Stable mixed donor/host hematopoietic chimerism can be accomplished in dog leukocyte antigen (DLA)-identical littermate dogs given sublethal (200 cGy) total-body irradiation (TBI) before and immunosuppression with mycophenolate mofetil (MMF) and cyclosporine (CSP) after transplant (Blood 89:3048, 1997). Studies were based on the hypothesis that drugs that prevent graft-versus-host disease (GVHD) after transplant also suppress host-versus-graft (HVG) reactions and thereby enhance engraftment. Here, we asked whether pretransplant TBI provided marrow space for the graft to home or caused host immunosuppression. To address the questions, recipients were given pretransplant irradiation to cervical, thoracic, and abdominal lymph nodes (except pelvis), DLA-identical littermate marrow grafts, and MMF/CSP posttransplant. Six dogs that received 450 cGy irradiation showed initial engraftment. Two rejected their grafts after 8 and 18 weeks, 1 died with GVHD and engraftment, and 3 are alive as mixed chimeras after 57 to 97 weeks. Four dogs given 200 cGy irradiation also showed initial engraftment, but rejected their grafts after 10 to 18 weeks. Mixed chimerism was present in nonirradiated marrow and lymph node spaces and involved granulocytes, T cells, and monocytes. While other explanations are possible, results seem consistent with the hypothesis that pretransplant radiation provides host immunosuppression, and grafts can create their own marrow space. These data set the stage for the development of novel transplant regimens that substitute immunosuppressive for cytotoxic agents.
CONVENTIONAL ALLOGENEIC hematopoietic stem-cell transplants involve conditioning of recipients by intense and toxic chemoradiation, which has the triple purpose of eradicating the underlying disease, creating marrow space for the graft to home, and destroying the host’s immune system in a broad and nonspecific manner for the graft to be accepted. To prevent graft-versus-host disease (GVHD), grafts are either depleted of T cells or, more often, recipients are given postgrafting immunosuppression. We have recently proposed a new concept for conducting allotransplants, which is founded on the knowledge that both host-versus-graft (HVG) and GVH reactions are T-cell–mediated in the major histocompatibility complex (MHC)-identical setting. Consequently, we sought to optimize postgrafting immunosuppression not only to prevent GVHD, but also to control HVG reactions. This enabled us to reduce markedly the dose of pretransplant total-body irradiation (TBI) needed for uniformly successful engraftment. Specifically, a short course of postgrafting mycophenolate mofetil (MMF) and cyclosporine (CSP) allowed the TBI dose to be lowered from the supralethal range of 920 cGy to the sublethal and nonmyeloablative level of 200 cGy.1 Dogs transplanted in this manner became stable mixed donor/host hematopoietic chimeras. The finding raised the questions whether low-dose (200 cGy) TBI in this model primarily served to create marrow space for the graft to home or whether its role was to provide host immunosuppression. To address these questions, the present study substituted lymph node irradiation for TBI before transplant and sought to determine whether stable mixed chimerism could be established in unirradiated marrow and lymph node spaces.
MATERIALS AND METHODS
Litters of harriers, beagles, Walker hounds, pit bull/beagle crossbreeds, and other mixed breeds were either raised at the Fred Hutchinson Cancer Research Center (Seattle, WA) or purchased from commercial kennels in the state of Washington. The dogs weighed from 7.0 to 13.3 (median, 9.6) kg and were 7 to 27 (median, 8) months old. They were observed for disease for at least 60 days before study. All were immunized for leptospirosis, papillomavirus, distemper, hepatitis, and parvovirus. Research was conducted according to the principles outlined in the Guide for Laboratory Animal Facilities and Care prepared by the National Academy of Sciences, National Research Council. The research protocols were approved by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center. The kennels are certified by the American Association for Accreditation of Laboratory Animal Care.
DLA-matched littermate donor/recipient pairs were chosen on the basis of identity by highly polymorphic MHC class I and class II microsatellite markers.2 In addition, specific DLA DRB1 allelic identity was determined by direct sequencing.3
For lymph node irradiation, a high-energy linear accelerator (Varian CLINAC 6, Palo Alto, CA) was used. The irradiation was delivered with a 6 million electron volt beam using equally weighted anterior and posterior ports to treat the central portion of the dogs. The field extended from the upper pelvis to the base of the skull. The pelvis itself, the lower extremities, the head, and the upper extremities, as well as most of the ribs, were blocked by lead shields. The limitation of the technique was that marrow in the vertebrae and sternum was irradiated, while, in turn, pelvic, splenic, splanchnic, medullary, and peripheral lymphoid tissues remained unirradiated. Irradiation doses studied were 450 and 200 cGy, respectively, and were delivered at 200 cGy/min in a single setting. Two dogs were given 450 cGy lymph node irradiation and no marrow grafts to assess the effect of irradiation on peripheral blood cell counts.
Marrow for transplantation was aspirated from the donors under general anesthesia through long needles inserted into humeri and femora.4 After appropriate screening, marrow was infused intravenously (IV) within 4 hours of lymphoid irradiation at doses of 2.1 to 4.8 (median, 4.0) × 108 nucleated cells/kg. The day of marrow infusion was designated as day 0. All dogs were given standard postgrafting care.5 This included twice-daily oral nonabsorbable antibiotics, polymyxin sulfate and neomycin sulfate, which were begun on day –1 and given until day 14 after transplant, and prophylactic systemic ceftazadime, injected twice daily from day 0 until day 14. None of the transplanted dogs required RBC or platelet transfusion support. The dogs’ clinical status was assessed twice daily. WBC counts, platelet counts, hematocrits, and differentials were performed daily through day 21 and twice weekly thereafter.
All marrow recipients were given MMF 10 mg/kg twice daily subcutaneously (SC), on days 0 to 27, and CSP 10 mg/kg twice daily orally on days −1 to 35 after transplant, 7.5 mg/kg twice daily on days 36 to 50, 5 mg/kg twice daily on days 51 to 75, and 3 mg/kg twice daily on days 76 to 100.1 Hematopoietic engraftment was assessed by sustained recoveries of granulocyte and platelet counts after the postirradiation nadir and by documentation of donor (CA)n repeat polymorphisms in cells from peripheral blood, popliteal lymph nodes, and marrow. Graft rejection was defined as complete disappearance of cells with donor (CA)n repeat polymorphisms. The (CA)n dinucleotide repeats were assessed using a polymerase chain reaction (PCR)-based assay.6 The assay has the sensitivity to reliably detect donor or host cells down to a level of 2.5% of the total cell population. Mixed hematopoietic chimerism was quantified by estimating the proportion of donor-specific DNA among host DNA using the storage phosphorimaging technique and defined as between 2.5% and 97.5% donor cells after transplant.7 The marrow aspiration (humeral head) and lymph node (popliteal area) biopsy sites used after transplant were outside of the irradiation field. Marrow aspirates and lymph node biopsies were performed under general anesthesia.
Cell separations were performed using monoclonal antibodies and a fluorescence-activated cell sorter (Vantage FACSSORT; Becton Dickinson, San Jose, CA). Granulocytes were first separated from mononuclear cells by a Ficoll cut (specific gravity, 1.074) and then further purified using the monoclonal antibody DM5, which recognizes a canine myeloid antigen.8 CD4 and CD8+ T cells were purified using monoclonal antibodies CA9.JD3 (anti-CD4) and CA13.1E4 (anti-CD8).9
When studies were completed, dogs were euthanized and underwent complete autopsies, which included histopathologic examinations.
RESULTS
Lymph node irradiation controls.
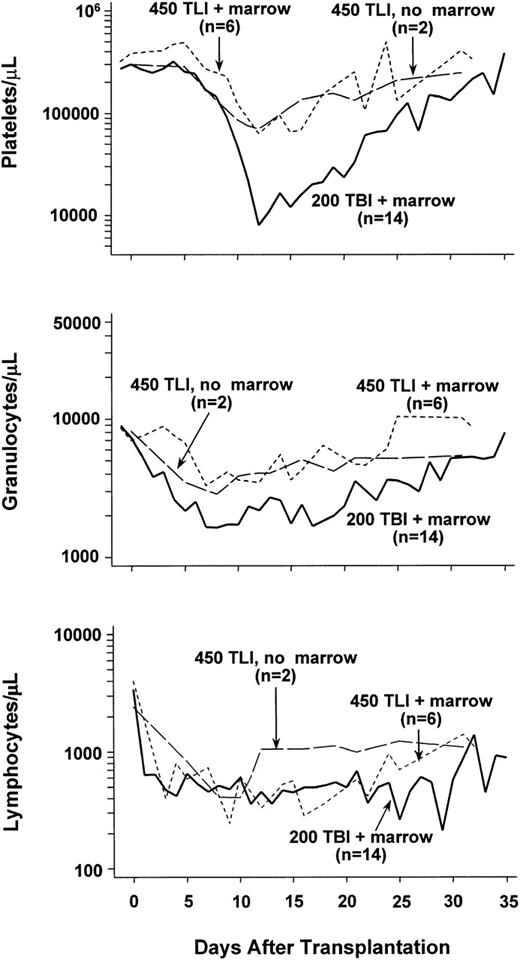
Two dogs were given 450 cGy lymph node irradiation, but neither marrow grafts nor MMF/CSP. Figure 1 shows their granulocyte, lymphocyte, and platelet changes over a period of 35 days following irradiation. Both dogs showed declines in platelet counts to less than 100,000/μL beginning on days 10 and 11. Thereafter, platelet counts slowly returned to the normal range. Similarly, moderate declines in granulocyte counts were noted with nadirs of 2,500 and 3,200 cells/μL, respectively, seen on day 8. Thereafter, counts slowly returned to the normal range. Lymphocyte counts declined dramatically, and nadirs were reached between days 8 and 10 with subsequent slow recovery. Hematocrits remained within the normal range (not shown).
Median peripheral blood platelet, granulocyte, and lymphocyte changes in dogs given 450 cGy total-lymphoid irradiation (TLI) and no marrow grafts (n = 2), 450 cGy TLI, marrow grafts, and postgrafting MMF/CSP (n = 6), or 200 cGy TBI, marrow grafts, and postgrafting MMF/CSP (n = 12).
Median peripheral blood platelet, granulocyte, and lymphocyte changes in dogs given 450 cGy total-lymphoid irradiation (TLI) and no marrow grafts (n = 2), 450 cGy TLI, marrow grafts, and postgrafting MMF/CSP (n = 6), or 200 cGy TBI, marrow grafts, and postgrafting MMF/CSP (n = 12).
DLA-identical marrow grafts.
Table 1 summarizes the results of allogeneic marrow transplants. All 6 recipients given 450 cGy lymph node irradiation showed initial evidence of allogeneic engraftment, which was manifested as mixed donor/host chimerism. Two of the 6 lost their grafts at 8 and 18 weeks after transplantation, respectively, and survived with complete autologous recovery. One of the 4 remaining dogs died with acute GVHD at slightly more than 6 weeks after transplant. This dog’s hematopoietic system was almost entirely replaced by donor cells. Three dogs have remained stable mixed chimeras between 57 and 97 weeks after transplant.
Marrow Grafts From DLA-Identical Littermates After Conditioning With Lymph Node Irradiation
| Lymph Node Irradiation Dose (cGy)* . | Recipient No. . | Sustained Allograft . | GVHD . | Complete Autologous Recovery . | Duration of Mixed Chimerism (wk)† . | Cause of Death . | |
|---|---|---|---|---|---|---|---|
| Acute . | Chronic . | ||||||
| 450 | E451 | No | — | — | Yes | 18 | ET2 |
| E535 | No | — | — | Yes | 8 | ET2 | |
| E481 | Yes | Yes | — | — | >6 | GVHD, ET1 | |
| E527 | Yes | No | No | — | >57 | Alive | |
| E400 | Yes | No | No | — | >97 | Alive | |
| E399 | Yes | No | No | — | >73 | ET2 | |
| 200 | E270 | No | — | — | Yes | 10 | ET2 |
| E329 | No | — | — | Yes | 10 | ET2 | |
| E490 | No | — | — | Yes | 11 | ET2 | |
| E464 | No | — | — | Yes | 18 | ET2 | |
| Lymph Node Irradiation Dose (cGy)* . | Recipient No. . | Sustained Allograft . | GVHD . | Complete Autologous Recovery . | Duration of Mixed Chimerism (wk)† . | Cause of Death . | |
|---|---|---|---|---|---|---|---|
| Acute . | Chronic . | ||||||
| 450 | E451 | No | — | — | Yes | 18 | ET2 |
| E535 | No | — | — | Yes | 8 | ET2 | |
| E481 | Yes | Yes | — | — | >6 | GVHD, ET1 | |
| E527 | Yes | No | No | — | >57 | Alive | |
| E400 | Yes | No | No | — | >97 | Alive | |
| E399 | Yes | No | No | — | >73 | ET2 | |
| 200 | E270 | No | — | — | Yes | 10 | ET2 |
| E329 | No | — | — | Yes | 10 | ET2 | |
| E490 | No | — | — | Yes | 11 | ET2 | |
| E464 | No | — | — | Yes | 18 | ET2 | |
All recipients were given MMF/CSP posttransplant.
Delivered at 200 cGy/min.
Determined by microsatellite marker studies.
Abbreviations: ET1, euthanized because of poor clinical condition; ET2, euthanized at completion of the study.
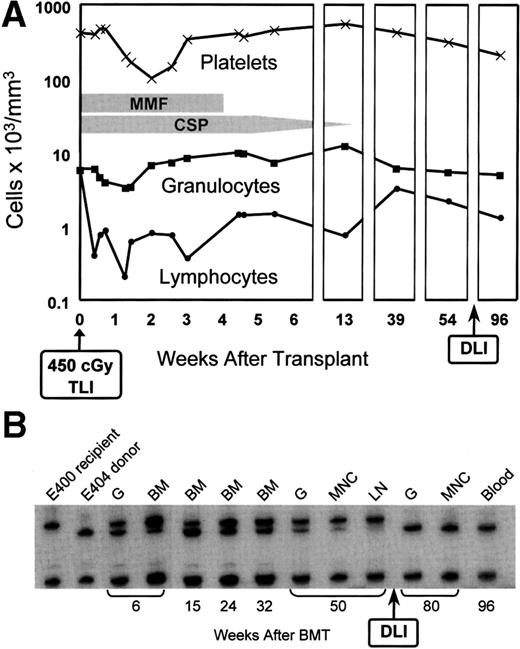
Figure 2 illustrates peripheral blood changes and microsatellite marker studies in dog E400 with sustained graft. As was seen in control dogs, the degree of myelosuppression from the lymph node irradiation in the transplanted dog was mild. The recipient’s granulocyte count reached a nadir of 3,400 cells/μL by day 9 after transplant, and the platelet count a nadir of 101,000 cells/μL by day 14. Counts returned rapidly to pretransplant levels. In contrast, lymphocyte counts declined to a nadir of 200 cells/μL on day 9; pretransplant levels have as yet not been reached by day 270. The microsatellite marker studies illustrate mixed chimerism not only among nucleated peripheral blood cells, but also among popliteal lymph node and marrow cells obtained from sites outside of the irradiation field. Mixed chimerism was present already in the first marrow sample, which was taken at 4 weeks after transplant. As part of a separate study,10 dog E400 was given an IV infusion of 6.1 × 107 alloreactive CD3+ T cells/kg from the marrow donor 72 weeks after the original transplant (Fig 2). Within 6 weeks of infusion, microsatellite markers of host origin disappeared from DNA of nucleated peripheral blood, marrow, and lymph node cells. Only cells of donor origin have been found during the subsequent period of observation, which now extends to 25 weeks. This conversion from mixed to all donor chimerism occurred in the absence of clinically evident GVHD.
(A) Peripheral blood granulocyte, platelet, and lymphocyte changes in dog E400 conditioned with 450 cGy TLI and given a marrow graft from a DLA-identical littermate on day 0, followed by postgrafting MMF/CSP for 4 and 14.3 weeks, respectively. (B) Results of testing for microsatellite markers of donor and recipient cells before transplantation (lanes 1 and 2) and recipient cells after marrow transplantation (lanes 3 to 13). G, granulocytes; BM, bone marrow; MNC, mononuclear cells; LN, lymph node; DLI, donor lymphocyte infusion.
(A) Peripheral blood granulocyte, platelet, and lymphocyte changes in dog E400 conditioned with 450 cGy TLI and given a marrow graft from a DLA-identical littermate on day 0, followed by postgrafting MMF/CSP for 4 and 14.3 weeks, respectively. (B) Results of testing for microsatellite markers of donor and recipient cells before transplantation (lanes 1 and 2) and recipient cells after marrow transplantation (lanes 3 to 13). G, granulocytes; BM, bone marrow; MNC, mononuclear cells; LN, lymph node; DLI, donor lymphocyte infusion.
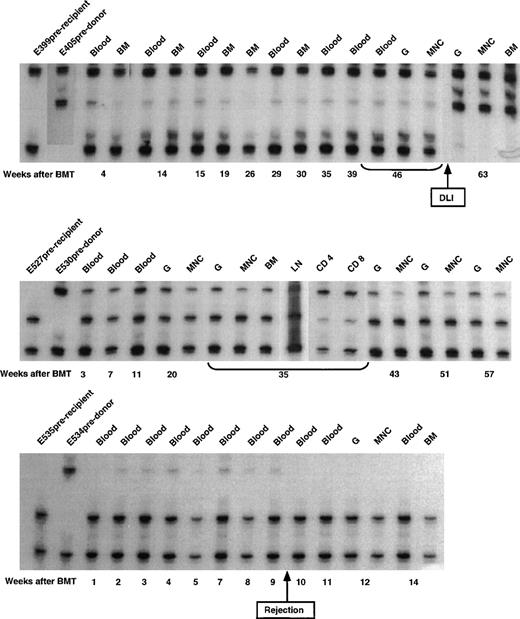
The microsatellite marker studies on 3 of the remaining dogs conditioned by 450 cGy lymph node irradiation are shown in Fig3. Initial allogeneic engraftment and subsequent rejection were seen in dog E535. Dogs E527 and E399 remained stable mixed chimeras. The initial donor contribution in E399 was estimated to be 5% to 10%. Within 6 weeks of infusion of donor lymphocytes at week 57 after transplant,10 the donor contribution increased to ≥90%.
Microsatellite marker studies of donor and recipient cells before transplantation and recipient cells after marrow transplantation in 3 dogs given 450 cGy TLI, marrow grafts from DLA-identical littermates on day 0, and postgrafting MMF/CSP for 4 and 14.3 weeks, respectively. CD4, CD4+ T cells; CD8, CD8+ T cells.
Microsatellite marker studies of donor and recipient cells before transplantation and recipient cells after marrow transplantation in 3 dogs given 450 cGy TLI, marrow grafts from DLA-identical littermates on day 0, and postgrafting MMF/CSP for 4 and 14.3 weeks, respectively. CD4, CD4+ T cells; CD8, CD8+ T cells.
Table 2 shows a more detailed study of microsatellite marker results in 1 of the transplanted dogs (E527) using flow cytometry and phosphorimage analysis. The analysis, performed 38 weeks after transplant, showed that mixed chimerism included all cell lineages studied. Peripheral blood granulocytes showed a donor contribution of 45%, mononuclear cells 35%, CD4+ cells 65%, and CD8+ cells 55%. Furthermore, 25% of lymph node cells and 45% of marrow cells were composed of donor cells.
Microsatellite Marker Results in Dog E527 38 Weeks After 450 cGy Lymphoid Irradiation and Marrow Transplant
| Source of Cells . | % Donor Cells* . |
|---|---|
| Peripheral blood | |
| Granulocytes | 45 |
| Mononuclear cells | 35 |
| CD4+ T cells | 65 |
| CD8+ T cells | 55 |
| Lymph node (popliteal) | |
| Nucleated cells | 25 |
| Marrow (humerus head) | |
| Nucleated cells | 45 |
| Source of Cells . | % Donor Cells* . |
|---|---|
| Peripheral blood | |
| Granulocytes | 45 |
| Mononuclear cells | 35 |
| CD4+ T cells | 65 |
| CD8+ T cells | 55 |
| Lymph node (popliteal) | |
| Nucleated cells | 25 |
| Marrow (humerus head) | |
| Nucleated cells | 45 |
Estimated by phosphorimage analysis.7
Figure 1 shows the median peripheral blood cell counts from all 6 dogs studied. Except for lower lymphocyte counts, blood cell changes were comparable to those in lymph node radiation controls. The lower lymphocyte values were likely due to MMF. The median platelet nadir was at 70,000 cells/μL and the granulocyte nadir at 3,000 cells/μL. Only the dog with GVHD (E481) experienced a platelet nadir less than 10,000 cells/μL, presumably the result of the elimination of host hematopoiesis by GVHD. By comparison, historical control dogs given 200 cGy TBI1 11 experienced more profound declines and more delayed recoveries of granulocyte and platelet counts, while their lymphocyte counts were comparable to those in lymph node radiation controls (Fig 1).
At a dose of 200 cGy lymph node irradiation, all 4 dogs so treated showed initial allogeneic engraftment. The 4 rejected their allografts after 10 to 18 weeks as judged by the disappearance of donor microsatellite marker bands in DNA of cells from marrow and peripheral blood. The maximum contributions of donor cells in the 4 dogs ranged from 5% to 10%. Their peripheral blood counts experienced only moderate changes after transplant, with a median platelet nadir of 108,000/μL (day 13), granulocyte nadir of 4,100/μL (day 12), and lymphocyte nadir of 900/μL (day 3). Counts recovered promptly.
DISCUSSION
Previous studies showed that stable mixed donor/host hematopoietic chimerism was reliably established in a canine model of DLA-identical marrow transplantation when recipients were conditioned by sublethal and nonmyeloablative TBI (200 cGy) and given a short course of MMF/CSP after transplant to control residual HVG and also GVH reactions.1 11 This raised the questions whether TBI was required to provide host immunosuppression or create marrow space for the allograft to home. Within the limitations of the experimental design, current findings of long-term mixed chimerism in dogs conditioned with 450 cGy lymph node irradiation are consistent with the concept that an important role of pretransplant irradiation was to effect host immunosuppression and that marrow space could be created by the grafts themselves, most likely through subclinical GVH reactions. Direct evidence for the GVH effect came from the stable presence of donor cells in marrow and lymph node spaces that were located outside of the irradiation field. Results support the hypothesis that allografts can be achieved by substituting effective host immunosuppression for cytotoxic pretransplant therapy including TBI. Effective pretransplant immunosuppression combined with posttransplant immunosuppression (MMF/CSP), which is aimed at controlling both host and donor immune cell activities would result in mutual graft/host tolerance that would be manifested as stable mixed donor/host chimerism.
The external-beam lymph node irradiation used here lacked specificity and excluded a substantial proportion of the dogs’ lymphoid tissues, eg, those in marrow, bowel, spleen, pelvis, and peripheral lymph nodes. This probably explains why 2 of the dogs given 450 cGy radiation and all dogs given 200 cGy ultimately rejected their allografts.
A number of preclinical studies in inbred strains of mice have also explored the development of less intensive conditioning programs for establishing allogeneic hematopoietic engraftment.12-22Most studies have included host T-cell depletion by in vivo administration of antibodies that were combined with TBI at high but, at least in mice, sublethal doses and dose rates. TBI was often delivered along with pretransplant thymic irradiation and postgrafting CSP or even high-dose posttransplant cyclophosphamide. Most studies that have involved allogeneic transplants did not address the questions of marrow space versus immunosuppression since all included TBI. However, in earlier studies, Slavin et al23,24 reported on allogeneic marrow engraftment in mice using 1,600 cGy total lymphoid irradiation. In another study, Leong et al25 used dimethylbusulfan “as a selective ‘space’-creating myelosuppressive agent and CD4 plus CD8 monoclonal antibodies as sole immunosuppressive agents” to condition inbred mice for hematopoietic grafts. They concluded that both creation of marrow space and host immunosuppression were needed for successful grafts. Their argument was based on the assumption that dimethylbusulfan was strictly myelosuppressive. However, their conclusions may have to be tempered somewhat by the earlier demonstration in a random-bred canine allograft model that high-dose dimethylbusulfan was not only myelosuppressive, but also immunosuppressive.26 Specifically, half of the dogs treated with a single dose of 10 mg dimethylmyleran/kg experienced sufficient immunosuppression for stable allogeneic hematopoietic engraftment, and the engraftment rate could be further increased to 90% with additional small doses of antithymocyte serum. The dimethylmyleran doses used in the most successful murine studies ranged from 8 to 20 mg/kg.25
There is recent evidence from studies in syngeneic or congenic mice that infusion of very large numbers of marrow cells (≥8 × 109 cells/kg) without conditioning of recipients can lead to engraftment of transplanted cells.27 In contrast with the observations in mice by Stewart et al and the current findings in dogs conditioned by lymphoid radiation, were reports on successfully transplanted, nonconditioned humans and dogs with severe combined immunodeficiency disease (SCID) in which either direct chromosome analysis of marrow cells or PCR-based testing for the genotype of the γ-chain gene in blood monocytes showed exclusively recipient cells,28,29 while T cells were either all or partially of donor type. An exception was a patient who developed myelosuppression and aplasia in the course of GVHD and then changed to all donor type hematopoiesis after a second transplant.30,31 The current canine results of mixed marrow chimerism and the subsequent conversion to all donor chimerism by lymphocyte infusion10 can also be explained by creation of marrow space through (subclinical) GVHD.
Based on the canine data with lymphoid irradiation, we have treated 2 patients with congenital T-cell deficiencies other than SCID by marrow grafts without conditioning but with MMF/CSP for 4 and 5 weeks, respectively, after transplant.32 This was done under the assumption that the inherited T-cell deficiencies were functionally equivalent to those induced in current dogs by lymphoid irradiation. Both patients have stably engrafted. The first patient, a 30-year-old man, is now more than 6 months after transplant. In addition to donor T- and B-cell engraftment, 95% of his granulocytes and 50% of his marrow cells are of donor origin. These results give indirect support to the notion that allografts that involve all cell lineages may be achievable solely with the help of immunosuppression, an approach that would avoid the toxicities associated with current high-dose transplant regimens and, thereby, be safe enough to be performed in the ambulatory care setting.
ACKNOWLEDGMENT
We are grateful to Dr George Sale from the Fred Hutchinson Cancer Research Center Pathology group for review of histopathology and to Lori Ausburn, Eric Bell, Alix Smith, and the technicians of the Canine Shared Resource and of the hematology and pathology laboratories for their technical assistance. Dr Barbara Johnston provided veterinary supervision. Bonnie Larson and Harriet Childs provided manuscript preparation and illustration. We would like to thank Dr Tom Matthew at Roche Bioscience (Palo Alto, CA) for providing MMF.
Supported in part by Grants No. CA15704, HL36444, HL03701, and DK42716 from the National Institutes of Health (NIH), Department of Health and Human Services, Bethesda, MD; by a grant from the Gabriella Rich Leukemia Foundation (P.M.); by NIH Grants No. DK09718 (G.G.) and RR12558 (J.L.W.); and by a prize from the Josef Steiner Krebsstiftung, Bern, Switzerland, and the Laura Landro Salomon Endowment Fund (R.S.). H.P.K. is a Markey Molecular Medicine Investigator.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal