (sp act 5.6 × 106 IU/mg (Amgen, Thousand Oaks, CA). Human recombinant soluble trimeric CD40-ligand (rhCD40L) was a generous gift from Immunex, Seattle, WA.
Myelogenous human cell line cultures.
The acute monocytic leukemia-derived THP-1 (TIB 202), the acute myelogenous leukemia-derived KG-1 (CCL 246), the chronic myelogenous leukemia line K562 (CCL 243), the macrophage-like histocytic lymphoma U937 (CRL 1593), and the promyelocytic leukemia-derived HL-60 (CCL 240) were obtained from American Type Culture Collection (Rockville, MD); BV173 was a kind gift from Dr Alan Gevirtz at the University of Pennsylvania (Philadelphia, PA). Cell lines were maintained in exponential growth by serial passage in 162 cm2 tissue culture flasks (Costar, Cambridge, MA) in culture medium (CM) consisting of RPMI-1640 with 10% heat-deactivated fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μg/mL gentamicin sulfate, .05 mmol/L 2-ME, 1 mmol/L sodium pyruvate, and 1 mmol/L nonessential amino acids (all reagents Biofluids, Rockville, MD) at 37o C, 5% CO2. Cell lines used in all experiments were split 5:1 every 2 to 3 days, and were carried no more than 2 to 3 months in culture from the original stocks. To assess the effects of treatments with CI and/or cytokines, cells were washed and seeded in fresh medium at 1 × 106 or 5 × 105 per well in 24-well tissue culture plates (Costar), then cultured for 18 hours before CI and/or cytokine addition.
CD34pos human myeloid cultures.
Normal bone marrow cells enriched for CD34pos cells (purity 91% to 98%) were obtained from three healthy donors (Poietic Technologies, Inc, Gaithersburg, MD) and in one instance from cadaveric vertebral bodies by positive immunomagnetic selection using a CD34 isolation kit (Miltenyi Biotech Inc, Auburn CA). To promote enrichment and expansion of intermediate DC precursors,20,21CD34pos cells were resuspended to 4 × 104cells/mL and cultured for 6 days in an enriched Iscove’s Modified Dulbecco’s Medium22 supplemented with 10% FCS (Hyclone, Logan, UT), 20 ng/mL rh-c-kit ligand, 10 ng/mL rhGM-CSF, and 10 ng/mL rhTNF-α; fresh medium and factors were added on day 3. After the 6-day expansion and enrichment phase, cells were harvested, washed, and replated in 48-well cluster plates (Costar) at 5 × 105 cells per well in fresh medium containing rhGM-CSF and rhTNF-α but not rh-c-kit ligand. Various doses of CI or rhCD40L were added 45 minutes later, and wells were harvested at various subsequent timepoints for analysis as described in Results.
Human peripheral blood monocyte cultures.
Human monocytes were prepared from four healthy volunteers using leukapheresis and countercurrent centrifugal elutriation as previously described.18 A serum-free modification of our prior culture method was used (Nguyen et al, manuscript in preparation); monocytes were washed and resuspended in serum-free medium (Macrophage-SFM, Gibco, Grand Island, NY) with 50 ng/mL added rhGM-CSF, and plated at 5 × 106 cells per well in 24-well cluster plates (Costar) overnight, after which various doses of CI or rhCD40L were added as described in Results.
Ab and fluorescence-activated cell sorter (FACS) analysis.
Multicolor FACS analysis was used as previously developed to analyze normal human monocytes and DC.18 PE-conjugated MoAb against human B7.1, monomorphic HLA-DR, and CD33 were obtained from Becton Dickinson (Mountain View, CA), B7.2, ICAM-1, and CD1a from Pharmingen (San Diego, CA), monomorphic HLA-ABC from Sigma (St. Louis, MO), and CD40 from Harlan (Oxford, UK); subclass-matched, conjugated control Ab were obtained from Becton Dickinson and Caltag Laboratories (San Francisco, CA). Unconjugated HB15a, a mouse MoAb recognizing human CD83 was a kind gift of Thomas Tedder (Duke University, Durham, NC). PE-conjugated goat antimouse IgG (GAMPE) as well as unconjugated mouse immunoglobulin (Ig) subclass MoAb controls were acquired from Caltag. Cultured cell lines were harvested, distributed into 12 × 15 mm polystyrene FACS tubes (Falcon, Lincoln Park, NJ), centrifuged, and supernatants removed. Cells were FcR blocked at 4°C for 20 min with unconjugated 1 mg/mL human IgG (Sigma) in FACS buffer (Ca++/Mg++-free Hanks’ Buffered Saline Solution [HBSS] containing 0.1% bovine serum albumin [BSA] and .02% sodium azide). Cells were then washed in FACS buffer, centrifuged, and stained with various Ab under identical conditions for 20 min. Cells were then washed and centrifuged as before and resuspended in 0.5 mL FACS buffer. In some experiments cells were counterstained with FITC-conjugated anti-CD14 or subclass matched MoAb (Becton Dickinson). Samples stained with CD83 or isotype-matched control Ab were washed, then counterstained with GAMPE. In later experiments PE-conjugated anti-CD83 antibody (Coulter) was substituted for indirect staining of CD83.
Cells were then washed and resuspended in 0.5 mL FACS buffer and subjected to three- or two-color analysis on a Becton Dickinson FACSort using CellQuest analysis software; 0.8 μg/mL propidium iodide (PI) was added to samples to enable exclusion of nonviable cells from analysis.
Microscopy.
Culture medium was carefully removed from cells cultured in 24- or 48-well plates, and fresh medium supplied. Cells were then resuspended and transferred onto Lab-Tek 8-glass chamber slides (Nunc, Naperville, IL) previously coated with 1% polylysine (Sigma, St Louis, Mo) and incubated for 30 min at 37oC, 5% CO2.18 Slides were then examined and photographed with an Olympus IX-70 inverted microscope (Melville, NY) using Nomarski differential interference contrast (DIC) optics. Cells were either photographed while viable, or first fixed in absolute ethanol and stained with Wright’s solution.18
Allosensitization studies.
Human T lymphocytes obtained from lymphocyte-rich elutriation fractions were purified using T-cell isolation columns (R&D, Minneapolis, MN).18 In some experiments non-naive T cells were depleted by incubating lymphocytes with 10 μg/mL anti-CD45RO (Caltag) before column application.23 HL-60 cells or cultured CD34pos bone marrow cells were subjected to various CI or CI/cytokine combination treatments, were harvested, washed 3 times in CM, γ-irradiated to 30 Gy, then cocultured with T lymphocytes in 96-well flat-bottomed plates (Costar). The T-cell number was held constant at 1 × 105 cells/well while the irradiated HL-60 cells were added to each well in graded numbers. Cocultures were incubated for 96 hours, then pulsed with 1 μCi/well [3H]-TdR, and harvested and counted 18 hours later.
RESULTS
Acute promyelocytic leukemia line HL-60 displays profound early and delayed responses to CI treatment.
Previous studies of human monocytes indicated that the optimal dose of CI for enhancing DC differentiation varied among culture media, with relatively higher CI concentrations required in media with higher protein content (not shown). Our preliminary screenings with human transformed myeloid cell lines, each cultured in RPMI-1640 medium containing 10% FCS, indicated that phenotypic responses to CI treatment varied among lines, but were maximal for each cell line tested in the same CI dose range (not shown). CI-reactive cell lines displayed varied upregulation of surface costimulatory molecules B7.1, B7.2, and/or ICAM-1 after CI treatment (HL-60>K562>THP-1>KG-1; BV173 and U937 nonreactive, Fig 1 and not shown); HL-60 additionally displayed marked upregulation of CD83, similarly to normal peripheral blood monocytes and immature DC (see below).18 The optimally activating A23187 dose selected for HL-60 in all subsequent experiments (conducted in 10% FCS) was 180 ng/mL, a concentration 2 to 5 times lower than that previously used by others to induce apoptosis of HL-60 in similar media.24 25This optimum A23187 concentration had a pronounced antiproliferative effect on HL-60 (not shown) but was accompanied by only modest (typically 15% to 20%) cell death (compared with untreated controls) during periods of observation (see below).
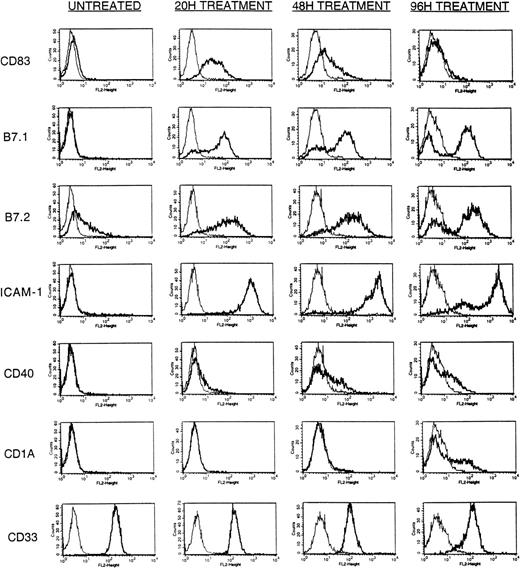
Time-course analysis of A23187-inducible surface Ag expression. HL-60 cells (5 × 105) were cultured in a final volume of 2 mL with or without an optimal dose (180 ng/mL) of A23187 as described in Materials and Methods. Cells were harvested at 20, 48, and 96 hours post addition of A23187 and stained with PE-conjugated mouse-antihuman CD83, B7.1, B7.2, ICAM-1, CD40, CD1a, CD33, or IgG subclass-matched control Ab, and analyzed by FACS as described in Materials and Methods. Results displayed are at 20 hours without A23187 treatment (UNTREATED), 20 hours with A23187 treatment (20H TREATMENT), 48 hours with A23187 treatment (48H TREATMENT), and 96 hours with A23187 treatment (96H TREATMENT). Cultures not treated with A23187 showed an identical immunophenotypic profile at 20, 48, and 96 hours (not shown). Histograms display staining intensity of viable (PI-excluding) cells, comparing control Ab (light lines) to specific Ab (heavy lines).
Time-course analysis of A23187-inducible surface Ag expression. HL-60 cells (5 × 105) were cultured in a final volume of 2 mL with or without an optimal dose (180 ng/mL) of A23187 as described in Materials and Methods. Cells were harvested at 20, 48, and 96 hours post addition of A23187 and stained with PE-conjugated mouse-antihuman CD83, B7.1, B7.2, ICAM-1, CD40, CD1a, CD33, or IgG subclass-matched control Ab, and analyzed by FACS as described in Materials and Methods. Results displayed are at 20 hours without A23187 treatment (UNTREATED), 20 hours with A23187 treatment (20H TREATMENT), 48 hours with A23187 treatment (48H TREATMENT), and 96 hours with A23187 treatment (96H TREATMENT). Cultures not treated with A23187 showed an identical immunophenotypic profile at 20, 48, and 96 hours (not shown). Histograms display staining intensity of viable (PI-excluding) cells, comparing control Ab (light lines) to specific Ab (heavy lines).
HL-60 cells were cultured during a 96-hour period with or without 180 ng/mL A23187 and evaluated periodically by FACS analysis. In the absence of CI treatment during this culture period a subpopulation of untreated HL-60 cells constitutively expressed B7.2 and/or HLA-DR, but no expression of CD83, B7.1, ICAM-1, CD40, CD1a, or CD14 was observed (Fig 1 and not shown). In addition, untreated cells uniformly expressed both MHC Class-I molecules and the myeloid marker CD33 (see below).
After initiation of A23187 treatment HL-60 cells rapidly developed de novo expression of CD83, a cell surface protein typically expressed during DC activation.19 Although already detectable within 4 hours of A23187 treatment (not shown) CD83 expression became maximal within 20 hours, declined after 48 hours, and was essentially undetectable by 96 hours (Fig 1). This early and transient expression of CD83 was similar to that reported previously for ionophore-treated peripheral blood monocytes.18 Marked de novo expression of cell surface B7.1 and ICAM-1, and markedly upregulated expression of B7.2 also developed within 20 hours of A23187 treatment. Although nearly uniform high expression of ICAM-1 was attained, B7.1 and B7.2 expression remained somewhat more heterogeneous, with a minority of cells remaining negative for expression of these molecules (Fig 1). However, in contrast to CD83 expression, B7.1, B7.2, and ICAM-1 continued to be maximally expressed on a majority of cells throughout the culture period (Fig 1).
Expression of CD40 and CD1a did not become prominent until 48 to 96 hours of CI treatment (Fig 1). Although A23187 treatment alone was insufficient to induce uniform upregulation of CD40, the proportion of cells expressing CD40 increased in magnitude after 24 hours and maintained this expression through the culture period (Fig 1). The de novo appearance of CD1a expression on a subset of cells occurred even later, being detectable only after 72 to 96 hours of A23187 treatment (Fig 1).
In contrast to the above modulations, levels of the constitutively expressed myeloid marker CD33 were not increased by A23187 treatment, but instead were slightly attenuated at 48 and 96 hours (Fig 1). In contrast to CI’s effect on normal monocytes and immature DC, A23187 treatment alone did not enhance HL-60 HLA-DR expression, and paradoxically downregulated HLA-ABC expression (see below).
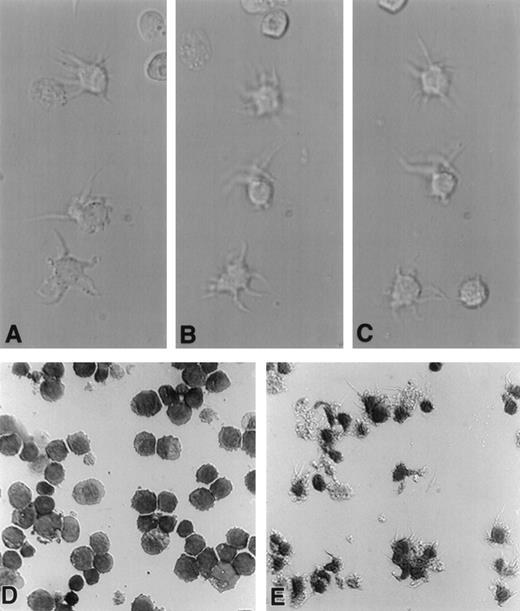
In synchronous morphologic studies, treatment of HL-60 cells for 24 hours with CI A23187 (180 ng/mL) caused many cells to develop motile dendritic processes when transferred to polylysine-coated glass slides (Fig 2A-C). Continued incubation with A23187 for 72 to 96 hours resulted in expression of dendritic processes on nearly all treated cells (Fig 2E), which were absent on untreated cells (Fig 2D).
A23187-treated HL-60 cells display progressive acquisition of dendritic processes. HL-60 cells (1 × 106) were cultured in a final volume of 2 mL with or without an optimal dose of A23187 (180 ng/mL) for up to 96 hours. Cells were harvested at 24 or 96 hours, resuspended in fresh medium, transferred to polylysine-coated glass chamber slides, and incubated an additional 20 minutes at 37°C. Cells were either immediately examined microscopically (600×) and photographed using DIC (Nomarski) optics (A, B, and C), or were fixed in 100% ethanol, stained in Wright’s solution, and then photographed (D and E). Photomicrographs A, B, and C were taken at consecutive 30-second intervals and depict living HL-60 cells treated with A23187 for 20 hours. Note motility of dendritic processes. Photomicrographs D and E depict fixed, stained, HL-60 cells previously left untreated (D) or treated with ionophore for 96 hours (E).
A23187-treated HL-60 cells display progressive acquisition of dendritic processes. HL-60 cells (1 × 106) were cultured in a final volume of 2 mL with or without an optimal dose of A23187 (180 ng/mL) for up to 96 hours. Cells were harvested at 24 or 96 hours, resuspended in fresh medium, transferred to polylysine-coated glass chamber slides, and incubated an additional 20 minutes at 37°C. Cells were either immediately examined microscopically (600×) and photographed using DIC (Nomarski) optics (A, B, and C), or were fixed in 100% ethanol, stained in Wright’s solution, and then photographed (D and E). Photomicrographs A, B, and C were taken at consecutive 30-second intervals and depict living HL-60 cells treated with A23187 for 20 hours. Note motility of dendritic processes. Photomicrographs D and E depict fixed, stained, HL-60 cells previously left untreated (D) or treated with ionophore for 96 hours (E).
Effect of cytokine treatments on HL-60 cells alone or in combination with CI.
Because several cytokines exert marked differentiating effects on human monocytes and DC,18,19,26-31 we investigated whether such cytokines could modulate the differentiating effect of CI on HL-60 cells. Granulocyte-macrophage colony-stimulating factor (rhGM-CSF) (20 ng/mL), rhIL-4 (1000 U/mL), and rhTNF-α (100 U/mL) were found to have no detectable effect on studied HL-60 cell surface markers during 7 days of observation when added singly or together in the absence of CI (data not shown). In contrast, single-agent rhIFN-γ (1000 U/mL), as previously reported by others,32 induced a majority of HL-60 cells to express HLA-DR at 72 hours (Fig 3), with such upregulation already apparent in some cells after 24 hours of treatment (not shown). Nonetheless, the upregulation of HLA-DR by rhIFN-γ was not completely uniform, and rhIFN-γ caused negligible modulations of the other studied surface molecules (Fig 3).
Effects of rhIFN-γ and rhGM-CSF on A23187-induced HL-60 surface Ag expression. HL-60 cells (5 × 105) were cultured in a final volume of 2 mL with or without an optimal dose (180 ng/mL) of CI A23187, rhIFN-γ (1000 U/mL), or rhGM-CSF (20 ng/mL). Cells were harvested 72 hours later, stained with PE-conjugated mouse antihuman B7.2, HLA-DR, HLA-ABC, CD40, CD1a, CD83, or IgG subclass-matched control Ab, and analyzed by FACS as described in Materials and Methods. Cell viabilities (percent) at time of analysis (72 hours of treatment) are indicated below histograms for each treatment group. Histograms display staining intensity of viable (PI-excluding) cells, comparing control Ab (light lines) to specific Ab (heavy lines).
Effects of rhIFN-γ and rhGM-CSF on A23187-induced HL-60 surface Ag expression. HL-60 cells (5 × 105) were cultured in a final volume of 2 mL with or without an optimal dose (180 ng/mL) of CI A23187, rhIFN-γ (1000 U/mL), or rhGM-CSF (20 ng/mL). Cells were harvested 72 hours later, stained with PE-conjugated mouse antihuman B7.2, HLA-DR, HLA-ABC, CD40, CD1a, CD83, or IgG subclass-matched control Ab, and analyzed by FACS as described in Materials and Methods. Cell viabilities (percent) at time of analysis (72 hours of treatment) are indicated below histograms for each treatment group. Histograms display staining intensity of viable (PI-excluding) cells, comparing control Ab (light lines) to specific Ab (heavy lines).
