Abstract
Circulating plasma cells in 10 cases of reactive plasmacytosis had a shared phenotype with early plasma cell (CD19+CD38+ CD138+ CD40+CD45+ CD11a+ CD49e−CD56−). In most cases, a minor subpopulation of CD28+ plasma cells was also detected. Reactive plasma cells were highly proliferative, suggesting the presence of circulating progenitors (plasmablasts). After CD138+ plasma cell removal, highly proliferative CD138− plasmablasts differentiated into CD138+ plasma cells within a few days. This differentiation, which was associated with increased CD38 and decreased HLA-DR expression, was further confirmed by a large increase in intracellular Ig content (associated with Ig secretion) and was concomitant with extensive secretion of interleukin-6 (IL-6). The addition of neutralizing anti–IL-6 and anti-CD126 (IL-6 receptor) monoclonal antibodies totally prevented Ig secretion and cell differentiation by inducing apoptosis of plasmablasts, which indicates that IL-6 is an essential survival factor for plasmablasts. This report provides the first characterization of normal plasmablasts and shows that their phenotype is not exactly that of multiple myeloma cells.
LONG-LIVED NONPROLIFERATING bone marrow plasma cells (PCs) are generated during immune response, essentially memory immune response.1,2 Memory B cells activated by the antigen in secondary lymphoid organs in close contact with dendritic cells differentiate into plasmablasts that migrate into the bone marrow and finally become PCs.1-3 Plasmablasts, unlike PCs, are short-lived proliferating cells.2 In well-controlled immune response, plasmablasts represent less than 0.1% of peripheral blood mononuclear cells, and no PCs are detected in normal peripheral blood. For these reasons, the study of terminal PC differentiation remains difficult, although it is especially critical if progress is to be made in the biology of myeloma cells, the malignant counterpart of normal PCs.4 Although several models of PC differentiation have been developed in vitro to overcome these difficulties,5-10most of them are limited by the low number of PCs obtained. Yet, recent studies have shown that CD27 and/or CD40 triggering of memory cells allows more than 50% of PCs to be obtained.9,10Unfortunately, the study of plasmablast differentiation into PCs is not easy with these models, because the culture is a mix of B cells, plasmablasts, and PCs. Interestingly, a large number of nonmalignant PCs can be obtained in cases of reactive plasmacytosis (RP), thereby allowing more detailed study of normal PCs. RP has been reported in a variety of infections and neoplastic conditions,11-14 and the PCs involved are polyclonal.11-13 However, data concerning the biology of reactive PCs remain sparse. In vivo, reactive plasmacytoses are known to be proliferative and to disappear spontaneously and rapidly, but no data are available concerning the mechanisms controlling their proliferation and cell survival or death. More recently, reactive plasmacytoses have been observed in patients bearing interleukin-6 (IL-6)–producing tumors in cases of cardiac myxoma and gastric carcinoma.12,14 Furthermore, the proliferation of reactive polyclonal PCs in cardiac myxoma has been shown to be IL-6–dependent.12 Because IL-6 has been extensively described as a B-cell differentiation factor15and as a growth factor for myeloma cells,4 the study of the mechanisms regulating the proliferation and death of PCs from RP could be of major interest in improving our understanding of myeloma cells.
We report here the phenotype of PCs from 10 reactive plasmacytoses. In all 4 cases evaluated, reactive plasmacytoses involved highly proliferating cells, suggesting that available circulating plasmablasts were present. In fact, plasmablasts in vitro differentiated into PCs, which facilitated studies of their phenotypic and functional characterization and of the differentiation process. The implications of plasmablast characterization are considered with respect to multiple myeloma (MM) as an expansion of malignant plasmablasts and plasma cells.
MATERIALS AND METHODS
Reactive Plasmacytoses
Ten cases of plasmacytosis were collected over a 3-year period. In all cases, transient polyclonal circulating PCs were observed in peripheral blood (from 8% to 79% PCs) in different contexts, mainly infections (n = 5, including 2 Epstein-Barr virus [EBV] infections in immunocompromised hosts, designated as RP5 and RP7) or tumors (n = 3; Table 1). A diagnosis of reactive plamacytosis was established initially by cytological identification of PCs on spins, followed by flow cytometry PC identification (intracellular κ and λ staining). For patient RP5, plasmacytosis was quite extensive in terms of the absolute number of cells and the percentage of plasma cells allowing the cryopreservation of peripheral blood mononuclear cells (PBMNCs).
Characteristics of Patients With RP
| . | Diagnosis . | More Specific Context . | Age/Sex . | Duration . | % of PCs . | CRP (mg/L)* . | Ig (g/L)* . |
|---|---|---|---|---|---|---|---|
| RP1 | T-cell lymphoma | — | 69/F | ND | 20† | 66 | ND |
| RP2 | B-cell lymphoma | — | 87/F | ND | 11‡ | ND | ND |
| RP3 | Acute myeloid leukemia | — | 66/M | ND | 11‡ | ND | ND |
| RP4 | Rheumatoid arthritis | At the time of cardiac surgery | 78/F | ND | 10† | ND | ND |
| RP5 | Infectious mononucleosis | Treatment with cyclosporin A | 8/M | 10 d | 50† | 21 | 36 |
| RP6 | Acute respiratory infection | B-cell lymphoma | 42/M | 7 d | 20† | 20 | 43 |
| RP71-153 | EBV reactivation | Aplasia1-155 | 27/M | NE | 79† | 270 | 34 |
| RP8 | Acute respiratory infection | — | 54/M | 6 d | 8† | ND | ND |
| RP9 | Acute respiratory infection | — | 70/M | ND | 12‡ | 108 | 28 |
| RP10 | Primary amyloidosis | Aplasia1-155 | 49/M | 5 d | 12† | ND | ND |
| . | Diagnosis . | More Specific Context . | Age/Sex . | Duration . | % of PCs . | CRP (mg/L)* . | Ig (g/L)* . |
|---|---|---|---|---|---|---|---|
| RP1 | T-cell lymphoma | — | 69/F | ND | 20† | 66 | ND |
| RP2 | B-cell lymphoma | — | 87/F | ND | 11‡ | ND | ND |
| RP3 | Acute myeloid leukemia | — | 66/M | ND | 11‡ | ND | ND |
| RP4 | Rheumatoid arthritis | At the time of cardiac surgery | 78/F | ND | 10† | ND | ND |
| RP5 | Infectious mononucleosis | Treatment with cyclosporin A | 8/M | 10 d | 50† | 21 | 36 |
| RP6 | Acute respiratory infection | B-cell lymphoma | 42/M | 7 d | 20† | 20 | 43 |
| RP71-153 | EBV reactivation | Aplasia1-155 | 27/M | NE | 79† | 270 | 34 |
| RP8 | Acute respiratory infection | — | 54/M | 6 d | 8† | ND | ND |
| RP9 | Acute respiratory infection | — | 70/M | ND | 12‡ | 108 | 28 |
| RP10 | Primary amyloidosis | Aplasia1-155 | 49/M | 5 d | 12† | ND | ND |
The percentage of plasma cells was either defined in the total population, ie, on blood smears (
) or in PBMNC, ie, on cytospins (
).
Abbreviations: ND, not done; NE, not evaluable (patient died during plasmacytosis).
Normal values: CRP, <3 mg/L; Ig, <15 g/L.
This case has been extensively described elsewhere.18
Aplasia after stem cell transplantation (RP7, allogeneic; RP10, autologous).
In vitro, cells were cultured in RPMI 1640 (GIBCO BRL, Cergy-Pontoise, France) supplemented with 10% heat-inactivated fetal calf serum (FCS; GIBCO BRL), 2 mmol/L glutamine, 100 μg/mL streptomycin, 100 U/mL penicillin, and 2 × 10−5 mol/L 2-mercaptoethanol.
Reagents and Antibodies
Recombinant human fibroblast growth factor basic (FGF basic) was obtained from R&D (Minneapolis, MN).
Except for anti-κ (κ), which were rabbit polyclonal antibodies, all others were mouse monoclonal antibodies (MoAbs). Anti-CD138 (B-B4), anti-CD40-phycoerythrin (PE), anti-CD95 (B-G27), and anti–IL-6 (B-E8) MoAbs were obtained from Diaclone Research (Besançon, France); APO2.7-PE, unconjugated or PE- or fluorescein isothiocyanate (FITC)-conjugated IgG1 control, anti-CD11a-PE, anti-CD19-FITC/-PE, anti-CD20, anti-CD21, anti-CD23-FITC, anti-CD45-FITC, anti-CD45RA-FITC, anti-HLADR-PE, anti-CD95 (CH-11), anti-CD126-PE, and FITC-conjugated goat antimouse (GAM) MoAbs from Immunotech (Marseille, France); anti-κ-PE and anti-CD45RO-FITC from Dako (Glostrup, Denmark); anti-CD20-FITC, anti-CD22-PE, anti-CD25-PE, anti-CD28-PE, anti-CD38-PE, anti-CD56-PE, and anti-CD80-PE MoAbs from Becton Dickinson (San Jose, CA); anti-CD86-PE MoAb from Pharmingen (San Diego, CA); anti-λ-PE (λ) MoAb from Tebu (Le Perray en Yvelines, France); anti-CD49e-PE (SAM-1) MoAb from Serotec Ltd (Oxford, UK); goat neutralizing antihuman FGF basic Ab from R&D; and Steptavidin-Quantum Red Conjugate (PECY5) from Sigma (St Louis, MO). Anti-CD138, anti-CD95 (B-G27), and IgG1 control MoAbs were conjugated to biotin, as previously described.16
IL-6 and Ig Enzyme-Linked Immunosorbent Assay (ELISA)
IL-6 concentrations were determined in cell supernatants using an ELISA from Diaclone Research. Ig (IgA + IgG + IgM) concentrations were also determined by an ELISA. Briefly, a 96-well plate (MaxiSorp Plate; Nunc, Glostrup, Denmark) was coated in phosphate-buffered saline (PBS) overnight at 4°C with 5 μg/mL mouse antihuman κ chain MoAb (clone 6E1; Immunotech) or antihuman λ chain MoAb (clone C4; Immunotech), washed 3 times in PBS 0.5% Tween 20, saturated for 1 hour at 4°C with PBS containing 3% bovine serum albumin (BSA), incubated with standards or samples for 2 hours at 20°C, washed 3 times in PBS 0.5% Tween 20, incubated with peroxidase-conjugated affinipure goat antihuman Ig (IgA + IgG + IgM; Jackson ImmunoResearch Laboratories, West Grove, PN) for 1 hour at 20°C, washed 3 times in PBS 0.5% Tween 20, and incubated for a few minutes with 50 μL peroxidase substrate (0.5 mg/mL o-phenylene diamine dihydrochloride; Sigma) in 0.1 mol/L sodium acetate (pH 4) containing 0.003% H2O2. The reaction was stopped by adding 50 μL of 1 N H2SO4, and absorbance was determined with a spectrophotometer at 490 nm. Standardization was performed with human gammaglobulin (Jackson ImmunoResearch Laboratories).
Cell Staining and Flow Cytometry
For immunostaining, cells were incubated with the different MoAbs in simple, double, or triple staining in PBS containing 1% BSA, 0.02% NaN3, and 20% AB serum at 4°C for 30 minutes, washed, and fixed in PBS 1% formaldehyde. For unconjugated and biotinylated MoAbs, a second incubation was performed before fixation with FITC-GAM and PECY5-conjugated streptavidin, respectively. κ and λ staining was performed without AB serum. For intracellular κ and λ staining, cells were fixed and permeabilized using IntraPrep Permeabilization Reagent (Immunotech), stained for 30 minutes at 4°C in PBS containing 1% BSA, washed, and fixed in 1% formaldehyde. Fluorescence-activated cell sorting (FACS) analysis was performed on a FACScalibur apparatus (Becton Dickinson). Acquisition and analysis were performed with Cell-Quest software (Becton Dickinson). The fluorescence intensity ratio (r) was obtained by dividing the fluorescence of each antigen by the fluorescence of the appropriate control. The levels of this ratio were defined as follows: −, r < 1.2; +/−, 1.2 < r < 2; +, 2 < r < 20; ++, 20 < r < 50; +++, r > 50.
Determination of Cell Proliferation by BrdU Incorporation
Cells were incubated for 2 hours with 50 μmol/L BrdU (5-bromo-2′-deoxyuridine; Sigma) at 37°C in culture medium, washed in PBS, and permeabilized overnight in PBS containing 0.01% Tween 20 and 1% paraformaldehyde. Double staining with FITC anti-BrdU- and PE-conjugated anti-λ or anti-κ Abs was performed as previously described.17
Immunomagnetic Bead Purification/Removal of Plasma Cells and B Cells
MACS LS+ separation columns (Miltenyi) were used for immunomagnetic bead depletion of PCs, according to the protocol of the supplier (Milteny: Biotec, Parish, France). Briefly, cells were labeled with anti-CD138 B-B4 IgG1 MoAb, washed, and then incubated with rat antimouse IgG1 MACS MicroBeads. Unlabeled cells were not retained by the column and constituted the CD138− fraction. Retained cells (CD138+cells) were then flushed out, consituting the CD138+fraction. These separation procedures allowed us to obtain pure populations (>95%): CD138+ purification was checked by cytological analysis on cell cytospins, and CD138−purification was monitored by CD138 expression analysis. When indicated in the text or legends to figures, B lymphocytes were removed by the same method, using B9E9 anti-CD20 IgG2 MoAb (Immunotech) and rat antimouse IgG2 MACS MicroBeads. The removal of B cells was performed before CD138+ population purification or removal.
RESULTS
Peripheral Blood Plasma Cells From RP Have a Shared Phenotype With the CD49e− Plasma Cell Precursor
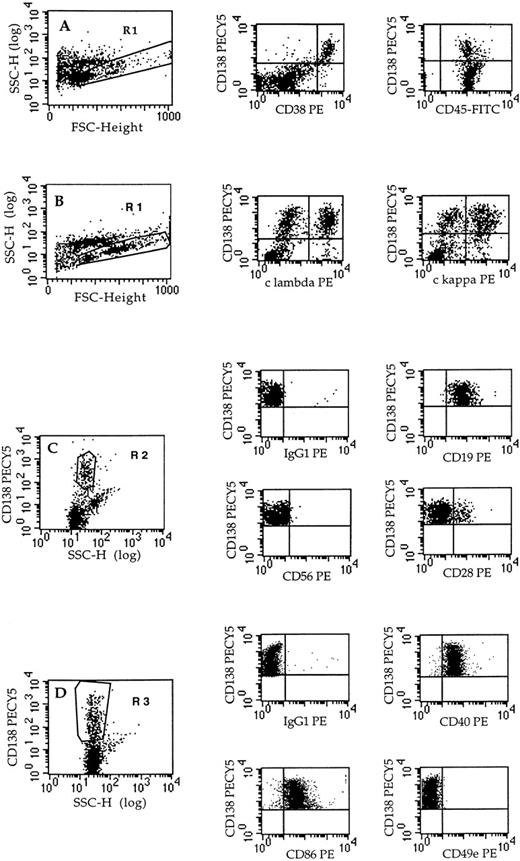
Ten reactive plasmacytoses were analyzed in the two different contexts of infections and tumors (Table 1). The diagnosis of RP was established by cytological detection of PCs in peripheral blood. In flow cytometry, PCs were identified by CD138++, CD38bright, and intracytoplasmic κ+ or λ+(Fig 1). The phenotype of PC was determined in double staining with CD138 (syndecan-1). As shown in Fig 1 and summarized in Table 2, circulating PCs were polyclonal and presented a shared phenotype: CD19+CD38+++ CD138+++ CD40+CD45int CD49e− CD56−CD11a+. Moreover, a very minor population of CD28+ PC (range, 3% to 12%) was observed in 6 of 9 evaluated cases. The expression of CD45 was intermediate, ie, inferior to lymphocyte expression but superior to malignant PCs from patients with MM (not shown). The phenotype of reactive PCs proved to be very similar to that of CD49− immature PCs, ie, PC precursor.19 20
Phenotype of plasma cells from RPs. The phenotype of plasma cells was determined in triple staining with anti-CD138-PECY5, anti-CD45-FITC, and the PE-conjugated MoAb indicated. Acquisition was performed on all cells (dot-plots A and B) or on CD138+gated cells (dot-plots C and D). Fluorescence analysis was performed on gated cells (R1 or R2 or R3), as indicated in each dot-plot. Dot-plots: A, RP4; B, RP1; C, RP4; D, RP7.
Phenotype of plasma cells from RPs. The phenotype of plasma cells was determined in triple staining with anti-CD138-PECY5, anti-CD45-FITC, and the PE-conjugated MoAb indicated. Acquisition was performed on all cells (dot-plots A and B) or on CD138+gated cells (dot-plots C and D). Fluorescence analysis was performed on gated cells (R1 or R2 or R3), as indicated in each dot-plot. Dot-plots: A, RP4; B, RP1; C, RP4; D, RP7.
Phenotype of Plasma Cells From 10 RPs
| . | RP1 . | RP2* . | RP3 . | RP4 . | RP5 . | RP6 . | RP7 . | RP8 . | RP9 . | RP10 . | Total (n = 10) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % of cells expressing | |||||||||||
| CD38+++ | 30% | 15% | 11% | 20% | 89% | 37% | 87% | 27% | 13% | 8% | +10/10 |
| κ | ++ (16%) | ++ (6%) | ND | ++ (16%) | ++ (63%) | ++ (20%) | ++ (49%) | ++ (14%) | ++ (8%) | +++ (7%) | +9/9 |
| λ | +++ (15%) | +++ (8%) | ND | +++ (4%) | +++ (28%) | +++ (17%) | +++ (40%) | +++ (13%) | +++ (4%) | +++ (3%) | +9/9 |
| CD138 | ++ (24%) | +++ (17%) | +++ (6%) | +++ (14%) | +++ (50%) | +++ (23%) | +++ (20%) | ++ (9%) | +++ (7%) | +++ (6%) | +10/10 |
| Phenotype of CD138+cells | |||||||||||
| CD11a | + (90%) | + | ND | ND | + | ND | ND | ND | ND | ND | +3/3 |
| CD19 | + | + | ND | + | ++ | + | + | + | + | + | +9/9 |
| CD28 | + (7%) | − | + (7%) | ++ (12%) | + (3%) | − | + (1%) | − | + (2%) | ND | +6/9† |
| CD40 | + | + | ND | ± | + | + | + | ND | + | ND | +7/7 |
| CD45 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +10/10 |
| CD49e | ND | ND | ND | − | − | − | − | ND | − | ND | −5/5 |
| CD56 | − | − | − | − | − | − | − | − | − | ND | −9/9 |
| CD80 | ND | ND | ND | ND | ND | ± | ± | ± | ± | ND | ±4/4 |
| CD86 | ND | ND | ND | ND | ND | + | + | ++ | + | ND | +4/4 |
| HLA-DR | ND | ND | ND | ND | + | ND | ND | + | + | ND | +3/3 |
| Apo2.7 | ND | ND | ND | ND | ND | − | − | − | − | ND | −4/4 |
| . | RP1 . | RP2* . | RP3 . | RP4 . | RP5 . | RP6 . | RP7 . | RP8 . | RP9 . | RP10 . | Total (n = 10) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % of cells expressing | |||||||||||
| CD38+++ | 30% | 15% | 11% | 20% | 89% | 37% | 87% | 27% | 13% | 8% | +10/10 |
| κ | ++ (16%) | ++ (6%) | ND | ++ (16%) | ++ (63%) | ++ (20%) | ++ (49%) | ++ (14%) | ++ (8%) | +++ (7%) | +9/9 |
| λ | +++ (15%) | +++ (8%) | ND | +++ (4%) | +++ (28%) | +++ (17%) | +++ (40%) | +++ (13%) | +++ (4%) | +++ (3%) | +9/9 |
| CD138 | ++ (24%) | +++ (17%) | +++ (6%) | +++ (14%) | +++ (50%) | +++ (23%) | +++ (20%) | ++ (9%) | +++ (7%) | +++ (6%) | +10/10 |
| Phenotype of CD138+cells | |||||||||||
| CD11a | + (90%) | + | ND | ND | + | ND | ND | ND | ND | ND | +3/3 |
| CD19 | + | + | ND | + | ++ | + | + | + | + | + | +9/9 |
| CD28 | + (7%) | − | + (7%) | ++ (12%) | + (3%) | − | + (1%) | − | + (2%) | ND | +6/9† |
| CD40 | + | + | ND | ± | + | + | + | ND | + | ND | +7/7 |
| CD45 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +10/10 |
| CD49e | ND | ND | ND | − | − | − | − | ND | − | ND | −5/5 |
| CD56 | − | − | − | − | − | − | − | − | − | ND | −9/9 |
| CD80 | ND | ND | ND | ND | ND | ± | ± | ± | ± | ND | ±4/4 |
| CD86 | ND | ND | ND | ND | ND | + | + | ++ | + | ND | +4/4 |
| HLA-DR | ND | ND | ND | ND | + | ND | ND | + | + | ND | +3/3 |
| Apo2.7 | ND | ND | ND | ND | ND | − | − | − | − | ND | −4/4 |
The phenotype of plasma cells was defined in double staining with CD138. The intensity level was defined as indicated in Materials and Methods. CD38, intracytoplasmic κ and λ, and CD138+cells were determined in the total population. The phenotype of plasma cells was defined in the CD138+ cell population.
Immunological staining was performed after cells were cultured for 72 hours at 37°C in RPMI plus 10% FCS.
CD28 was either not expressed or in a minor subpopulation of plasma cells.
Peripheral Blood Plasma Cells From RP Result From the Maturation of Plasma Cell Progenitors (Plasmablasts) In Vivo and In Vitro
Maturation of polyclonal reactive plasma cells in vitro.
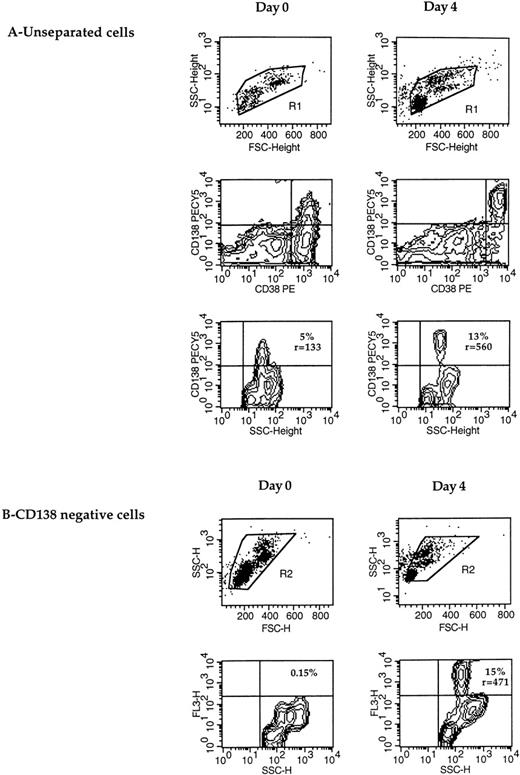
In most cases of RP, CD138 was not expressed by all PCs (Table 2). As shown in Fig 2, not all CD38 bright cells (PCs)21 expressed CD138. Furthermore, the absolute number of cytoplasmic κ plus λ positive cells was always superior to that of CD138+ cells, which confirms that CD138 was not expressed by all PCs (Table 2). This failure of anti-CD138 B-B4 MoAb to stain all circulating PCs prompted us to search for the presence of putative plasmablasts. When PBMC were cultured for a few days in RPMI containing 10% FCS (Fig2), most CD38 bright cells spontaneously acquired CD138 expression within a few days (Fig 2A). The fraction of CD138+ cells (13% at day 4 v 5% at day 0) and the fluorescence intensity ratio (560 v 133) were clearly enhanced. To exclude any possibility that the increase in the CD138+ population resulted from the death of CD138− cells, CD138+ cells were removed at day 0 before in vitro culture. As shown in Fig 2B, 15% of cells expressed CD138 (ratio [r] = 471) after 4 days, compared with none at day 0. This upregulation of CD138 was accompanied by a significant enhancement of the CD38 expression level (Fig 2A).
CD138 expression is upregulated during maturation of plasma cells in vitro. (A) CD138 expression was determined in double staining with CD38 in unseparated cells at day 0 and after a 4-day culture. The percentage and fluorescence intensity ratio of CD138 expression were defined in the R1-gated cells and are indicated within the dot-plots. (B) After immunomagnetic removal of CD138+cells, CD138− cells were cultured for 4 days, and the expression of CD138-PECY5 (FL3-H) (percentage and fluorescence intensity ratio r) was determined in the R2-gated population. CD138+ and CD138− cell separation was performed as described in Materials and Methods (RP8).
CD138 expression is upregulated during maturation of plasma cells in vitro. (A) CD138 expression was determined in double staining with CD38 in unseparated cells at day 0 and after a 4-day culture. The percentage and fluorescence intensity ratio of CD138 expression were defined in the R1-gated cells and are indicated within the dot-plots. (B) After immunomagnetic removal of CD138+cells, CD138− cells were cultured for 4 days, and the expression of CD138-PECY5 (FL3-H) (percentage and fluorescence intensity ratio r) was determined in the R2-gated population. CD138+ and CD138− cell separation was performed as described in Materials and Methods (RP8).
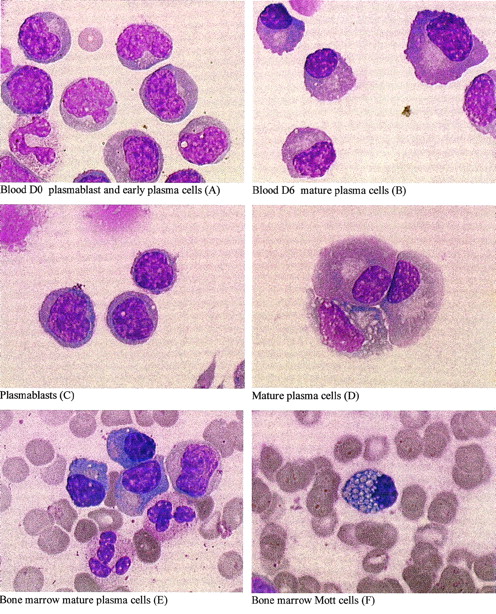
These phenotypic modifications were associated with morphogical maturation into PCs, as indicated by dense chomatin clumping, well-developed cytoplasm, and an eccentrically placed nucleus (shown for patient RP5 in Fig 3A and B). In vivo, mature PCs (E) as well as occasional cells with prominent Russell bodies (F), known as Mott cells,22 were only seen within the bone marrow. In vitro, mature PCs, as well as occasional Mott cells, were seen, but only after 6 days of culture (B). These data suggest that PC precursors were present in peripheral blood and were able to differentiate in vitro and in vivo.
Plasma cell differentiation in vitro and in vivo. May-Grünwald-Giemsa staining of cell cytospins at day 0 (A and C) or 6 (B and D) from fresh (A and B) or thawed (C and D) PBMNCs or of bone marrow smears (E and F) from patient RP5. From day 0, fresh or thawed PBMNCs were cultured for 6 days in 10% FCS RPMI 1640. After thawing, plasma cells (CD138+) and B cells (CD20+) were removed, as described in Materials and Methods. The CD19+ plasmablastic population (C) accounted for 95% of cells; the remaining cells were T lymphocytes and monocytes. (Original magnification × 1,000.)
Plasma cell differentiation in vitro and in vivo. May-Grünwald-Giemsa staining of cell cytospins at day 0 (A and C) or 6 (B and D) from fresh (A and B) or thawed (C and D) PBMNCs or of bone marrow smears (E and F) from patient RP5. From day 0, fresh or thawed PBMNCs were cultured for 6 days in 10% FCS RPMI 1640. After thawing, plasma cells (CD138+) and B cells (CD20+) were removed, as described in Materials and Methods. The CD19+ plasmablastic population (C) accounted for 95% of cells; the remaining cells were T lymphocytes and monocytes. (Original magnification × 1,000.)
Maturation correlated with the extinction of proliferation.
The high proliferation of freshly explanted cells was apparent in the 4 cases evaluated (RP5, 7, 8, and 9). Figure4 shows that 15% to 50% of cκ+ and 15% to 50% of cλ+ incorporated BrdU in the case of RP7 and RP9. However, cell maturation correlated with a dramatic extinction of proliferation when cells were cultured in vitro. After only 48 hours of culture, the percentage of BrdU+ cells was reduced sixfold (dot-plot A), whereas no BrdU+ cells were detected after 3 days (dot-plot B). Taken together, the immature phenotype and the high proliferation argue for the presence of a highly proliferative plasmablastic compartment.
High-rate proliferation of reactive plasma cells is rapidly downregulated. Freshly explanted cells were incubated for 2 hours with 1 mmol/L BrdU at day 0 and at day 2 or 3, as indicated in the figure. BrdU+ cells were determined in the intracytoplasmic (c) λ or κ population by double staining with anti-λ-PE or anti-κ-PE MoAb versus FITC-anti-BrdU MoAb, as described in Materials and Methods. For each dot-plot, 10,000 events were acquired. Dot-plots: A, RP9; B, RP7.
High-rate proliferation of reactive plasma cells is rapidly downregulated. Freshly explanted cells were incubated for 2 hours with 1 mmol/L BrdU at day 0 and at day 2 or 3, as indicated in the figure. BrdU+ cells were determined in the intracytoplasmic (c) λ or κ population by double staining with anti-λ-PE or anti-κ-PE MoAb versus FITC-anti-BrdU MoAb, as described in Materials and Methods. For each dot-plot, 10,000 events were acquired. Dot-plots: A, RP9; B, RP7.
Isolation and Characterization of Plasmablasts
Isolation and phenotypic characterization of plasmablasts.
To achieve their isolation, plasmablasts were negatively purified by eliminating PCs using magnetic beads coupled to anti-CD138 (RP 5, 7, 8, and 9). RP5 and RP7 were observed in the context of EBV (re)activation. To avoid any confusion between B lymphocytes (CD19+CD20+) and plasmablasts (CD19+CD20−), the former were removed using magnetic beads coupled to anti-CD20. Because plasmacytosis was quite extensive for RP5 (>89% of PBMC), the removal of plasma cells and B cells allowed us to obtain a very pure population of plasmablasts (95%; Fig 3). The remaining cells were T lymphocytes and monocytes. The plasmablasts were unrecognized cytologically either as B lymphocytes or as PCs (Fig 3C). Moreover, these cells spontaneously differentiated into PCs, which was the only cell population present within a few days (Fig 3D, day 6). Cytological analysis showed that cells first became plasmacytoid (from day 0 to day 2) and then PCs (after day 2), progressively reaching the 100% level at day 4 (data not shown). Few plasmablasts were also found within bone marrow (not shown).
The phenotype of the remaining population obtained by negative purification was defined in double staining with CD19 (Table 3). Table 2 clearly shows that these cells were neither resting nor activated B lymphocytes, because they did not express CD20, CD21, CD23, or CD25 and only weakly expressed CD40 and CD80. They were not PCs, because CD138 was not expressed, CD38 was not very brightly expressed, and intracellular Ig was not highly expressed.16,19-21 23 These data show that the plasmablast phenotype was distinct from both resting and activated B-lymphocyte phenotypes and from the plasma cell phenotype. Moreover, plasmablasts expressed surface Ig.
Phenotypic Analysis of the CD19+Plasmablastic Population
| . | RP5 . | RP7 . | RP8 . | RP9 . |
|---|---|---|---|---|
| Antigens (Ag) | ||||
| PC Ag | ||||
| CD19 | ++ | + | + | ++ |
| CD38 | +++ | ++ | +++ | ++ |
| CD138 | − | − | − | − |
| cIg | + | ++ | +++ | +++ |
| B cells Ag | ||||
| CD19 | ++ | + | + | ++ |
| CD20 | − | − | ND | − |
| CD21 | − | − | ND | − |
| CD22 | +/− | − | ND | +/− |
| CD23 | − | − | ND | − |
| sIg | + | + | ND | + |
| CD40 | + | + | ND | + |
| CD45 | +++ | +++ | +++ | +++ |
| CD45RA | + | ++ | ND | + |
| CD45RO | − | − | ND | − |
| CD80 | +/− | +/− | − | − |
| CD86 | + | + | + | + |
| Adhesion and activation Ag | ||||
| CD25 | − | − | ND | ND |
| CD28 | + (3%) | − | − | ND |
| CD49e | − | − | − | − |
| CD56 | − | − | ND | − |
| CD95 | + | + | + | + |
| HLA-DR | + | +++ | + | ++ |
| . | RP5 . | RP7 . | RP8 . | RP9 . |
|---|---|---|---|---|
| Antigens (Ag) | ||||
| PC Ag | ||||
| CD19 | ++ | + | + | ++ |
| CD38 | +++ | ++ | +++ | ++ |
| CD138 | − | − | − | − |
| cIg | + | ++ | +++ | +++ |
| B cells Ag | ||||
| CD19 | ++ | + | + | ++ |
| CD20 | − | − | ND | − |
| CD21 | − | − | ND | − |
| CD22 | +/− | − | ND | +/− |
| CD23 | − | − | ND | − |
| sIg | + | + | ND | + |
| CD40 | + | + | ND | + |
| CD45 | +++ | +++ | +++ | +++ |
| CD45RA | + | ++ | ND | + |
| CD45RO | − | − | ND | − |
| CD80 | +/− | +/− | − | − |
| CD86 | + | + | + | + |
| Adhesion and activation Ag | ||||
| CD25 | − | − | ND | ND |
| CD28 | + (3%) | − | − | ND |
| CD49e | − | − | − | − |
| CD56 | − | − | ND | − |
| CD95 | + | + | + | + |
| HLA-DR | + | +++ | + | ++ |
Fluorescence staining and analysis were performed as described in Materials and Methods. The phenotype of plasmablastic cells was defined in double staining with CD19 in the CD138− population (after removal of the CD138+ population).
Abbreviations: cIg, cytoplasmic Ig; sIg, surface Ig; ND, not done.
Plasmablasts (CD138−) are more proliferative than early plasma cells (CD138+).
Because plasmablasts and plasma cells could be separated on the basis of CD138 expression, the labeling index of CD138− and CD138+ cells was determined. As shown in Fig 5 for the λ+ population, BrdU+ cells were mainly located within the CD138− fraction (25% for CD138−cells v 8% for CD138+ cells). The same result was obtained for the κ+ population (data not shown). Because the survival of reactive plasma cells was dependent on paracrine IL-6, 3 ng/mL of IL-6 was added in each fraction at the time of initial culture.
Proliferative reactive plasma cells are mainly located in the CD138− plasmablastic fraction. The labeling index was determined by BrdU incorporation, as described in Fig 4. The labeling index was determined in the unseparated population and the CD138+ and CD138− populations. CD138+ and CD138− cell isolation was performed as described in Materials and Methods (RP10). Acquisition was performed either in R1 gated cells (A and B) or R2 gated cells (C and D). (A and C) λ-PE versus BrdU-FITC; (B and D) λ-PE versus control IgG1-FITC.
Proliferative reactive plasma cells are mainly located in the CD138− plasmablastic fraction. The labeling index was determined by BrdU incorporation, as described in Fig 4. The labeling index was determined in the unseparated population and the CD138+ and CD138− populations. CD138+ and CD138− cell isolation was performed as described in Materials and Methods (RP10). Acquisition was performed either in R1 gated cells (A and B) or R2 gated cells (C and D). (A and C) λ-PE versus BrdU-FITC; (B and D) λ-PE versus control IgG1-FITC.
Characterization of Plasmablast Differentiation Into Plasma Cells
CD138 expression is upregulated during differentiation.
To determine critical phenotypic changes during the differentiation process, we looked at the expression of different molecules, ie, CD38, CD49e, CD138, HLA-DR, and cytoplasmic Ig. Plasma cells (CD138+) and B lymphocytes (CD20+) were removed before culture.
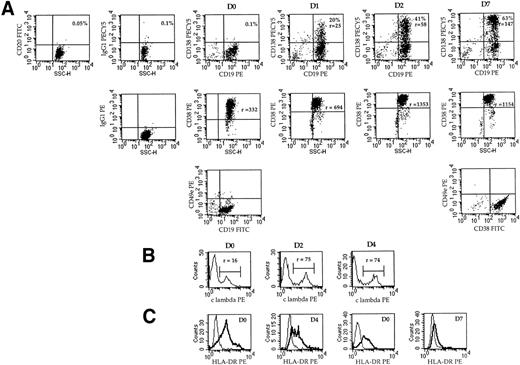
Figure 6A shows that CD138 was not expressed on plasmablasts (D0 dot-plots), but was quickly upregulated from day 1 (20%, r = 25). Both the percentage and fluorescence intensity ratio increased progressively (41%, r = 58 at day 2), reaching 63% (r = 147) at day 7. The CD38 intensity ratio was highly enhanced during the differentiation process, ie, from 332 at day 0 to 1,353 at day 4 (4-fold increase), as shown with RP5. The expression of CD49e was negative in CD19+ plasmablastic cells and remained negative in CD38+++ PCs (Fig 6). The intensity of cytoplasmic Ig was enhanced, as shown in Fig 6B for λ light-chain expression. The fluorescence intensity ratio increased from 16 to 75 within 2 days and then remained stable for 7 days. HLA-DR expression was decreased from r = 6 to 3 within 4 days (RP8) and from r = 4.7 to 1.6 within 7 days (RP5; Fig 6C).
Phenotypic changes during the differentiation process of plasmablasts into plasma cells in vitro. (A) Analysis of CD138 and CD19 expression in double staining with anti-CD138-biotin-streptavidin-PECy5 and anti-CD19-PE MoAbs and analysis of CD38 expression in simple staining during time-course differentiation (RP5). At day 0, CD138+ and CD20+ cells were removed as described in Materials and Methods. The percentage of positive cells and the fluorescence intensity ratio (r) are indicated. Intracellular light-chain staining was performed, as described in Materials and Methods. The histograms show the fluorescence profiles for λ or κ expression as indicated. (B) Analysis of CD49e expression at day 0 or 7 by double staining with anti-CD49e MoAb and anti-CD19-FITC MoAb or anti-CD38-FITC MoAb, respectively (RP5). (C) Analysis of the expression of HLA-DR by simple staining with anti-HLA-DR-PE MoAb. Overlay histograms represent the immunofluorescence of HLA-DR and the control. (Left) RP8; (right) RP5.
Phenotypic changes during the differentiation process of plasmablasts into plasma cells in vitro. (A) Analysis of CD138 and CD19 expression in double staining with anti-CD138-biotin-streptavidin-PECy5 and anti-CD19-PE MoAbs and analysis of CD38 expression in simple staining during time-course differentiation (RP5). At day 0, CD138+ and CD20+ cells were removed as described in Materials and Methods. The percentage of positive cells and the fluorescence intensity ratio (r) are indicated. Intracellular light-chain staining was performed, as described in Materials and Methods. The histograms show the fluorescence profiles for λ or κ expression as indicated. (B) Analysis of CD49e expression at day 0 or 7 by double staining with anti-CD49e MoAb and anti-CD19-FITC MoAb or anti-CD38-FITC MoAb, respectively (RP5). (C) Analysis of the expression of HLA-DR by simple staining with anti-HLA-DR-PE MoAb. Overlay histograms represent the immunofluorescence of HLA-DR and the control. (Left) RP8; (right) RP5.
Investigations in all 4 cases analyzed showed that CD138, CD38, cIg, and HLA-DR were the only molecules whose expression was modulated during the differentiation process. Our results indicate that CD138 was the only antigen able to discriminate plasmablasts from PCs.
Because FGF basic has been reported to induce CD138 expression in mesenchymal cells,24 we investigated whether FGF basic was involved in the upregulation of CD138 expression in PCs. Neither the addition of FGF basic (10 ng/mL) nor that of neutralizing anti-FGF Ab (50 μg/mL) modified CD138 upregulation or Ig secretion, indicating that FGF basic was not involved in this process (Table 4).
Neutralization of IL-6 (BE-8) and Its Receptor CD126 (PM-1) Prevented Differentiation by Inducing Apoptosis of Plasmablasts
| . | RP5 . | RP8 . | RP9 . | |||
|---|---|---|---|---|---|---|
| CD138+ . | Apo2.7+ . | CD138+ . | Apo2.7+ . | CD138+ . | Apo2.7+ . | |
| Treatment | ||||||
| Nil | 25% | 13% | 14% | 8% | 6% | 4% |
| Anti–IL-6 MoAb | 9% | 42% | ND | ND | ND | ND |
| Anti–IL-6 + anti-CD126 MoAbs | ND | ND | 0.5% | 25% | 0.2% | 10% |
| Anti-FGF basic Ab | 24% | 15% | ND | ND | 7.5% | ND |
| FGF basic | 26% | ND | ND | ND | 6.8% | ND |
| . | RP5 . | RP8 . | RP9 . | |||
|---|---|---|---|---|---|---|
| CD138+ . | Apo2.7+ . | CD138+ . | Apo2.7+ . | CD138+ . | Apo2.7+ . | |
| Treatment | ||||||
| Nil | 25% | 13% | 14% | 8% | 6% | 4% |
| Anti–IL-6 MoAb | 9% | 42% | ND | ND | ND | ND |
| Anti–IL-6 + anti-CD126 MoAbs | ND | ND | 0.5% | 25% | 0.2% | 10% |
| Anti-FGF basic Ab | 24% | 15% | ND | ND | 7.5% | ND |
| FGF basic | 26% | ND | ND | ND | 6.8% | ND |
Fluorescence analysis at day 3 of CD138 and APO2.7 expression in untreated or treated cells, as indicated (anti–IL-6, B-E8: 50 μg/mL; anti-CD126, PM-1: 10 μg/mL; anti-FGF: 50 μg/mL; rFGF basic: 10 ng/mL). The percentages of positive cells for CD138 or APO2.7 were determined in the total population.
Differentiation of plasmablasts into plasma cells is characterized by enhanced Ig secretion and extensive apoptosis.
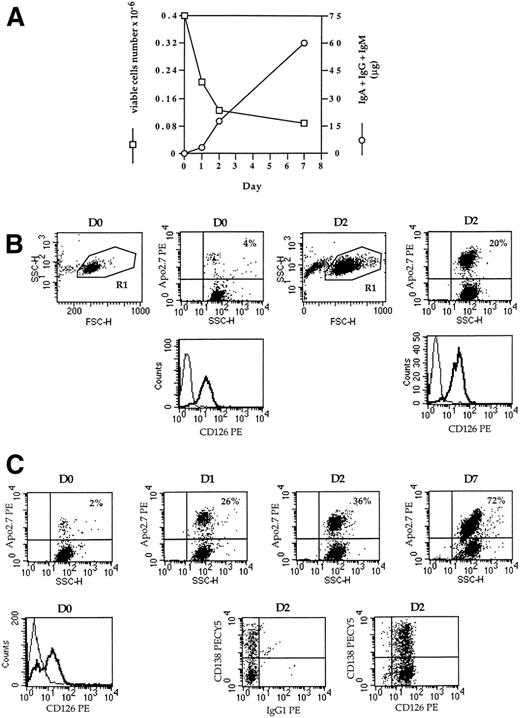
Cytologic and phenotypic differentiation was confirmed by studies of cell capacity to secrete Ig. Figure 7A shows that Ig accumulated rapidly in supernatant for 7 days. At day 0, 98% of cells were viable, but only 28% were viable at day 7. Thus, the capacity of cells to secrete Ig was enhanced from day 0 to day 7.
Kinetics of IgG secretion, apoptosis, and IL6-R (CD126) expression. After thawing (RP5), CD20+cells were removed and the population was separated into CD138+ and CD138− cells. (A) CD20− CD138− cells were cultured in RPMI 1640 10% FCS with 3 ng/mL IL-6 for 7 days. At days 0, 1, 2, and 7, supernatant was harvested and the amount of secreted Ig was determined by ELISA. The number of viable cells was determined by trypan-blue exclusion. (B and C) CD20− CD138+cells (B) and CD20− CD138− (C) cells were cultured in RPMI 1640 10% FCS with 3 ng/mL IL-6. Apoptotic cells were determined by Apo2.7-PE staining. CD138-PECY5 and CD126-PE expression was determined in double staining in the viable cell population (R1 dot-plot FSC v SSC).
Kinetics of IgG secretion, apoptosis, and IL6-R (CD126) expression. After thawing (RP5), CD20+cells were removed and the population was separated into CD138+ and CD138− cells. (A) CD20− CD138− cells were cultured in RPMI 1640 10% FCS with 3 ng/mL IL-6 for 7 days. At days 0, 1, 2, and 7, supernatant was harvested and the amount of secreted Ig was determined by ELISA. The number of viable cells was determined by trypan-blue exclusion. (B and C) CD20− CD138+cells (B) and CD20− CD138− (C) cells were cultured in RPMI 1640 10% FCS with 3 ng/mL IL-6. Apoptotic cells were determined by Apo2.7-PE staining. CD138-PECY5 and CD126-PE expression was determined in double staining in the viable cell population (R1 dot-plot FSC v SSC).
IL-6 Is an Essential Survival Factor for Plasmablasts
This model of RP proved to be of particular interest, because differentiation was spontaneous and required no exogeneous factor. In 3 evaluated cases, the determination of cytokines produced in vitro showed that a large amount of IL-6 was secreted (range, 2 to 20 ng/0.2 × 106 cells × 24 hours). Purification of each peripheral blood cell population (plasma cells, B lymphocytes, T lymphocytes, and monocytes) indicated that IL-6 was produced essentially by monocytes (data not shown). To determine its role in the differentiation process, IL-6 was neutralized with an anti–IL-6 MoAb (B-E8) or B-E8 and a blocking anti-CD126 MoAb (PM-1) at the time of initial culture, which induced a dramatic inhibition of IgG secretion (data not shown). CD138 expression was then evaluated to correlate inhibition of IgG secretion with a lack of differentiation. It was found that the proportion of CD138+ cells was highly reduced among B-E8–treated cells (Table 4). Compared with untreated cells, the level of CD138 staining was decreased, and CD138++ cells were reduced from 25% to 9% at day 3 (RP5). The lack of CD138 induction was associated with a large increase in the number of apoptotic cells. APO2.7 MoAb, which recognizes 7A6 antigen,25 was used to characterize and count apoptotic cells. Table 4 shows that the proportion of apoptotic cells was enhanced threefold by B-E8 treatment (42% v 13% at day 3, RP5). When both B-E8 and PM-1 were added, a total inhibition of CD138 induction was observed (RP8 and 9). Once again, the absence of CD138 expression was clearly associated with an increase in apoptotic cells (3-fold at day 3).
In view of the reduced upregulation of CD138 and the increase in apoptosis, our data indicate that IL-6 is an essential survival factor for plasmablasts.
Despite the presence or addition of IL-6, reactive plasma cells were subject to apoptosis. Figure 7B and C show that both CD138− and CD138+ cells died by apoptosis (as determined by Apo2.7 staining). In CD138+ cells, positive CD126 expression was not modulated in culture. Although only approximately 30% of CD138− cells expressed CD126 at day 0, they were all CD126+ after 2 days. Despite this overall expression of CD126, apoptotic cells kept accumulating, reaching 72% at day 7. This suggests that loss of cell viability and apoptosis were not related to CD126 expression.
DISCUSSION
In 10 cases of RP, circulating PC had a shared phenotype (CD11a+ CD19+ CD281-12%+CD38+++ CD40+ CD45intCD49e− CD56− CD138+++). Circulating PCs can be regarded as PC precursors or early plasma cells, as defined by Kawano et al,19 because they lack the CD49e expression characteristic of bone marrow PCs, the reference mature PCs.16,19,26 CD28 was expressed in a minor subpopulation of cells from 6 of 9 of the reactive plasmacytoses, whereas we previously showed that CD28 is not expressed in bone marrow PCs.16CD138 expression, as compared with that of bone marrow PCs, was weaker and heterogeneous, ie, CD38+-reactive PCs were not all CD138+.16 However, when cells were cultured a few days in vitro, CD38+ CD138− PCs all became CD138+, and the level of CD38 expression was significantly enhanced (n = 4). Because CD138 expression is lost by apoptotic malignant PCs (unpublished data and Jourdan et al27), the disappearance of CD38+CD138− cells could be due to apoptosis. In all 4 cases evaluated, CD138− cells were not stained by Apo2.7 MoAb (data not shown).
In all investigated RPs (n = 4), circulating PCs proliferated highly ex vivo. Proliferation was downregulated spontaneously and rapidly (within 4 to 7 days) after cytological maturation of cells in vitro, suggesting that PC progenitors (plasmablasts) were present in peripheral blood and able to differentiate into PCs in vivo and in vitro. To further demonstrate this hypothesis, we removed CD138+ plasma cells and CD20+ B cells and determined the phenotype of plasmablasts in double staining with CD19. The CD19+CD20− CD21− CD23−CD38+ CD40low CD45intCD56− CD80+/− CD86+CD138− HLA-DR+ sIg+cIg+ phenotype clearly distinguished plasmablasts from B lymphocytes and PCs. When the plasmablasts were cultured in vitro, they spontaneously differentiated into PCs (Figs 2, 3, and 6). Quite interestingly, CD138 was not expressed in plasmablasts but induced early and progressively during the differentiation process. Our data indicate that CD138 was the only differentiation antigen allowing PCs to be distinguished from plasmablasts. The differentiation of plasmablasts into plasma cells was also characterized by an increase in CD38 and cytoplasmic Ig expression and a decrease in HLA-DR expression. The upregulation of CD38 during the differentiation of immature PCs into mature PCs was in good agreement with a previous study, showing that early blood PC CD49e− CD38++differentiated into mature PC CD49+ CD38+++upon addition of IL-6 or stromal cells.19 Nevertheless, no upregulation of CD49e was observed during RP, even in the presence of stromal cells and/or IL-6 (data not shown). The phenotype of reactive short-lived bone marrow PCs remains undefined. In 2 cases (RP5 and RP7), bone marrow smears were available and mature PCs were observed. However, their phenotype, especially CD49e expression, could not be determined. The expression of CD49e on plasma cells remains controversial, requiring further investigation. Bone marrow and tonsil plasma cells were found to be either CD49e+19,20,26 or CD49e−,3,28 even though the same MoAb was used in all these studies (Sam-1, IgG2b isotype). In our hands, mature reactive plasma cells (obtained in vitro after 6 days of culture) were always found to be CD49e−, regardless of the anti-CD49e MoAb used (from the Fifth International Workshop on White Cell Differentiation Antigens; data not shown). The decrease in HLA-DR expression during differentiation is reminiscent of the previously described downregulation of HLA-DR during IL-6–induced differentiation of a few B cells into PCs (CD38++) in the CESS EBV+ cell line.29 Nevertheless, the precise role of IL-6 in this model is still uncertain, because IgG secretion was found to increase when proliferation was inhibited by hydroxyurea.6
Our data provide the first evidence that IL-6 (mainly produced by monocytes) is the survival factor for human plasmablasts. Previous studies demonstrated the critical role of IL-6 in PC differentiation but did not indicate its precise role.15 More recently, Kawano et al19 demonstrated that IL-6 was an essential survival factor for early circulating PC CD38+CD49e−. The ability of IL-6 to rescue plasmablasts from apoptosis could explain why reactive plasmacytoses are observed in vivo mainly in cases of excessive IL-6 production.12,14 We confirmed that reactive plasmacytoses, as reported in 2 sparse cases,11,14 are associated with detectable circulating IL-6 (4 of 4 cases). In fact, PCs were observed as long as IL-6 was detectable in vivo, ie, with high C-reactive protein values. This disappearance of circulating PCs was not simply the result of migration (eg, to bone marrow), because the significant increase in total serum Ig (eg, IgG >40 g/L) disappeared within 2 to 4 weeks (data not shown). This rapid decrease in Ig serum is a further indication that PCs are not long-lived, which is contrary to a recent report claiming that bone marrow PCs in the mouse are very long-lived, ie, t1/2 = 100 days.30
The majority of cells died in vitro within 1 week despite the addition of IL-6 or stromal cells (data not shown). The inescapable nature of this apoptosis prompted us to investigate the major molecules involved in PC survival and apoptosis, ie, Bcl-2 family proteins and CD95.31,32 We found that plasmablasts and PCs expressed CD95 (Table 3), and observed in 2 of 3 cases that CD95 triggering induced massive apoptosis of plasmablasts and PCs (data not shown). Plasmablasts and PCs from reactive plasmacytoses expressed Bcl-2 very weakly as compared with bone marrow PCs.33 Tonsillar PCs have been reported to be Bcl-2+ CD95+ and sensitive to CD95-induced apoptosis, whereas bone marrow PCs were Bcl-2+ CD95−.31,32 In this context, Bcl-2+/− CD95+ reactive PCs were distinct from both tonsillar and bone marrow PCs. In contrast to tonsillar PCs, those from RP were not rescued from spontaneous apoptosis by bone marrow fibroblasts, which suggests that the mechanisms of cell death regulation were distinct (data not shown). The very weak expression of Bcl-2 in RP could explain both the proliferation and the apoptosis.33 Although reactive plasma cells were CD95+, their phenotype was similar to that of plasma cells generated in the EL4 system, ie, Bcl-2low and CD49e−.7
The characterization of nonmalignant plasmablast expansions has major implications for the understanding of MM. MM is thought to be an expansion of malignant plasmablasts and PCs, although the phenotype of myeloma cells and cell lines, ie, CD19−CD28+ CD138+, is in total contrast with that of the nonmalignant plasmablasts defined here, ie, CD19+CD28− CD138−.16,34 How can this discrepancy be explained? It is now well known that the lack of CD19 expression is directly related to that of Pax5.35The expression of CD138 by myeloma cells and cell lines suggests that the former could be expansions of early PCs rather than plasmablasts.16,17,20,23,34 Concerning CD28, we have shown that its expression is a hallmark of myeloma cells from patients with advanced and terminal disease and of all MM cell lines. This would suggest that CD28 expression is characteristic of the proliferative malignant compartment.16,17,34 In reactive plasmacytoses, we found that PCs were highly proliferative (Figs 4 and5),11,12 although the population of CD28+ PCs remained very low or even inexistent, which would indicate that no correlation exists between proliferation and CD28 expression in plasmablasts and PCs. The role of CD28 expression in malignant PCs is still uncertain, although the constant expression of CD28 and its ligand CD86 in myeloma cells could give them an advantage for survival rather than growth.17
In conclusion, our study shows that reactive plasmocytoses are expansions of PC progenitors (plasmablasts) and plasma cell precursors or early plasma cells. Plasmablasts, in particular, are highly proliferative in vivo, whereas progenitors and precursors are both short-lived in vivo. However, plasmablasts in vitro stopped proliferating and differentiated into mature plasma cells. The survival of plasmablasts and their differentiation were totally dependent on endogenous paracrine IL-6. Maturation was characterized by a mature plasma-cell morphology (including the appearance of Mott cells and crystal-like structures within the cytoplasm), increased secretion of Ig, and expression of plasma-cell antigens, mainly CD138. More precisely, CD138 was the only antigen able to discriminate plasmablasts (CD138−) from early and mature plasma cells (CD138+). It is noteworthy that this maturation process was not observed in vivo in peripheral blood, although mature plasma cells were seen within bone marrow. The phenotype of plasmablasts and early plasma cells and the demonstration of IL-6 as their survival factor are important to an understanding of the process of plasma-cell generation and the biology of MM resulting from the malignant transformation of this lineage.
ACKNOWLEDGMENT
The authors thank Dr J. Wijdenes (Diaclone Research) for providing B-B4, B-E8, and B-G27 MoAbs and Dr T. Kishimoto for providing PM-1 MoAb.
Supported by grants from the Ligue Nationale Contre le Cancer. G.J. is supported by the Ligue Régionale des Deux-Sèvres de Recherche Contre le Cancer and C.P.-D. is supported by the Association pour le Développement de l’Etude des Leucémies et Maladies du Sang (Adelmas).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Catherine Pellat-Deceunynck, PhD, INSERM U463, Institut de Biologie, 9, quai Moncousu, 44093 Nantes Cedex 01, France; e-mail: cpellat@nantes.inserm.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal