Abstract
Among snake venom procoagulant proteins, group II prothrombin activators are functionally similar to blood coagulation factor Xa. We have purified and partially characterized the enzymatic properties of trocarin, the group II prothrombin activator from the venom of the Australian elapid, Tropidechis carinatus (rough-scaled snake). Prothrombin activation by trocarin is enhanced by Ca2+, phospholipids, and factor Va, similar to that by factor Xa. However, its amidolytic activity on peptide substrate S-2222 is significantly lower. We have determined the complete amino acid sequence of trocarin. It is a 46,515-Dalton glycoprotein highly homologous to factor Xa and shares the same domain architecture. The light chain possesses an N-terminal Gla domain containing 11 γ-carboxyglutamic acid residues, followed by two epidermal growth factor (EGF)-like domains; the heavy chain is a serine proteinase. Both chains are likely glycosylated: the light chain at Ser 52 and the heavy chain at Asn 45. Unlike other types of venom procoagulants, trocarin is the first true structural homologue of a coagulation factor. It clots snake plasma and thus may be similar, if not identical, to snake blood coagulation factor Xa. Unlike blood factor Xa, it is expressed in high quantities and in a nonhepatic tissue, making snake venom the richest source of factor Xa-like proteins. It induces cyanosis and death in mice at 1 mg/kg body weight. Thus, trocarin acts as a toxin in venom and a similar, if not identical, protein plays a critical role in hemostasis.
THE ACTIVATION OF prothrombin to thrombin is a reaction central to the blood coagulation cascade. This is accomplished in vivo by the prothrombinase complex consisting of a serine proteinase factor Xa and a cofactor factor Va formed on negatively charged phospholipid membranes in the presence of Ca2+ ions.1 Prothrombin is also specifically targeted by various exogenous hemostatic factors that affect blood coagulation.2 Over the years, many such exogenous proteins from snake venom sources have been identified.3,4 These proteins proteolytically activate prothrombin and are grouped into four categories based on structural characteristics, functional properties, and cofactor requirements in prothrombin activation.5,6Group IA activators, such as ecarin,7 are metalloproteinases that efficiently convert prothrombin into meizothrombin. Their activity is not enhanced by the addition of the nonenzymatic cofactors of the prothrombinase complex: factor Va, phospholipids, and Ca2+ ions. On the other hand, group IB activators are metalloproteinase–C-type-lectin complexes whose activity is Ca2+-dependent. They are exemplified by carinactivase-1 and multactivase.6,8 The ability of group II prothrombin activators, such as notecarin,9 to convert prothrombin to thrombin is greatly enhanced by factor Va in the presence of negatively charged phospholipids and Ca2+ions. This group of proteins has been found exclusively in Australian elapid venoms. Group III activators also activate prothrombin to thrombin by cleaving both the necessary peptide bonds. However, their prothrombin-converting abilities are enhanced by only Ca2+ ions and phospholipids, not by factor Va. Such prothrombin activators have been isolated from Oxyuranus scutellatus (oscutarin) and Pseudonaja textilisvenoms.10 11
Functionally, group II prothrombin activators strongly resemble blood coagulation factor Xa.2,5,9 Like factor Xa, these serine proteinases cleave both peptide bonds (Arg 274-Thr 275 and Arg 323-Ile 324) of bovine prothrombin, meizothrombin and prethrombin-2 being formed as intermediates.9 Their prothrombin-converting activities are stimulated to an extent comparable to that of factor Xa by the addition of the other cofactors required for the formation of the prothrombinase complex.9 As with factor Xa, factor Va forms a tight 1:1 complex with these prothrombin activators in the presence of Ca2+ ions and phospholipids.9 The molecular size and subunit compositions of these proteinases and factor Xa are also comparable. The molecular masses of group II proteinases range from 54 to 64.5 kD,2 12 whereas that of factor Xa is 45 kD. All of these activators, like factor Xa, have two disulfide-linked subunits, a light chain (molecular weight [Mr], ∼23 kD) and a heavy chain (Mr range, 32 to 37 kD). Furthermore, the active site of these serine proteinases is also found in the heavy chain.
Although functional similarities between group II prothrombin activators and factor Xa have been well documented, structural details, including sequence information on this group, are not yet available. Structural aspects of these proteinases should contribute significantly towards understanding structure-function relationships and mechanisms of factor Xa-mediated prothrombin activation. Furthermore, the phylogenetic distance and functional similarities between these proteinases and factor Xa could help define molecular details of interactions among the protein components of the prothrombinase complex. Therefore, we initiated studies on trocarin, a group II prothrombin activator from the venom of the Australian rough-scaled snake, Tropidechis carinatus. We describe here its purification, characterization, and complete amino acid sequence. (The SWISS-PROT accession no. of trocarin is P81428.) The data presented here indicate that trocarin is the first exogenous procoagulant protein produced in a nonhepatic source, namely, the venom gland, which shares structural and functional similarities with coagulation factor Xa.
MATERIALS AND METHODS
Materials.
T carinatus venom was obtained from Venom Supplies Pty Ltd (Tanunda, South Australia). Lysyl endopeptidase, endoproteinase Glu C, and endoproteinase Asp N were purchased from Wako Pure Chemicals (Osaka, Japan). β-Mercaptoethanol was purchased from Nacalai Tesque (Kyoto, Japan). Trypsin, 4-vinylpyridine, phosphatidylcholine (PC), phosphatidylserine (PS), ovalbumin, bovine serum albumin (BSA), and soybean trypsin inhibitor were bought from Sigma Chemical Co (St Louis, MO). Cyanogen bromide was bought from JT Baker (Phillipsburg, NJ). BNPS-skatole was obtained from Fluka Chemika-BioChemika (Buchs, Switzerland). S-2222 and S-2238 were purchased from Chromogenix (Mölndal, Sweden). Bovine prothrombin, bovine factor Xa, and bovine factor Va were obtained from Haematologic Technologies Inc (Essex Junction, VT). Human plasma was donated by healthy volunteers.Python reticulatus plasma was obtained by heart puncture of pythons anaesthetized with sodium phenobarbital (60 mg/kg body weight). All other chemicals and reagents were of the highest quality available.
Procoagulant activity.
The procoagulant activity of T carinatus venom and its fractions was determined by their effect on the recalcification time of human plasma using a BBL fibrometer (Becton-Dickinson Microbiology Systems, Sparks, MD). Clotting of a mixture of 100 μL human plasma and 250 μL (50 mmol/L Tris-HCl buffer, pH 7.5, plus sample) at 37°C was initiated by adding 50 μL of 50 mmol/L CaCl2, and the recalcification time was measured. The procoagulant effect of the purified prothrombin activator, trocarin, onP reticulatus plasma was also determined. Dose responses for trocarin and human factor Xa using both human and P reticulatus plasmas were performed.
Reconstitution of the prothrombinase complex.
The effect of the various cofactors of the prothrombinase complex (phospholipids, factor Va, and Ca2+ ions) on prothrombin activation by trocarin was determined by measuring the generation of thrombin. The prothrombinase complex was reconstituted as follows. Appropriate dilutions of trocarin (or bovine and human factor Xa, for comparison) were preincubated for 15 minutes at 25°C in a buffer containing 50 mmol/L Tris-HCl, 100 mmol/L NaCl, 0.5 mg/mL ovalbumin, pH 7.5, in the presence or absence of various cofactors such as phospholipid vesicles (9:1 PC:PS; prepared according to de Kruiff et al13; 100 μmol/L), factor Va (10 nmol/L), and CaCl2 (5 mmol/L). When CaCl2 was not present in the mixture, EDTA was added (final concentration, 10 mmol/L). Activation was initiated with the addition of prothrombin (final concentration, 5.6 μmol/L). After different time intervals, aliquots of 1 μL were withdrawn from the reaction mixture (total volume, 50 μL) and added to 190 μL of 50 mmol/L Tris-HCl, 100 mmol/L NaCl, pH 7.5, containing 10 μg/mL soybean trypsin inhibitor, and 25 mmol/L EDTA (to inhibit further conversion of prothrombin). The thrombin-specific chromogenic substrate H-D-Phe-pipecolyl-Arg-p-nitroaniline hydrochloride (S-2238) was subsequently added (10 μL; final concentration, 100 μmol/L), and the amidolytic activity of the thrombin formed was determined by measuring its hydrolysis at 405 nm. Measurements were made in triplicate using 96-well microtiter plates on a Ceres UV 900C ELISA plate reader (Bio-Tek Instruments Inc, Winooski, VT). Soybean trypsin inhibitor inhibits the low amidase activity of factor Xa on S-2238 but does not affect the activity of thrombin on the substrate.14
Determination of cleavage site(s) of prothrombin by trocarin.
The cleavage products formed during prothrombin activation by trocarin were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Prothrombin (5 μg) was incubated with trocarin or human factor Xa (5 ng each) in the presence of Ca2+ ions (5 mmol/L), 9:1 PC:PS phospholipid vesicles (100 μmol/L), and factor Va (10 nmol/L) in 50 mmol/L Tris-HCl, 100 mmol/L NaCl, pH 7.5, at 37°C for 4 hours. The reaction mixtures were electrophoresed on a nonreducing gradient gel (4% to 20%), according to Laemmli.15 The gels were stained with Coomassie Brilliant Blue R-250.
To determine the cleavage site(s) of prothrombin by trocarin, we activated prothrombin in the presence of factor Va and Ca2+ions. Bovine prothrombin (1 μmol/L), trocarin (or human factor Xa, for comparison; 10 nmol/L), and bovine factor Va (5 nmol/L) were dissolved in a buffer of pH 7.5, containing 50 mmol/L Tris-HCl, 100 mmol/L NaCl, 5 mmol/L CaCl2, and 1 μmol/L BSA, and incubated at 37°C overnight. The cleavage products were separated by reverse-phase high pressure liquid chromatography (RP-HPLC) on a Sephasil C18 (100 × 2.1 mm) column. The amino terminal sequence of thrombin was determined by Edman degradation, as described below.
Amidolytic activity on chromogenic substrate.
The amidolytic activity of trocarin on the factor Xa-specific chromogenic substrate N-benzoyl-L-Ile-L-Glu-L-Gly-L-Arg-p-nitroaniline hydrochloride (S-2222) was studied as follows. Varying amounts of S-2222 were added to 50 mmol/L Tris-HCl, 100 mmol/L NaCl, pH 7.5, containing trocarin (1 μmol/L) or factor Xa (10 nmol/L) in disposable cuvettes (total volume, 1 mL) at 25°C. The release of p-nitroaniline was monitored at 405 nm using a Jasco V-560 UV/Vis spectrophotometer (Jasco Corp, Tokyo, Japan). The Km and Vmax were estimated from Lineweaver-Burk plots.
Protein purification.
Trocarin was purified by gel filtration of T carinatus crude venom, followed by anion exchange and RP-HPLC. Crude venom (200 mg in 2 mL distilled water) was applied to a Superdex 75 gel filtration column (1.6 × 60 cm) equilibrated with 50 mmol/L Tris-HCl buffer, pH 7.5, using a Pharmacia FPLC system (Pharmacia, Uppsala, Sweden). The pooled procoagulant fraction was subfractionated on an anion exchange column UNO Q-1 (Bio-Rad, Hercules, CA; column volume, 1 mL) also using the FPLC system. Approximately 20 mg of protein was loaded on to the column equilibrated with 50 mmol/L Tris-HCl buffer, pH 7.5. Bound proteins were eluted with a linear gradient of 1 mol/L NaCl in the same buffer. In the final step, trocarin was purified by RP-HPLC on a Nucleosil C18 (250 × 10 mm) column using a Vision Workstation (Perkin-Elmer Biosystems, Foster City, CA). The column was equilibrated with 0.1% trifluoroacetic acid (TFA), and a linear gradient of 80% acetonitrile in 0.1% TFA was used for elution.
Capillary zone electrophoresis.
Homogeneity of the protein was assessed by capillary electrophoresis essentially as described earlier.16
Electrospray ionization mass spectrometry.
Precise masses (±0.01%) of the native protein and peptides were determined by electrospray ionization mass spectrometry using a Perkin-Elmer Sciex API 300 LC/MS/MS System. Theoretical masses of the peptides were calculated by a custom software written by Chiew Fook Loong (School of Computing, National University of Singapore, Singapore).
Separation of subunits.
The protein subunits were separated by reduction and pyridylethylation. The native protein (1 mg) was dissolved in 500 μL of 0.13 mol/L Tris HCl, 1 mmol/L EDTA, 6 mol/L guanidine HCl, pH 8.5. After adding 20 μL of the reducing agent, β-mercaptoethanol, this solution was incubated under N2 for 2 hours at 37°C. The alkylating reagent, 4-vinylpyridine (200 μL), was subsequently added and the mixture was incubated under N2 for another 2 hours at room temperature. The reaction mixture was immediately desalted and the two pyridylethylated chains separated by RP-HPLC on a Nucleosil C18 (250 × 4.6 mm) column using a linear gradient of acetonitrile in 0.1% TFA.
Enzymatic cleavage.
Peptides of the light and heavy chains were generated by digestions with the enzymes lysyl endopeptidase (Lys C), endoproteinase Glu C, and endoproteinase Asp N. In each case, approximately 200 μg of protein was dissolved in 200 μL of 50 mmol/L Tris-HCl buffer, 4 mol/L urea, 5 mmol/L EDTA, pH 7.5. The enzymes Lys C, Glu C, or Asp N (5 μg each) were added and digestions were performed overnight at 37°C.
Chemical cleavage.
Heavy chain peptides were also obtained by chemical cleavage using cyanogen bromide (Met-specific) and 3-bromo-3-methyl-2-(2-nitrophenylmercapto)-3H-indole (BNPS-skatole; Trp-specific) performed essentially as described in Current Protocols in Protein Science,17 except that, in the former procedure, 70% TFA was used, instead of 70% formic acid. For cleavage with CNBr, the heavy chain (500 μg) was dissolved in 250 μL of 70% TFA to which 5 μL of β-mercaptoethanol was added. A 200-fold molar excess (over Met residues) of CNBr in 70% TFA was added to make a final protein concentration of 1 mg/mL. After 24 hours of incubation in the dark at room temperature, 10 vol of Milli-Q water was added to the mixture and lyophilized. Tryptophanyl bond cleavage was performed as follows. The heavy chain (500 μg) was dissolved in 500 μL 0.1% TFA. Approximately 1.5 mL of 1 mg/mL BNPS-skatole solution in glacial acetic acid was added, and the mixture was incubated overnight in the dark at room temperature for 24 hours. An equal volume of Milli-Q water was subsequently added and the digest was centrifuged at 10,000g to precipitate residual reagent and reaction byproducts.
The peptides generated by enzymatic and chemical cleavage were separated by RP-HPLC using a Sephasil C18 (100 × 2.1 mm), a Jupiter C18 (250 × 4.6 mm), or a Hypersil BDS C18 (150 × 1 mm) column, using linear gradients of acetonitrile in 0.1% TFA.
Amino terminal sequencing.
Amino terminal sequencing of the native protein, its light and heavy chains, and the peptides generated by digesting them was performed by automated Edman degradation using a Perkin-Elmer Applied Biosystems 494 pulsed-liquid phase protein sequencer (Procise) with an on-line 785A PTH-amino acid analyzer.
Decarboxylation of light chain.
The light chain was decarboxylated according to Poser and Price.18 The reduced and alkylated light chain was lyophilized to dryness from 50 mmol/L HCl and then heated at 110°C overnight under vacuum. Before sequencing, the sample was cleaned up by RP-HPLC using a Jupiter C18 (250 × 4.6 mm) column.
Carbohydrate detection.
The method of Dubois et al19 was used to detect the presence of carbohydrate in individual chains of the protein. Concentrated sulphuric acid (5 mL) was dropped directly into 1 mL of 5% phenol containing 400 μg of the light chain or 480 μg of the heavy chain, and the absorbance at 490 nm was measured. Glucose was used as the standard; BSA and insulin were negative controls.
RESULTS AND DISCUSSION
Purification of prothrombin activator from T carinatus venom.
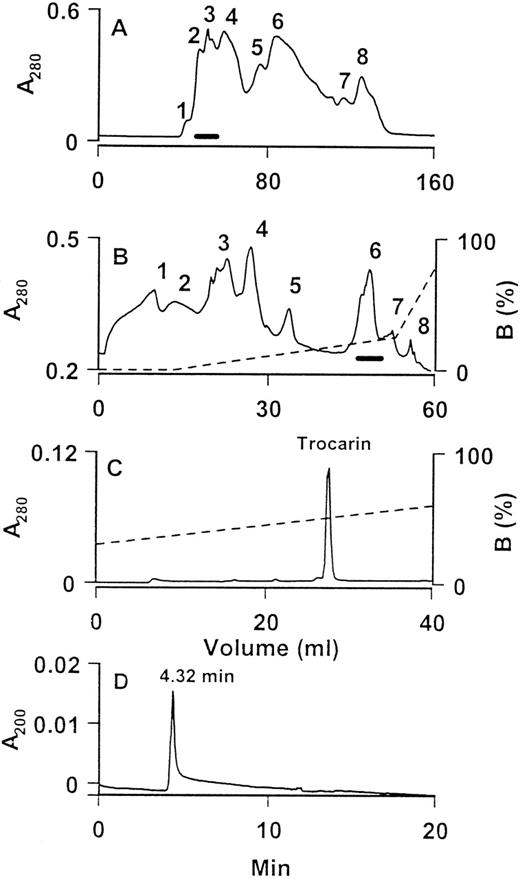
The procoagulant effects of the venom of T carinatus are well known.20,21 A factor Xa-like activity responsible for these procoagulant effects was partially purified by Morrison et al.22 Recently, Marsh et al12 isolated and partially characterized the prothrombin activator and showed that it belongs to group II. We purified it to homogeneity from T carinatus venom as follows. The crude venom was separated into eight fractions on a Superdex 75 gel filtration column (Fig 1A). Procoagulant fractions were pooled and subfractionated using anion exchange chromatography on a UNO Q-1 column (Fig 1B). Of the eight fractions obtained, procoagulant activity was found only in fraction 6. This fraction was homogeneous, as judged from its RP-HPLC profile (Fig 1C). This procoagulant protein was named trocarin in accordance with the recommendations of the Subcommittee for the Nomenclature of Exogenous Hemostatic Factors.23 It comprised approximately 5% of total venom protein (wt/wt). Trocarin is not highly soluble in aqueous buffers (pH 3.0 to 7.5). When pure, it dissolves only up to approximately 1 mg/mL. Its late elution on a Nucleosil C18 column also indicates its hydrophobic nature.
Purification and determination of homogeneity of trocarin. (A) Gel chromatography of T carinatus venom on a Superdex 75 column. Flow rate: 1.5 mL/min. Procoagulant activities of all fractions were tested and fractions indicated by a horizontal bar were pooled. (B) Subfractionation of pooled procoagulant fraction by anion exchange chromatography on a UNO Q-1 column. Bound proteins were eluted by a linear gradient of NaCl. Flow rate: 2 mL/min. Procoagulant activities of all fractions were tested and fractions indicated by a horizontal bar were pooled. (C) RP-HPLC of fraction 6 from UNO Q-1 column. Column: Nucleosil C18. Bound proteins were eluted by a linear gradient of buffer B (80% ACN in 0.1% TFA). Flow rate: 2 mL/min. The procoagulant protein peak is labeled as trocarin. (D) Capillary zone electropherogram of trocarin. Electrophoresis runs of the protein (1 mg/mL) were performed on a BioFocus 3000 HPCE system (Bio-Rad) using a coated capillary (24 cm × 25 μm) from positive to negative polarities at 12 kV. Buffer: 100 mmol/L phosphate buffer, pH 2.5. Temperature: 18°C. Duration: 20 minutes. Sample injected using pressure mode 10 psi/s. Trocarin migrates as a single peak at 4.32 min.
Purification and determination of homogeneity of trocarin. (A) Gel chromatography of T carinatus venom on a Superdex 75 column. Flow rate: 1.5 mL/min. Procoagulant activities of all fractions were tested and fractions indicated by a horizontal bar were pooled. (B) Subfractionation of pooled procoagulant fraction by anion exchange chromatography on a UNO Q-1 column. Bound proteins were eluted by a linear gradient of NaCl. Flow rate: 2 mL/min. Procoagulant activities of all fractions were tested and fractions indicated by a horizontal bar were pooled. (C) RP-HPLC of fraction 6 from UNO Q-1 column. Column: Nucleosil C18. Bound proteins were eluted by a linear gradient of buffer B (80% ACN in 0.1% TFA). Flow rate: 2 mL/min. The procoagulant protein peak is labeled as trocarin. (D) Capillary zone electropherogram of trocarin. Electrophoresis runs of the protein (1 mg/mL) were performed on a BioFocus 3000 HPCE system (Bio-Rad) using a coated capillary (24 cm × 25 μm) from positive to negative polarities at 12 kV. Buffer: 100 mmol/L phosphate buffer, pH 2.5. Temperature: 18°C. Duration: 20 minutes. Sample injected using pressure mode 10 psi/s. Trocarin migrates as a single peak at 4.32 min.
The homogeneity of trocarin was determined by capillary zone electrophoresis and electrospray ionization mass spectrometry. The electropherogram (Fig 1D) shows a single peak, indicating a single molecular species. The mass spectrum (data not shown) showed peaks of mass/charge ratios ranging from 19 to 38 charges and the molecular mass of trocarin was found to be 46,515 based on the reconstructed spectrum. Some preparations showed a variation in molecular mass (±0.1%). This is most likely due to heterogeneity in glycosylation or fragmentation of carbohydrate groups during mass spectrometry, because, during sequence determination, no heterogeneity in any amino acid residue was observed.
Procoagulant activity and catalytic properties of trocarin.
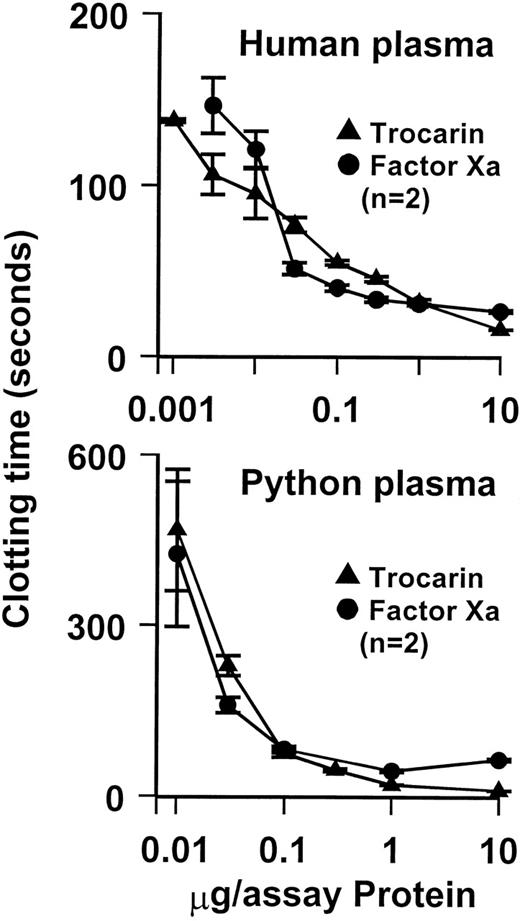
Trocarin exhibits potent procoagulant effects. It shortened the recalcification time of human plasma (control clotting times of 161 seconds) to 22 seconds at a concentration of 16.9 nmol/L (Fig 2). A similar procoagulant protein has been isolated from T carinatus venom,12which is known to clot factor X-deficient plasma, but not factor V- and II-deficient plasmas, suggesting that it activates prothrombin and requires factor V for optimum activity. Furthermore, like all group II prothrombin activators and factor Xa, its blood clotting activity was enhanced by the addition of phospholipids and Ca2+ions.12 We determined the effect of Ca2+ ions, negatively charged phospholipid vesicles (9:1 PC:PS), and bovine factor Va on the rate of bovine prothrombin activation by trocarin and factor Xa (bovine and human). The addition of Ca2+ ions, phospholipids, and factor Va greatly stimulates the activity of trocarin (Table 1), confirming that it is a group II prothrombin activator. Trocarin has a higher basal activity in the absence of all cofactors compared with bovine and human factor Xa. The stimulation of trocarin by the cofactors is generally about an order of magnitude lower than that of bovine and human factor Xa. The complete complex containing trocarin is twofold and fourfold less active than prothrombinase complexes containing bovine and human Xa, respectively (Table 1). This could be due to subtle differences in the interaction between reptilian and mammalian enzymes with mammalian factor Va. In addition, optimal cofactor concentrations for trocarin may be different from those for mammalian enzymes.
Effect of trocarin on coagulation of human and python plasma. Procoagulant activity measured by determining recalcification time of plasmas in the presence of various amounts of trocarin or human factor Xa using BBL fibrometer at 37°C. Clotting of plasma was initiated by the addition of CaCl2. Trocarin shows very similar procoagulant effects to human factor Xa on both human and python plasma.
Effect of trocarin on coagulation of human and python plasma. Procoagulant activity measured by determining recalcification time of plasmas in the presence of various amounts of trocarin or human factor Xa using BBL fibrometer at 37°C. Clotting of plasma was initiated by the addition of CaCl2. Trocarin shows very similar procoagulant effects to human factor Xa on both human and python plasma.
Effect of Ca2+ Ions, Phospholipids, and Bovine Factor Va on Trocarin- and Factor Xa-Mediated Prothrombin Activation
| Activator . | v (pmol prothrombin/ min/μg) . | Relative Rate . |
|---|---|---|
| Trocarin | 0.81 | 1 |
| Trocarin, Ca2+ | 93 | 115 |
| Trocarin, Ca2+, PL | 3,617 | 4,465 |
| Trocarin, Ca2+, Va | 2,091 | 2,581 |
| Trocarin, Ca2+, PL, Va | 12,809 | 15,814 |
| Bovine Xa | 0.19 | 1 |
| Bovine Xa, Ca2+ | 241 | 1,268 |
| Bovine Xa, Ca2+, PL | 9,687 | 50,984 |
| Bovine Xa, Ca2+, Va | 2,757 | 14,511 |
| Bovine Xa, Ca2+, PL, Va | 26,155 | 137,658 |
| Human Xa | 0.08 | 1 |
| Human Xa, Ca2+ | 205 | 2,563 |
| Human Xa, Ca2+, PL | 20,407 | 255,088 |
| Human Xa, Ca2+, Va | 4,668 | 58,350 |
| Human Xa, Ca2+, PL, Va | 49,132 | 614,150 |
| Activator . | v (pmol prothrombin/ min/μg) . | Relative Rate . |
|---|---|---|
| Trocarin | 0.81 | 1 |
| Trocarin, Ca2+ | 93 | 115 |
| Trocarin, Ca2+, PL | 3,617 | 4,465 |
| Trocarin, Ca2+, Va | 2,091 | 2,581 |
| Trocarin, Ca2+, PL, Va | 12,809 | 15,814 |
| Bovine Xa | 0.19 | 1 |
| Bovine Xa, Ca2+ | 241 | 1,268 |
| Bovine Xa, Ca2+, PL | 9,687 | 50,984 |
| Bovine Xa, Ca2+, Va | 2,757 | 14,511 |
| Bovine Xa, Ca2+, PL, Va | 26,155 | 137,658 |
| Human Xa | 0.08 | 1 |
| Human Xa, Ca2+ | 205 | 2,563 |
| Human Xa, Ca2+, PL | 20,407 | 255,088 |
| Human Xa, Ca2+, Va | 4,668 | 58,350 |
| Human Xa, Ca2+, PL, Va | 49,132 | 614,150 |
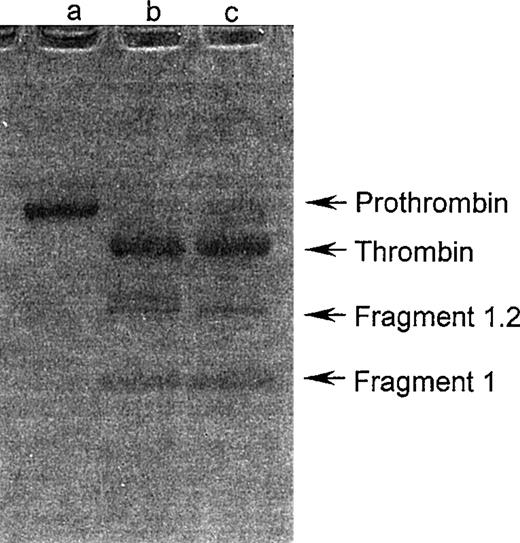
The cleavage products obtained by trocarin-mediated prothrombin activation were examined by SDS-PAGE on a nonreducing gel. The electrophoretic pattern was identical to that for prothrombin cleavage products by human Xa (Fig 3). Human factor Xa cleaves bovine prothrombin at two sites, Arg 274-Thr 275 and Arg 323-Ile 324. We determined the cleavage site(s) of prothrombin by trocarin in the presence of factor Va and Ca2+ ions. Amino-terminal sequencing of the first four residues of one of the cleavage products (Mr, 38 kD) yielded two parallel sequences, Thr-Ser-Glu-Asp and Ile-Val-Glu-Gly. These correspond to the amino-termini of the light and heavy chains of thrombin generated when cleavage of prothrombin occurs at Arg 274-Thr 275 and Arg 323-Ile 324. This indicates that trocarin has identical cleavage specificities to mammalian factor Xa, cleaving both peptide bonds of bovine prothrombin required for conversion to physiological thrombin. This is in contrast to group IA and IB activators, which cleave only one peptide bond, converting prothrombin to meizothrombin.5 6
SDS-PAGE analysis of prothrombin activation. Prothrombin (5 μg) was activated with trocarin or human factor Xa (5 ng each) in the presence of Ca2+ ions (5 mmol/L), 9:1 PC:PS phospholipid vesicles (100 μmol/L), and factor Va (10 nmol/L) at 37°C for 4 hours. The reaction mixture was loaded on a nonreducing 4% to 20% gradient gel. Untreated prothrombin (lane a), cleavage products of prothrombin by trocarin (lane b), and factor Xa (lane c) were electrophoresed according to Laemmli.15
SDS-PAGE analysis of prothrombin activation. Prothrombin (5 μg) was activated with trocarin or human factor Xa (5 ng each) in the presence of Ca2+ ions (5 mmol/L), 9:1 PC:PS phospholipid vesicles (100 μmol/L), and factor Va (10 nmol/L) at 37°C for 4 hours. The reaction mixture was loaded on a nonreducing 4% to 20% gradient gel. Untreated prothrombin (lane a), cleavage products of prothrombin by trocarin (lane b), and factor Xa (lane c) were electrophoresed according to Laemmli.15
We tested the amidolytic activity of trocarin on a synthetic substrate specific for factor Xa. Trocarin did cleave S-2222, indicating identical sequence specificity of cleavage to factor Xa. We determined the Km and Vmax for the S-2222 hydrolysis by trocarin and human and bovine factor Xa (Table 2). Whereas the Km’s for the three enzymes are comparable, the Vmax of trocarin is approximately 800 and 1,400 times lower than that of human and bovine factor Xa, respectively. Similar results were reported earlier with notecarin. It showed approximately 120-fold lower amidolytic activity than bovine factor Xa on CBS 31.39, the best synthetic substrate among those that were tested.9 It is interesting to note that trocarin has a lower amidolytic activity on synthetic substrate S-2222, but a higher activity on a macromolecular substrate, prothrombin, in the absence of Ca2+ ions than mammalian factor Xa (Table 1).
Kinetic Parameters for Hydrolysis of S-2222 by Trocarin and Factor Xa
| Enzyme . | Vmax (Δabs/min/μg) . | Km (mol/L) . |
|---|---|---|
| Trocarin | 0.0055 | 2.5 × 10−4 |
| Bovine FXa | 7.8 | 3.1 × 10−4 |
| Human FXa | 4.5 | 4.0 × 10−4 |
| Enzyme . | Vmax (Δabs/min/μg) . | Km (mol/L) . |
|---|---|---|
| Trocarin | 0.0055 | 2.5 × 10−4 |
| Bovine FXa | 7.8 | 3.1 × 10−4 |
| Human FXa | 4.5 | 4.0 × 10−4 |
Amino terminal sequencing of trocarin.
Amino terminal sequencing of the native protein yielded two parallel sequence signals of equivalent intensity, indicating that it consisted of two chains. These two sequences were highly homologous to the light and heavy chains of factor Xa. Upon reduction and pyridylethylation, the native protein yielded two chains: a light and a heavy chain 17 and 30 kD in mass, respectively. The individual chains were sequenced separately.
The light chain.
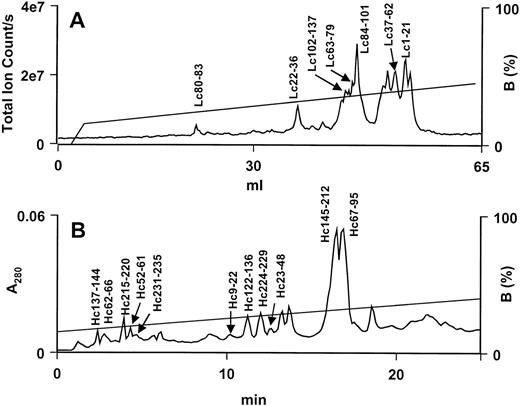
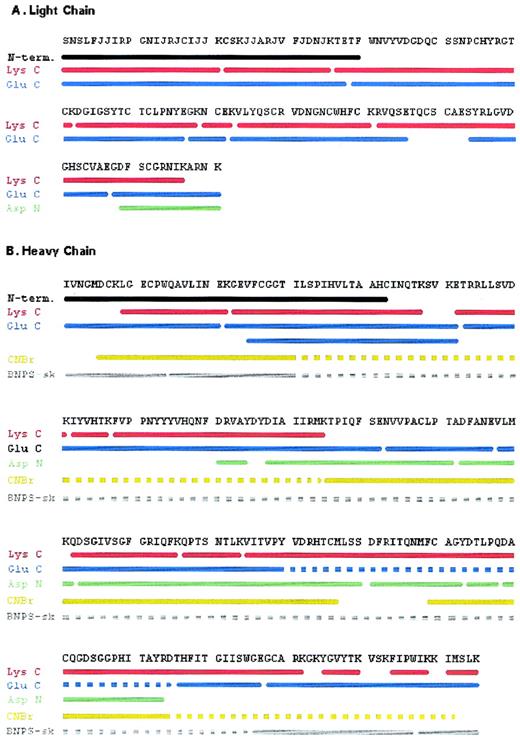
Amino-terminal sequencing of the light chain yielded the first 40 residues. Eleven residues at positions 6, 7, 14, 16, 18, 19, 25, 26, 29, 32, and 35 could not be identified. These positions correspond to those of Gla residues in factor Xa. Most of the sequence of the light chain was completed with peptides generated by a Lys C digest (Fig 4A). Seven Lys C peptides (Lc1-21, Lc22-36, Lc37-62, Lc63-79, Lc80-83, Lc84-101, and Lc102-137) covered the first 137 residues (Fig 5A). The C-terminal peptide, Lc128-151, and other overlapping peptides (Lc39-77, Lc78-82, Lc83-106, and Lc114-127) were obtained and sequenced from a Glu C digest (data not shown). Lysine was confirmed to be the C-terminal residue of the light chain, because it was the last residue sequenced on peptides, Lc129-141 and Lc128-141, generated by Asp N (data not shown) and Glu C, respectively (Fig 5A). The sequences of all peptides were unambiguously identified and their masses corroborate the sequence data (Table 3). In addition to the Gla residues, residue 52 could not be identified during sequencing, indicating that there may be some posttranslational modification at this location (see below).
RP-HPLC separation of peptides from Lys C digests of trocarin subunits. (A) LC-MS profile of peptides from light chain digest on a Hypersil BDS C18 column. Flow rate: 50 μL/min. (B) HPLC profile of peptides from heavy chain digest on a Jupiter C18 column. Flow rate: 1 mL/min. Peptides were eluted with a linear gradient of buffer B (80% ACN in 0.1% TFA).
RP-HPLC separation of peptides from Lys C digests of trocarin subunits. (A) LC-MS profile of peptides from light chain digest on a Hypersil BDS C18 column. Flow rate: 50 μL/min. (B) HPLC profile of peptides from heavy chain digest on a Jupiter C18 column. Flow rate: 1 mL/min. Peptides were eluted with a linear gradient of buffer B (80% ACN in 0.1% TFA).
Complete amino acid sequence of trocarin. Strategy for determination of the sequence of light (A) and heavy chains (B). N-term.: Amino terminal sequence of intact chain. Peptides were derived from a digest of light or heavy chains by endoproteinase Lys C (Lys C), endoproteinase Glu C (Glu C), endoproteinase Asp N (Asp N), cyanogen bromide (CNBr), and BNPS-skatole (BNPS-sk). Solid lines represent regions whose sequences were determined by Edman degradation and/or confirmed by mass spectrometry. Broken lines represent regions of peptides that were not sequenced.
Complete amino acid sequence of trocarin. Strategy for determination of the sequence of light (A) and heavy chains (B). N-term.: Amino terminal sequence of intact chain. Peptides were derived from a digest of light or heavy chains by endoproteinase Lys C (Lys C), endoproteinase Glu C (Glu C), endoproteinase Asp N (Asp N), cyanogen bromide (CNBr), and BNPS-skatole (BNPS-sk). Solid lines represent regions whose sequences were determined by Edman degradation and/or confirmed by mass spectrometry. Broken lines represent regions of peptides that were not sequenced.
Theoretical and Experimentally Determined Masses of Peptides of Trocarin
| . | Molecular Mass . | |
|---|---|---|
| Calculated3-151 . | Observed3-152 . | |
| Light chain | ||
| Lys C peptides | ||
| Lc1-21 | 2,861.90 | 2,861.53 ± 0.56 |
| Lc22-36 | 2,138.10 | 2,137.58 ± 0.33 |
| Lc37-62* | 3,340.64 | 3,664.06 ± 0.57 |
| Lc63-79 | 2,031.25 | 2,031.84 ± 0.67 |
| Lc80-83 | 597.68 | 597.6 |
| Lc84-101 | 2,487.87 | 2,488.38 ± 0.42 |
| Lc102-137 | 4,313.80 | 4,314.93 ± 0.40 |
| Glu C peptides | ||
| Lc1-38 | 5,194.96 | 5,193.25 ± 1.36 |
| Lc39-77* | 5,259.54 | 5,262.27 ± 1.92 |
| Lc78-82 | 654.59 | 653.60 |
| Lc83-106 | 3,215.26 | 3,215.27 ± 0.60 |
| Lc128-141 | 1,670.76 | 1,670.82 ± 0.25 |
| Heavy chain | ||
| Lys C peptides | ||
| Hc137-144 | 887.97 | 888.50 ± 0.96 |
| Hc145-212 | 7,946.94 | 7,948.50 ± 1.29 |
| Glu C peptides | ||
| Hc1-21 | 2,542.98 | 2,542.80 ± 0.33 |
| Hc53-102 | 6,125.95 | 6,125.64 ± 0.51 |
| Hc112-122 | 1,194.36 | 1,194.01 ± 0.28 |
| Hc196-207 | 1,360.50 | 1,360.08 ± 0.25 |
| Hc208-235 | 3,336.09 | 3,336.49 ± 0.27 |
| Asp N peptides | ||
| Hc81-85 | 622.68 | 622.50 |
| Hc88-111 | 2,833.37 | 2,833.38 ± 0.24 |
| Hc112-122 | 1,194.36 | 1,194.01 ± 0.28 |
| Hc123-160 | 4,288.92 | 4,288.20 ± 0.86 |
| Hc161-173 | 1,670.91 | 1,670.88 ± 0.43 |
| Hc174-178 | 572.61 | 572.50 |
| Hc179-194 | 1,752.89 | 1,753.32 ± 0.28 |
| Hc103-117 | 1,665.85 | 1,665.77 ± 0.94 |
| Hc195-235 | 4,793.66 | 4,793.01 ± 0.20 |
| CNBr peptides3-153 | ||
| Hc95-120 | 2,892.26 | 2,892.63 ± 0.02 |
| Hc121-157 | 4,209.77 | 4,209.44 ± 0.24 |
| Hc169-232 | 7,353.33 | 7,353.48 ± 0.62 |
| . | Molecular Mass . | |
|---|---|---|
| Calculated3-151 . | Observed3-152 . | |
| Light chain | ||
| Lys C peptides | ||
| Lc1-21 | 2,861.90 | 2,861.53 ± 0.56 |
| Lc22-36 | 2,138.10 | 2,137.58 ± 0.33 |
| Lc37-62* | 3,340.64 | 3,664.06 ± 0.57 |
| Lc63-79 | 2,031.25 | 2,031.84 ± 0.67 |
| Lc80-83 | 597.68 | 597.6 |
| Lc84-101 | 2,487.87 | 2,488.38 ± 0.42 |
| Lc102-137 | 4,313.80 | 4,314.93 ± 0.40 |
| Glu C peptides | ||
| Lc1-38 | 5,194.96 | 5,193.25 ± 1.36 |
| Lc39-77* | 5,259.54 | 5,262.27 ± 1.92 |
| Lc78-82 | 654.59 | 653.60 |
| Lc83-106 | 3,215.26 | 3,215.27 ± 0.60 |
| Lc128-141 | 1,670.76 | 1,670.82 ± 0.25 |
| Heavy chain | ||
| Lys C peptides | ||
| Hc137-144 | 887.97 | 888.50 ± 0.96 |
| Hc145-212 | 7,946.94 | 7,948.50 ± 1.29 |
| Glu C peptides | ||
| Hc1-21 | 2,542.98 | 2,542.80 ± 0.33 |
| Hc53-102 | 6,125.95 | 6,125.64 ± 0.51 |
| Hc112-122 | 1,194.36 | 1,194.01 ± 0.28 |
| Hc196-207 | 1,360.50 | 1,360.08 ± 0.25 |
| Hc208-235 | 3,336.09 | 3,336.49 ± 0.27 |
| Asp N peptides | ||
| Hc81-85 | 622.68 | 622.50 |
| Hc88-111 | 2,833.37 | 2,833.38 ± 0.24 |
| Hc112-122 | 1,194.36 | 1,194.01 ± 0.28 |
| Hc123-160 | 4,288.92 | 4,288.20 ± 0.86 |
| Hc161-173 | 1,670.91 | 1,670.88 ± 0.43 |
| Hc174-178 | 572.61 | 572.50 |
| Hc179-194 | 1,752.89 | 1,753.32 ± 0.28 |
| Hc103-117 | 1,665.85 | 1,665.77 ± 0.94 |
| Hc195-235 | 4,793.66 | 4,793.01 ± 0.20 |
| CNBr peptides3-153 | ||
| Hc95-120 | 2,892.26 | 2,892.63 ± 0.02 |
| Hc121-157 | 4,209.77 | 4,209.44 ± 0.24 |
| Hc169-232 | 7,353.33 | 7,353.48 ± 0.62 |
Glycopeptides. Calculated mass of Lc39-77 includes carbohydrate mass (335.12).
Masses calculated from their sequences. Cysteines were pyridylethylated.
Masses were determined by electrospray ionization mass spectrometry.
Met residues converted to homoserine lactone by CNBr treatment.
The heavy chain.
The first 43 residues were identified by amino-terminal sequencing of intact, reduced, and alkylated heavy chain. The sequence was completed using peptides generated with Lys C (Fig 4B) and Glu C (data not shown) digests. Eleven Lys C peptides, Hc9-22, Hc23-48, Hc52-61, Hc62-66, Hc67-95, Hc122-136, Hc137-144, Hc145-212, Hc215-220, Hc224-229, and Hc231-235, were sequenced (Fig 5B). Long peptide Hc145-212 was subdigested with trypsin (data not shown) and a resultant peptide, Hc195-211, was sequenced. An additional eight peptides obtained from the Glu C digest, Hc1-21, Hc22-52, Hc25-52, Hc53-102, Hc103-117, Hc118-195, Hc196-207, and Hc208-235, were needed to complete the sequence (Fig 5B). Endoproteinase Glu C also cleaved after Asp207 and resulted in the peptide Hc208-235. To confirm the heavy chain sequence, peptides were also generated by chemical cleavage with CNBr and BNPS-skatole (data not shown). Cyanogen bromide peptides, Hc6-94, Hc95-120, Hc121-157, and Hc169-232, and BNPS-skatole peptides, Hc1-14, Hc15-205, Hc206-227, and Hc228-235, were sequenced (Fig 5B). Only the N-termini of peptides Hc6-94 and Hc15-205 were sequenced. The C-terminal Lys was the final residue on Glu C peptide, Hc208-235, and BNPS-skatole peptide, Hc228-235. Verification of the sequence of the heavy chain was obtained from the masses of peptides from various digests (Table 3). The masses of seven peptides (Hc81-85, Hc88-111, Hc112-122, Hc123-160, Hc161-173, Hc174-178, and Hc179-194) obtained from an Asp N digest of the heavy chain (data not shown) provided further verification of sequence data (Table 3). The identity of residue 45 could not be ascertained by sequencing, indicating posttranslational modification (see below).
Posttranslational modifications in trocarin: γ-Carboxylation of glutamate residues.
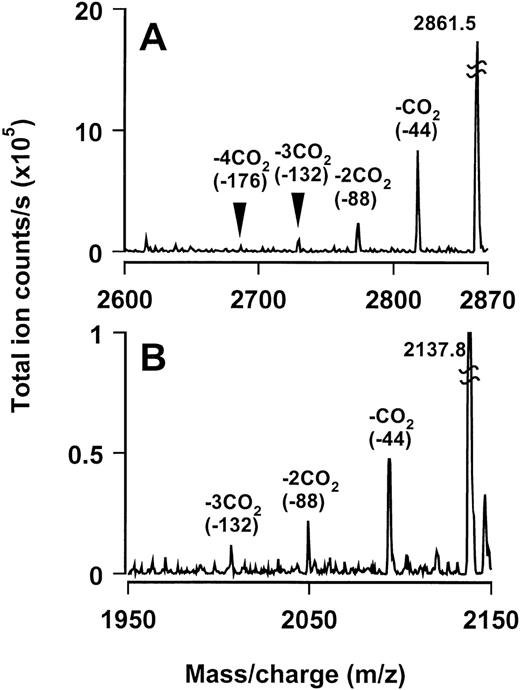
As mentioned above, residues 6, 7, 14, 16, 18, 19, 25, 26, 29, 32, and 35 of the light chain were unidentifiable by sequencing and corresponded to positions of Gla residues in factor Xa. Both mass spectrometric and sequencing approaches were used to identify and confirm the presence of Gla residues in these positions. The masses of two Lys C-generated peptides, Lc1-21 and Lc22-36, were consistent with the presence of Gla residues in the unidentified positions (Table 3). In the mass spectra of these peptides, we could identify partially decarboxylated peptides with masses corresponding to successive loss of up to 3 or 4 carbon dioxide molecules (Fig6). For further confirmation, we decarboxylated18 the light chain and sequenced its amino terminus. Glutamate residues were identified in positions 6, 7, 14, 16, 18, 19, 25, 26, 29, 32, and 35 that were blank during the sequencing of the untreated light chain. Thus, these results confirm the presence of Gla residues at these positions of the light chain.
Evidence for γ-carboxylation of glutamic acid residues in the Gla domain. Reconstructed mass spectra of peptides spanning the Gla domain: (A) Lc1-21 and (B) Lc22-36. Total masses of these peptides are consistent with the presence of Gla residues in them. Partially decarboxylated peptides with masses corresponding to successive loss of up to 4 and 3 carbon dioxide molecules (∼44 mass units), respectively, can also be identified.
Evidence for γ-carboxylation of glutamic acid residues in the Gla domain. Reconstructed mass spectra of peptides spanning the Gla domain: (A) Lc1-21 and (B) Lc22-36. Total masses of these peptides are consistent with the presence of Gla residues in them. Partially decarboxylated peptides with masses corresponding to successive loss of up to 4 and 3 carbon dioxide molecules (∼44 mass units), respectively, can also be identified.
The existence of Gla residues is documented in venom proteins. Tans et al9 earlier showed the presence of Gla residues in notecarin, the group II prothrombin activator from N scutatus scutatus. They estimated eight Gla residues per molecule, in contrast to trocarin’s 11 Gla residues. The group III prothrombin activator, oscutarin (Oxyuranus scutellatus), has also been shown to possess Gla residues.10
O-glycosylation.
As mentioned earlier, light chain residue 52 could not be identified during sequencing. During preliminary characterization, we identified the presence of carbohydrate moieties in both light and heavy chains using the method of Dubois et al19 (data not shown). Thus, we predicted that position 52 is probably glycosylated. Mass spectrometric data on a peptide containing residue 52 indicates that it is a Ser that is O-glycosylated with a disaccharide moiety (unpublished observations). Characteristically, O-glycosylation sites are found in Pro/Ser/Thr-rich regions. In trocarin, Ser 51 and Pro 54 are found in the immediate vicinity of Ser 52 (51-SSNP-54). Although Ser or Thr occupies position 52 in several factor Xa proteins (Fig 7) and these regions are similar to that of trocarin, these residues are not glycosylated. However, disaccharide (xylose-glucose) and trisaccharide [xylose(2)-glucose] units have been found to be O-linked to corresponding Ser residues on the light chains of human and bovine factors VII and IX and human protein Z.24 25
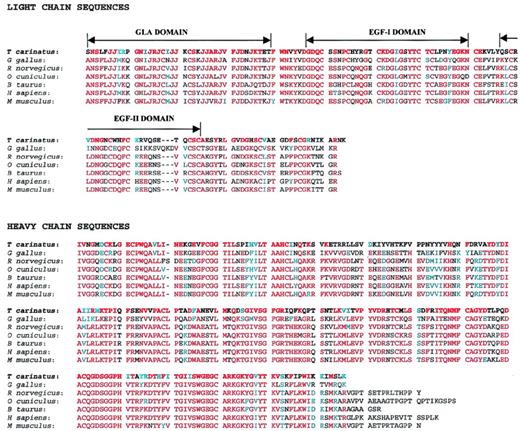
Alignment of amino acid sequence of trocarin with factor Xa sequences. The sequences are taken from the following sources.T carinatus: Tropidechis carinatus (rough-scaled snake, this report, SWISS-PROT no. P81428); G gallus: Gallus gallus (chicken, SWISS-PROT no. P25155); R norvegicus:Rattus norvegicus (rat, Genbank no. 2144491); O cuniculus: Oryctolagus cuniculus (rabbit, SWISS-PROT no.O19045); B taurus: Bos taurus (bovine, SWISS-PROT no.P00743); H sapiens: Homo sapiens (human, SWISS-PROT no.P00742); and M musculus: Mus musculus (mouse, Genbank no. 2664220). Identical and homologous residues are shaded in red and blue, respectively. Gla, EGF-I, and EGF-II domain boundaries are indicated above light chain sequences. Gaps (−) are inserted for optimal alignment.
Alignment of amino acid sequence of trocarin with factor Xa sequences. The sequences are taken from the following sources.T carinatus: Tropidechis carinatus (rough-scaled snake, this report, SWISS-PROT no. P81428); G gallus: Gallus gallus (chicken, SWISS-PROT no. P25155); R norvegicus:Rattus norvegicus (rat, Genbank no. 2144491); O cuniculus: Oryctolagus cuniculus (rabbit, SWISS-PROT no.O19045); B taurus: Bos taurus (bovine, SWISS-PROT no.P00743); H sapiens: Homo sapiens (human, SWISS-PROT no.P00742); and M musculus: Mus musculus (mouse, Genbank no. 2664220). Identical and homologous residues are shaded in red and blue, respectively. Gla, EGF-I, and EGF-II domain boundaries are indicated above light chain sequences. Gaps (−) are inserted for optimal alignment.
N-glycosylation.
As mentioned above, the heavy chain was also found to be glycosylated. Because only position 45 could not be identified during sequencing of the heavy chain, it was recognized as the glycosylation site. Furthermore, it falls within the consensus sequence N-X-T, where N is glycosylated. Based on mass difference, we estimate the size of carbohydrate moiety to be approximately 3 kD. Deconvolution of the mass spectra of Lys C peptide Hc23-48, Glu C peptides Hc22-52 and Hc25-52, CNBr peptide Hc6-94, and BNPS-skatole peptide Hc15-205 was difficult, probably due to either heterogeneity in glycosylation or fragmentation of the carbohydrate during ionization. Further studies are underway to determine the structure of the carbohydrate moiety.
Lack of β-hydroxylation of Aspartate 63.
In vitamin K-dependent blood coagulation factors containing epidermal growth factor (EGF) domains, including factor X, Asp 63 (64), is modified to erythro-β-hydroxyaspartate (Hya).26,27However, during sequencing of Lys C peptide Lc63-79 of trocarin, we identified Asp at position 63. Mass spectrometric data on peptide Lc63-79 also indicated the lack of β-hydroxylation of Asp 63 (data not shown). According to Stenflo et al,28 hydroxylation is associated with the consensus sequence Cys-X-Hya/Hyn-X-X-X-X-Tyr/Phe-X-C-X-C. Because trocarin fulfills these sequence requirements, perhaps additional secondary and/or tertiary structural features required for hydroxylation are missing.
Homology and domain structure of trocarin.
Trocarin shares high identity (53% to 60.0%) and homology (62% to 70%) with factor Xa (Fig 7). Based on homology, we propose similar domain architecture for trocarin. The light chain is homologous to the light chains of vitamin K-dependent coagulation factors, especially that of factor Xa (53% to 59% identity, 60% to 67% homology with factor Xa). It consists of an N-terminal Gla domain (residues 1-39), followed by two EGF-like domains, EGF-I (residues 50-81) and EGF-II (residues 89-124). Cysteine residues are completely conserved. By homology, intrachain disulfide linkages should be between cysteines 17 and 22, 50 and 61, 55 and 70, 72 and 81, 89 and 100, 96 and 109, and 111 and 124. Cysteine 132 most probably links the light chain to Cys 108 of the heavy chain.
The amino-terminal domain of trocarin has 11 Gla residues. The first 10 lie in positions conserved in the Gla domains of vitamin K-dependent coagulation factors. The eleventh Gla residue in position 35 of trocarin is conserved in other factor Xas but is replaced by Asp in human factor Xa. On the other hand, Gla 39 found in other factor Xas is missing in trocarin. This indicates that, although the positions of the first 10 Gla residues (6, 7, 14, 16, 19, 20, 25, 26, 29, and 32) are critical, either one of the additional Glas in positions 35 and 39 can be dispensed without severely compromising activity.
The organization of Gla residues into a domain homologous to the Gla domains of vitamin K-dependent coagulation factors has been shown for the first time in a protein isolated from venom. A number of peptide toxins that have been sequenced from the venoms of the Conusspecies of mollusks also contain Gla residues.29,30However, the organization of these Gla residues does not resemble Gla domains of vitamin K-dependent coagulation factors. Although Gla residues are involved in calcium binding, they play different overall functions in Conus peptide toxins and vitamin K-dependent coagulation factors. In many Conus toxins, Gla residues, upon binding calcium, contribute to the formation of a functionally important amphipathic helix.31-34 On the other hand, the Gla domains of factor Xa and other vitamin K-dependent coagulation factors mediate Ca2+-dependent binding to negatively charged phospholipids. Calcium and negatively charged phospholipids have a stimulatory effect on trocarin’s prothrombin-converting ability (Table 1). Other Gla-containing proteins from snake venom, notecarin9 and oscutarin,10also possess the same property (ie, Ca2+- and phospholipid-stimulated activity).
All factor Xas have a short hydrophobic (helical) stack of residues forming a linker between the Gla and EGF-I domains and so does trocarin. As mentioned above, it is known that the first EGF domain of factor Xa is involved in calcium binding. This domain of trocarin shares 56% to 75% identity and 66% to 84% homology with those of other factor Xas, and the residues that act as calcium ligands in factor Xa EGF-I domains are completely conserved. Hence, it is probable that the EGF-I domain of trocarin also binds calcium. In factor Xa, there is some evidence that the second EGF module may mediate factor Va binding, which may be further enhanced by the presence of EGF-I domain.35 The second EGF domain of trocarin has only a 39% to 53% identity and a 44% to 58% homology with factor Xa EGF-II domains, which is significantly lower than that between their EGF-I domains. However, factor Va is still able to enhance the activity of trocarin. Thus, it would be interesting to examine the structure-function relationships of this domain.
The heavy chain is the catalytic subunit and is homologous to serine proteinases. It contains all functional residues, including the triad of the charge-relay system: His 42, Asp 88, and Ser 185. Comparison with other factor Xas show identities and homologies ranging from 50% to 60% and 62% to 71%, respectively. Cysteine residues are completely conserved even in the heavy chain. By homology, intrachain disulfide bonds are probably formed between cysteines 7 and 12, 27 and 43, 156 and 170, and 181 and 209. The residues forming the substrate-binding pocket (Asp 179, Ala 180, Cys 181, and Gln 182) and those involved in forming the hydrogen-bonded structure antiparallel to the substrate (Ser 204, Trp 205, Gly 206, and Gly 216) are identical to those found in factor Xa. We noted that the region from residues 48 to 84 shows very little homology with factor Xa. Here, identities and homologies range from only 6% to 14% and 11% to 25%, respectively. However, synthetic peptides based on the sequence of this region in human factor Xa (heavy chain residues 60 to 75 and 69 to 80) were highly effective in preventing factor Xa–factor Va interaction.36 The role of the corresponding segments of trocarin in its interaction with factor Va would be of interest.
Probable physiological role of trocarin.
Snake venom contains up to 11.5 mg/mL of trocarin, whereas human blood contains only 10 μg/mL of factor X. Thus, snake venom is the richest source of factor Xa-like proteins. It is important to note that mammalian coagulation proteins are synthesized primarily in the liver. Trocarin, on the other hand, is expressed in a nonhepatic tissue, the venom gland. The only other known exogenous (ie, nonblood) factor Xa is the virus-activating protease isolated from chick embryo allantoic and amniotic fluid, but this is found at concentrations of only about 2 ng/mL37 and 165 ng/mL,38 respectively. Trocarin induced cyanosis and death in mice within approximately 320 and 150 minutes at 1 and 10 mg/kg (intraperitoneal), respectively. A prothrombin activator from P textilis venom causes death in rats within several minutes of intravenous injection at doses as low as 23 μg/kg body weight.11 Preinjection of heparin (intravenous) neutralized the lethal effects of the procoagulant,11 indicating that lethality was due to disseminated intravascular coagulopathy.4 Thus, trocarin appears to play the role of a toxin in the venom of T carinatus.
Trocarin induces clotting in snake plasma (Fig 2). Based on its structural and functional similarities to coagulation factor Xa and the existence of a similar extrinsic coagulation cascade in snake blood,39 it is logical to propose that trocarin is similar, if not identical, to snake blood coagulation factor Xa. However, blood coagulation factor X will be in the zymogen form, unlike active trocarin found in the venom. Furthermore, this protein would play a critical role in hemostasis. Thus, two closely related, if not identical, proteins play distinctly different physiological roles.
ACKNOWLEDGMENT
The authors thank Prof P. Gopalakrishnakone for providing P reticulatus plasma and Chiew Fook Loong for writing the peptide analysis software.
Supported by Academic Research Grants No. RP 960304 A and RP 950377 to R.M.K.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to R. Manjunatha Kini, PhD, Bioscience Centre, Faculty of Science, National University of Singapore, Singapore 117600; e-mail: bsckinim@nus.edu.sg.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal