Abstract
The catalytically inactive precursor of urokinase-type plasminogen activator (pro-u-PA) induced a chemotactic response in rat smooth muscle cells (RSMC) through binding to the membrane receptor of urokinase (u-PA receptor [u-PAR]). A soluble form of u-PAR activated by chymotrypsin cleavage as well as a peptide located between domain 1 and 2 of u-PAR reproduced the effect of pro-u-PA on cell migration. The chemotactic pro-u-PA effect correlates with a dramatic reorganization of actin cytoskeleton, of adhesion plaques, and with major cell shape changes in RSMC. Pro-u-PA induced a decrease in stress fiber content, membrane ruffling, actin ring formation, and disruption leading to the characteristic elongated cell shape of motile cells with an actin semi-ring located close to the leading edge of cells. u-PAR effects on both chemotaxis and cytoskeleton were sensitive to pertussis toxin and, hence, possibly require G proteins. u-PAR effects are accompanied by a relocation of u-PAR, vitronectin receptor (VNR) vβ3, β1 integrin subunit, and Src tyrosine kinase to the leading membrane of migrating cells. In conclusion, our data show that pro-u-PA, via binding to u-PAR, controls a signaling pathway, regulated by tyrosine kinases and possibly G proteins, leading to cell cytoskeleton reorganization and cell migration.
UROKINASE (u-PA) IS INVOLVED in a number of physiological and pathological processes: fibrin degradation, tissue involution, wound healing, inflammation, angiogenesis, rheumatoid arthritis, and cancer invasion (for reviews, see Fazioli and Blasi1 and Blasi2). These effects may be reproduced in culture with catalytically active or inactive derivatives of u-PA but require binding to the u-PA receptor (CD87/u-PAR). For instance, cell adhesion and cell migration regulation mediated by the membrane u-PA receptor may not require u-PA catalytic activity, because either pro-u-PA, high molecular weight (HMW)-u-PA, or its amino-terminal fragment (ATF) can exert the same influence on cells.3-5 In myeloid cells, u-PAR–mediated chemotaxis requires heterotrimeric G-proteins and tyrosine kinase activity.5
The structure of u-PAR is organized in three different domains. The first domain is involved in the binding of u-PA,6 whereas domains 2 and 3 are endowed with binding activity for vitronectin.3 However, an intact receptor is important for the high-affinity binding to both u-PA and vitronectin.7,8u-PAR is anchored to the plasma membrane through a glycosyl-phosphatidyl-inositol moiety and does not possess a cytoplasmic domain. Thus, the problem arises of how u-PAR can mediate intracellular signal transduction. A transmembrane adaptor, not yet identified, has been hypothesized for the chemotactic effect4,5; a direct binding to integrins and/or caveolin may mediate its cell adhesion effects.9
Factors acting on cell motility regulate cytoskeleton organization by polymerization and depolarization of actin filaments. Chemoattractants induce reorganization of actin cytoskeleton concomitantly with induction of cell motility.10,11 For instance, nonmotile fibroblasts exhibit higher stress fibers content than do motile fibroblats.12-14 CD 87/u-PAR has been proposed to be a membrane-associated chemokine, because it can directly induce chemotaxis2,5 as well as modify cell adhesion by undergoing ligand-mediated conformational changes.3 9 Thus, like chemokines, u-PAR might induce reorganization of the actin cytoskeleton.
Smooth muscle cells (SMC) represent the most abundant cell type in the arterial blood vessels and appear to exist in two different states. In intact arteries, SMC are in a contractile state and do not divide. They are involved in the maintenance of the elasticity and rigidity of the vessel wall and control blood pressure. When the endothelial wall is damaged, these cells undergo a transition to a synthetic state characterized by cell division and cell migration. The migration of SMC from the tunica media to the neointima represents a key event in the development and progression of vascular diseases participating to intimal thickening in atherosclerotic lesions and to restenosis after angioplasty (for reviews, see Casscells,15 Van Leeuwen,16 and Schwartz17). In vivo, SMC are embedded in the extracellular matrix. Migration from the tunica media to the neointima needs disruption of contacts between SMC and extracellular matrix proteins and also crossing of the basement membrane that separates SMC from endothelium. In SMC, u-PA stimulates migration and signal transduction through its specific receptor.18 19
The importance of u-PA in these effects is shown by the phenotype of u-PA−/− recombinant mice in which SMC do not divide or migrate to cause restenosis. Activation of plasminogen into plasmin, an enzyme able to degrade extracellular matrix and basement membrane proteins, explains the role of u-PA in SMC migration in vivo that does not appear to require binding to u-PAR.20However, u-PAR and u-PA are required for basic fibroblast growth factor (bFGF)-induced migration of murine SMC.21 Moreover, the effect of u-PA could depend on its ability to activate growth factors such as bFGF,22 pro-transforming growth factor-β (pro-TGF-β),23 and pro-hepatocyte growth factor (pro-HGF).24 25
The role of u-PAR in SMC migration is not known. Indeed, u-PAR−/− mice do not reproduce the phenotype of u-PA−/−20,26,27 with respect to restenosis, suggesting that its role may be not essential. On the other hand, because the u-PAR−/−/t-PA−/− double knockout mice, unlike the t-PA−/− mice, display major fibrin deposits (K. Danø, personal communication, 1998), u-PAR must be involved in mediating u-PA functions in vivo. Thus, the data from knockout mice may not give the absolute answer and, even in the absence of a u-PAR phenotype on SMC migration, the role of u-PAR is still far from being elucidated. In any case, the elucidation of the molecular mechanisms of u-PA functions is extremely important, and SMC represent an exceptional tool to investigate the chemokine action of u-PA and u-PAR, because they possess an impressive cytoskeleton and good migration properties in vitro.
The aim of the present study is to determine the influence and mechanisms of u-PA and u-PAR in rat SMC (RSMC) migration and RSMC cytoskeleton organization. We show that pro-u-PA, through u-PAR, induces cell migration, cell shape changes, and cytoskeleton reorganization. Pro-u-PA/u-PAR also induce a redistribution of integrins and u-PAR to the leading edge of migrating RSMC. The presence of Src is essential in this process. Because Bordetella pertussis toxin (BPT) blocks all pro-u-PA/u-PAR effects on cell migration and cytoskeleton, we suggest that the pathway may include a G protein-regulated step.
MATERIALS AND METHODS
Materials.
Rat smooth muscle cells were a kind gift of Dr M. Bertulli (Bayer Research Laboratory, Milan, Italy). BPT and its mutant were a kind gift of Dr M.G. Pizza (Sienna, Italy). Receptor-associated protein (RAP) and human ATF were generously provided respectively by Dr M. Nielsen (University of Aarhus, Denmark) and Dr J. Henkin (Abbott Research Laboratories, Chicago, IL). 3T3 Fibroblasts from wild-type and Src−/− mice, obtained through Dr K.B. Kaplan (M.I.T., Cambridge, MA), were transfected with full-size human u-PAR cDNA by standard procedures to generate 3T3/u-PAR clones. The presence of cell surface u-PA–binding u-PAR was determined by ligand-binding assays. Collagen I and fibronectin were purchased from Boehringer Mannheim (Mannheim, Germany). Primary antibodies used are the following: rabbit anti–c-Src (polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA), mouse antivitronectin receptor αvβ3 (monoclonal LM 609; Chemicon, Temecula, CA), mouse anti-β1 integrin subunit (kind gift of Dr P.C. Marchisio, DIBIT, Milan, Italy), and mouse antivinculin (monoclonal; Sigma, St Louis, MO). For the production of rat u-PAR antibodies, cDNA encoding amino acids 25-114 of rat u-PAR was subcloned into the expression vector pTrcHis A (InVitro, San Diego, CA), and the recombinant protein was expressed and purified to use for immunization of rabbits.28 Antirat u-PAR IgG detects rat u-PAR by immunofluorescence and competes for the binding of125I-labeled rat u-PA in receptor binding assay (S. Rabbani, data not shown). The secondary antibodies were rhodamine-conjugated F(ab)′2 fragments of antirabbit Ig (Protos Immunoresearch, San Francisco, CA) and fluorescein-conjugated F(ab)′2 fragments of antimouse Ig (Dako, Copenhagen, Denmark). Nonspecific rabbit polyclonal Igs, non-specific monoclonal mouse IgG1κ (MOPC-21), tetramethylrhodamine isothiocyanate (TRITC), and fluorescein isothiocyanate (FITC)-conjugated phalloidin and fMLP were from Sigma. Peptide 1 (AVTYSRSRYLEC) and its scrambled form (TLVEYYSRASCR) have been described previously.5Peptide D, a shorter version of peptide H: of rat origin, has the following sequence: PRGRY. Human su-PAR was purified by affinity chromatography from conditioned media of Chinese hamster ovary cells transfected with a mutant of u-PAR.29Chymotrypsin-cleaved su-PAR was prepared as previously described.6
The cDNA encoding rat pro-u-PA was isolated from rat kidney cDNA library and inserted into the baculovirus expression vector pFast Bac1 (GIBCO/BRL, Gaithersburg, MD).30 Rat pro-u-PA was expressed according to manufacturer’s instructions and purified by affinity chromatography using antihuman u-PA antibody. Purity of rat pro-u-PA was confirmed by sodium dodecyl sulfate (SDS)-gel electrophoresis and sequence analysis.
Cell culture.
RSMC monolayers and mouse Src+ or Src−/− 3T3 fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS). In some chemotaxis or immunofluorescence experiments, cells were pretreated with blocking antibodies. Before cell treament with antibodies, RSMC were washed once with phosphate-buffered saline (PBS) and detached at 37°C from support using 0.05% (wt/vol) EDTA in Ca2+-Mg2+-free PBS. The cells were then washed in serum-free medium and cell suspensions were treated in serum-free DMEM either with antibodies against u-PAR (10 μg/mL), with antivitronectin receptor antibodies (0.5 μg/mL), or with unspecific control IgG (10 μg/mL) for 1 hour at 4°C, and chemotaxis assay was performed as described below.
Chemotaxis assay.
Chemotaxis assay was performed as previously described.4Briefly, modified Boyden chambers were used with polyvinylpyrrolidone-free polycarbonate filters (13-mm diameter; 5-μm pore size; Nucleopore-Costar, Cambridge, MA). These filters were treated with collagen I (100 μg/mL in 0.5 N acetic acid) and fibronectin (10 μg/mL). Each step was followed by a wash with serum-free DMEM containing 0.2% bovine serum albumin (BSA). RSMC, grown to confluence, were detached from support (see above) and washed twice in serum-free medium. Then, 20,000 to 40,000 cells in serum-free DMEM were added to the upper well of the Boyden chamber. The molecules to be tested as chemoattractant were diluted in serum-free medium and added to the lower well of the Boyden chamber. When chemotaxis was performed in the presence of antibodies or BPT, these molecules were added in both wells of the Boyden chamber. Overnight migration was allowed at 37°C in humidified conditions with 95% air/5% CO2. The cells remaining on the upper surface of filters were then scraped off, and filters were fixed in methanol and stained in a solution of 10% (wt/vol) crystal violet in 20% (vol/vol) methanol. Random cell migration, ie, migration in the absence of chemoattractant, was given the arbitrary value of 100%. All experiments were performed in triplicate. Results are the mean ± standard deviation of the number of cells counted in 10 high power fields per filter and expressed as fold over control. The number of cells that crossed the filter in random cell migration was approximately 20 to 40 per field.
Wounding assay.
For wounding experiments, RSMC were grown to confluence in DMEM supplemented with 10% FCS on a glass coverslip in a 2-cm2well in 4-well plates. Monolayers were washed once with PBS and cultured for 24 hours in serum-free medium. Single-cross wounds were then made by dragging a sterile pipette tip accross the monolayer to create a cell-free space. Injured monolayers were washed with PBS and allowed to recover for a further 24 hours in serum-free medium supplemented or not with the molecule to be tested. RSMC were then fixed and actin cytoskeleton was visualized with rhodamine-conjugated phalloidin as described below. Quantification was made by taking photographs at lower magnification and by counting the number of cells that had migrated from the wound edge into the cell-free space.
Immunofluorescence microscopy.
Cells were seeded on glass coverslips at a density of 15,000 to 25,000 cells (20% to 40% confluence) per 2-cm2 well in 4-well plates (Nunc, Roskilde, Denmark) cultured for 24 hours in DMEM plus 10% FCS. Monolayers were then washed with PBS and cultured for another 24 hours in DMEM without FCS. Cells were challenged for different time periods with the appropriate stimulator. After incubation, cells were fixed for 20 minutes at room temperature with a solution of 3% paraformaldehyde and 2% sucrose in PBS, pH 7.5. After 3 washes with PBS-BSA 0.2%, cells were permeabilized with 20 mmol/L HEPES, pH 7.4, 300 mmol/L saccharose, 50 mmol/L NaCl, 3 mmol/L MgCl2, 0.5% (vol/vol) Triton X-100 for 3 minutes at 4°C. Monolayers were again washed three times with PBS-BSA 0.2% and incubated with PBS-BSA 2% for 15 minutes at 37°C. Cells were incubated for 30 minutes at 37°C with primary antibodies, washed three times with PBS-BSA 0.2%, and further incubated with PBS-BSA 2% for 15 minutes at 37°C. The cells were then stained with secondary antibodies. For visualization of filamentous actin, coverslips were incubated with fluorescein-conjugated phalloidin for 30 minutes at 37°C. After incubation of secondary antibodies, cells were washed three times with PBS-BSA 0.2% and once with distilled water. Coverslips were mounted with 20% (wt/vol) mowiol in PBS and analyzed on a Zeiss Axiophot microscope (Carl Zeiss, Oberkochen, Germany). Fluorescence photographs were taken on T-Max 400 film (Eastman Kodak Co, Rochester, NY). Cytoskeleton pictures were taken with a Zeiss 100 neofluar lens, whereas quantification was performed using a Zeiss 40 neofluar lens.
RESULTS
Pro-u-PA induces RSMC migration through a mechanism requiring a conformational change of u-PAR.
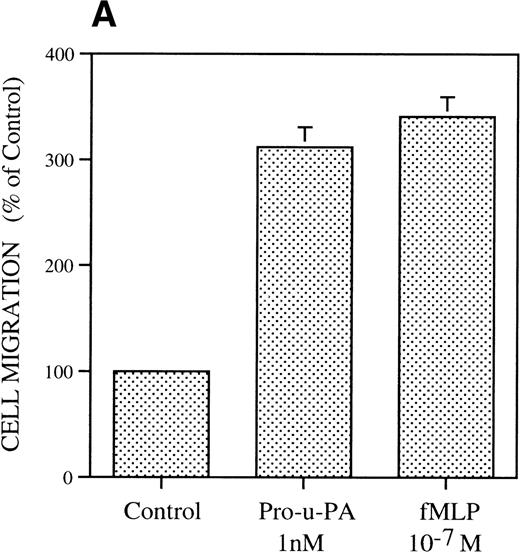
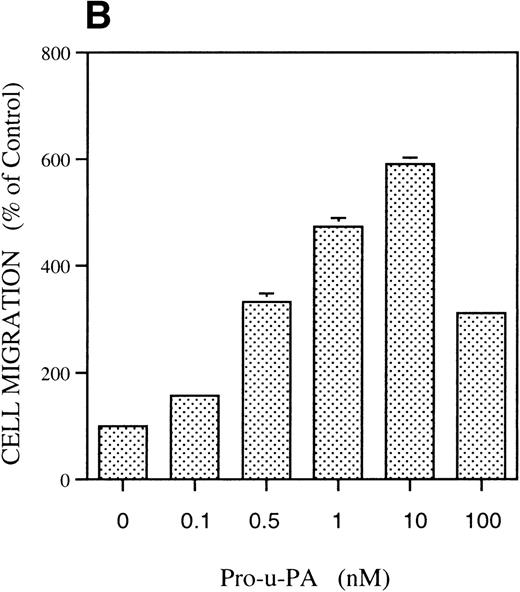
The migratory response of RSMC was determined with a chemotaxis assay using modified Boyden chambers. In the first experiment (Fig 1A), rat pro-u-PA was compared with the well-characterized attractant fMLP.31 Rat pro-u-PA (1 nmol/L) induced RSMC to migrate threefold above control, an effect comparable to that of 10−7 mol/L fMLP (Fig 1A). Concentration-dependence showed a bell-shaped dose-response curve with stimulation at concentrations as low as 0.1 nmol/L, a maximum at 10 nmol/L reaching a value sixfold above control, and inhibition at higher concentrations (100 nmol/L; Fig 1B). The stimulation of migration by pro-u-PA (as well as other attractants) varied with the age of the culture and in some experiments was rather low (50% to 60% over background), whereas with cells kept in culture for a shorter time, it reached levels greater than 300% to 600%. However, none of the properties described in the following part was ever qualitatively affected.
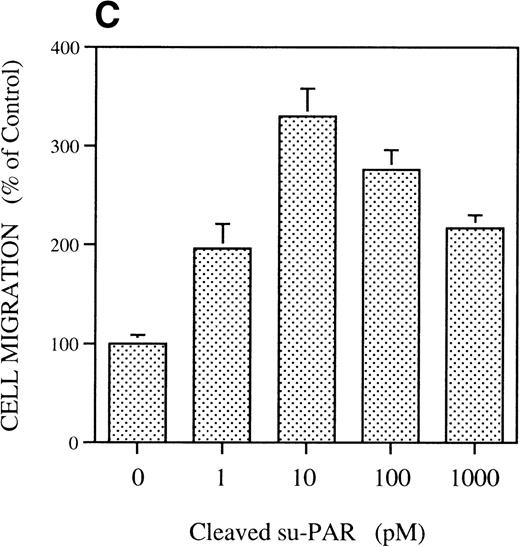
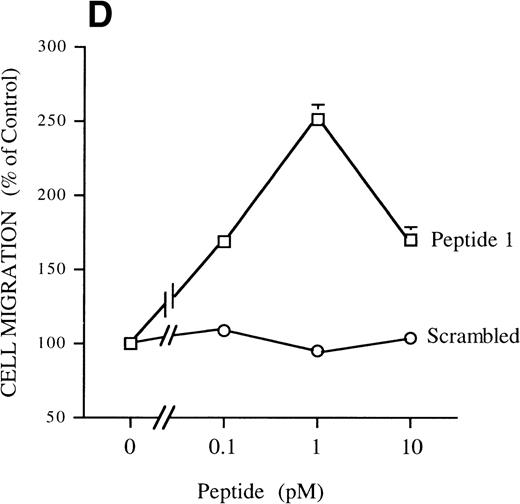
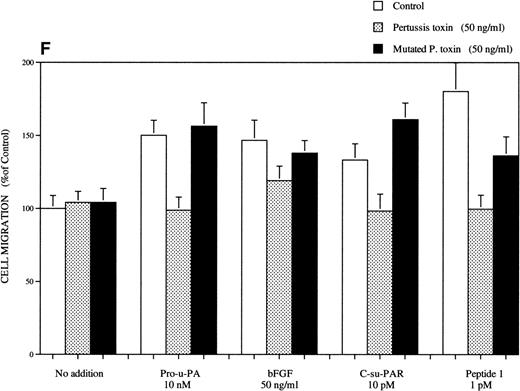
Chemotactic response of RSMC to pro-u-PA, C-su-PAR, and peptide 1. Chemotaxis assay was performed as described in methods using modified Boyden chambers. (A) Comparison of the chemotactic effects of pro-u-PA (1 nmol/L) and fMLP (10−7 mol/L) on RSMC. (B) Effects of increasing doses of pro-u-PA on RSMC migration. (C) Effects of increasing concentrations of C-su-PAR on RSMC migration. (D) Chemotactic activity of synthetic peptides on RSMC migration. Peptide 1 (AVTYSRSRYLEC), which corresponds to amino acids 84-95 of human u-PAR, and a scrambled version of peptide 1 (TLVEYYSRASCR) were tested as chemoattractant. Cells migrated towards increasing doses of peptide 1 or its scrambled version, as indicated. (E) Effect of the LRP antagonist RAP on RSMC migration and its effect on pro-u-PA stimulation. (F) Influence of either wild-type BPT or mutated BPT on pro-u-PA–induced or bFGF-induced chemotactic response of RSMC. Toxins were present in both chambers of the Boyden apparatus. Random cell migration of unstimulated cells is considered to be 100% migration. Results are the mean ± SD (n = 3).
Chemotactic response of RSMC to pro-u-PA, C-su-PAR, and peptide 1. Chemotaxis assay was performed as described in methods using modified Boyden chambers. (A) Comparison of the chemotactic effects of pro-u-PA (1 nmol/L) and fMLP (10−7 mol/L) on RSMC. (B) Effects of increasing doses of pro-u-PA on RSMC migration. (C) Effects of increasing concentrations of C-su-PAR on RSMC migration. (D) Chemotactic activity of synthetic peptides on RSMC migration. Peptide 1 (AVTYSRSRYLEC), which corresponds to amino acids 84-95 of human u-PAR, and a scrambled version of peptide 1 (TLVEYYSRASCR) were tested as chemoattractant. Cells migrated towards increasing doses of peptide 1 or its scrambled version, as indicated. (E) Effect of the LRP antagonist RAP on RSMC migration and its effect on pro-u-PA stimulation. (F) Influence of either wild-type BPT or mutated BPT on pro-u-PA–induced or bFGF-induced chemotactic response of RSMC. Toxins were present in both chambers of the Boyden apparatus. Random cell migration of unstimulated cells is considered to be 100% migration. Results are the mean ± SD (n = 3).
The effect was u-PAR–dependent as shown by the inhibition by antibodies against u-PAR (see below), in agreement with previous results obtained with human and murine cells.4Chymotrypsin-cleaved soluble u-PAR (C-su-PAR) was previously reported to substitute for u-PA to stimulate cell migration, even in cells devoid of u-PAR, indicating that u-PAR acts by interacting with other cell surface molecules.4,5 We tested C-su-PAR and found that it stimulates RSMC migration with a dose-dependent effect and maximal response at 10 pmol/L. Higher concentrations decreased cell migration (Fig 1C). These results are in perfect agreement with the chemotactic effects of u-PA or ATF on myeloid and other cells that causes a conformational change in u-PAR and exposes a chemotactic epitope.4 Indeed, synthetic u-PAR peptides, corresponding to a sequence located between domain 1 and domain 2 of u-PAR, can reproduce the effects of both pro-u-PA and C-su-PAR on cell migration.5 Peptide 1, a 12 amino acids peptide of human u-PAR located between amino acids 84 and 95, greatly increases RSMC migration with maximal effect at 1 pmol/L (Fig 1D). In the same experiment, we determined the effect of a peptide that has the same amino acid composition but a scrambled sequence. This peptide did not induce a migratory response. Because the amount of u-PA and u-PAR is controlled by PAI-1 and LDL-receptor related protein (LRP), which internalize u-PA-PAI-1 complexes and u-PAR as well,32 33 we tested whether the antagonist of LRP, RAP, had any effect on chemotaxis of SMC. However (Fig 1E), RAP did not have an effect on its own and did not affect the migration induced by pro-u-PA.
BPT (50 ng/mL), which inhibits the activity of receptors dependent on heterotrimeric G proteins, was found to inhibit pro-u-PA–dependent chemotaxis. As shown in Fig 1F, BPT inhibited the effect pro-u-PA on RSMC migration. A similar effect was also noticed with fMLP (not shown), as expected.31 34 However, BTP had no effect on random cell migration in the absence of stimulation. These data show that also in RSMC the migratory response to pro-u-PA is pertussis toxin-sensitive and, hence, follows the same rules as in myeloid cells, ie, being reproduced with soluble u-PAR fragments and being sensitive to BPT. In fact, C-su-PAR–dependent chemotaxis was also BPT-sensitive. We tested the effect of BPT on bFGF-induced migration; in this case, the inhibitory effect was almost absent (Fig 1F). The differential effect of BPT on pro-u-PA–dependent and bFGF-dependent migration further supports the idea that pro-u-PA acts by mechanisms different from those of bFGF.
Having defined the migratory response of rat SMC to pro-u-PA, we then tested the effect on the cytoskeleton organization of these cells, because they are particularly suitable for this study, due to their well-developed cytoskeletal apparatus.
u-PA/u-PAR induce time-dependent cell shape changes and cytoskeleton reorganization.
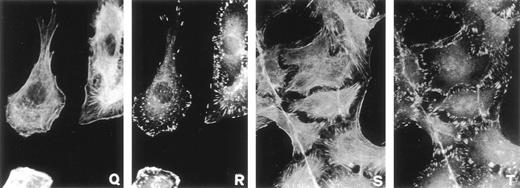
Cell shape change and cytoskeleton reorganization are observed as part of a chemotactic motility response.10 11 To examine cell shape changes and cytoskeleton reorganization, subconfluent cultures of serum-starved RSMC were challenged with rat pro-u-PA (1 nmol/L) for increasing incubation times from 5 to 120 minutes. Control conditions were represented by unstimulated cells kept at 37°C for 120 minutes. Actin filaments were observed using fluorescein-conjugated phalloidin as an actin staining reagent. Cell-substratum contact sites were visualized by immunofluorescence with antivinculin antibodies. Upon stimulation with pro-u-PA, several time-dependent changes in cytoskeleton occurred. The lower magnification pictures of Fig 2 show the general changes that appeared after the addition of pro-u-PA. Within 30 minutes, cell shape, size, and actin cytoskeleton were affected, but the effect reversed after 120 minutes (Fig 2). Higher magnification pictures give more detailed information (Fig 3). In control cultures, most cells show a high number of stress fibers, showing a well-developed cytoskeleton (Fig 3A). Focal adhesions containing vinculin were evenly distributed over the bottom surface of RSMC (Fig 3B). Five minutes after the addition of pro-u-PA, membrane ruffling responses were observed but cell-cell contacts were not broken. A decrease in stress fibers content was also observed (Fig 3C), and vinculin started to be redistributed at the periphery of cells (Fig3D). Subsequently, actin was reorganized into a ring-like circular structure with some of the actin filaments radially disposed (Fig 3E) in contact with vinculin-containing focal contacts (Fig 3F). These pictures also suggest that, through actin ring contractility, this change of shape might serve to break cell-cell contacts (Fig 3G and H), allowing the cell to move. Figure 3I and J show an actin ring in the process of being cleaved and Fig 3K and L show a ring that has been actually cleaved. Cleavage of the ring of actin correlated with a shape resembling that of motile cells in which actin was organized in semicircular structures. These stages are shown in Fig 3M through P. In pro-u-PA–stimulated cells, this typical morphology of motile cells was observed within 30 minutes. Close to the leading edge of the cell, a semi-ring of actin filaments was formed concomitant with the generation of membrane ruffles (Fig 3Q). In many cells, distribution of vinculin also changed becoming organized in a double row, at the leading edge of the cell, flanking the actin filament semi-rings (Fig 3R). These effects gradually declined, and after 2 hours, the number of actin stress fibers and the cell shape returned to levels similar to those observed in control cells (compare Fig 3S and A). However, at this time, the distribution of vinculin did not return to that of unstimulated RSMC, appearing to be still organized in rows at the periphery of cells (compare Fig 3A and T). Cells were aggregated anew and new cell-cell contacts were formed (Fig 3S and T). These data show that the effects of pro-u-PA on cell morphology and cytoskeleton structure are transient.
Effect of pro-u-PA on actin cytoskeleton organization and on morphology of RSMC. Subconfluent (50% to 70%) cells were incubated in the absence (A) or in the presence of 1 nmol/L pro-u-PA for 30 (B) or 120 minutes (C). Actin filaments were visualized using FITC-coupled phalloidin as described.
Effect of pro-u-PA on actin cytoskeleton organization and on morphology of RSMC. Subconfluent (50% to 70%) cells were incubated in the absence (A) or in the presence of 1 nmol/L pro-u-PA for 30 (B) or 120 minutes (C). Actin filaments were visualized using FITC-coupled phalloidin as described.
Effect of pro-u-PA on actin cytoskeleton (A, C, E, G, I, K, M, O, Q, and S) and vinculin distribution (B, D, F, H, J, L, N, P, R, and T) on RSMC. Cells were incubated in the absence (A and B) or in the presence of 1 nmol/L pro-u-PA for 5 minutes (C and D), for 10 to 15 minutes (E, F, G, H, I, and J), for 30 minutes (K, L, M, N, O, P, Q, and R), or for 120 minutes (S and T). The cells were then fixed and double-stained with fluorescein-conjugated phalloidin to visualize actin filaments and with antibody against vinculin, which was visualized with a rhodamine-conjugated secondary antibody.
Effect of pro-u-PA on actin cytoskeleton (A, C, E, G, I, K, M, O, Q, and S) and vinculin distribution (B, D, F, H, J, L, N, P, R, and T) on RSMC. Cells were incubated in the absence (A and B) or in the presence of 1 nmol/L pro-u-PA for 5 minutes (C and D), for 10 to 15 minutes (E, F, G, H, I, and J), for 30 minutes (K, L, M, N, O, P, Q, and R), or for 120 minutes (S and T). The cells were then fixed and double-stained with fluorescein-conjugated phalloidin to visualize actin filaments and with antibody against vinculin, which was visualized with a rhodamine-conjugated secondary antibody.
To test whether the shapes observed really represent intermediates between a resting and a migrating morphology, we measured the frequency of the different types of cellular shapes and cytoskeleton organization at differents times. Photographs were taken at low magnification and cells with different morphology were counted and classified (Fig 4). Three stages were arbitrarily defined. Stage 1 is a cell shape exhibiting numerous stress fibers with vinculin distributed under the surface of the cell in contact with substratum, as shown in Fig 3A and B. These are features of unstimulated cells. Stage 2 includes cells with membrane ruffling and decreased stress fibers content, redistribution of vinculin, formation or disruption of actin ring, or a motile cell shape with actin semi-ring at the leading edge of the cell (Fig 3C through R). Cells were classified in stage 3 when they showed actin stress fibers reformation while vinculin remained still distributed at the periphery of cells (see Fig 3S and T). In Fig 4A, the phalloidin-staining of the three exemplified stages are shown. In unstimulated cultures, 50% of RSMC were in stage 1 and 50% in stage 2 (Fig 4B). Within 5 minutes, the proportion of cells in stage 1 decreased to approximately 25%, whereas stage 2 cells increased by a similar value. No stage 3 cells were observed (Fig 4B). At t = 30 minutes, the percentage of cells in stage 1 remained constant, whereas cells in stage 2 slighty decreased. At this time, approximately 20% of RSMC were classified in stage 3 (Fig 4B). After 120 minutes, the proportion of stage 2 cells decreased to approximately 40% while the percentage of cells in stage 3 increased to approximately 40% (Fig 4B). These data therefore suggest that the addition of pro-u-PA causes changes in cell morphology and cytoskeleton structure that represent modification from a resting to a migrating state.
Pro-u-PA–induced actin cytoskeleton reorganization in RSMC. Cells were stimulated with 1 nmol/L pro-u-PA for different time periods at 37 °C, after which cells were fixed and stained with fluorescein-conjugated phalloidin. Quantification of actin cytoskeleton reorganization was performed by taking photographs at low magnification and by counting cells in each stage of cytoskeleton organization. (A) Examples of these stages are given (for more details see the text) briefly. Stage 1 corresponds to resting cells that exhibit stress fibers. Stage 2 coresponds to cells showing a reorganization of cytoskeleton: a decrease of stress fibers content, membrane ruffling, actin ring or actin semi-ring with elongated cell shape characteristic of motile cells. Stage 3 corresponds to cells that reform stress fibers but with a vinculin distribution different from that of cells in stage 1 (see Fig 3). (B) The percentage of cells exhibiting actin cytoskeleton reorganization was recorded after incubation for 5, 30, and 120 minutes with pro-u-PA.
Pro-u-PA–induced actin cytoskeleton reorganization in RSMC. Cells were stimulated with 1 nmol/L pro-u-PA for different time periods at 37 °C, after which cells were fixed and stained with fluorescein-conjugated phalloidin. Quantification of actin cytoskeleton reorganization was performed by taking photographs at low magnification and by counting cells in each stage of cytoskeleton organization. (A) Examples of these stages are given (for more details see the text) briefly. Stage 1 corresponds to resting cells that exhibit stress fibers. Stage 2 coresponds to cells showing a reorganization of cytoskeleton: a decrease of stress fibers content, membrane ruffling, actin ring or actin semi-ring with elongated cell shape characteristic of motile cells. Stage 3 corresponds to cells that reform stress fibers but with a vinculin distribution different from that of cells in stage 1 (see Fig 3). (B) The percentage of cells exhibiting actin cytoskeleton reorganization was recorded after incubation for 5, 30, and 120 minutes with pro-u-PA.
Because cytoskeletal and shape changes occur during a chemotactic response, we tested whether pro-u-PA effects on chemotaxis and cell shape occurred through the same molecular mechanisms. To test this point, we used u-PAR fragments or synthetic peptides. Both cleaved su-PAR (C-su-PAR) and peptide 1 were able to reproduce the effects of pro-u-PA on cell shape and cytoskeleton. Within the same time-range, 10 pmol/L C-su-PAR (Fig 5B and C) or 1 pmol/L peptide 1 (Fig 5D) induced membrane ruffling, reorganization of actin filaments, and motile morphology, essentially as described above for pro-u-PA. Taken together, these results confirm that pro-u-PA induces cell shape changes and cytoskeleton reorganization through u-PAR using the same signaling pathway that controls cell migration. Because the experiments of Figs 3, 4, and 5 were performed under chemokinetic conditions, the results indicate that the exposure of the same chemotactic u-PAR epitope is involved in both chemotaxis and chemokinesis.
Effects of chymotrypsin-cleaved su-PAR and peptide 1 on actin cytoskeleton on RSMC. Cells were incubated in the absence of chemoattractant (A) or with 10 pmol/L C-su-PAR for 5 (B) or 30 minutes (C) or with 1 pmol/L peptide 1 for 30 minutes. Effects of BPT on the pro-u-PA–induced actin cytoskeleton reorganization on RSMC. Cells were untreated or pretreated with 50 ng/mL pertussis toxin or with 50 ng/mL of mutated pertussis toxin for 6 hours; the cells were then incubated with or without 1 nmol/L pro-u-PA in the absence or in the presence of toxin. (A) Untreated and unstimulated cells. (E) Unstimulated cells treated with 50 ng/mL pertussis toxin. (F) Unstimulated cells treated with 50 ng/mL mutated pertussis toxin. (G) Untreated cells stimulated for 30 minutes with 1 nmol/L pro-u-PA. (H) Pertussis toxin (50 ng/mL) -treated cells stimulated for 30 minutes with 1 nmol/L pro-u-PA. (I) Mutated pertussis toxin (50 ng/mL)- treated cells stimulated for 30 minutes with 1 nmol/L pro-u-PA. Actin filaments were visualized using fluorescein-conjugated phalloidin.
Effects of chymotrypsin-cleaved su-PAR and peptide 1 on actin cytoskeleton on RSMC. Cells were incubated in the absence of chemoattractant (A) or with 10 pmol/L C-su-PAR for 5 (B) or 30 minutes (C) or with 1 pmol/L peptide 1 for 30 minutes. Effects of BPT on the pro-u-PA–induced actin cytoskeleton reorganization on RSMC. Cells were untreated or pretreated with 50 ng/mL pertussis toxin or with 50 ng/mL of mutated pertussis toxin for 6 hours; the cells were then incubated with or without 1 nmol/L pro-u-PA in the absence or in the presence of toxin. (A) Untreated and unstimulated cells. (E) Unstimulated cells treated with 50 ng/mL pertussis toxin. (F) Unstimulated cells treated with 50 ng/mL mutated pertussis toxin. (G) Untreated cells stimulated for 30 minutes with 1 nmol/L pro-u-PA. (H) Pertussis toxin (50 ng/mL) -treated cells stimulated for 30 minutes with 1 nmol/L pro-u-PA. (I) Mutated pertussis toxin (50 ng/mL)- treated cells stimulated for 30 minutes with 1 nmol/L pro-u-PA. Actin filaments were visualized using fluorescein-conjugated phalloidin.
Because cytoskeleton reorganization correlated with cell migration, we examined the effect of BPT on the reorganization of actin filaments. Treatment of control (untreated and unstimulated) cells either with BPT or with a mutated BPT, which cannot ADP-ribosylate G proteins, had no effect (Fig 5A, E, and F). However, pro-u-PA–induced actin cytoskeleton reorganization of RSMC (Fig 5G) was inhibited by active BPT (Fig 5H). In contrast, mutated pertussis toxin did not inhibit pro-u-PA–induced cell shape change and cytoskeleton reorganization (Fig 5I). The qualitative data of Fig 5 are representative of those quantitatively obtained in cell migration assays. The pertussis-toxin sensitivity of pro-u-PA–mediated cell shape change and cytoskeleton reorganization, like chemotaxis, suggests that the u-PAR signaling pathway may require G proteins.
Wounding experiments.
Using two different assays, chemotaxis and chemokinesis, we have shown that pro-u-PA stimulates cell shape change, cytoskeleton reorganization, and cell migration through binding to u-PAR. An in vitro wound-healing assay was used to confirm these results.
Single-cross wounds were made in confluent monolayers of serum-starved RSMC grown on glass coverslips and cells were stimulated with 1 pmol/L peptide D containing the rat u-PAR minimal chemotactic epitope. Peptide D increased the number of migrating cells by approximately 50% compared with control conditions (Fig 6A). However, medium containing 1% FCS, which was used as positive control, gave a more than 100% increment. RSMC migrating from the edge of the wound exhibited the same change in morphology and cytoskeleton organization described above in chemokinesis assays, like formation of an actin ring with some actin filaments located radially to the ring (Fig 6B), or motile morphology with cytoskeleton organized into an actin semi-ring and membrane ruffles located at the leading edge of cells (Fig 6C).
Effect of peptide D on RSMC migration into a wound. Single-cross wounds were made in confluent monolayers grown on glass coverslip. After a wash with PBS, monolayers were then allowed to recover for 24 hours in the absence or in the presence of 1 pmol/L peptide D. Peptide D contains the minimum chemotactic epitope of rat u-PAR, corresponding to the human peptide 1. One percent FCS was used as a positive control. Quantification of migration was performed by taking photographs at low magnification and counting cells that had migrated into the cell-free space (A). Two examples of actin cytoskeleton organization in migrating cells, visualized using rhodamine-conjugated phalloidin, are shown in (B) and (C).
Effect of peptide D on RSMC migration into a wound. Single-cross wounds were made in confluent monolayers grown on glass coverslip. After a wash with PBS, monolayers were then allowed to recover for 24 hours in the absence or in the presence of 1 pmol/L peptide D. Peptide D contains the minimum chemotactic epitope of rat u-PAR, corresponding to the human peptide 1. One percent FCS was used as a positive control. Quantification of migration was performed by taking photographs at low magnification and counting cells that had migrated into the cell-free space (A). Two examples of actin cytoskeleton organization in migrating cells, visualized using rhodamine-conjugated phalloidin, are shown in (B) and (C).
We conclude, therefore, that pro-u-PA can activate chemotaxis, chemokinesis, and wound-healing in vitro through the same mechanism(s) that includes a modification of u-PAR. However, the effect in the wound-healing assay is only minor.
Pro-u-PA induces membrane relocation of integrins, u-PAR, and c-Src.
We next examined the effects of pro-u-PA on the subcellular localization of u-PAR, VNR αvβ3, β1 integrin subunit, and c-Src, using immunofluorescence microscopy.
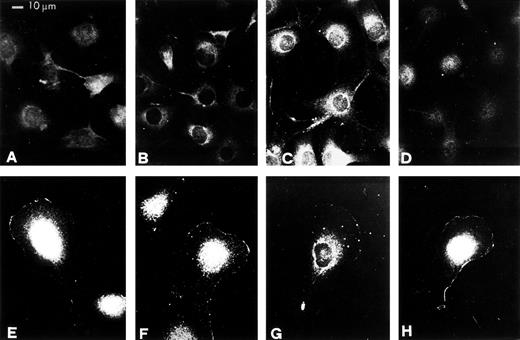
In control cells, u-PAR, like VNR and integrin β1 subunit, was widely distributed over the surface of the cell in contact with the substratum (Fig 7A, B, and C). In pro-u-PA–stimulated RSMC exhibiting motile morphology, u-PAR redistributed to the leading edge of the cell (Fig 7E). Similarly, VNR αvβ3 and β1 subunit were redistributed to the leading edge of migrating cells (Fig 7F and G). Whereas in nonstimulated cells c-Src was distributed in the cytoplasm (Fig 7D), pro-u-PA caused a c-Src redistribution to plasma membrane. In motile RSMC, c-Src was mainly located at the leading edge of cells (Fig 7H).
Relocations of u-PAR, VNR, β1, and c-Src proteins to membranes at the leading edge of migrating RSMC in response to pro-u-PA. Subconfluent (50% to 70%) cultures were incubated in the absence (A, B, C, and D) or in the presence of 1 nmol/L pro-u-PA for 30 minutes (E, F, G, and H). The cells were then fixed and stained with antibodies against u-PAR (A and E), VNR (B and F), β1 (C and G), or c-Src (D and H).
Relocations of u-PAR, VNR, β1, and c-Src proteins to membranes at the leading edge of migrating RSMC in response to pro-u-PA. Subconfluent (50% to 70%) cultures were incubated in the absence (A, B, C, and D) or in the presence of 1 nmol/L pro-u-PA for 30 minutes (E, F, G, and H). The cells were then fixed and stained with antibodies against u-PAR (A and E), VNR (B and F), β1 (C and G), or c-Src (D and H).
In conclusion, these data show that pro-u-PA induces relocation of u-PAR, integrins, and c-Src to the leading edge of motile RSMC. The physiological relevance of this finding is supported by the observation that, unlike Src+ 3T3/u-PAR fibroblasts, Src−/− 3T3/u-PAR fibroblasts do not respond to ATF by cell shape changes and cytoskeletal reorganization (Fig 8).
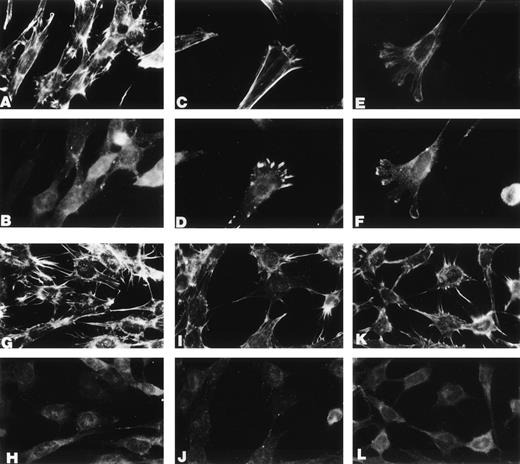
Src is required for u-PA–dependent cell shape changes. Mouse 3T3 cells from Src−/− and wild-type mice were transfected with a human u-PAR cDNA. u-PAR–positive cells (determined by direct binding and immunofluorescence assays) were then supplemented with 10 nmol/L ATF (the amino terminal fragment of human u-PA) and incubated for 0 or 30 minutes at 37°C; at the end of the incubation, the cells were washed, stained with phalloidin (A, C, E, G, I, and K) or with antivinculin antibodies (B, D, F, H, J, and L), and viewed and photographed under the immunofluorescence microscope. From (A) to (F): Src+ cells incubated in the absence (A and B) or in the presence of 10 nmol/L ATF for 30 minutes (C, D, E, and F). From (G) to (L): Src−/− cells incubated in the absence (G and H) or in the presence of 10 nmol/L ATF for 30 minutes (I, J, K, and L).
Src is required for u-PA–dependent cell shape changes. Mouse 3T3 cells from Src−/− and wild-type mice were transfected with a human u-PAR cDNA. u-PAR–positive cells (determined by direct binding and immunofluorescence assays) were then supplemented with 10 nmol/L ATF (the amino terminal fragment of human u-PA) and incubated for 0 or 30 minutes at 37°C; at the end of the incubation, the cells were washed, stained with phalloidin (A, C, E, G, I, and K) or with antivinculin antibodies (B, D, F, H, J, and L), and viewed and photographed under the immunofluorescence microscope. From (A) to (F): Src+ cells incubated in the absence (A and B) or in the presence of 10 nmol/L ATF for 30 minutes (C, D, E, and F). From (G) to (L): Src−/− cells incubated in the absence (G and H) or in the presence of 10 nmol/L ATF for 30 minutes (I, J, K, and L).
Cooperation of u-PAR and vitronectin receptor in pro-u-PA–induced cell migration.
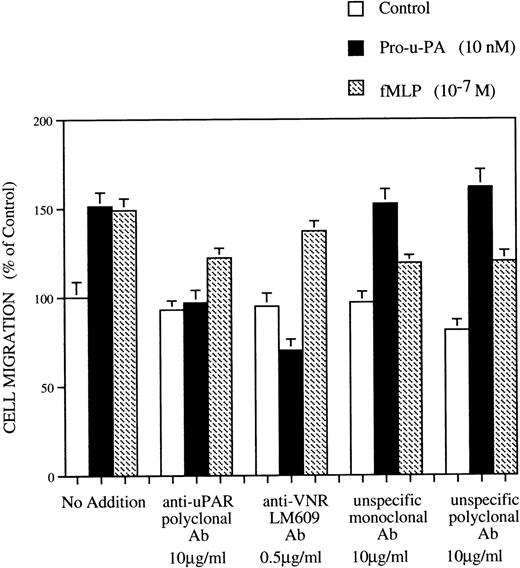
Polyclonal antibodies against domain 1 of rat u-PAR inhibit pro-u-PA binding (S. Rabbani, data not shown). RSMC were first incubated with either anti-u-PAR or control antibodies (10 μg/mL) and then subjected to chemotaxis assay. Treatment with anti-u-PAR but not unspecific control antibodies inhibited the response to pro-u-PA (Fig 9). In addition, we found that pro-u-PA–induced RSMC migration could also be blocked with LM 609, a monoclonal antibody against VNR αvβ3 (Fig 9). None of these treatments altered the fMLP-dependent chemotaxis. Control monoclonal or polyclonal antibodies had, respectively, no or slight inhibitory effect on RSMC migration (Fig 9).
Effect of antibodies anti-u-PAR and anti-VNR on RSMC chemotaxis in response to pro-u-PA and fMLP. Cells were detached from support and pretreated for 1 hour at 4°C in serum-free medium without or with antibodies against u-PAR (10 μg/mL) or with antibodies anti-VNR (LM 609) (0.5 μg/mL) or with unspecific isotype-matched control antibodies (10 μg/mL). The cells were then subjected to chemotaxis assay and migrated towards medium alone (□), 10 nmol/L pro-u-PA (▪), or 10−7 mol/L fMLP (▨). Antibodies at the same concentrations as in the pretreatment were added in both chambers of the Boyden apparatus and were present during the whole assay. Random cell migration of RSMC towards medium alone without any chemoattractants or antibodies was considered to be 100% migration. Results are the mean mean ± SD (n = 3).
Effect of antibodies anti-u-PAR and anti-VNR on RSMC chemotaxis in response to pro-u-PA and fMLP. Cells were detached from support and pretreated for 1 hour at 4°C in serum-free medium without or with antibodies against u-PAR (10 μg/mL) or with antibodies anti-VNR (LM 609) (0.5 μg/mL) or with unspecific isotype-matched control antibodies (10 μg/mL). The cells were then subjected to chemotaxis assay and migrated towards medium alone (□), 10 nmol/L pro-u-PA (▪), or 10−7 mol/L fMLP (▨). Antibodies at the same concentrations as in the pretreatment were added in both chambers of the Boyden apparatus and were present during the whole assay. Random cell migration of RSMC towards medium alone without any chemoattractants or antibodies was considered to be 100% migration. Results are the mean mean ± SD (n = 3).
These results suggest that the relocation of u-PAR and integrins might have also functional implication and that both u-PAR and vitronectin receptor functions are involved in u-PAR–mediated RSMC migration.
DISCUSSION
Chemokines are proteins controlling leukocyte migration.31,34 However, many cell types are required to migrate and hence may use similar mechanisms as leukocytes. The u-PA/u-PAR system, which has been shown to be essential in the migration not only of T lymphocytes and macrophages but also of many tumor cells, appears to control migration of many cell types. Indeed, mice lacking u-PA are extremely sensitive to bacterial infections,35,36 and metastasis in nude mice xenograft models can be prevented by blocking the activity of u-PA or its interaction with u-PAR.37-41
In this report, we show that in rat smooth muscle cells pro-u-PA can induce chemotaxis, chemokinesis, and wound healing in vitro, along with cytoskeletal and adhesion plaques rearrangements and rapid relocalization of u-PAR, integrins, and c-Src to the leading edge of the migrating cells. At least the migration and the cytoskeletal rearrangement are blocked by pertussis toxin, implying that a heterotrimeric G protein-coupled signal transduction mechanism is at the basis of these effects. Addition of pro-u-PA can be substituted by the exogenous supply of u-PAR fragments or of synthetic peptides that contain the u-PAR chemotactic epitope and that have been shown to mimic u-PA–induced chemotaxis in myelomonocytic cells.5 Thus, the basic mechanisms involved are the same in RSMC, myelomonocytic cells, and other cells. For all of these considerations, the real inducer of migration is the membrane molecule, u-PAR. Because u-PAR has neither transmembrane nor intracytoplasmic domain, it needs to contact a transmembrane-signal transducer (the adaptor).5 Thus, u-PAR is a cell-surface chemokine that becomes activated upon pro-u-PA binding. The term chemokine, although improper for the lack of a structural basis, is also justified by the inhibitory activity exerted by BTP, ie, by the possible involvement of heterotrimeric G proteins in u-PAR–dependent signaling.
The initial mechanism appears again to be the exposure of a specific u-PAR epitope as previously demonstrated in nonadherent myeloid cells, because pro-u-PA can be substituted by soluble u-PAR fragments or synthetic peptides.5 That pro-u-PA induces a dose-dependent chemotactic response in RSMC through a conformational modification of u-PAR is shown by two sets of data. First, anti-u-PAR antibodies can block the chemotactic effect of u-PA and, second, cleaved su-PAR as well as peptides 1 and D can substitute for pro-u-PA and induce cell migration, in agreement with previous data.4,5 This report also shows that the same mechanism applies to different migration models, chemotaxis, chemokinesis, and, to a lesser extent, wound assays. Indeed, not only u-PAR dependent chemotaxis, but also the cytoskeletal changes are induced by u-PAR fragments or peptides and are blocked by BPT. This suggests that a u-PAR signaling pathway is mediated by a Gi-coupled receptor. This is the case for the fMLP-induced chemotaxis, which is mediated by a chemoattractant receptor coupled to G proteins.31,32 However, we have found no direct evidence of cross-talk between fMLP and u-PAR signaling. It is also well known that G proteins play a key role in the regulation of the actin cytoskeleton through intermediates signaling molecules of the rho subfamily.42,43 In particular, membrane ruffles appear to be regulated by the rac GTP-binding protein, whereas rho stimulates actin stress fibers and focal adhesion formation.43-45
Clusters of integrins bound to proteins of the extracellular matrix form adhesion plaques linked to intracellular proteins and constitute the site of attachment of actin stress fibers.46,47 c-Src, normally localized to endosomal membranes, is redistributed to focal adhesions upon cell activation, where it regulates cell adhesion.48-52 Molecular interactions of u-PAR with integrins and tyrosine kinases of the Src family has been reported and functional cooperation has been suggested.4,9 53-55 We demonstrate here that c-Src, αvβ3, and β1 integrins, as well as u-PAR, are redistributed to the leading edge upon pro-u-PA–induced migration of RSMC.
Moreover, antibodies to αvβ3, as well to u-PAR, inhibit pro-u-PA–dependent chemotaxis, suggesting a functional cooperation between these molecules in promoting migration. Indeed, in SMC, adhesion depends on β1 integrins, whereas migration depends on αvβ3.56,57 It is now well established that u-PAR is able to provide cell adhesion by binding directly to vitronectin.3,58 However, u-PAR also interacts with integrins.9,53-55 Integrins may play different roles in adhesion and migration,56,59-61 and u-PAR interaction with integrins appears to modulate their functions.9 55Important questions need to be answered to understand u-PAR–regulated cell migration. In particular, it is not known whether u-PAR affects chemotaxis and cell adhesion through consecutive, parallel, or connected pathways.
The presence of c-Src at the leading edge of RSMC demonstrates its activation upon u-PAR–signaling. Involvement of c-Src in the signaling pathway of u-PAR is supported by our finding that 3T3/u-PAR fibroblasts from Src−/− mice show an altered response to ATF challenge. Compared with wild-type, 3T3/u-PAR Src−/− fibroblasts do not reorganize their actin cytoskeleton or show shape changes.
Finally, the question still remains on whether cleavage of u-PAR in vivo is required for signaling. Although many of the effects of u-PA can be obtained with catalytically inactive derivatives (such as pro-u-PA or ATF), nevertheless, the position of the chemotactic epitope coincides with a u-PA–sensitive site of u-PAR.5 Indeed, at physiological concentrations, u-PA cleaves u-PAR at residue 83 very efficiently (50% in 30 minutes).8 Moreover, the linker region between domain D1 and D2 may not only be cleaved by u-PA, but also by chymotrypsin, plasmin, and other proteolytic enzymes. It is therefore possible that cleavage of u-PAR, even though required for activity, may also occur after conformational change induced by catalytically inactive u-PA derivatives, because it can be performed by other proteases produced by the cell system used. Further studies will be required to solve this problem.
ACKNOWLEDGMENT
The authors thank Drs M. Bertulli, M.G. Pizza, M. Nielsen, K.B. Kaplan, and J. Henkin for providing cells and reagents. We are very grateful to Dr P.C. Marchisio for helpful advice and comments. We also give our friendly thanks to Drs M. Mazzotti, G. Cecchini, L. Spinardi, and T. Teesalu for technical support and stimulating discussion.
Supported by grants from the Italian Association for Cancer research (AIRC), The Italian Ministry of Education (MURST), the Istituto Superiore di Sanità (ISS), and the EU (Biomed Grant No. BMH4-CT96-0017).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Francesco Blasi, MD, Molecular Genetics, DIBIT, H. S. Raffaele, via Olgettina, 58, 20132 Milano, Italy; e-mail:blasi.francesco@hsr.it.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal