Abstract
A clinical relationship between dose-intensity of melphalan and response rate has been demonstrated in multiple myeloma. Promising results have been reported after 200 mg/m2 melphalan, especially in younger patients. It is uncertain whether 100 mg/m2 melphalan (MEL100) can offer similar results in older patients. To address this issue, patients were treated with 2 or 3 MEL100 courses followed by stem cell support. Seventy-one patients (median age, 64 years) entered the protocol at diagnosis. Their clinical outcome was compared with that of 71 pair mates (median age, 64 years) selected from patients treated at diagnosis with oral melphalan and prednisone (MP) and matched for age and β2-microglobulin. Complete remission was 47% after MEL100 and 5% after MP. Median event-free survival was 34 months in the MEL100 group and 17.7 months in the MP group (P < .001). Median overall survival was 56+ months for MEL100 and 48 months for MP (P< .01). In a multivariate analysis, β2-microglobulin levels and MEL100 were independent risk factors associated with outcome: superior event-free and overall survival were observed in patients presenting low β2-microglobulin levels at diagnosis and receiving MEL100 as induction regimen. In conclusion, MEL100 was superior to MP in terms of complete remission rate, event-free survival, and overall survival.
THE CLINICAL IMPACT of dose-intensive chemotherapy has been evaluated in several hematologic tumors. Dose escalation is now a standard approach in acute leukemia, aggressive lymphoma, and multiple myeloma.1 For the past 30 years, oral melphalan and prednisone (MP) has been the treatment of choice for myeloma.2-4 Several randomized trials comparing MP versus other drug combinations have not shown any major improvement in clinical outcome.2,5-9 Autologous transplantation (AT) was superior to conventional chemotherapy and improved response rate, event-free survival, and overall survival.10-12 Further improvement has been obtained by the use of peripheral blood progenitor cells, harvested after chemotherapy and growth factor priming to allow the delivery of high-dose melphalan with rapid hematopoietic recovery and low mortality.13-18
All major clinical trials have reported a median age for transplanted patients ranging from 49 to 52 years (57 years in the French trial).12,19-22 In the largest series, 496 patients were enrolled in clinical trials to receive 2 AT within 6 months: 54% were older than 50 years of age and 73% (363 subjects) received the second transplant.19 In the French randomized trial, only 74 of the 100 patients enrolled in the AT arm received the transplant.12 These exclusions were closely related to age: 18% less than 60 years of age did not receive the AT, compared with 42% of those more than 60 years of age.12 Older patients constitute more than 50% of the total.23 Thus, the development of new dose-intensive chemotherapies with lower toxicity is essential.
A simplified procedure in which melphalan dose is reduced from 200 to 100 mg/m2 (MEL100), repeated every 2 months, has been designed. In this report, we evaluate the results of this approach in elderly myeloma patients.
MATERIALS and METHODS
Patients
Seventy-one patients at diagnosis entered the protocol between November 1993 and November 1997. They received 4 g/m2cyclophosphamide (CY) followed by 100 mg/m2 melphalan every 2 months for up to 3 courses. The Southwest Oncology Group (SWOG) diagnostic criteria24 and Durie and Salmon staging system were used.25 Patients 55 to 75 years of age were eligible. Inclusion criteria included normal cardiac, renal, pulmonary, and hepatic function on the basis of routine clinical and laboratory examinations, echocardiography, and lung-function tests. Patients were excluded if a positive serological test for hepatitis B virus (HBV), hepatitis C virus (HCV), or human immunodeficiency virus (HIV) was detected. The institutional review board approved the protocol and written informed consent was obtained from all patients.
To compare the clinical outcome of MEL100, 71 patients were selected among 161 untreated patients with symptomatic myeloma registered at our institution between February 1990 and June 1995 and were treated at diagnosis with oral MP. They met the same eligibility criteria as for the MEL100 regimen. They were pairs matched for age (within 1 year,P = .9) and β2-microglobulin levels (within 1 mg/L,P = 1.0). Patient characteristics are listed in Table 1.
Patient Characteristics
| . | MEL100 . | MP . | . |
|---|---|---|---|
| No. of patients | 71 | 71 | |
| Age | |||
| <60 yrs | 18 | 18 | |
| >60 yrs | 53 | 53 | |
| β2-microglobulin | Matching criteria | ||
| <3 mg/L | 19 | 19 | |
| >3 mg/L | 52 | 52 | |
| % of patients | |||
| Stage at diagnosis | |||
| II | 25 | 28 | |
| III | 75 | 72 | |
| Isotype | |||
| IgG | 58 | 68 | |
| IgA | 21 | 22 | |
| Bence Jones protein | 21 | 10 | |
| Creatinine >2 mg/dL | 6 | 11 | |
| Hemoglobin <10 g/dL | 40 | 47 | |
| Labeling index >1.2% | 28 | 30 | |
| Bone marrow plasmocytosis >30% | 62 | 64 | |
| Performance status >3 | 54 | 56 |
| . | MEL100 . | MP . | . |
|---|---|---|---|
| No. of patients | 71 | 71 | |
| Age | |||
| <60 yrs | 18 | 18 | |
| >60 yrs | 53 | 53 | |
| β2-microglobulin | Matching criteria | ||
| <3 mg/L | 19 | 19 | |
| >3 mg/L | 52 | 52 | |
| % of patients | |||
| Stage at diagnosis | |||
| II | 25 | 28 | |
| III | 75 | 72 | |
| Isotype | |||
| IgG | 58 | 68 | |
| IgA | 21 | 22 | |
| Bence Jones protein | 21 | 10 | |
| Creatinine >2 mg/dL | 6 | 11 | |
| Hemoglobin <10 g/dL | 40 | 47 | |
| Labeling index >1.2% | 28 | 30 | |
| Bone marrow plasmocytosis >30% | 62 | 64 | |
| Performance status >3 | 54 | 56 |
Treatment Regimens
MEL100 regimen.
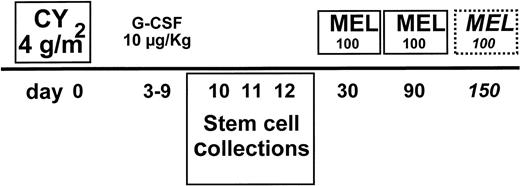
All patients received 2 or 3 DAV courses as debulking (dexamethasone-doxorubicin [adriamycin]-vincristine; 50 mg/m2 adriamycin on day 1, 1 mg vincristine on day 1, and 40 mg dexamethasone on days 1, 2, 3, and 4, with each course repeated every 28 days). CY at 4 g/m2 was administered at day 0 in 2 doses, with subsequent 4 g/m2 2-mercaptoethanesulphonic acid (MESNA) in 5 divided doses. Granulocyte colony-stimulating factor (G-CSF) was administered at 10 μg/kg from day 3 to the last day of leukapheresis initiated upon recovery of leukocytes to 2 × 109/L (Fig 1). All stem cell harvests were collected before the first MEL100. The percentage of circulating CD34 cells was evaluated as previously described.26 Three harvest procedures were performed. A Fresenius Cell Separator AS 104 (MTS, Schweinfurt, Germany) was used. At day 30, MEL100 was infused in 30 minutes. At day 31, stem cells were reinfused. G-CSF was administered at 5 μg/kg from day 33 until the neutrophil count was greater than 500/μL in 2 consecutive tests. MEL100 was repeated every 2 months for a total of 2 courses in patients reaching complete remission (CR) and 3 courses in those reaching partial remission (PR) after the second course.
MEL100 regimen: treatment plan. CY at 4 g/m2and 10 μg/kg G-CSF were used to mobilize stem cells collected and cryopreserved at days 10 to 12. MEL100 was infused at day 30 and repeated at day 90. The third course was only delivered to patients in partial remission at day 150.
MEL100 regimen: treatment plan. CY at 4 g/m2and 10 μg/kg G-CSF were used to mobilize stem cells collected and cryopreserved at days 10 to 12. MEL100 was infused at day 30 and repeated at day 90. The third course was only delivered to patients in partial remission at day 150.
Oral MP regimen.
All patients received six 7-day courses of 6 mg/m2melphalan and 60 mg/m2 prednisone at 4-week intervals.
Supportive Care
All patients receiving MEL100 were discharged 7 days after chemotherapy infusion. Blood cell counts were performed every other day and weekly clinical appointments were scheduled. Patients received standard support care measures. Careful oral hygiene and oral nystatin suspension were suggested. Oral cyprofloxacin or cotrimoxazole was prescribed as antimicrobial prophylaxis. Acyclovir prophylaxis was instituted in the event of previous herpes infection. Patients who developed neutropenic pyrexia greater than 38°C received ceftriaxone at home. Those whose fever lasted longer than 24 to 48 hours after ceftriaxone were admitted for intravenous broad-spectrum antimicrobial therapy. Blood product support was used when the hemoglobin concentration decreased to less than 8 g/dL or when the platelet count decreased to less than 15,000/μL.
Response Criteria and Statistics
PR was defined as 50% reduction of serum myeloma protein, 90% decrease of Bence Jones proteinuria, and 50% reduction of bone marrow infiltration. CR required disappearance of serum or urine myeloma protein analyzed by standard electrophoresis and marrow plasmacytosis less than 1% for at least 2 months. All other results were regarded as failures. Early death included any death within 100 days for both MEL100 and MP. Statistical methods included χ2 tests for comparison of rates27 and Kaplan Meier estimates.28 Event-free and overall survival curves were plotted from the beginning of treatment for all regimens. Event-free and overall survival among categorical prognostic variables was compared using the log-rank test.29 A multivariate analysis was performed using a Cox regression model to measure the effects of several variables on outcome.30
RESULTS
MEL100
On an intent to treat basis, 89% of patients completed the entire program. Seventy-one received the first MEL100, 68 reached the second, and 63 were eligible for the third. Twenty-four reached CR after the second MEL100. The third was administered to 39 patients only. All patients completed the second and third MEL100 within a maximum of 3 months from the previous course. The median time between the first and second MEL100 was 2.3 months and that between the second and third was 2.2 months. On an intent to treat basis, the frequencies of PR (CR) were 36% (2%) after DAV and increased to 43% (3%) after CY, 77% (19%) after the first MEL100, 86% (34%) after the second, and 88% (47%) after the third. For patients attaining PR after DAV, the incidence of CR after MEL100 was 72% and for those attaining PR after CY it was 63%.
No toxic death occurred. After a median follow-up of 30 months for survivors, 55% were alive in remission, 13% had died after relapse due to progression (11%) or infections (2%), 17% were alive after relapse, 4% were alive with progressive disease, and 11% were alive but registered as failures due to adverse events. Among the 11% failures (8/71), 6 patients were in remission and 2 had not responded and were alive after salvage therapy; 3 did not complete the second MEL100 (1 gastrointestinal toxicity, 1 secondary neoplasia, and 1 inadequate collection of CD34+ cells) and 5 did not complete the third (1 gastrointestinal toxicity, 1 heart failure, and 3 inadequate collection of CD34+ cells).
MP
Sixty-eight patients completed 3 courses of MP, and 66 received 6 courses. Three patients died after the second or the third MP course (1 sepsis, 1 heart failure, and 1 disease progression). The time interval between the first and the sixth course of MP ranged from 6 to 9 months (median, 6.7 months). After a median follow up of 39.4 months, 15% were alive in remission, 20% were alive after relapse or with progressive disease, and 65% had died.
MEL100 Versus MP
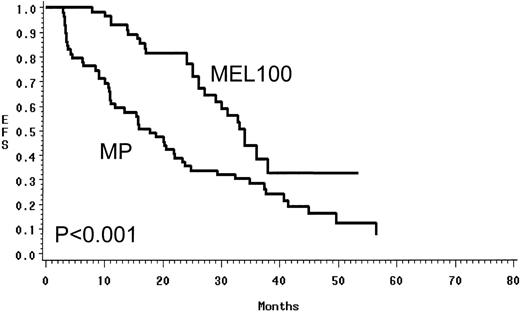
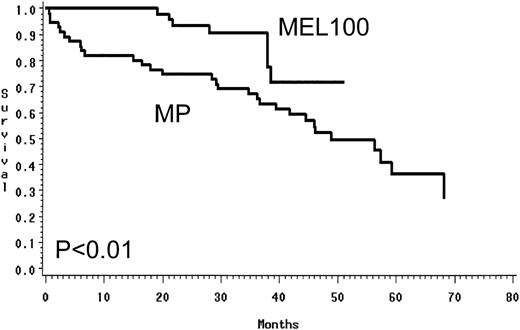
Using an intent to treat approach, MEL100 was superior to MP and resulted in a higher PR rate of 88% versus 49% (P < .01) and a CR rate of 47% versus 5% (P < .01; Table 2). In the MEL100 group, median event-free survival was 34 months, compared with 17.7 months in the MP group. MEL100 had a significantly longer event-free survival (P< .001) than the MP group (Fig 2). The median overall survival was not reached for patients receiving MEL100 (56+ months) and was 48 months for those receiving MP; MEL100 was superior to MP (P < .01; Fig 3). The probabilities of event-free survival (overall survival) at 4 years after diagnosis were 33% (71%) after MEL100 and 14% (52%) after MP.
Prognostic Factors
In univariate analysis of 9 pretreatment and posttreatment variables, 5 were significantly associated with event-free survival and 3 with overall survival (Table 3). In the multivariate analysis of risk factors affecting the outcome, the presence of β2-microglobulin levels less than 4 mg/L at diagnosis and the administration of MEL100 retained independent significance (Table 4). Low levels of β2-microglobulin at diagnosis and the administration of MEL100 as induction regimen significantly influenced event-free and overall survival. CR was significant in univariate analysis, but not when MEL100 therapy was included in the multivariate analysis.
Factors Influencing Outcome
| Parameter . | No. of Patients . | EFS (mo) . | P . | OS (mo) . | P . |
|---|---|---|---|---|---|
| β2-microglobulin (mg/L) | |||||
| <4.0 | 52 | 30 | .002 | 62 | .003 |
| >4.0 | 90 | 17 | 28 | ||
| Labeling index (%) | |||||
| <1.2 | 69 | 28 | .05 | 54 | .12 |
| >1.2 | 41 | 16 | 48 | ||
| Creatinine (mg/dL) | |||||
| <2.0 | 110 | 28 | .05 | 57 | .33 |
| >2.0 | 16 | 21 | 50 | ||
| Hemoglobin (g/dL) | |||||
| <10.0 | 53 | 26 | .43 | 58 | .84 |
| >10.0 | 75 | 30 | 54 | ||
| Age (yrs) | |||||
| <60 | 60 | 28 | .21 | 56 | .72 |
| >60 | 82 | 24 | 57 | ||
| Stage | |||||
| II | 43 | 24 | .63 | 56 | .45 |
| III | 99 | 27 | 51 | ||
| Isotype | |||||
| Non-Ig | 115 | 26 | .71 | 57 | .58 |
| IgA | 27 | 28 | 52 | ||
| Treatment | |||||
| MEL100 | 71 | 34 | .001 | 56+ | .01 |
| MP | 71 | 17, 7 | 48 | ||
| CR | |||||
| Yes | 38 | 40 | .01 | 56+ | .02 |
| No | 104 | 28 | 44 |
| Parameter . | No. of Patients . | EFS (mo) . | P . | OS (mo) . | P . |
|---|---|---|---|---|---|
| β2-microglobulin (mg/L) | |||||
| <4.0 | 52 | 30 | .002 | 62 | .003 |
| >4.0 | 90 | 17 | 28 | ||
| Labeling index (%) | |||||
| <1.2 | 69 | 28 | .05 | 54 | .12 |
| >1.2 | 41 | 16 | 48 | ||
| Creatinine (mg/dL) | |||||
| <2.0 | 110 | 28 | .05 | 57 | .33 |
| >2.0 | 16 | 21 | 50 | ||
| Hemoglobin (g/dL) | |||||
| <10.0 | 53 | 26 | .43 | 58 | .84 |
| >10.0 | 75 | 30 | 54 | ||
| Age (yrs) | |||||
| <60 | 60 | 28 | .21 | 56 | .72 |
| >60 | 82 | 24 | 57 | ||
| Stage | |||||
| II | 43 | 24 | .63 | 56 | .45 |
| III | 99 | 27 | 51 | ||
| Isotype | |||||
| Non-Ig | 115 | 26 | .71 | 57 | .58 |
| IgA | 27 | 28 | 52 | ||
| Treatment | |||||
| MEL100 | 71 | 34 | .001 | 56+ | .01 |
| MP | 71 | 17, 7 | 48 | ||
| CR | |||||
| Yes | 38 | 40 | .01 | 56+ | .02 |
| No | 104 | 28 | 44 |
Abbreviations: EFS, event-free survival; OS, overall survival.
Multivariate Analysis
| EFS . | RR . | P . | OS . | RR . | P . |
|---|---|---|---|---|---|
| β2-microglobulin <4 mg/L | .824 | .04 | β2-microglobulin <4 mg/L | .795 | .04 |
| Any MEL100 | .348 | .04 | Any MEL100 | .471 | .05 |
| Labeling index <1.2% | .947 | .55 | Any first CR | 1.031 | .56 |
| Any first CR | 1.061 | .75 | Labeling index <1.2% | 1.024 | .67 |
| EFS . | RR . | P . | OS . | RR . | P . |
|---|---|---|---|---|---|
| β2-microglobulin <4 mg/L | .824 | .04 | β2-microglobulin <4 mg/L | .795 | .04 |
| Any MEL100 | .348 | .04 | Any MEL100 | .471 | .05 |
| Labeling index <1.2% | .947 | .55 | Any first CR | 1.031 | .56 |
| Any first CR | 1.061 | .75 | Labeling index <1.2% | 1.024 | .67 |
Abbreviations: EFS, event-free survival; OS, overall survival; RR, relative risk.
The presence of labeling index greater than 1.2% identified a subgroup of 41 patients (21 for MP and 20 for MEL100) with poor outcome after MP, but relatively good outcome after MEL100. Their median event-free and overall survival was 13 and 32 months after MP, compared with 29 and 50+ after MEL100. These data suggested that MEL100 could abrogate the negative prognostic factor of high labeling index and explained why it did not influence outcome in univariate and multivariate analysis.
Mobilization Regimen and Toxicity
After CY, toxicity was mild: the median duration of severe granulocytopenia (neutrophils <500/μL) was 3 days, the platelet count was never less than 70,000/μL, and only 2 fevers of unknown origin were observed. After 2 or 3 leukaphereses, 90% of patients mobilized at least 6 × 106/kg (Table 5). An adequate number of CD34 cells was available to support the first course for all patients, the second for 98%, and the third for 94%. The number of CD34 cells reinfused was correlated with the median duration of severe thrombocytopenia (platelet count <25,000/μL): 5, 3, and 1 day after reinfusion of less than 2 × 106/kg, 2 to 3 × 106/kg, and greater than 3 × 106/kg CD34+ cells, respectively. Neutropenia was not related. The corresponding median duration of neutropenia was 6, 5, and 5 days.
Toxicity After the First, Second, and Third MEL100
| . | 1st MEL100 . | 2nd MEL100 . | 3rd MEL100 . |
|---|---|---|---|
| CD34+ reinfused (1 × 106/kg) | 3.8 (1.2-9) | 3.5 (1-8) | 4 (1.1-14) |
| Days with ANC <500/μL | 5 (2-8) | 4 (2-9) | 4 (2-8) |
| Days with Hb <8 g/dL | 1 (0-5) | 0 (0-5) | 0 (0-1) |
| Days with platelets <25,000/μL | 2 (0-8) | 2 (0-7) | 1 (0-7) |
| No. of RBC transfusions | 2 (1-6) | 1 (1-3) | 1 (1-2) |
| No. of platelet transfusions | 1 (1-5) | 1 (1-5) | 1 (1-3) |
| Days with fever >38°C | 4 (2-8) | 3 (1-9) | 2 (1-3) |
| Days with antibiotics use | 7 (4-11) | 6 (5-14) | 7 (5-10) |
| Days of hospitalization | 12 (5-28) | 13 (6-24) | 8 (6-19) |
| Patients transfused with RBC (%) | 54 | 26 | 9 |
| Patients transfused with platelets (%) | 69 | 68 | 55 |
| Patients with fever >38°C (%) | 27 | 18 | 14 |
| Patients hospitalized (%) | 25 | 24 | 23 |
| . | 1st MEL100 . | 2nd MEL100 . | 3rd MEL100 . |
|---|---|---|---|
| CD34+ reinfused (1 × 106/kg) | 3.8 (1.2-9) | 3.5 (1-8) | 4 (1.1-14) |
| Days with ANC <500/μL | 5 (2-8) | 4 (2-9) | 4 (2-8) |
| Days with Hb <8 g/dL | 1 (0-5) | 0 (0-5) | 0 (0-1) |
| Days with platelets <25,000/μL | 2 (0-8) | 2 (0-7) | 1 (0-7) |
| No. of RBC transfusions | 2 (1-6) | 1 (1-3) | 1 (1-2) |
| No. of platelet transfusions | 1 (1-5) | 1 (1-5) | 1 (1-3) |
| Days with fever >38°C | 4 (2-8) | 3 (1-9) | 2 (1-3) |
| Days with antibiotics use | 7 (4-11) | 6 (5-14) | 7 (5-10) |
| Days of hospitalization | 12 (5-28) | 13 (6-24) | 8 (6-19) |
| Patients transfused with RBC (%) | 54 | 26 | 9 |
| Patients transfused with platelets (%) | 69 | 68 | 55 |
| Patients with fever >38°C (%) | 27 | 18 | 14 |
| Patients hospitalized (%) | 25 | 24 | 23 |
Values are the median, with the range in parentheses, unless otherwise specified.
Abbreviations: ANC, absolute neutrophil count; Hb, hemoglobin; RBC, red blood cell.
Effect on neutropenia.
After the first, second, and third MEL100, the median duration of severe neutropenia was 5, 4, and 4 days, respectively. Severe neutropenia lasting more than 7 days occurred in 15% (first course), 17% (second), and 13% (third) of patients.
Effect on thrombocytopenia.
After the first, second, and third MEL100, the median duration of severe thrombocytopenia was 2, 2, and 1 day, respectively. Severe thrombocytopenia lasting more than 7 days occurred only after the first course in 5% of patients.
Transfusion requirement.
The percentage of patients requiring red blood cell transfusion was 54% after the first course, 26% after the second, and 9% after the third, whereas those requiring platelets ranged from 69% to 55% (Table 5).
Extrahematologic toxicity.
Cases of extrahematologic toxicity were: 2 pneumonias, 17 fevers of unknown origin, 10 mucosites, and 1 gastrointestinal toxicity after the first course; 13 fevers of unknown origin, 5 mucosites, 1 gastrointestinal toxicity, and 1 heart failure after the second; 10 fevers of unknown origin, and 4 mucosites after the third.
DISCUSSION
Cytokines and stem cell support have drastically changed the chemotherapy approach to cancer patients, allowing both dose intensification and reduction of myelotoxicity. Hematopoietic growth factors have significantly improved neutropenia after conventional chemotherapy.31 However, thrombocytopenia and cumulative myelotoxicity have limited further dose intensification.31Peripheral blood progenitor cells after high-dose chemotherapy have induced faster neutrophil and platelet recovery and reduced blood product support and therapy-related morbidity.13,15 32
Chemotherapy followed by G-CSF is the most widely used approach. CY at 1.2 g/m2 efficiently mobilizes stem cells and increasing doses enhance their number proportionally.14 CY at 7 g/m2 is the optimal regimen,14,33 but infections and long-lasting trombocytopenia are predictable. We have previously reported that 3 g/m2 CY has negligible toxicity in an outpatient setting with an adequate CD34 cell harvest.34 We show here excellent CD34 mobilization after 4 g/m2 CY. In a recent study, mobilization with G-CSF alone was compared with 6 g/m2 CY plus G-CSF: higher morbidity and greater CD34 cell mobilization, but comparable hematopoietic recovery after transplantation was observed.35 A higher CD34 harvest is needed to support intensive chemotherapy at diagnosis and cryopreserve enough for intensive salvage therapy at relapse. All of these data suggest that 3 to 4 g/m2 CY plus G-CSF should be used as a standard mobilization regimen. This approach combines a high CD34 harvest with low toxicity.
Encouraging results with high-dose melphalan followed by stem cell support have been reported in selected series of myeloma patients.11,12,19,20,34 In the randomized study of Attal et al,12 AT was superior to standard treatment, as in a retrospective case-matched study by the SWOG.11 In refractory patients, 60 mg/m2 melphalan improved response rate and outcome compared with 30 mg/m2melphalan.34 We show here that MEL100 is superior to MP. In view of difficulty of drawing definitive conclusions from historical controls, differences in main prognostic factors were eliminated by matching patients for 2 pretreatment factors affecting clinical outcome: age and β2-microglobulin. Both groups of 71 patients were highly selected and all showed comparable clinical conditions. Significantly higher response rates and prolonged event-free and overall survival were observed in the MEL100 group.
Multivariate analysis was performed to evaluate the prognostic effect of disease sensitivity to treatment (attainment of CR) and dose intensity (MEL100 v MP). When β2-microglobulin levels and labeling index were combined with CR and MEL100 administration, MEL100 emerged as a significant factor, influencing both event-free and overall survival in addition to β2-microglobulin. Neither CR nor labeling index was a significant factor. This might be due to the low number of patients enrolled. The greater importance of MEL100 rather than obtaining CR (which was significant on univariate analysis), as well as low β2-microglobulin levels rather than high labeling index has interesting implications. MEL100 significantly increased both the CR and the PR rate. Does this global increased rate of response influence outcome and reduce the influence of CR? High labeling index negatively affected outcome of patients receiving MP, but not those receiving MEL100. Thus, MEL100 seems to abrogate the negative prognostic features of high labeling index.
Siegel et al recently reported that age is not a prognostic variable and should not constitute an exclusion criterion for AT protocols. Patients who where 67 years old showed similar toxicity and clinical outcome when compared with 52-year-old pair mates, but only 49 elderly subjects could be selected from 550 myeloma patients receiving AT.36 In several large clinical trials, 25% of patients did not complete the planned high-dose regimen.11,12 19Even if treatment-related mortality is low after transplantation, toxicity is not trivial. Highly selected elderly patients can certainly receive AT. In our opinion, stem cell-supported therapy should be delivered to the majority of older patients, but should be tailored to account for their age, general clinical condition, and frequent concomitant diseases.
The third MEL100 course was not administered to patients reaching CR after the second. It was administered to only 55% of patients. The third course increased the CR rate from 34% to 47%. This approach was chosen to increase CR rate, because, in a previous report, the best outcome was accomplished in patients reaching CR after treatment.12 In a preliminary analysis of the IFM-94 trial randomizing patients to 1 versus 2 transplants, no differences in CR rate, event-free survival, and overall survival were observed.37 In a multivariate analysis, the early completion of 2 transplants emerged as a highly significant factor for both event-free and overall survival, showing the greater importance of the time to second transplant rather than attaining CR.22Whether a similar outcome can be achieved with a less intense regimen, repeated every 2 months, remains an open question. Clinical trial comparing intermediate melphalan doses versus high-dose melphalan (ie, MEL100 versus double transplant with 200 mg/m2 melphalan) are needed to answer this important issue.
MEL100 was well tolerated, and patients were not isolated, were admitted for 7 days, and were then discharged. After 60 mg/m2 melphalan, mucositis was absent34; after MEL100, it ranged from 6% to 14%, but continuous hydration for 5 to 7 days was mandatory. When hydration was shortened, severe mucositis was the rule. The incidence of fever ranged from 14% to 27%. Patients were discharged before neutropenia occurred and returned home. In a more recent pilot trial with 120 mg/m2 melphalan, all patients were hospitalized throughout the neutropenic period, antibiotic prophylaxis was instituted, and the incidence of infections drastically decreased in comparison with patients admitted for 7 days only.
In conclusion, MEL100 was superior to MP in terms of response rate, event-free survival, and overall survival. We feel that AT should be offered to all younger patients in excellent clinical condition. MEL100 should be offered to older patients in good clinical condition or those who might suffer some kind of transplant-related clinical complications.
Supported in part by Associazione Italiana Ricerca Cancro (AIRC), Associazione Italiana Leucemie (AIL), and Ministero Università e Ricerca Scientifica e Tecnologica (MURST).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mario Boccadoro, MD, Divisione di Ematologia dell’Università di Torino, Ospedale Molinette, Via Genova 3, 10126 Torino, Italy; e-mail: mario.boccador@unito.it.