More striking modulatory effects were observed, however, when either rhIFN-γ or rhGM-CSF was added to HL-60 cultures in conjunction with CI treatment. Adjunct rhGM-CSF prevented the appearance of a subpopulation of cells negative for B7.2 expression that became apparent in CI-treated cells by 72 hours (Fig 3), and had a similar effect on B7.1 expression (not shown). Although CI alone had no effect on HLA-DR expression, CI plus rhIFN-γ modestly enhanced upregulation of HLA-DR compared with rhIFN-γ treatment alone (Fig 3). Although rhGM-CSF and rhIFN-γ each modestly enhanced CI-induced CD40 expression, CD1a expression was enhanced only by rhGM-CSF, with rhIFN-γ displaying a dominant suppressive activity for the expression of this surface molecule (Fig 3). Cytokines in conjunction with CI displayed no profound effects on the transient expression of CD83.
As noted above, an unanticipated finding was that treatment of HL-60 cells with CI alone induced a drop in MHC Class-I expression that approached background levels by 72 to 96 hours (Fig 3). Whereas rhGM-CSF did not prevent CI-induced downregulation of MHC Class I, the addition of rhIFN-γ–restored MHC Class-I expression in CI-treated cells almost to untreated control levels (Fig 3).
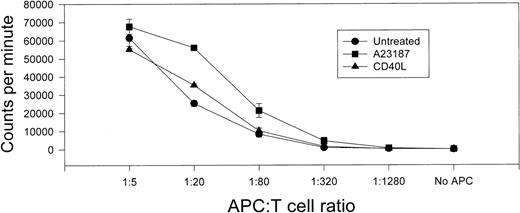
To determine whether CI or CI plus adjunct cytokines enhanced APC function in HL-60 cells, treated or untreated cells were irradiated and cocultured with MHC-unmatched T lymphocytes in an allosensitization assay. Neither untreated HL-60 cells nor HL-60 cells treated with rhGM-CSF alone proved effective for allosensitizing T cells (Fig 4). As previously reported by others,32 treatment with rhIFN-γ alone (or rhIFN-γ plus rhGM-CSF) enhanced HL-60’s allosensitizing capacity (Fig 4), but only two- to threefold. In contrast, marked enhancement of T-cell allosensitization capacity was observed when HL-60 cells were treated with CI alone (more than 25-fold increased T-cell [3H]-TdR uptake v untreated group at a 5:1 T-cell:APC ratio). Still further augmentation of allosensitization capacity was observed when HL-60 cells were treated both with CI and adjunct cytokines. Combination treatment with CI, rhGM-CSF, and rhIFN-γ was most effective (74-fold [3H]-TdR uptakev untreated group at a 5:1 T-cell:APC ratio) followed by CI plus rhIFN-γ (50-fold) and CI plus rhGM-CSF (39-fold).
Treatment with CI A23187 or A23187 with adjunct cytokines enhances T-cell allosensitizing capacity of HL-60 cells. HL-60 cells (5 × 105/well) were cultured in 24-well plates in 2 mL CM (Untreated) or CM supplemented with rhGM-CSF (20 ng/mL, GM-CSF), rhIFN-γ (1000 U/mL, IFN-γ) or combined rhGM-CSF plus rhIFN-γ, each of these conditions with or without CI A23187 (180 ng/mL). HL-60 cells were harvested 72 hours later, washed three times in CM, γ-irradiated (30 Gy), and cocultured at various HL-60:T-cell ratios with freshly prepared allogeneic human T lymphocytes (1 × 105/well) in triplicate 96-well tissue culture plate wells in 200 μL final volume CM. Cells were maintained in culture for 96 hours, pulsed with 1 μCi [3H]-TdR, harvested 18 hours later, and [3H]-TdR incorporation assessed by liquid scintillation spectrometry. Ordinate displays [3H]-TdR incorporation as thousands of CPM per well during the final 18-hour culture period; abscissa displays ratio of APC:T cells. Not shown: irradiated HL-60 cells alone, regardless of treatment, generated < 750 cpm when seeded at 20,000 cells per well; T cells alone generated < 250 cpm. Bars indicate SEM from triplicate wells.
Treatment with CI A23187 or A23187 with adjunct cytokines enhances T-cell allosensitizing capacity of HL-60 cells. HL-60 cells (5 × 105/well) were cultured in 24-well plates in 2 mL CM (Untreated) or CM supplemented with rhGM-CSF (20 ng/mL, GM-CSF), rhIFN-γ (1000 U/mL, IFN-γ) or combined rhGM-CSF plus rhIFN-γ, each of these conditions with or without CI A23187 (180 ng/mL). HL-60 cells were harvested 72 hours later, washed three times in CM, γ-irradiated (30 Gy), and cocultured at various HL-60:T-cell ratios with freshly prepared allogeneic human T lymphocytes (1 × 105/well) in triplicate 96-well tissue culture plate wells in 200 μL final volume CM. Cells were maintained in culture for 96 hours, pulsed with 1 μCi [3H]-TdR, harvested 18 hours later, and [3H]-TdR incorporation assessed by liquid scintillation spectrometry. Ordinate displays [3H]-TdR incorporation as thousands of CPM per well during the final 18-hour culture period; abscissa displays ratio of APC:T cells. Not shown: irradiated HL-60 cells alone, regardless of treatment, generated < 750 cpm when seeded at 20,000 cells per well; T cells alone generated < 250 cpm. Bars indicate SEM from triplicate wells.
Similar results were obtained in four separate experiments, and were comparable whether or not CD45R0pos (ie, non-naive) T cells were depleted before culture (data not shown). The maximal T-cell alloproliferation induced by treated HL-60 cells, however, always remained lower than that stimulated by normal peripheral blood DC.18
CI treatment rapidly induces many DC characteristics in cultured CD34pos cells that are not identical to the effects of rhCD40L treatment.
Highly enriched CD34pos cells obtained from four normal bone marrow donors were initially cultured for 6 days in rh-c-kit ligand, rhGM-CSF, and rhTNF-α to promote enrichment and expansion of DC precursors. Cells were then centrifuged and suspended in identical fresh medium except for the absence of rh-c-kit ligand. At this time, individual replated groups were also treated with varying doses of CI, and each group was harvested for analysis 20 or 40 hours after replating. We also studied in two of these experiments the effects of rhCD40L, a ligand that has been reported to have differentiating effects on CD34pos myeloid progenitors as well as on incompletely differentiated peripheral DC.13 14
At the time of initial replating, the day six cultured CD34pos cells were always highly heterogeneous in their surface protein expression, with a majority of cells expressing modest amounts of B7.2, a minority expressing B7.1 or CD83, and only a relatively small fraction manifesting DC-like morphology (not shown); this heterogeneous and predominantly nonactivated profile persisted without alteration when these cells were replated for 20 or 40 hours in medium containing only rhGM-CSF and rhTNF-α (Figs 5 and 6). However, including either rhCD40L (2.5 to 40 μg/mL) or CI (180 to 750 ng/mL) in culture caused the great majority of cells to acquire dendritiform morphologic characteristics within 20 hours of replating (Fig 5). Despite this profound morphologic effect of rhCD40L, the latter caused negligible immunophenotypic activation of the replated cells during this culture period at each of the tested concentrations (Fig 6). In contrast, similar to previous observations in peripheral blood monocytes, CI treatment of day 6 cultured CD34poscells resulted in a dose-dependent marked and rapid upregulation of B7.1, B7.2, and CD83 and a marked downregulation of CD14 in the CD14pos subpopulation. In addition, modest and/or more variable upregulation of ICAM-1, HLA-DR, CD1a, CD40, and HLA-ABC were observed (Figs 6 and 7 and not shown). CI’s effect was essentially complete in three of four donors within 20 hours of replating, but required an additional 20 hours to generalize in the fourth donor. The allosensitizing capacity of CD34pos cultured cells was modestly enhanced by such CI treatment, though even without CI treatment, cells displayed considerable allostimulatory potential at this time in culture (Fig 8), consistent with the marked phenotypic activation observed in a significant subpopulation of the cells (Fig 6). Prolonging CI treatment for an additional 5 days resulted in sustainedly high CD80 and CD86 expression and low CD14 expression, but as similarly observed in monocytes and HL-60, a decline in CD83 expression (not shown).
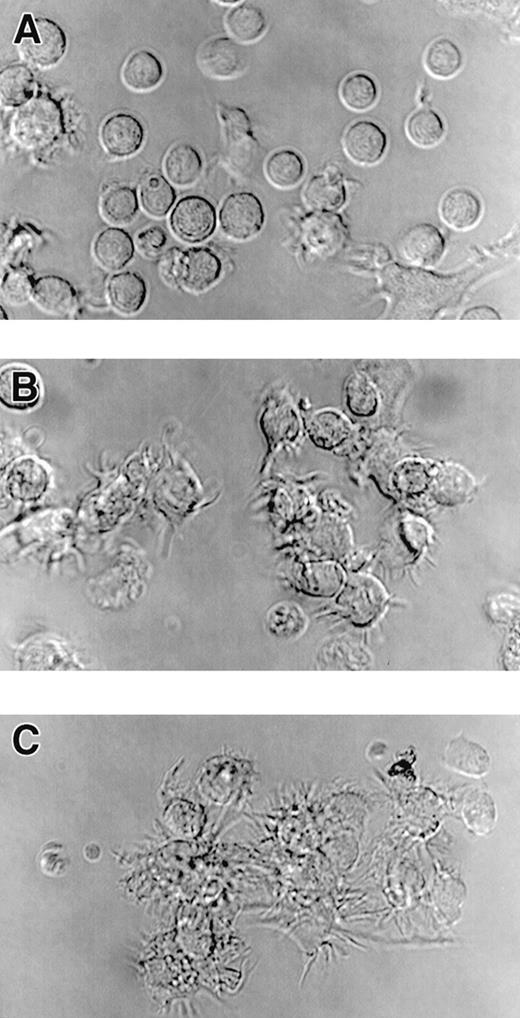
Cultured CD34pos bone marrow cells acquire the capacity to form dendritic processes in response to treatment with A23187 or rhCD40L. Cultures of CD34pos bone marrow cells initially expanded for 6 days in the presence of rh-c-kit ligand, rhGM-CSF, and rhTNF- to induce numerical expansion. Cells were then centrifuged and replated in identical fresh medium except for the absence of rh-c-kit ligand, with individual wells also receiving CI A23187 or rhCD40L. Twenty hours after replating, cells were harvested and centrifuged, resuspended in fresh medium and transferrred onto polylysine-coated glass chamber slides, incubated for an additional 20 minutes at 37oC, and photomicrographed (600×) using DIC (Nomarski) optics. Photomicrographs are representative of multiple CI and CD40L doses tested in two separate experiments: untreated cells (A); cells treated for 20 hours with 375 ng/mL CI A23187 (B); cells treated for 20 hours with 10 μg/mL rhCD40L (C). Note dendritic processes exhibited on cells in panels B and C that are largely absent in A. Similar results were observed after CI treatment at 180 ng or 750 ng/mL or rhCD40L at 2.5 or 40 μg/mL (not shown).
Cultured CD34pos bone marrow cells acquire the capacity to form dendritic processes in response to treatment with A23187 or rhCD40L. Cultures of CD34pos bone marrow cells initially expanded for 6 days in the presence of rh-c-kit ligand, rhGM-CSF, and rhTNF- to induce numerical expansion. Cells were then centrifuged and replated in identical fresh medium except for the absence of rh-c-kit ligand, with individual wells also receiving CI A23187 or rhCD40L. Twenty hours after replating, cells were harvested and centrifuged, resuspended in fresh medium and transferrred onto polylysine-coated glass chamber slides, incubated for an additional 20 minutes at 37oC, and photomicrographed (600×) using DIC (Nomarski) optics. Photomicrographs are representative of multiple CI and CD40L doses tested in two separate experiments: untreated cells (A); cells treated for 20 hours with 375 ng/mL CI A23187 (B); cells treated for 20 hours with 10 μg/mL rhCD40L (C). Note dendritic processes exhibited on cells in panels B and C that are largely absent in A. Similar results were observed after CI treatment at 180 ng or 750 ng/mL or rhCD40L at 2.5 or 40 μg/mL (not shown).
Alterations in immunophenotype of cultured human CD34pos bone marrow cells and peripheral blood monocytes in the presence of CI A23187 or rhCD40L. Cultures of CD34posbone marrow cells previously expanded for 6 days in the presence of cytokines (see Fig 5) were replated and cultured for an additional 20 hours in the same medium without rh-c-kit ligand with various doses of CI A23187 or rhCD40L added. In synchronous experiments, elutriated monocytes were washed and plated overnight as described in Materials and Methods, then cultured for an additional 20 hours with no additive or CI A23187 or rhCD40L. After the 20-hour treatment bone marrow progenitors and monocytes were harvested and stained with PE-conjugated antibodies against human B7.1, B7.2, and CD83 and analyzed by FACS (see Materials and Methods). Representative displayed data depict untreated cells, cells treated with an optimal dose of CI (375 ng/mL for CD34pos cells and 225 ng/mL for monocytes), or cells treated with 10 μg/mL rhCD40L. Similar results were observed in either cultured CD34pos progenitors or peripheral blood monocytes treated with 2.5 or 40 μg/mL doses of rhCD40L (not shown).
Alterations in immunophenotype of cultured human CD34pos bone marrow cells and peripheral blood monocytes in the presence of CI A23187 or rhCD40L. Cultures of CD34posbone marrow cells previously expanded for 6 days in the presence of cytokines (see Fig 5) were replated and cultured for an additional 20 hours in the same medium without rh-c-kit ligand with various doses of CI A23187 or rhCD40L added. In synchronous experiments, elutriated monocytes were washed and plated overnight as described in Materials and Methods, then cultured for an additional 20 hours with no additive or CI A23187 or rhCD40L. After the 20-hour treatment bone marrow progenitors and monocytes were harvested and stained with PE-conjugated antibodies against human B7.1, B7.2, and CD83 and analyzed by FACS (see Materials and Methods). Representative displayed data depict untreated cells, cells treated with an optimal dose of CI (375 ng/mL for CD34pos cells and 225 ng/mL for monocytes), or cells treated with 10 μg/mL rhCD40L. Similar results were observed in either cultured CD34pos progenitors or peripheral blood monocytes treated with 2.5 or 40 μg/mL doses of rhCD40L (not shown).
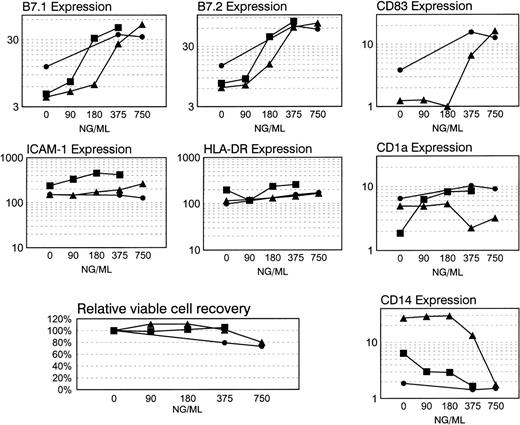
Dose-response analyses of immunophenotypic modulations of cultured CD34pos bone marrow cells during CI A23187 treatment. Six-day cultured CD34pos myeloid cells enriched from normal volunteer bone marrow were cultured in medium containing rhGM-CSF and rhTNF- with graded doses of CI A23187 (0 to 750 ng/mL). Cells were harvested and analyzed by flow cytometry with added propidium iodide (PI) as described in Fig 6 and Methods. Abscissa displays ng/mL of A23187 present during CI treatment. Two or three separate dose-response experiments were performed and plotted for each cell surface marker analyzed (B7.1, B7.2, CD83, ICAM-1, HLA-DR, CD1a, and CD14). Also portrayed is the viable cell recovery relative to control for three dose-response experiments (0 ng/mL group = 100%). Cell surface Ag expression data are portrayed as Specific Mean Fluorescence quotients (mean fluorescent intensity of cells stained with specific Ab/mean fluorescent intensity of cells stained with subclass-matched control Ab); a minimum of 5000 cells with background-level PI staining was assayed per determination.
Dose-response analyses of immunophenotypic modulations of cultured CD34pos bone marrow cells during CI A23187 treatment. Six-day cultured CD34pos myeloid cells enriched from normal volunteer bone marrow were cultured in medium containing rhGM-CSF and rhTNF- with graded doses of CI A23187 (0 to 750 ng/mL). Cells were harvested and analyzed by flow cytometry with added propidium iodide (PI) as described in Fig 6 and Methods. Abscissa displays ng/mL of A23187 present during CI treatment. Two or three separate dose-response experiments were performed and plotted for each cell surface marker analyzed (B7.1, B7.2, CD83, ICAM-1, HLA-DR, CD1a, and CD14). Also portrayed is the viable cell recovery relative to control for three dose-response experiments (0 ng/mL group = 100%). Cell surface Ag expression data are portrayed as Specific Mean Fluorescence quotients (mean fluorescent intensity of cells stained with specific Ab/mean fluorescent intensity of cells stained with subclass-matched control Ab); a minimum of 5000 cells with background-level PI staining was assayed per determination.
Treatment with CI A23187 enhances T-cell allosensitizing capacity of cultured CD34pos bone marrow cells. The latter were treated and harvested as in Fig 6, then cocultured at various APC:T lymphocyte ratios with fixed numbers of freshly prepared allogeneic human T lymphocytes as in Fig 4. Ordinate displays CPM/105 input T cells during the last 18 hours in culture. Groups are “Untreated” (continued culture in rhGM-CSF and rhTNF-), “CD40L” (10 μg/mL rhCD40L) and “A23187” (375 ng/mL). Abscissa displays ratio of APC: T cells. Not shown: CD34pos cells alone, regardless of treatment, generated < 250 cpm when seeded at 20,000 cells per well; T cells alone generated < 250 cpm. Bars indicate SEM from triplicate wells.
Treatment with CI A23187 enhances T-cell allosensitizing capacity of cultured CD34pos bone marrow cells. The latter were treated and harvested as in Fig 6, then cocultured at various APC:T lymphocyte ratios with fixed numbers of freshly prepared allogeneic human T lymphocytes as in Fig 4. Ordinate displays CPM/105 input T cells during the last 18 hours in culture. Groups are “Untreated” (continued culture in rhGM-CSF and rhTNF-), “CD40L” (10 μg/mL rhCD40L) and “A23187” (375 ng/mL). Abscissa displays ratio of APC: T cells. Not shown: CD34pos cells alone, regardless of treatment, generated < 250 cpm when seeded at 20,000 cells per well; T cells alone generated < 250 cpm. Bars indicate SEM from triplicate wells.
Despite the contrasting effects of CI and rhCD40L on CD80, CD86, and CD83 expression in cultured CD34pos bone marrow cells, both agents were capable of inducing upregulated expression of these molecules by peripheral blood monocytes within a 20-hour exposure period (Fig 6).
DISCUSSION
Our data are consistent with the hypothesis that agents known to mobilize intracellular calcium can affect myeloid cells at apparently differing stages of ontogeny and can serve as a decisive differentiation stimulus, leading to rapid acquisition of many characteristics often associated with mature DC. In certain cases such calcium mobilization appears to result in true DC differentiation,18 whereas in other cases (such as the established leukemic cell line HL-60) it induces a somewhat more circumscribed set of characteristics associated with mature DC. In either case, treatments with such calcium mobilizing agents may rapidly preempt alternate courses of differentiation that would have occurred in its absence. In addition to our previous demonstrations of CI’s effects in peripheral blood monocytes and immature DC,18the present report describes commensurate effects, including striking rapid upregulation of CD83 and B7 costimulatory molecule expression and acquisition of dendritic processes, when CD34pos bone marrow cells and the leukemic cell line HL-60 are treated with CI. We have recently found, in addition, that CI treatment of freshly obtained chronic myelogenous leukemia (CML) cells from 10 out of 10 patients induced many similar responses (Engels et al, manuscript submitted). Although membrane signal transduction pathways may not be operative at every stage of myeloid ontogeny to physiologically trigger sufficient calcium mobilization to achieve differentiation, the myeloid cell’s inherent potential for such differentiation is readily demonstrable with CI treatment. Although CI treatment appears to supply signals leading to the acquisition of DC characteristics by bypassing at least the initial requirements for receptor/ligand-mediated membrane signal transduction, it remains a formal possibility that CI acts indirectly, through induction of secretion of biologic agents by the treated myeloid cells. These agents (presumably cytokines or chemokines) could in turn act in an autocrine fashion, by binding to specific receptors on the cell surface, and thus supply such signals that induce DC differentiation/activation.
The cell line HL-60’s response to CI treatment is of considerable interest for several reasons. Originally isolated from a 36-year-old female patient with acute promyelocytic leukemia,33,34HL-60 has the demonstrated capacity to differentiate in vitro into cells expressing many characteristics of different myeloid-lineage cell types, including granulocytes in the presence of dimethyl sulfoxide (DMSO) or retinoic acid,35,36 monocytes in the presence of 1,25 dihydroxy vitamin D3 or rhIFN-γ,37-40macrophages in the presence of phorbol myristate acetate (PMA),41 or eosinophils in the presence of alkalinity.42 Because of the extraordinary multipotency of HL-60 cells compared with other culture-adapted human myelogenous leukemia lines, it appeared possible that appropriate signals delivered to these cells would induce differentiation along a myeloid DC pathway. However, previous studies that have attempted to use cytokines alone to promote DC differentiation in HL-60 have not been successful,2 even though such strategies successfully promoted DC development in CML and AML cells from individual patients.2,10 11 Our laboratory also has been unable to induce differentiation in HL-60 with such cytokines (see above) or with rhCD40L (data not shown). Nonetheless, calcium mobilization treatment of HL-60 results in DC-like differentiation, showing the cell line’s inherent potential for such differentiation even when it cannot be induced with putative biologic agents.
The phenotypic alterations induced by CI in HL-60 cells in many ways paralleled previously shown effects in human peripheral blood monocytes and immature DC,18 including rapid upregulation of B7.1, B7.2, ICAM-1, and early peaking expression of CD83. Like peripheral blood monocytes, HL-60 cells treated with CI acquired the tendency to elaborate motile cellular processes that caused HL-60 cells to morphologically resemble myeloid DC; as also observed in monocytes this effect was maximal after 72 to 96 hours in culture. In addition, as in monocyte studies (reference 18 and manuscript in preparation), cytokines that had limited impact when added alone to HL-60 cultures had more substantial impact when included in the context of CI treatment.
Several characteristics of HL-60 cells and their response to CI treatment differed markedly, however, from normal (nontransformed) myeloid-lineage cells. HL-60 cells largely failed to express HLA-DR constitutively, and in contrast to normal peripheral blood monocytes or immature DC failed to upregulate HLA-DR expression after CI treatment unless IFN-γ was also present in the culture. CI also had the anomalous action of downregulating HL-60’s MHC Class-I expression, which was in contrast to the modest enhancement of MHC Class-I expression typically observed when normal blood monocytes or immature DC are treated with CI.18 Aberrant MHC Class-II expression is a frequently reported characteristic of acute promyelocytic leukemias such as HL-60.43-45 There also exist both physiologic and pathologic precedents for somatic cell downregulation of MHC Class I: for example, thyroid-stimulating hormone downregulates MHC Class-I expression in normal thyroid cells,46 and adenovirus transformation downregulates MHC Class-I expression in a variety of infectable somatic targets.47 Paralleling such precedents, HL-60’s MHC Class-I downregulation during CI treatment could largely be countered by rhIFN-γ treatment (Fig 3).
In contrast to HL-60, CI treatment of fresh CML cells from 10 patients has resulted in upregulation of both MHC Class-I and -II expression in addition to costimulatory molecule, CD40 and CD83 upregulation; in no case was paradoxical downregulation of MHC Class I induced by CI (Engels et al, manuscript submitted). It remains to be determined whether HL-60’s anomalous MHC Class-I and -II regulation will be observed among freshly obtained AML samples.
Similarly to peripheral blood myeloid cells and to HL-60 cells, 6-day cultured, CD34pos bone marrow cells displayed prompt responses to CI treatment, including rapid acquisition of CD83, B7.1, and B7.2 expression. In contrast to peripheral monocytes and HL-60, however, cultured CD34pos cells treated with CI displayed much more rapid acquisition of dendritic processes; these differences may reflect an artifact of the CD34pos cells’ sustained exposure to exogenous cytokines both before and after CI treatment. Because CI treatment promotes rapid and near-uniform DC-like differentiation of 6-day cultured CD34pos cells, it preempts the subsequent heterogenous differentiation, predominantly to neutrophils and monocytes, which is typically observed in such cells between days 6 and 12 of culture.20
Given that myeloid cells respond to calcium mobilization at various stages of ontogeny by acquiring elements of DC-like differentiation, it is of great interest to determine which factors and/or ligands present in normal bone marrow and peripheral tissues can induce such calcium mobilization and differentiation via physiologic signal transduction. Such calcium-mobilizing signals may vary during ontogeny with the myeloid cell’s expression (or lack) of receptors for individual agents, and may include CD40L and/or IL-4, because other investigators have reported that exposure of B lymphocytes to these agents can result in significant calcium mobilization, and, at least in the case of CD40L, apparent calcineurin-mediated cell activation.48,49Because many of CI’s activating effects on myeloid cells can be blocked by calmodulin and calcineurin antagonists,50 we are presently assessing the role of these enzymes in the capacity of CD40L, IL-4, and other agents to induce myeloid cell differentiation.
However, it is apparent that most IL-4, GM-CSF, and/or TNF-α–based myeloid differentiating regimens require many more days than CI treatment (typically 1 week or longer) to induce uniform DC-like activation, as evidenced by the late appearance of upregulated costimulatory molecule expression, CD83 expression, and/or enhanced T-cell sensitizing efficiency during such treatments.2,10,11,19,29,30,51-57 It is possible that such cytokine treatments may not operate through a calcium-dependent differentiation pathway; alternatively, their activation of the calcium-dependent pathway may be indirect and less efficient than CI treatment, due to complex signal transduction issues. This is well illustrated by CD40L. Although previously published reports indicate that prolonged (6 day) CD40L exposure can induce immunophenotypic activation of cultured CD34pos myeloid cells,14it is apparent from the present studies that shorter (20 hour) treatments with rhCD40L or CI do not have the same effects on cultured CD34pos cells: rhCD40L induces profound morphologic effects without immunophenotypic activation within the tested 20-hour window of exposure (Figs 5 and 6), whereas CI induces both effects simultaneously (Figs 5, 6, and 7). In contrast, 20-hour treatment of peripheral blood monocytes with either CI or rhCD40L can promote equivalent immunophenotypic activation (Fig 6). We are investigating the possibility that CD40L signal transduction displays variable coupling to calcium-dependent signaling pathways during myeloid maturation.
In these studies, we showed that the HL-60 myeloid leukemia line had the capacity to respond to CI in ways similar to those that we have observed in normal myeloid cells,18 resulting in the development of a mature, activated APC phenotype that in many regards resembled activated myeloid DC. Anomalies in its response to CI compared with normal myeloid cells could be normalized with rhIFN-γ treatment, and cytokines (rhIFN-γ and rhGM-CSF) that independently had modest effects on HL-60’s antigen-presenting efficiency had more potent effects in the context of concomitant CI treatment. These data suggest that CI treatment can serve as an alternative means to enhance the immunogenicity of myeloid leukemias in vaccine strategies in which leukemias are unresponsive to cytokine treatment alone, but in which an endogenous calcium-dependent activation pathway is pharmacologically inducible. We are currently analyzing the effects of CI and adjunct cytokine treatments on leukemic cells isolated freshly from patients with various classes of myelogenous leukemia. Efforts are directed to using CI-treated fresh leukemia cells to efficiently sensitize autologous T cells to leukemia-associated antigens.
ACKNOWLEDGMENT
The authors are indebted to Drs Nirbhay Kumar, Alan Scott, and Frances Hakim for helpful suggestions and encouragement, as well as to Charles Carter and Drs Susan Leitman, E.J. Read, and Harvey Kleinman of the NIH Department of Transfusion Medicine. We also wish to thank Dr Kathy Picha at Immunex for her helpful support and discussions in regards to rhCD40L, as well as Cathy Bare for excellent technical support.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests and correspondence to Peter A. Cohen, MD, Center for Surgery Research, FF-50, Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland, OH 44195; email: cohenp@ccf.org.


![Fig. 4. Treatment with CI A23187 or A23187 with adjunct cytokines enhances T-cell allosensitizing capacity of HL-60 cells. HL-60 cells (5 × 105/well) were cultured in 24-well plates in 2 mL CM (Untreated) or CM supplemented with rhGM-CSF (20 ng/mL, GM-CSF), rhIFN-γ (1000 U/mL, IFN-γ) or combined rhGM-CSF plus rhIFN-γ, each of these conditions with or without CI A23187 (180 ng/mL). HL-60 cells were harvested 72 hours later, washed three times in CM, γ-irradiated (30 Gy), and cocultured at various HL-60:T-cell ratios with freshly prepared allogeneic human T lymphocytes (1 × 105/well) in triplicate 96-well tissue culture plate wells in 200 μL final volume CM. Cells were maintained in culture for 96 hours, pulsed with 1 μCi [3H]-TdR, harvested 18 hours later, and [3H]-TdR incorporation assessed by liquid scintillation spectrometry. Ordinate displays [3H]-TdR incorporation as thousands of CPM per well during the final 18-hour culture period; abscissa displays ratio of APC:T cells. Not shown: irradiated HL-60 cells alone, regardless of treatment, generated < 750 cpm when seeded at 20,000 cells per well; T cells alone generated < 250 cpm. Bars indicate SEM from triplicate wells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/4/10.1182_blood.v94.4.1359/4/m_blod41625004x.jpeg?Expires=1765892229&Signature=N2AinyMdqyCD-c4Tb7p-SLyxn72jNrfbFqQJCcoy8Paftda4qFwqdc64aurtvR9vEN6Tan62vXbwXOhs~RW6wM88ia-4kM-r9s3YSmeeG3OFVvQxnaqKqSvzh8fSqqibk68d9gofdUqahY2WfCSfrE~-gtRIyR77w5Bi~iQAwGqrvCW0Jq0Lenbj-QKgb7PU5MFZ8dJagkpt-Q2f49sMHS6lJE-xbJijH5o83HqfioMpMtKarkbKet9l2ysu8nNRk5m5TXducSWF0G8GnPy75BXLS0MoWF~w8Zehe2NIZht-haVYLn~Jhlj1SamYDHchiX-BKNbPK0TMO~xoJE2WLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal