C-Jun amino terminal kinase/stress-activated protein kinases (JNK/SAPK) and p38 subgroups of mitogen-activated protein kinases have been suggested to play a critical role in apoptosis, cell growth, and/or differentiation. We found that a short exposure of SKT6 cells, which respond to erythropoietin (Epo) and induce erythroid differentiation, to osmotic or heat shock induced transient activation of JNK/SAPK and p38 and inactivation of ERK and resulted in erythroid differentiation without Epo, whereas long exposure of the cells to these stresses induced prolonged activation/inactivation of the same kinases and caused apoptosis. Inhibition of JNK/SAPK and p38 resulted in inhibition of stress-induced erythroid differentiation and apoptosis. Inhibition of ERK had no effect on stress-induced erythroid differentiation, but stimulated apoptosis. Activation of p38 and/or JNK/SAPK for a short time caused erythroid differentiation without Epo, although its prolonged activation induced apoptosis. Activation of ERK suppressed stress-induced apoptosis. These results indicate that short cellular stresses, inducing transient activation of JNK/SAPK and p38, lead to cell differentiation rather than apoptosis. Furthermore, activation of JNK/SAPK and p38 is required for both cell differentiation and apoptosis, and the duration of their activation may determine the cell fate, cell differentiation, and apoptosis. In contrast, inactivation of ERK is required for stress-induced apoptosis but not cell differentiation.
C-JUN AMINO TERMINAL kinase (JNK) or stress-activated protein kinases (SAPK),1,2 p38 MAP kinase or cytokine suppressive anti-inflammatory drug binding protein (p38),3,4 and classical mitogen-activated protein (MAP) kinases (ERK) are subgroups of a large MAP kinase family. ERK is a protein-serine/threonine kinase that is rapidly activated by a variety of cell growth and differentiation stimuli5-7 and plays a central role in mitogenic signaling.8 The p38 and JNK/SAPK cascades are primarily activated by various environmental stresses: osmotic shock, UV radiation, heat shock, x-ray radiation, hydrogen peroxide, and protein synthesis inhibitors and by the proinflammatory cytokines, tumor necrosis factor-α (TNF-α), and interleukin-1 (IL-1).1-4,9-14These cellular stresses and proinflammatory cytokines induce apoptotic cell death.13 Stimulation of Fas also induces activation of p38 and JNK/SAPK.15 16 Thus, it has been suggested that JNK/SAPK and p38 have a critical role in signal transduction of apoptotic cell death.
Expression of a constitutively active mutant of MEK kinase-1 (MEKK1), an upstream activator of JNK/SAPK, has been shown to induce apoptosis in mouse fibroblasts.17 Similarly, expression of the constitutively activated form of MKK3,18 which is an upstream kinase of p38, induced apoptosis in PC12 cells. Activation of another upstream kinase of p38, MKK6, also induced apoptosis in Jurkat T cells.19 Furthermore, overexpression of ASK120 or TAK1,21 both of which are MAPK kinases and activate both JNK/SAPK and p38, also caused apoptotic cell death in COS7 cells and Xenopus embryos, respectively. Conversely, cells expressing dominant-inhibitory mutants within the JNK/SAPK pathway become highly resistant to stress-induced apoptosis.13,18,22-24 Expression of dominant-negative SEK1, which is an upstream kinase of JNK/SAPK, suppressed TNF-α–induced apoptosis.13 The dominant-negative form of JNK1, but not of p38, inhibited UV and γ radiation-induced apoptosis.23The dominant-negative mutants of c-Jun, SEK1 and MKK3,18and anti-c-Jun antibody25 blocked apoptosis when nerve growth factor was removed in PC12 cell culture. Disruption of JNK3, which is predominantly expressed in neurons, prevented neuronal apoptosis.26 These results support that JNK/SAPK and p38 play a vital part in apoptotic cell death.
There are conflicting reports concerning the involvement of JNK/SAPK and p38 in apoptosis.27-31 Induction of JNK/SAPK activity by ligation of Fas was a result rather than a cause.27TNF-α receptor-mediated JNK/SAPK activation was not linked to apoptosis.28 CD40 ligand, which is known to counteract apoptosis in B cells, activates JNK/SAPK.29 Disruption of SEK1 did not affect the induction of stress-induced apoptosis, but instead protected thymocytes from Fas-mediated and CD3-mediated apoptosis.30 TRAF2-deficient mice experienced severe reduction of TNF-α–induced JNK/SAPK activation, but were highly sensitive to TNF-α–induced apoptosis.31 These results suggest that JNK/SAPK and p38 are not always involved in stress-induced, TNF-α–induced, or Fas-induced apoptosis.
JNK/SAPK and p38 can also be activated by such mitogenic factors as epidermal growth factor and phorbol esters11 and by T-cell activation signaling.32 It was recently reported that various hematopoietic cytokines, interleukins, and colony-stimulating factors, which regulate hematopoietic cell growth, survival, and differentiation, activate not only classical MAP kinase ERK, but also p38 and JNK/SAPK.33-37 It was further reported that T-cell proliferation in response to IL-2 and IL-7 requires p38 activation38 and that JNK/SAPK and p38 are required for erythropoietin (Epo)-induced growth signal.39 Recently, we also showed that Epo induces transient activation of JNK/SAPK, p38, and ERK in SKT6 cells, which can respond to Epo and induce hemoglobinization and that activation of JNK/SAPK and p38, but not ERK, is required for Epo-induced erythroid differentiation of SKT6 cells.39 It was also shown that GTPase-deficient G proteins induce persistent activation of JNK and PC12 cell differentiation, and overexpression of c-Jun induces neurite outgrowth.40 In P19 embryonic carcinoma cells, ectopic expression of c-Jun leads to cell differentiation,41 and overexpression of the dominant negative form of JNK blocks the induction of endodermal differentiation by retinoic acid.42 Differentiation of WEHI-3B(D+) myelomonocytic leukemia cells is also induced by ectopic expression of c-Jun.43 These reports indicate that JNK/SAPK and p38 pathways are involved in not only apoptotic, but also mitogenic and differentiation signalings, at least, in some cell lineages.
To explore the roles of JNK/SAPK, p38, and ERK, we examined whether or not environmental stresses such as osmotic or heat shock could mimic Epo-induced erythroid differentiation of SKT6 cells. Surprisingly, we found that a short exposure of the cells to these cellular stresses induced transient activation of JNK/SAPK and p38 and transient inactivation of ERK. This resulted in erythroid differentiation without Epo stimulation, whereas long exposure to these stresses induced persistent activation of JNK/SAPK and p38 and inactivation of ERK and caused apoptotic cell death. Activation of JNK/SAPK and p38 was also found to be required for stress-induced erythroid differentiation as well as apoptotic cell death, and activation of p38 alone was sufficient to induce erythroid differentiation to some extent without any stimulation. Furthermore, inhibition of ERK was found to be required for stress-induced apoptotic cell death but not erythroid differentiation. We discuss here the possible roles of JNK/SAPK, p38, and ERK during stress-induced cell differentiation and apoptotic cell death.
MATERIALS AND METHODS
Cell culture and stress-induced erythroid differentiation.
Epo-responsive mouse erythroleukemia SKT6 cells44 were maintained in Ham F-12 medium supplemented with 10% fetal calf serum. SKT6 cells were induced to differentiate by the addition of 0.5 U/mL of recombinant human Epo (2.6 × 105 U/mg; Kirin Brewery) followed by incubation for 4.5 days, and hemoglobin-positive cells were stained by 0.05% 2,7-diaminofluorene (DAF).39 Cells were treated with osmotic shock (0.1 mol/L NaCl) or heat shock (42°C) for 1 hour, then cultured in normal culture medium with or without 0.5 U/mL of Epo for 4.5 days, and hemoglobinized cells were counted.
S-oligos, inhibitors, and activators of MAP kinases.
Various concentrations of antisense, sense, or scrambled phosphothioester oligonucleotides (S-oligos) (BEX, Tokyo, Japan), p38-specific inhibitor SB203580 (SmithKline Beacham, King of Prussia, PA) in dimethyl sulfoxide (DMSO), MEK-specific inhibitor PD98059 (Calbiochem, La Jolla, CA) in ethanol, or p38 and JNK/SAPK activators, anisomycin (Calbiochem)45 and C2-ceramide (Calbiochem),46 were mixed in the culture medium before stress treatment, and the effect on cell differentiation and apoptotic cell death was examined. The S-oligos were added again 24 hours after stress treatment. The antisense S-oligos used were described previously.39 As control, the corresponding sense S-oligos and scrambled S-oligos were used.
In vitro protein kinase assay.
Analysis of apoptotic cell death and DNA fragmentation.
Apoptotic cell death was measured by Tdt-mediated dUTP nick-end labeling (TUNEL) methods as followed by the manufacturer’s protocol (fluorescein apoptosis detection system) (Promega, Madison, WI). DNA fragmentation was assayed by the modified method of Sellins and Cohen.47 SKT6 cells (1 × 106 cells) were washed with phosphate-buffered saline and suspended in a lysis buffer (10 mmol/L Tris-HCl, pH 7.5, 10 mmol/L ethylenediamine tetraacetate, 0.5% Triton X-100) for 10 minutes on ice. The suspension was centrifuged at 16,000 rpm for 20 minutes and the fragmented DNA was recovered from the supernatant. The supernatant was subjected to digestion with ribonuclease A (0.5 mg/mL) for 1 hour at 37°C followed by incubation with proteinase K (0.5 mg/mL) for 1 hour at 37°C. Equal amounts of the precipitated DNA were separated by 1.8% agarose gel electrophoresis and visualized by ethidium bromide staining.
Caspase-3 assay.
Caspase-3 activity was measured by CPP32 colorimetric assay kit (Clontech, Palo Alto, CA). The cell extracts (100 μg protein) were incubated at 37°C for 60 minutes with labeled specific substrate (DEVD-pNA) in reaction buffer. The colorimetry of the cleaved substrates was measured in a spectrophotometer at a wavelength of 405 nm. One unit is defined as the amount of enzyme required to cleave 1 pmol of the substrate at 37°C for 60 minutes under the standard reaction conditions.
Plasmid construction.
An HindIII-XbaI fragment of pcDNA3-flag-MKK6(Glu)48 was ligated to an ecdysone-inducible mammalian expression vector pIND (Invitrogen, Carlsbad, CA).49 The pIND/MKK6(Glu) and pVgRXR were transfected into SKT6 cells. Several double resistant (Zeocin and G418) clones were isolated, and the expression of MKK6(Glu) was induced by 2 μmol/L muristerone A, which was confirmed by antiflag-tag antibody (M2). The hemagglutinin (HA) epitope-tagged constitutively active MKK1 (ΔN3-S218E/S222D)50 was subcloned into theKpnI/EcoRV sites in pCDNA3 and transfected into SKT6 cells. The expression of HA-MKK1 mutant was detected by immunoblotting with monoclonal antibody to HA.
RESULTS
A short exposure of SKT6 cells to heat or osmotic shock induces erythroid differentiation without Epo.
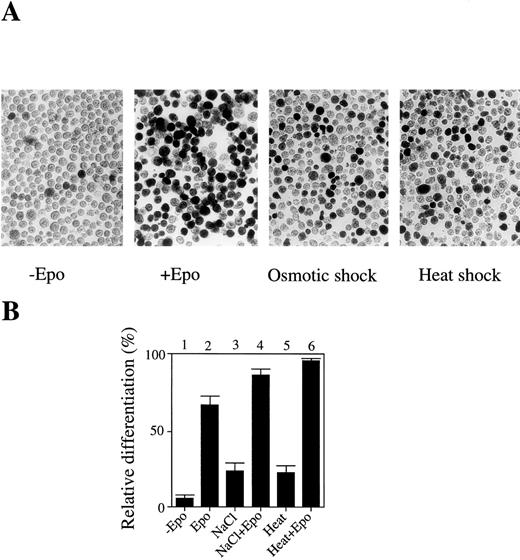
In an attempt to determine the roles of JNK/SAPK and p38 in apoptotic and cell differentiation signals, we examined whether environmental stresses such as osmotic shock and heat shock could induce cell differentiation rather than apoptotic cell death. Mouse erythroleukemia SKT6 cells can respond to Epo and induce erythroid differentiation. These cells are not dependent on Epo to proliferate and their proliferation is neither enhanced nor impaired by the presence of Epo. We found that a short exposure (less than 1 hour) of SKT6 cells, which can be induced to differentiate into hemoglobinized cells in response to Epo (Fig 1A, left two panels), to osmotic shock (0.1 mol/L NaCl) or heat shock (42°C) induced erythroid differentiation without Epo stimulation (Fig 1A, right two panels), although the number of hemoglobinized cells was about one third of that stimulated with Epo (Fig 1B, lanes 3 and 5). In the presence of Epo, these stresses additively stimulated the Epo-induced erythroid differentiation (Fig 1B, lanes 4 and 6) compared with Epo alone (Fig1B, lane 2). The best conditions to induce erythroid differentiation, but not to induce apoptotic cell death, were found to be with the addition of 0.1 mol/L NaCl to the cells for 1 hour or incubation of the cells at 42°C for 1 hour.
A short exposure of SKT6 cells to osmotic or heat shock induces erythroid differentiation rather than apoptotic cell death. (A) Left two panels: the hemoglobinized SKT6 cells stained with DAF after culture with or without Epo for 4.5 days. Right two panels: the hemoglobinized cells after treatment with 0.1 mol/L NaCl or 42°C for 1 hour, followed by incubation without Epo for 4.5 days. (B) The relative differentiation of SKT6 cells. Cells treated with 0.1 mol/L NaCl for 1 hour (lanes 3, 4), cells incubated at 42°C for 1 hour (lanes 5, 6), or untreated cells (lanes 1, 2) were cultured with (lanes 2, 4, 6) or without (lanes 1, 3, 5) Epo for 4.5 days and stained. The percentage of hemoglobinized cells in the presence of Epo is defined as 100%.
A short exposure of SKT6 cells to osmotic or heat shock induces erythroid differentiation rather than apoptotic cell death. (A) Left two panels: the hemoglobinized SKT6 cells stained with DAF after culture with or without Epo for 4.5 days. Right two panels: the hemoglobinized cells after treatment with 0.1 mol/L NaCl or 42°C for 1 hour, followed by incubation without Epo for 4.5 days. (B) The relative differentiation of SKT6 cells. Cells treated with 0.1 mol/L NaCl for 1 hour (lanes 3, 4), cells incubated at 42°C for 1 hour (lanes 5, 6), or untreated cells (lanes 1, 2) were cultured with (lanes 2, 4, 6) or without (lanes 1, 3, 5) Epo for 4.5 days and stained. The percentage of hemoglobinized cells in the presence of Epo is defined as 100%.
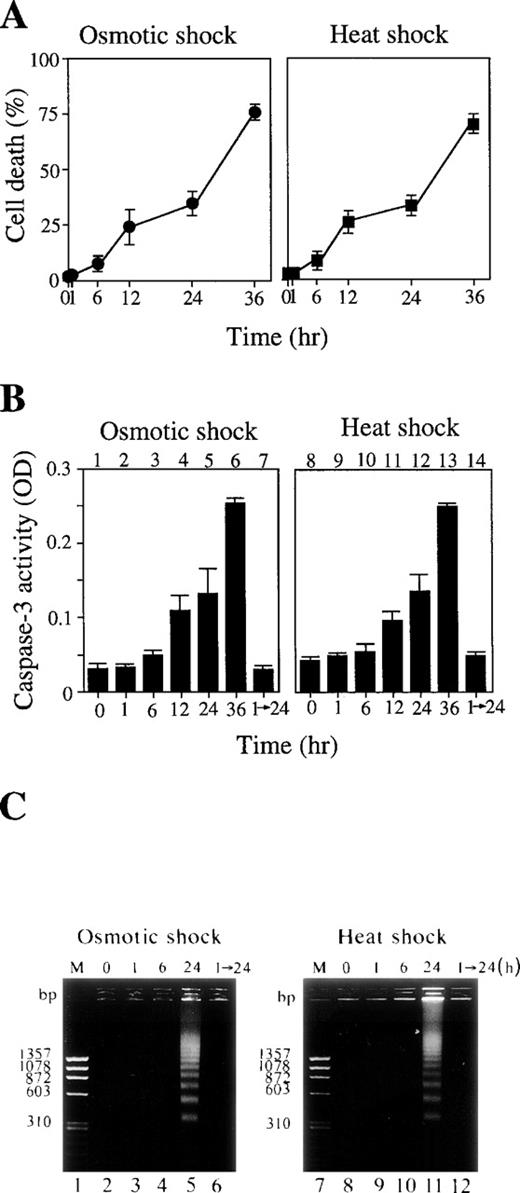
The majority of the cells treated with these cellular stresses for up to 1 hour survived (Fig 2A), and neither caspase-3 activation (Fig 2B, lanes 2 and 9) nor DNA fragmentation (Fig2C, lanes 3 and 9) was observed. This was true even 24 hours after release of these short stresses (Fig 2B, lanes 7 and 14; Fig 2C, lanes 6 and 12). However, a longer exposure of the cells to these stresses actually activated caspase-3 (Fig 2B, lanes 3 to 6 and 10 to 13) and DNA fragmentation (Fig 2C, lanes 5 and 11), leading to apoptotic cell death (Fig 2A).
(A) Cell death rate (%) after osmotic (left panel) or heat (right panel) shock for various periods as indicated. (B) Caspase-3 activity in cells treated with 0.1 mol/L NaCl (lanes 1 to 7) or incubated at 42°C (lanes 8 to 14) for the periods indicated. Lanes 7 and 14: activity in the cells treated with NaCl (lane 7) or heat (lane 14) for 1 hour followed by incubation for an additional 24 hours under normal conditions. (C) DNA fragmentation of the cells treated with 0.1 mol/L NaCl (left panel) or 42°C (right panel) for various periods as indicated. Lanes 1 and 7: molecular weight marker. Lanes 6 and 12: cells treated with the stresses for 1 hour followed by a further 24-hour incubation under normal conditions.
(A) Cell death rate (%) after osmotic (left panel) or heat (right panel) shock for various periods as indicated. (B) Caspase-3 activity in cells treated with 0.1 mol/L NaCl (lanes 1 to 7) or incubated at 42°C (lanes 8 to 14) for the periods indicated. Lanes 7 and 14: activity in the cells treated with NaCl (lane 7) or heat (lane 14) for 1 hour followed by incubation for an additional 24 hours under normal conditions. (C) DNA fragmentation of the cells treated with 0.1 mol/L NaCl (left panel) or 42°C (right panel) for various periods as indicated. Lanes 1 and 7: molecular weight marker. Lanes 6 and 12: cells treated with the stresses for 1 hour followed by a further 24-hour incubation under normal conditions.
Transient activation of JNK/SAPK and p38 and inactivation of ERK by a short osmotic or heat shock.
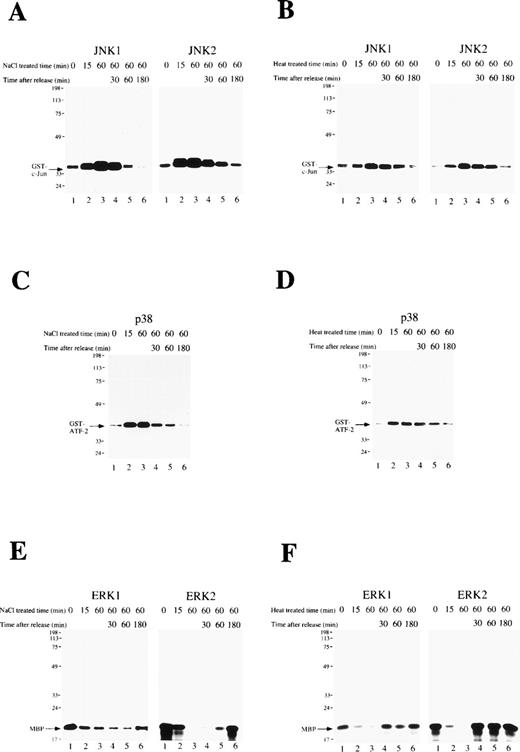
The activity of the MAP kinase family (JNK1, JNK2, p38, ERK1, and ERK2) was measured during osmotic or heat shock-induced erythroid differentiation of SKT6 cells. Cells were exposed to these cellular stresses for 1 hour and released. In vitro kinase assays in the immunoprecipitates with each specific antibody at the indicated time point showed that the activities of JNK1 (Figs 3A and B, left panels), JNK2 (Figs 3A and B, right panels), and p38 (Figs 3C and D) were only weakly detected before osmotic-treatment or heat-treatment (Figs 3A-D, lane 1), but rapidly and dramatically increased within 15 minutes after stress treatment (Figs 3A-D, lane 2). They remained at the maximum levels until stress release (Figs 3A-D, lane 3), but returned to the basal levels within 3 hours thereafter (Figs 3A-D, lane 6).
A short exposure of SKT6 cells to osmotic or heat shock induces transient activation of JNK/SAPK and p38, but suppresses activity of ERK. SKT6 cells were treated with osmotic shock (0.1 mol/L NaCl) (A, C, E) or heat shock (42°C) (B, D, F) for 0, 15, or 60 minutes, and cultured for a further 0, 30, 60, or 180 minutes under normal conditions. (A and B) The JNK1 (left panels) and JNK2 (right panels) activity in the immunoprecipitates at the indicated time points after stress treatment was assayed with GST-c-Jun as a substrate. Arrows indicate the phosphorylated GST-c-Jun. (C and D) The p38 activity in the immunoprecipitates at the indicated time points after stress treatment was assayed with GST-ATF-2 as a substrate. Arrows indicate the phosphorylated GST-ATF-2. (E and F) The ERK1 (left panels) or ERK2 (right panels) activity in the immunoprecipitates at the indicated time points after stress treatment was assayed with major basic protein (MBP) as a substrate. Arrows indicate the phosphorylated MBP.
A short exposure of SKT6 cells to osmotic or heat shock induces transient activation of JNK/SAPK and p38, but suppresses activity of ERK. SKT6 cells were treated with osmotic shock (0.1 mol/L NaCl) (A, C, E) or heat shock (42°C) (B, D, F) for 0, 15, or 60 minutes, and cultured for a further 0, 30, 60, or 180 minutes under normal conditions. (A and B) The JNK1 (left panels) and JNK2 (right panels) activity in the immunoprecipitates at the indicated time points after stress treatment was assayed with GST-c-Jun as a substrate. Arrows indicate the phosphorylated GST-c-Jun. (C and D) The p38 activity in the immunoprecipitates at the indicated time points after stress treatment was assayed with GST-ATF-2 as a substrate. Arrows indicate the phosphorylated GST-ATF-2. (E and F) The ERK1 (left panels) or ERK2 (right panels) activity in the immunoprecipitates at the indicated time points after stress treatment was assayed with major basic protein (MBP) as a substrate. Arrows indicate the phosphorylated MBP.
In contrast, the activities of ERK1 (Figs 3E and F, left panels) and ERK2 (Figs 3E and F, right panels) were clearly detected before stress treatment (Figs 3E and F, lane 1), but rapidly decreased with this treatment (Figs 3E and F, lanes 2 and 3). They remained low until stress release (Figs 3E and F, lane 3) had returned to the original levels within 3 hours thereafter (Figs 3E and F, lane 6). In-gel kinase assays confirmed the specific activation of JNK/SAPK and inactivation of ERK (data not shown). These results indicate that a short exposure of SKT6 cells to these cellular stresses induces transient activation of JNK/SAPK as well as p38, but transiently suppresses the activity of ERK.
Inhibition of p38 results in a block of stress-induced erythroid differentiation and delay of apoptotic cell death, and inhibition of MEK stimulates stress-induced apoptotic cell death.
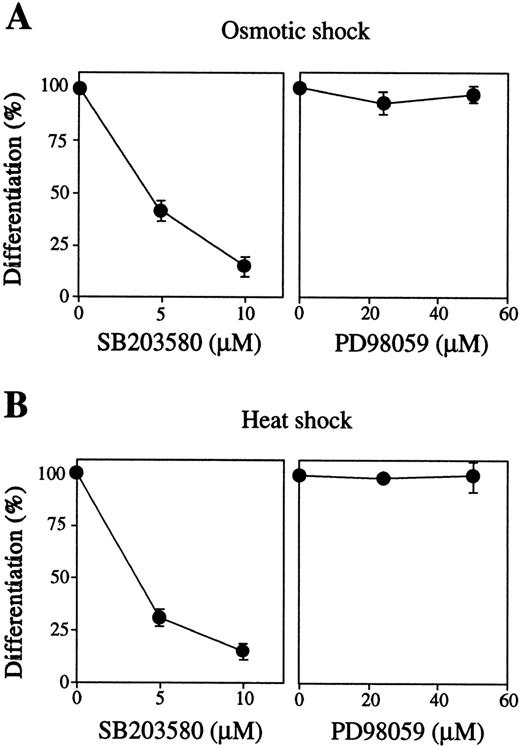
We next examined the effects of inhibition of these MAP kinases by their specific inhibitors on stress-induced erythroid differentiation. The p38-specific inhibitor SB203580 strongly blocked both osmotic-induced (Fig 4A, left panel, closed circles) and heat-induced (Fig 4B, left panel, closed circles) hemoglobinization of SKT6 cells in a dose-dependent manner. SB203580 actually inhibited p38 activity as mentioned previously.39 The MEK-specific inhibitor PD98059 did not affect these stress-induced erythroid differentiation at all (Figs 4A and B, right panels), although PD98059 (50 μmol/L) completely blocked activation of both ERK1 and ERK2 as described.39 Neither the inhibitor alone nor the solvents used to dissolve the inhibitors (0.1% DMSO or 0.1% ethanol) affected cell differentiation (data not shown). These results indicate that, at least, activation of p38, although not ERK, is essential for stress-induced erythroid differentiation.
Activation of p38 but not MEK is required for stress-induced erythroid differentiation. SKT6 cells mixed with various concentrations of SB203580 (left panel) or PD98059 (right panel) were treated by (A) osmotic or (B) heat shock for 1 hour, and the percentage of hemoglobinized cells (%) was counted after 4.5 days. The percentage of hemoglobinized cells after stress treatment without inhibitor is defined as 100%. Values shown are the mean of five experiments.
Activation of p38 but not MEK is required for stress-induced erythroid differentiation. SKT6 cells mixed with various concentrations of SB203580 (left panel) or PD98059 (right panel) were treated by (A) osmotic or (B) heat shock for 1 hour, and the percentage of hemoglobinized cells (%) was counted after 4.5 days. The percentage of hemoglobinized cells after stress treatment without inhibitor is defined as 100%. Values shown are the mean of five experiments.
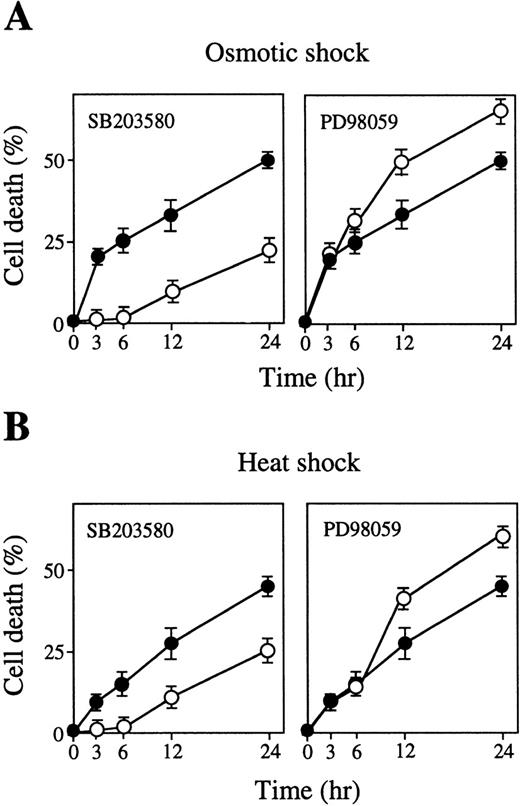
The effects of these inhibitors on osmotic-induced and heat-induced apoptotic cell death were also tested. Addition of SB203580 clearly caused delay of osmotic-induced (Fig 5A, left panel, open circles) and heat-induced (Fig 5B, left panel, open circles) apoptotic cell death. In contrast, inhibition of ERK activation by PD98059 clearly stimulated osmotic-induced (Fig 5A, right panel) or heat-induced (Fig 5B, right panel) apoptotic cell death. Neither the inhibitor alone (Figs 5A and B, closed circles) nor the solvents used to dissolve the inhibitors (data not shown) affected cell differentiation. Thus, activation of p38 and inhibition of ERK are required for stress-induced apoptotic cell death.
Inhibition of p38 causes delay of stress-induced apoptotic cell death, but inhibition of MEK stimulates stress-induced apoptotic cell death. Cell death rate (%) after (A) osmotic or (B) heat shock with (○) or without (•) SB203580 (left panel) or PD98059 (right panel) for various periods as indicated. Values shown are the mean of five experiments.
Inhibition of p38 causes delay of stress-induced apoptotic cell death, but inhibition of MEK stimulates stress-induced apoptotic cell death. Cell death rate (%) after (A) osmotic or (B) heat shock with (○) or without (•) SB203580 (left panel) or PD98059 (right panel) for various periods as indicated. Values shown are the mean of five experiments.
Antisense S-oligos of p38 and JNK/SAPK block stress-induced erythroid differentiation and cause delay of apoptosis, and those of ERK stimulate stress-induced apoptotic cell death.
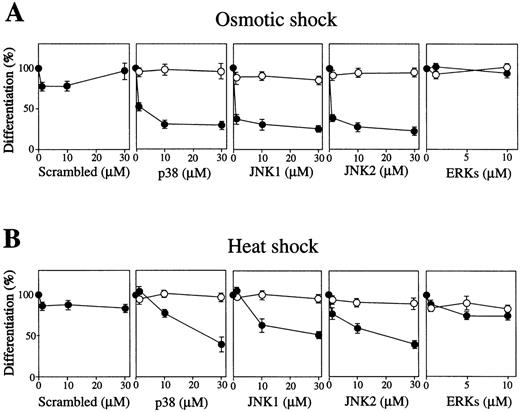
We also tested the effects of inhibition of these MAP kinases by their specific antisense S-olios on stress-induced erythroid differentiation. Antisense S-oligos of p38, JNK1, and JNK2 strongly inhibited the osmotic-induced (Fig 6A) or heat-induced (Fig 6B) hemoglobinization of SKT6 cells in a dose-dependent manner (Figs 6A and B, middle three panels, closed circles). All of these antisense S-oligos specifically inhibited the expression of each corresponding kinase as described.39 In contrast, antisense S-oligos of ERKs (commonly effective to both ERK1 and ERK2) did not affect stress-induced erythroid differentiation at all (Figs 6A and B, right end panels, closed circles). Neither scrambled S-oligos (Figs 6A and B, left end panels) nor sense S-oligos (Figs 6A and B, open circles) had any effect on it. Environmental stresses, thus, induce erythroid differentiation without Epo through activation of JNK1, JNK2, and p38, but not ERK.
Activation of p38 and JNK/SAPK, but not ERK, is required for stress-induced erythroid differentiation. SKT6 cells mixed with various concentrations (0 to 30 μmol/L) of antisense S-oligos (•) or sense S-oligos (○) of p38, JNK1, JNK2, ERKs (common to both ERK1 and ERK2), or scrambled S-oligos were (A) osmotic shocked or (B) heat shocked for 1 hour, and the percentage of hemoglobinized cells was counted after 4.5 days. The percentage of hemoglobinized cells after stress treatment without S-oligos is defined as 100%. Each point represents the mean of five replicates.
Activation of p38 and JNK/SAPK, but not ERK, is required for stress-induced erythroid differentiation. SKT6 cells mixed with various concentrations (0 to 30 μmol/L) of antisense S-oligos (•) or sense S-oligos (○) of p38, JNK1, JNK2, ERKs (common to both ERK1 and ERK2), or scrambled S-oligos were (A) osmotic shocked or (B) heat shocked for 1 hour, and the percentage of hemoglobinized cells was counted after 4.5 days. The percentage of hemoglobinized cells after stress treatment without S-oligos is defined as 100%. Each point represents the mean of five replicates.
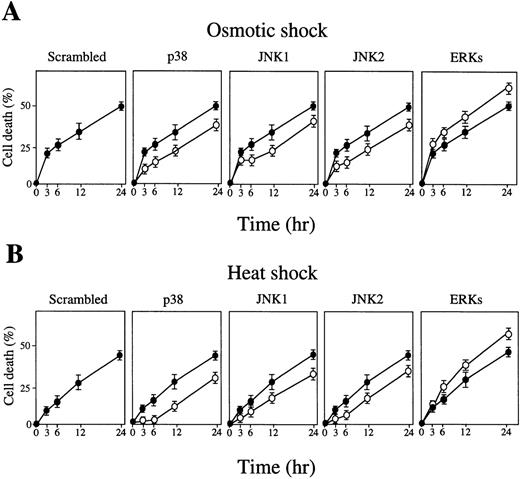
Similarly, addition of antisense S-oligos of p38, JNK1, and JNK2 caused delay of osmotic-induced (Fig 7A) and heat-induced (Fig. 7B) apoptotic cell death (Figs 7A and B, middle three panels, open circles). In contrast, addition of antisense S-oligos of ERK-stimulated apoptotic cell death (Figs 7A and B, right end panels). Neither scrambled S-oligos (Figs 7A and B, left end panels) nor sense S-oligos (Figs 7A and B, closed circles) had any effect on it. Taken together, we concluded that the activation of JNK/SAPK and p38 plays a critical role in stress-induced erythroid differentiation, and that activation of JNK/SAPK and p38 and the inhibition of ERK are critical for induction of stress-induced apoptotic cell death in SKT6 cells.
Inhibition of p38 or JNK/SAPK causes delay of stress-induced apoptotic cell death, but inhibition of ERK stimulates stress-induced apoptotic cell death. SKT6 cells mixed with 10 μmol/L of antisense S-oligos (○) or sense S-oligos (•) of p38, JNK1, JNK2, ERKs (common to both ERK1 and ERK2), or scrambled S-oligos were (A) osmotic shocked or (B) heat shocked for 1 hour, and the cell death rate (%) was measured at the indicated time points. Each point represents the mean of five replicates.
Inhibition of p38 or JNK/SAPK causes delay of stress-induced apoptotic cell death, but inhibition of ERK stimulates stress-induced apoptotic cell death. SKT6 cells mixed with 10 μmol/L of antisense S-oligos (○) or sense S-oligos (•) of p38, JNK1, JNK2, ERKs (common to both ERK1 and ERK2), or scrambled S-oligos were (A) osmotic shocked or (B) heat shocked for 1 hour, and the cell death rate (%) was measured at the indicated time points. Each point represents the mean of five replicates.
Activation of p38 and/or JNK/SAPK induces erythroid differentiation.
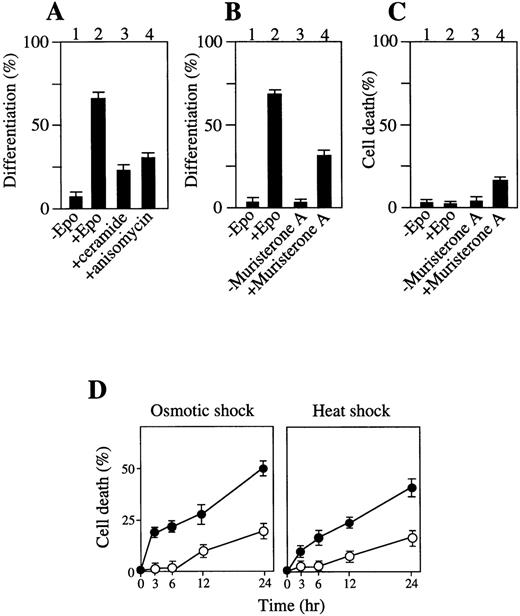
The effect of chemical activators on p38 and/or JNK/SAPK on erythroid differentiation without Epo stimulation was also examined. Incubation of SKT6 cells for 1 hour with C2-ceramide (100 μmol/L), which is an analog of a stress-induced physiologic activator of p38 and JNK/SAPK, ceramide,46 or with a protein biosynthesis inhibitor anisomycin (377 μmol/L), which is known to activate p38 and JNK/SAPK and induce apoptosis,45 resulted in specific activation of p38 and JNK/SAPK (data not shown) and led to production of hemoglobinized cells without Epo stimulation (Fig8A, lanes 3 and 4). Incubation of the cells with these activators for a longer period or at higher concentrations caused apoptotic cell death (data not shown). These data are consistent with the previous finding that low concentrations of ceramide induced HL60 cell differentiation.51
Activation of p38 and/or JNK/SAPK induces erythroid differentiation or apoptotic cell death to some extent, and activation of ERK inhibits stress-induced apoptotic cell death. (A) Addition of C2-ceramide or anisomycin for 1 hour induces erythroid differentiation. (B) Transient expression of MKK6 (Glu) induces erythroid differentiation. Lanes 1 and 2: transfectants of MKK6 (Glu) in pIND expression vector incubated with or without Epo. Lanes 3 and 4: transfectants incubated with or without muristerone A for 1 day. The percentage of hemoglobinized cells was counted after 4.5 days. Each value represents the mean of six independent clones. (C) A prolonged MKK6 expression slightly causes apoptotic cell death. Lanes 1 and 2: cell death rate of the transfectants with or without Epo. Lanes 3 and 4: cell death rate of the transfectants incubated with muristeron A for 4.5 days. (D) Expression of constitutively active MKK1 inhibits stress-induced apoptosis. The transfectants constitutively expressing MKK1 active mutant (▵N3-S218E/S222D) (○) or mock-transfectants (•) were treated with osmotic (left panel) or heat (right panel) shock for various periods as indicated, and the cell death rate (%) was measured. Values shown are the mean of five experiments.
Activation of p38 and/or JNK/SAPK induces erythroid differentiation or apoptotic cell death to some extent, and activation of ERK inhibits stress-induced apoptotic cell death. (A) Addition of C2-ceramide or anisomycin for 1 hour induces erythroid differentiation. (B) Transient expression of MKK6 (Glu) induces erythroid differentiation. Lanes 1 and 2: transfectants of MKK6 (Glu) in pIND expression vector incubated with or without Epo. Lanes 3 and 4: transfectants incubated with or without muristerone A for 1 day. The percentage of hemoglobinized cells was counted after 4.5 days. Each value represents the mean of six independent clones. (C) A prolonged MKK6 expression slightly causes apoptotic cell death. Lanes 1 and 2: cell death rate of the transfectants with or without Epo. Lanes 3 and 4: cell death rate of the transfectants incubated with muristeron A for 4.5 days. (D) Expression of constitutively active MKK1 inhibits stress-induced apoptosis. The transfectants constitutively expressing MKK1 active mutant (▵N3-S218E/S222D) (○) or mock-transfectants (•) were treated with osmotic (left panel) or heat (right panel) shock for various periods as indicated, and the cell death rate (%) was measured. Values shown are the mean of five experiments.
Transient expression of constitutively active MKK6 causes erythroid differentiation, although its prolonged expression slightly induces apoptotic cell death.
We further examined whether the transient activation of p38 is enough to induce erythroid differentiation without Epo stimulation and whether the persistent activation of p38 induces apoptosis. Transfectants of a constitutively active form of MKK6, MKK6 (Glu), which can specifically activate p38,48 in the inducible expression vector pIND, were hemoglobinized in response to Epo without MKK6 (Glu) expression (Fig 8B, lane 2). Incubation of the transfectants with muristerone A (and without Epo) for 1 day resulted in specific activation of p38 (data not shown), and led to production of hemoglobinized cells within 4.5 days (Fig 8B, lane 4) and no DNA fragmentation (data not shown). In contrast, a longer exposure (4.5 days) of the cells to muristerone A caused apoptotic cell death to some extent (Fig 8C, lane 4). Transfection of vector alone had no effect on cell differentiation (data not shown). Thus, the transient activation of p38 is sufficient to induce some level of erythroid differentiation without Epo stimulation, although its prolonged activation induces apoptotic cell death to some extent.
Expression of MKK1 inhibits stress-induced apoptotic cell death.
Expression of constitutively active form of MKK1, a protein kinase that phosphorylates and activates ERK, prevented the cells from induction of apoptotic cell death by osmotic shock (Fig 8D, left panel, open circles) and heat shock (Fig 8D, right panel, open circles) compared with mock transfectants (Fig 8D, closed circles), demonstrating that direct and selective activation of the ERK cascade suppresses apoptotic cell death and leads to survival of SKT6 cells.
DISCUSSION
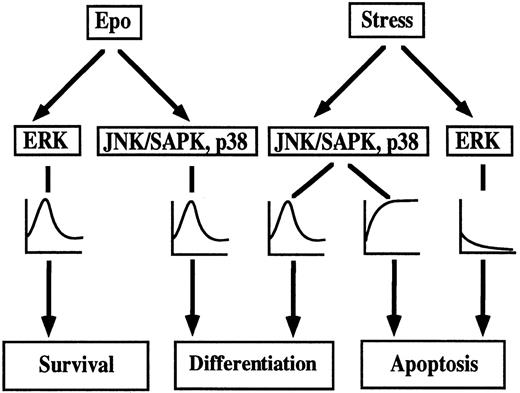
A model of environmental stress-induced erythroid differentiation and apoptotic cell death in SKT6 cells is depicted in Fig9. Epo induces transient activation of all MAP kinase family, ERK, JNK/SAPK, and p38,35,36 which, in turn, leads to erythroid differentiation. Actually, activation of JNK/SAPK and p38, but not ERK, is required for Epo-induced erythroid differentiation,39 and Epo-induced ERK activation may act on cell survival. Short exposure of SKT6 cells to environmental stress such as osmotic shock or heat shock, induces transient activation of JNK/SAPK and p38 (Fig 3), which partly mimics Epo stimulation and causes erythroid differentiation to some extent (Fig 1), whereas ERK activity is transiently suppressed (Fig 3). Inhibition of JNK/SAPK and/or p38, but not of ERK, strongly suppresses Epo-induced39 and environmental stress-induced erythroid differentiation (Figs 4 and 6). Activation of JNK/SAPK and/or p38 promotes erythroid differentiation to some extent (Fig 8). Thus, JNK/SAPK and p38, but not ERK, was confirmed to be essential for erythroid differentiation. A longer exposure of the cells to the environmental stresses leads to persistent activation of JNK/SAPK and p38 and inactivation of ERK (Fig 3), which finally induces apoptotic cell death (Fig 2). Inhibition of JNK/SAPK and/or p38 causes delay of stress-induced apoptotic cell death (Figs 5 and 7), but inhibition of ERK stimulates stress-induced apoptosis (Figs 5 and 7). Furthermore, activation of JNK/SAPK and/or p38 stimulates stress-induced apoptotic cell death, although activation of ERK strongly suppresses it (Fig 8). Thus, prolonged activation of JNK/SAPK and p38 as well as suppression of ERK activity is required for stress-induced apoptotic cell death.
Model of stress-induced cell differentiation and apoptotic cell death through MAPK family in SKT6 cells.
Model of stress-induced cell differentiation and apoptotic cell death through MAPK family in SKT6 cells.
We have shown that environmental stresses for a short time induce erythroid differentiation rather than apoptotic cell death without Epo stimulation through p38 and JNK/SAPK, but not ERK, cascades in SKT6 cells, and that signal leading to cell differentiation are, at least in part, shared with those leading to apoptotic cell death in SKT6 cells. We also performed in vitro erythroid colony-formation assays of mouse fetal liver cells of 13.5-day-old embryos in semisolid culture. The fetal liver cells were treated with osmotic shock (0.1 mol/L NaCl for 30 minutes) or heat shock (40°C for 40 minutes) and cultured in Methocell (Tokyo Chem., Tokyo, Japan) with or without Epo for 2 days. The milder stress treatment was used for these primary cells because the conditions used for SKT6 cells caused apoptotic cell death. The hemoglobinized colonies formed were counted. It was clearly demonstrated that these environmental stresses actually induced erythroid differentiation of primary mouse fetal liver cells without Epo, whereas the numbers of erythroid colonies formed by osmotic or heat treatment were about one third of those of Epo stimulation (data not shown). Furthermore, human promyelocytic HL60 cells, which can be differentiated into macrophage-like and granulocyte-like cells with 12-O-tetradecanoylphorbol-13-acetate and DMSO, respectively, similarly differentiated into granulocyte-like cells by comparable stress treatment, and the differentiation was blocked by inhibition of p38 (data not shown). Therefore, induction of differentiation by these cellular stresses for a short time is not a characteristic of SKT6 cells. The results obtained with SKT6 cells can be more generalized in normal erythropoiesis.
We showed here that the prolonged and persistent activation of JNK/SAPK and p38 and inactivation of ERK induced apoptotic cell death, while transient activation of JNK/SAPK and p38 led to erythroid differentiation of SKT6 cells. Thus, we concluded that JNK/SAPK and p38 serve an important function in both cell differentiation and apoptosis and that duration of activation of these kinases may partly contribute to determining the cell fate, cell differentiation, and apoptotic cell death. Chen et al23 also reported that the T-cell activation signals through CD28 induced a rapid and transient JNK1 activation in Jurkat T-cells, which in turn stimulated cell growth, whereas γ radiation or UV caused delayed and persistent JNK1 activation, which led to apoptotic cell death. These results also support our conclusion. The delay of JNK/SAPK activation may be caused by the time needed for accumulation of irreparable damage to certain threshold levels. The different timing and/or duration of JNK/SAPK and p38 activation may alter the outcome, proliferation, differentiation, or apoptosis. The prolonged JNK/SAPK and p38 activation after stress treatment may be caused by the lack or low level of dual-specific Thr/Tyr phosphatases, which dephosphorylate and inactivate the phophorylated ERK, JNK/SAPK, and/or p38.51-55 Thus, the prolonged and persistent activation of JNK/SAPK and p38 may overcome a certain threshold level to trigger activation of the factors required for apoptosis such as caspase family and specific DNases and/or to induce inhibition of apoptosis inhibitors such as Bcl-2, Bcl-XL, and Hsp family, which finally lead to apoptotic cell death.
We show here that environmental stresses not only trigger activation of JNK/SAPK and p38, but also concurrently suppresses ERK activity. The inactivation of ERK together with activation of JNK/SAPK and p38 may be critical for apoptosis. Xia et al18 reported that concurrent activation of JNK/SAPK and p38 and inhibition of ERK induces apoptosis, whereas activation of ERK prevents apoptosis in PC12 cells. Actually, we also observed here that inhibition of ERK clearly stimulates stress-induced apoptotic cell death and that activation of ERK prevents SKT6 cells from stress-induced apoptotic cell death. Thus, JNK/SAPK and p38 cascades may also act in an opposite way from ERK in SKT6 cells. The dynamic balance between MAP kinases and phosphates, and/or ERK and JNK/SAPK-p38 may also contribute to determine the cell fate, whether the cells undergo proliferation, differentiation, or apoptosis. The target molecules of these MAP kinases for apoptotic cell death as well as cell differentiation have to be identified to further understand the molecular mechanism of apoptosis and cell differentiation.
Inhibition of either JNK/SAPK or p38 suppressed stress-induced differentiation as well as apoptosis in SKT6 cells. However, the caspase-3 specific inhibitor, DEVD-CHO, caused delay of stress-induced apoptosis, but did not affect stress-induced erythroid differentiation (data not shown). The activation of caspase-3 appears to be essential for the apoptotic process and occurs after irreversible commitment to cell death. As shown in Fig 2B, caspase-3 was activated between 1 hour and 6 hours after stress treatments, suggesting that the commitment to cell death took place during this period. DNA fragmentation occurred around 24 hours after stress treatments; most of the cells fell into irreversible crisis at around 36 hours. Therefore, differentiation and cell death processes appear to occur in the following order: activation of JNK/SAPK and p38 and inactivation of ERK, commitment of differentiation or cell death, caspase-3 activation, DNA fragmentation, and finally total cell death.
The level of stress-induced differentiation did not reach to that of Epo-stimulated differentiation, indicating that transient activation of only JNK/SAPK and p38 may not be enough for full erythroid differentiation. We noticed that ERK activation inhibits stress-induced apoptotic cell death but has nothing to do with erythroid differentiation. It has been described, however, that JAK-STAT signaling pathway may be involved in Epo-induced erythroid differentiation.56 57 Therefore, activation of p38 and JNK/SAPK is required for erythroid differentiation, but other independent signaling pathways such as JAK-STAT pathway may also play a role in full erythroid differentiation.
ACKNOWLEDGMENT
We thank R.J. Davis for MKK6 (Glu), N.G. Ahn for MKK1 constructs, E. Nishida for discussion, J.S. Lee for SB203580, and N. Takahashi and J. Iita for technical assistance.
Supported in part by a Special Grant for Promotion of Research from The Institute of Physical and Chemical Research (RIKEN), and grants from the Ministry of Education, Science and Culture of Japan, from the Uehara Memorial Foundation, and from the Suzuken Memorial Foundation.
REFERENCES
Author notes
Address reprint requests to Kazuo Todokoro, PhD, Tsukuba Life Science Center, The Institute of Physical and Chemical Research (RIKEN), 3-1, Koyadai, Tsukuba, Ibaraki 305-0074, Japan; e-mail:todokoro@rtc.riken.go.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal