Abstract
Decreased adhesion of neutrophils to endothelial cells and delayed transendothelial cell migration of neutrophils have been consistently reported in neonatal animals and humans and contribute to their susceptibility to infection. The delayed transmigration of neutrophils is especially prevalent in premature neonates. To define the nature of this defect, we used an in vivo animal model of inflammation and found that radiolabeled leukocytes from adult rats transmigrated into the peritoneum of other adult rats 5 times more efficiently than they did in neonatal rats (P = .05). This indicated that defects in neonatal neutrophils could not completely account for the delayed transmigration. Delayed transmigration in the neonatal rats correlated with a defect in the expression of P-selectin on the surface of their endothelial cells. We found a similar P-selectin deficiency in endothelial cells lining mesenteric venules and umbilical veins of human premature infants when compared with term human infants. The decreased P-selectin in premature infants was associated with decreased numbers of P-selectin storage granules and decreased P-selectin transcription. Decreased P-selectin expression on the surface of endothelial cells in preterm infants may contribute to delayed neutrophil transmigration and increased susceptibility to infection.
THE PREVALENCE OF SEPSIS in newborn infants is 1 per 1,000 live births, with a mortality rate of 20% to 75%.1 In premature infants, the prevalence is estimated to be as high as 1 in 230 infants, with an even greater mortality.2 Because there are in excess of 75,000 infants less than 32 weeks gestation born prematurely each year in the United States,3 the morbidity and mortality from sepsis in premature infants continues to be a major health problem.
One reason for the increased susceptibility to infection in neonates is a defect in the neonatal inflammatory response that results in delayed accumulation of neutrophils at sites of microbial invasion. This defect is related to the decreased adhesion of neutrophils to endothelial cells and delayed transendothelial cell migration, both of which are among the most consistently reported defects in the neonatal immune system.1,4-6 Delayed transendothelial cell migration of neutrophils has been demonstrated in vivo in neonatal animals by intraperitoneal inoculation with various inflammatory mediators. Between 4 and 6 hours after the inoculation, neonatal animals have a markedly diminished peritoneal neutrophil count compared with adults.7-9 Because neutrophils are the first cells to arrive at the site of microbial invasion,10 delayed neutrophil transmigration allows the invading microorganisms to multiply unchallenged and ultimately predisposes newborns to an increased bacterial load and overwhelming sepsis.11 12
The recruitment of neutrophils to sites of microbial invasion is regulated by the sequential interaction of different classes of adhesion molecules, resulting in an adhesion and activation cascade.13 The first step in adhesion is rolling of neutrophils along the vessel wall. This is mediated by a class of adhesion molecules termed the selectins. Three selectins have been identified: L-, P-, and E-selectin. Selectins are critical in neutrophil transmigration by virtue of their ability to tether leukocytes in a reversible fashion under conditions of shear and flow, a process that mediates their rolling on the endothelium of inflamed vessels.14 Although all three selectins have been implicated in mediating neutrophil rolling, P-selectin is most important during the initial induction of neutrophil rolling after endothelial cell stimulation.15 It is constitutively synthesized by endothelial cells and stored in Weibel-Palade bodies along with von Willebrand factor (vWF).16 Within minutes of activation with certain agonists (phorbol myristate acetate, histamine, thrombin, c5a, or oxidants), P-selectin is translocated to the plasma membrane, where it supports rapid neutrophil adhesion.14
Investigations of neutrophil adhesion and transmigration in neonates have focused on neonatal neutrophils as the defective cell type responsible for delayed neutrophil adhesion to endothelial cells. The deficient adhesion of neonatal neutrophils to endothelial cells has been attributed to decreased expression of integrins,4diminished expression of L-selectin,5 and a rigid cytoskeleton that prevents redistribution of adhesion sites and normal deformability and movement of neutrophils.17 However, the premise that neonatal neutrophils are deficient in their expression of cell adhesion molecules has recently been challenged.18-22To date, studies have not examined endothelial cells despite the fact that adhesion molecules expressed by endothelial cells are required for neutrophil adhesion and transmigration.13 We hypothesized that decreased or delayed expression of cell adhesion molecules on the endothelial cell surface in newborns is a crucial factor in the delayed transmigration of neutrophils. To test this hypothesis, we used an in vivo model of peritoneal inflammation in newborn and adult rats. We found that neutrophil transmigration in neonatal rats was delayed. This delay was associated with a defect in P-selectin expression on the surface of neonatal endothelial cells. These studies were extended to humans. We found that the expression of P-selectin on endothelial cells is less in preterm neonatal infants compared with full-term neonates.
MATERIALS AND METHODS
In vivo transmigration studies.
Adult and 1-day-old Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) received an intraperitoneal injection of either 0.01 mL/g body weight thioglycollate (Becton Dickinson, Cockeysville, MD) or endotoxin tested-sterile phosphate-buffered saline (PBS; Sigma Chemical Co, St Louis, MO). Thioglycollate was prepared per the manufacturer’s instructions, autoclaved, and kept at room temperature for 1 to 3 weeks before use. At various times after the injection, the rats were killed by cervical dislocation and the peritoneum was lavaged. The lavage was performed 3 times with 0.1 mL/g 37°C PBS, 0.1% bovine serum albumin, and 10 U/mL heparin. The total number of leukocytes in the peritoneal fluid was measured with a Coulter Counter (Coulter Electronics, Inc, Hialeah, FL), and the percentage of neutrophils was determined by differential Wright stain. Neutrophil accumulation in the lavage fluid was also estimated with a myeloperoxidase assay using the tetramethylbenzidine technique.23 We compared the neutrophil count in the peritoneum between rats of the same age that received PBS with those receiving thioglycollate. The percentage increase in peritoneal neutrophils was calculated using the following equation: % increase = (count in thioglycollate lavage-count in PBS lavage)/(count in PBS lavage) × 100.
For the studies measuring transmigration of homologous leukocytes, we collected blood from adult rats, isolated the leukocytes by hypotonic lysis, and labeled them with 111Indium (111In), as previously described.24 Total leukocytes were used in these studies, rather than isolated neutrophils, to avoid the possibility of neutrophil activation during additional isolation procedures. The leukocytes were resting as demonstrated by quantification of surface L-selectin compared with leukocytes in whole blood and by leukocyte morphology. They were round with no aggregation or rosetting with platelets. Labeled leukocytes (6.5 × 105 per gram body weight) were injected into newborn and adult rats by an intracardiac puncture. Peripheral blood samples were then collected from the rats every hour for a white blood cell count and measurement of radioactivity. There was approximately a 50% increase in the white blood cell count of both the neonatal and adult rats. The amount of radioactivity in the blood was stable up to 2 hours after the injection and the increase in the white blood cell count remained elevated by 50% in both sets of animals. The rats then received intraperitoneal PBS or thioglycollate, as described above. Four hours after the intraperitoneal injection, the rats were killed, the peritoneum was lavaged, and the radiolabeled adult leukocytes that had transmigrated into the peritoneum and retrieved in the lavage fluid were counted (Beckman Gamma 5500; Beckman Instruments, Inc, Irvine, CA).
Immunohistochemistry.
Rat mesentery was collected at various times after intraperitoneal injection and fixed in 4% paraformaldehyde. Human mesentery was obtained at autopsy and fixed in Histochoice tissue fixative (Amresco, Solon, OH). Tissue from individuals who died from an infectious process or had an ongoing infection at the time of death was excluded from the study (Table 1). Mesentery from rats and humans was dehydrated in a graded series of acetone at 4°C and embedded (Immunobed; Polysciences Inc, Warrington, PA) at 4°C. Four-micrometer–thick sections were cut using glass knives and transferred to coated slides (Vectabond; Vector Laboratories, Burlingham, CA). P-selectin was detected on rat mesenteric endothelial cells using a 1:200 dilution of the monoclonal antibody (MoAb) PB1.3 (Cytel Corp, San Diego, CA) that has been used extensively for immunohistochemical analysis in other animal models of inflammation and recognizes P-selectin expressed on the endothelial cell surface.25,26 The MoAb S12 (16 μg/mL) was used to detect the expression of P-selectin in human mesenteric endothelial cells (provided by Rodger McEver, University of Oklahoma, Oklahoma City, OK). S12 has been well characterized and used extensively for immunohistochemical localization of P-selectin in humans.16 Unlike MoAb PB1.3, MoAb S12 recognizes P-selectin stored intracellularly as well as expressed on the endothelial cell surface. A rabbit antibovine antiserum to PECAM-1 was used at a 1:1,000 dilution to detect PECAM-1 expression on rat endothelial cells (provided by Steven Albelda, University of Pennsylvania Medical Center, Philadelphia, PA). An MoAb was used to detect human PECAM-1 (1:200 dilution; Genosys Biotechnologies Inc, Cambridge, UK). Tissue sections were incubated with the primary antibody overnight at room temperature. A biotinylated IgG was used as the secondary antibody (Vector Laboratories) at a 1:200 dilution for 1 hour at room temperature. The avidin-biotin immunoperoxidase technique (Vectastain ABC Reagent; Vector Laboratories) was used to detect biotinylated secondary antibody. Immunostaining negative controls included omission of the primary antibody or secondary antibody.
Patients From Whom Mesentery Was Obtained for Immunohistochemistry
| Age . | Cause of Death . |
|---|---|
| 18 wks gestation | Siamese twins |
| 18 wks gestation | Trisomy 18 |
| 22 wks gestation | Incompetent cervix |
| 27 wks gestation | Discordant twins |
| 32 wks gestation | Wide complex arrythmia |
| Term infant | Congential heart defect |
| 3 yrs | Cornelia de Lange syndrome |
| 4 yrs | Acute lymphoblastic leukemia |
| 6 yrs | Trauma |
| 10 yrs | Asthma |
| Age . | Cause of Death . |
|---|---|
| 18 wks gestation | Siamese twins |
| 18 wks gestation | Trisomy 18 |
| 22 wks gestation | Incompetent cervix |
| 27 wks gestation | Discordant twins |
| 32 wks gestation | Wide complex arrythmia |
| Term infant | Congential heart defect |
| 3 yrs | Cornelia de Lange syndrome |
| 4 yrs | Acute lymphoblastic leukemia |
| 6 yrs | Trauma |
| 10 yrs | Asthma |
Procurement of human umbilical vein endothelial cells.
Thirty umbilical cords were collected from preterm deliveries, excluding those suspected of having chorioamnionitis. Infants were delivered preterm because of pregnancy induced hypertension (5), placental abruption (6), an incompetent cervix (4), multiple gestation (7), placental insufficiency (3), a motor vehicle accident (1), precipitous delivery in the emergency room (2), maternal cholecystectomy (1), and posterior urethral valves with oligohydramnios (1). Of the preterm deliveries, 16 were vaginal and 14 were by cesarean section. Term deliveries were all of healthy infants, none of whom were admitted to an intensive care nursery. Mothers of preterm infants were exposed to various medications such as betamethasone, tocolytics, antihypertensives, and antibiotics. The only medication consistently administered to mothers delivering preterm was betamethasone.
Analysis of P-selectin, vWF, and ICAM-1 by enzyme-linked immunosorbent assay (ELISA).
Endothelial cells were isolated from the umbilical vein with collagenase and counted using a hemocytometer. An equal number of endothelial cells were lysed in a detergent lysis buffer (Tris-buffered saline, pH 7.4, 1% Triton-x, 1% 0.1 mol/L phenylmethylsulfonyl fluoride). The cell lysates were centrifuged, protein assays (Bio-Rad Laboratories, Hercules, CA) were performed on the supernatant, and equal concentrations of protein were used for sandwich ELISAs. The ELISAs for P-selectin and vWF were performed as previously described.27 The antihuman P-selectin MoAbs W-40 and S-12 and purified membranous P-selectin were used for the P-selectin ELISA (all provided by Rodger McEver). Rabbit antihuman vWF (Dako Corp, Carpinteria, CA), goat antihuman factor VIII-related antigen (Atlantic Antibodies, Scarborough, ME), and purified von Willebrand factor (provided by Jerry Roth, University of Washington, Seattle, WA) were used for the vWF ELISAs. The ICAM-1 ELISAs were performed with the PREDICA kit (Genzyme, Cambridge, MA).
Transmission electron microscopy.
Fresh umbilical cords from preterm and term infants were cut in cross-section, fixed with 2.5% glutaraldehyde and 1% paraformaldehyde in cacodylate buffer, postfixed with 1% osmium tetroxide, and embedded in epoxy resin. Thin sections were cut across the umbilical vein’s endothelial cells, counterstained with uranyl acetate and lead citrate, and observed with an Hitachi H-7100 transmission electron microscope (Hitachi Instruments Inc, Mountainview, CA). For each cord, 10 nonoverlapping, calibrated fields were evaluated for the number of Weibel-Palade bodies per cubic micrometer of nonnuclear cytoplasm (numerical density), as previously described.28Briefly, the numerical density (Nv) of Weibel-Palade bodies was determined by the formula Nv=nA/A(D + T),29 where nAis the number of Weibel-Palade bodies counted in an area (A) of a section (2.5 μm2), D is the mean diameter of Weibel-Palade bodies, and T is the section thickness (75 nm). The dimensions (long and short axis) of Weibel-Palade bodies were determined for 10 Weibel-Palade bodies per cord. The Weibel-Palade body dimensions and shape were the same between groups (preterm and term). The average dimensions were 116 ± 19 × 148 ± 30 nm (mean ± SD), meaning that the bodies are elliptical.
RNAse protection assay.
Endothelial cells were isolated from freshly collected human umbilical cords by incubation with collagenase and then lysed in TRIzol reagent (GIBCO BRL, Grand Island, NY). Total RNA was isolated in TRIzol according to the manufacturer’s instructions. The RNA probe was generated by in vitro transcription using T7 polymerase and a template containing a P-selectin cDNA insert extending from bp 316 to 647. This region encodes the entire lectin-like domain of P-selectin necessary for binding.30 A full-length human P-selectin cDNA in pGEM-9Zf(−) vector was cut by the restriction enzyme SacI, which leaves a 647-bp cDNA insert in the vector. The resulting plasmid was linearized with BamHI. An RNAse protection assay was performed with the RPAII kit (Ambion Inc, Austin, TX) to detect and quantitate P-selectin mRNA per the manufacturer’s instructions.
Statistics.
The percentage increase in peritoneal neutrophils in newborn and adult rats was compared using an unpaired t-test. Unpairedt-tests also were used to compare the means of the ELISAs. A Mann-Whitney rank sum test was performed on the mean numerical density of Weibel-Palade bodies. Significance was defined at the P < .05 level.
The animal studies were approved by the institutional animal care and use committee. The institutional review board approved all studies using human tissue and endothelial cells.
RESULTS
Delayed accumulation of neutrophils in the inflamed peritoneum of neonatal rats is not accounted for by a neutrophil defect.
Four hours after intraperitoneal injection of thioglycollate, there were significantly fewer neutrophils in the fluid obtained by peritoneal lavage of neonatal rats compared with fluid retrieved from adults. Both the absolute neutrophil number and the percentage increase in neutrophil count were greater in the adult rats. In 9 experiments, there was a fivefold greater increase in peritoneal neutrophils in adults compared with newborns (P = .01). Additionally, the percentage increase in myeloperoxidase activity in lavage fluid from adults was 23 times greater than that in samples from the newborn rats. These results are similar to those in previous studies7-9and confirmed that there is impaired accumulation of neutrophils at extravascular sites in response to inflammatory stimuli in neonatal animals. The defect in accumulation of neutrophils in the peritoneum of neonatal rats in our experiments was not due to lower circulating neutrophil numbers or impaired release of neutrophils from the bone marrow. In the 9 experiments, there was initially no significant difference in blood neutrophil number in samples from adults vs. neonates, and 4 hours after intraperitoneal injection of thioglycollate, the number of blood neutrophils was actually about twofold higher in samples from neonates compared with adults (P= .03).
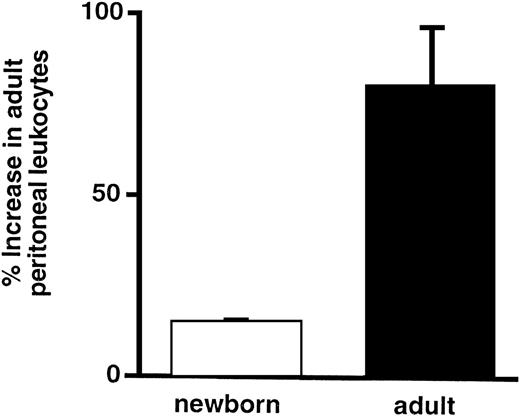
To determine whether leukocytes from an adult animal can accumulate normally in the peritoneum of a neonatal animal challenged with thioglycollate, leukocytes from adult rats were isolated, pooled, radiolabeled with 111In, and injected into allogenic adult and newborn rats. Rats then received an intraperitoneal injection of thioglycollate or PBS, as described above. The percentage increase in radiolabeled leukocytes in the peritoneal fluid from adults was fivefold greater than that in peritoneal fluid from newborns (Fig 1) and was almost exclusively neutrophils. This experiment indicated that the neonatal defect could not be overcome by transfusion of adult leukocytes that were competent to target and transmigrate and that the phenotype of the neonatal leukocyte alone does not account for the impaired accumulation.
Transfused leukocytes from adult rats do not transmigrate efficiently in neonatal rats. The ordinate indicates the percentage increase (see Materials and Methods) in cpm of radiolabeled adult leukocytes that transmigrated into the peritoneum of neonatal and adult rats. The bars represent the mean ± SEM of values from 4 rats in each group. The difference in the percentage increase in peritoneal leukocytes between the newborn and the adult is statistically significant (P = .05).
Transfused leukocytes from adult rats do not transmigrate efficiently in neonatal rats. The ordinate indicates the percentage increase (see Materials and Methods) in cpm of radiolabeled adult leukocytes that transmigrated into the peritoneum of neonatal and adult rats. The bars represent the mean ± SEM of values from 4 rats in each group. The difference in the percentage increase in peritoneal leukocytes between the newborn and the adult is statistically significant (P = .05).
In the original experiments measuring the transmigration of autologous neutrophils, the number of neutrophils in peritoneal fluid of neonatal rats increased with time after injection with thioglycollate and by 24 hours equaled the number in samples from adult rats. This early defect in accumulation of neutrophils with preserved ability to accumulate at later time points was similar to that reported in previous studies of neonatal animals and also to that in adult mice genetically deficient in P-selectin that were challenged with intraperitoneal thioglycollate.31
P-selectin is not expressed on the surface of endothelial cells from newborn rat mesentery.
The expression of P-selectin was examined in situ in endothelium in neonatal and adult rat mesentery at various times after the intraperitoneal injection of PBS or thioglycollate. By 15 minutes after the injection, P-selectin was detectable on the surface of endothelial cells in adult rats injected with thioglycollate but not in adult rats injected with PBS. The maximum expression of P-selectin in the adults was 30 minutes after the injection (Fig2A). The expression was sustained, but weaker, from 60 minutes to 4 hours after the injection. In contrast to the tissue from adult rats, the endothelial cells lining the mesenteric vessels of newborn rats injected with thioglycollate did not stain for P-selectin early after challenge (Fig 2B). Endothelial cells in mesentery from 1 newborn rat of the 11 studied stained weakly positive for P-selectin 120 minutes after thioglycollate injection. As in the adult rats, no newborn rats injected with PBS stained positively for P-selectin. Therefore, the delay in the intraperitoneal accumulation of neutrophils in neonatal rats coincided with decreased expression of P-selectin on endothelial cells from neonatal rats.
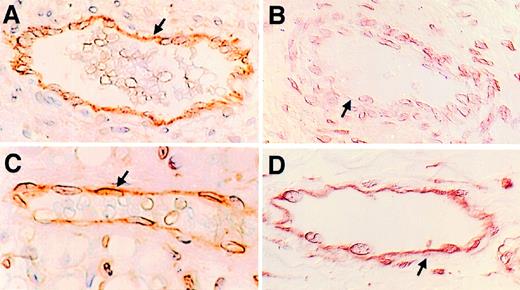
Newborn rats have a defect in expression of P-selectin on endothelial cells after an inflammatory stimulus. The photomicrographs in this figure are of samples collected 30 minutes after an intraperitoneal injection of thioglycollate and are representative of the 11 rats studied in each group. Postcapillary mesenteric venules were identified by their morphology. The sections were viewed with Nomarski differential interference contrast optics. All four panels were magnified 500×. (A and B) P-selectin expression. P-selectin expression was detected with the MoAb PB1.3. (A) is representative of mesenteric venules in the adult rat. The arrows demonstrate positive brown immunostaining of endothelial cells lining the vessels. (B) is representative of the newborn rat. The arrow points to endothelial cells lining newborn mesenteric venules that did not stain. (C and D) PECAM-1 expression. (C) is a section of adult mesentery and (D) is a section of newborn mesentery. Positive brown immunostaining for PECAM-1 is demonstrated covering the full height of the cytoplasm of endothelial cells (arrows).
Newborn rats have a defect in expression of P-selectin on endothelial cells after an inflammatory stimulus. The photomicrographs in this figure are of samples collected 30 minutes after an intraperitoneal injection of thioglycollate and are representative of the 11 rats studied in each group. Postcapillary mesenteric venules were identified by their morphology. The sections were viewed with Nomarski differential interference contrast optics. All four panels were magnified 500×. (A and B) P-selectin expression. P-selectin expression was detected with the MoAb PB1.3. (A) is representative of mesenteric venules in the adult rat. The arrows demonstrate positive brown immunostaining of endothelial cells lining the vessels. (B) is representative of the newborn rat. The arrow points to endothelial cells lining newborn mesenteric venules that did not stain. (C and D) PECAM-1 expression. (C) is a section of adult mesentery and (D) is a section of newborn mesentery. Positive brown immunostaining for PECAM-1 is demonstrated covering the full height of the cytoplasm of endothelial cells (arrows).
Platelet endothelial cell adhesion molecule-1 (PECAM-1) was used as a control adhesion molecule that is expressed by endothelial cells, because it is expressed early in development.32 Endothelial cells from both newborn and adult rats constitutively expressed PECAM-1 on their surface and the intensity of the staining was equivalent in tissues from rats of the two age groups (Fig 2C and D). In contrast, neither the newborn nor the adult rats expressed intercellular adhesion molecule-1 (ICAM-1) on the surface of endothelial cells lining mesenteric venules 4 hours after the intraperitoneal injection of thioglycollate. However, adult rats did exhibit surface expression of ICAM-1 when endothelial cells in skin were examined 24 hours after local injection of lipopolysaccharide as a positive control (not shown).
P-selectin expression is decreased on the surface of endothelial cells from human newborn mesentery.
To determine whether endothelial cells from human neonates have the same deficiency in P-selectin expression as observed in neonatal rats, mesenteric tissue was obtained at autopsy from patients of various ages for immunohistochemistry. Eleven samples from individuals ranging in age from 18 weeks gestation to 10 years of age were studied. There was intense staining for P-selectin in endothelial cells from older children ranging in age from 3 to 10 years, and there was consistent staining of the endothelial cells from term human neonates (Fig 3A). Faint staining for P-selectin was seen in endothelial cells at 27 weeks of gestation. Endothelial cells from fetuses ranging in age from 18 to 22 weeks of gestation did not stain positively for P-selectin (Fig 3B). As in the animal model, the age of the subject did not affect the intensity of staining for PECAM-1 on endothelial cells lining the mesenteric venules (Fig 3C and D). By immunohistochemical analysis of human tissues, P-selectin was not expressed early in development and the expression of P-selectin increased with advanced gestational age.
Expression of P-selectin is developmentally regulated in humans. Mesentery was collected during the autopsy of individuals of varying age and processed as in Fig 2. The sections were viewed with Nomarski differential interference contrast optics. All four panels are magnified 165×. (A and B) P-selectin expression. P-selectin expression was detected with the MoAb S12. (A) is from a term infant who died because of a congenital heart defect and (B) from a 22-week gestation infant delivered prematurely because of an incompetent cervix. There is immunostaining for P-selectin on mesenteric endothelial cells of the term infant (arrows), but no immunostaining was detected on the endothelial cells from the 22-week gestation infant. The arrow in (B) points to endothelial cells lining newborn mesenteric vessels that did not stain. (C and D) PECAM-1 expression: These sections are from the same tissue blocks as in (A) and (B). (C) is a section of mesentery from the term infant and (D) is from the 22-week gestation infant. The arrows in both figures indicate endothelial cells immunostained for PECAM-1.
Expression of P-selectin is developmentally regulated in humans. Mesentery was collected during the autopsy of individuals of varying age and processed as in Fig 2. The sections were viewed with Nomarski differential interference contrast optics. All four panels are magnified 165×. (A and B) P-selectin expression. P-selectin expression was detected with the MoAb S12. (A) is from a term infant who died because of a congenital heart defect and (B) from a 22-week gestation infant delivered prematurely because of an incompetent cervix. There is immunostaining for P-selectin on mesenteric endothelial cells of the term infant (arrows), but no immunostaining was detected on the endothelial cells from the 22-week gestation infant. The arrow in (B) points to endothelial cells lining newborn mesenteric vessels that did not stain. (C and D) PECAM-1 expression: These sections are from the same tissue blocks as in (A) and (B). (C) is a section of mesentery from the term infant and (D) is from the 22-week gestation infant. The arrows in both figures indicate endothelial cells immunostained for PECAM-1.
Endothelial cell stores of P-selectin and von Willebrand factor increase with gestational age in humans.
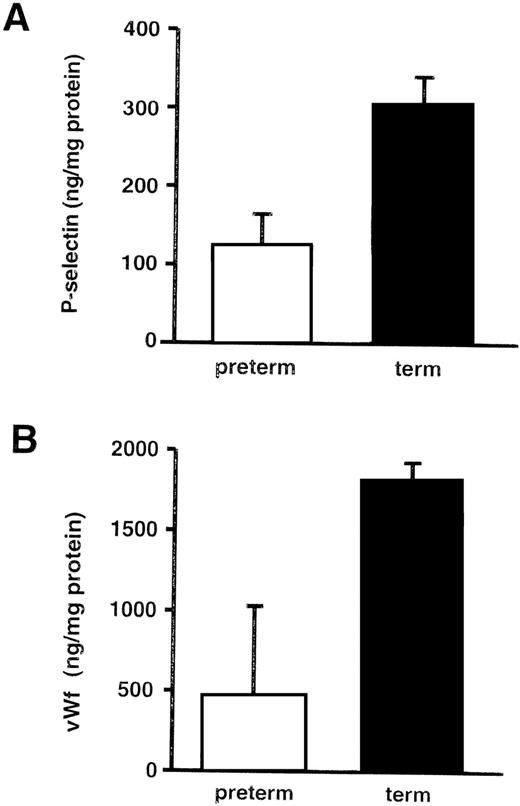
Endothelial cells from umbilical veins of preterm infants contained approximately half the amount of P-selectin as those from term infants (Fig 4A). As with P-selectin, the endothelial cell stores of vWF were diminished in the human umbilical vein endothelial cells (HUVECs) from preterm infants (Fig4B). In contrast, ICAM-1 levels were not lower when measured in the same cellular lysates (142.6 ng/mg protein in cells from preterm infants v 78.6 ng/mg protein in endothelial cells from term infants).
Endothelial cell stores of P-selectin and von Willebrand factor increase with gestational age in human neonates. ELISAs were performed on HUVEC lysates and normalized to total cellular protein. These graphs summarize the data from 12 term infants (>38 weeks) and 15 preterm infants (18 to 31 weeks). (A) P-selectin. The bars represent the mean ± SD. The amount of P-selectin was significantly less in preterm HUVECs compared with term HUVECs (P = .003). When individual samples were examined, the amount of P-selectin generally correlated with gestational age, although there was variation from sample to sample. (B) von Willebrand factor. The bars represent the mean ± SD. The amount of vWf was significantly less in preterm HUVECs compared with term HUVECs (P = .005).
Endothelial cell stores of P-selectin and von Willebrand factor increase with gestational age in human neonates. ELISAs were performed on HUVEC lysates and normalized to total cellular protein. These graphs summarize the data from 12 term infants (>38 weeks) and 15 preterm infants (18 to 31 weeks). (A) P-selectin. The bars represent the mean ± SD. The amount of P-selectin was significantly less in preterm HUVECs compared with term HUVECs (P = .003). When individual samples were examined, the amount of P-selectin generally correlated with gestational age, although there was variation from sample to sample. (B) von Willebrand factor. The bars represent the mean ± SD. The amount of vWf was significantly less in preterm HUVECs compared with term HUVECs (P = .005).
Maternal betamethasone was administered to hasten lung maturity in 77% of the preterm infants whose HUVECs were used in this study. To determine the effect of betamethasone on endothelial cell stores of P-selectin, HUVECs from umbilical cords of term infants were grown in the presence of either 0.02 μg/mL of betamethasone (the concentration predicted to be present in cord blood after the mother receives 12 mg intramuscular betamethasone33), 10 μg/mL betamethasone, or 100 ng/mL oncostatin M. Oncostatin M is an endothelial cell agonist that has been shown to increase P-selectin mRNA and protein in human endothelial cells.34 Both concentrations of betamethasone decreased the amount of ICAM-1 in HUVECs (53% and 49% decrease in cells treated with 0.02 or 10 μg/mL betamethasone, respectively) and increased P-selectin in HUVECs (not shown). The increase when the HUVECs were exposed to 0.02 μg/mL betamethasone (76% increase) was comparable to that upon exposure to oncostatin M (79% increase).
Decreased P-selectin in endothelial cells from premature human infants is associated with fewer Weibel-Palade bodies.
HUVECs were collected from 6 preterm infants ranging from 23 to 26 weeks of gestation and from 6 term infants greater than 38 weeks of gestation. Ultrastructural observation was performed by an observer blinded to the gestational age of the infants from whom the endothelial cells were collected and showed that intact endothelial cells lined all of the umbilical cords. Weibel-Palade bodies were morphologically identified35 36 as small, membrane-bound, rod-shaped organelles of moderate density that were located in the cytoplasm of the umbilical cord endothelial cells. There were few Weibel-Palade bodies in endothelial cells of very premature infants and the number increased with increasing gestational age (Figs 5 and 6).
Preterm umbilical vein endothelial cells have fewer Weibel-Palade bodies than term cells. HUVECs were viewed by transmission electron microscopy for quantitative morphology. The mean numerical density (NV) represents the number of Weibel-Palade bodies per cubic micrometer of nonnuclear cytoplasm. The difference between term and preterm endothelial cells is significant (P = .01).
Preterm umbilical vein endothelial cells have fewer Weibel-Palade bodies than term cells. HUVECs were viewed by transmission electron microscopy for quantitative morphology. The mean numerical density (NV) represents the number of Weibel-Palade bodies per cubic micrometer of nonnuclear cytoplasm. The difference between term and preterm endothelial cells is significant (P = .01).
The number of Weibel-Palade bodies per HUVEC is less in endothelial cells from preterm infants. This figure is representative of the data in Fig 5. (A) is from a 23-week gestation infant and (B) is from a term infant. Here there are 4 to 5 times as many Weibel-Palade bodies (arrows) in the cytoplasm of the term infant compared with the preterm infant.
The number of Weibel-Palade bodies per HUVEC is less in endothelial cells from preterm infants. This figure is representative of the data in Fig 5. (A) is from a 23-week gestation infant and (B) is from a term infant. Here there are 4 to 5 times as many Weibel-Palade bodies (arrows) in the cytoplasm of the term infant compared with the preterm infant.
mRNA for P-selectin is decreased in endothelial cells from premature neonates.
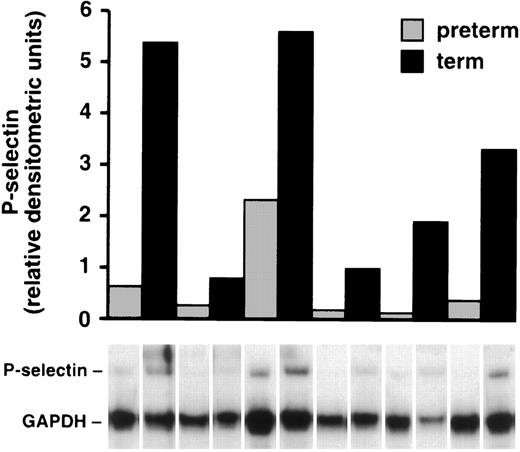
Umbilical cords were collected from 6 preterm infants ranging in age from 22 to 26 weeks and were paired with umbilical cords from 6 term infants born at approximately the same time, and P-selectin mRNA levels were measured. Endothelial cells were isolated from the veins and lysed, and the amount of P-selectin mRNA in each sample was quantitated with an RNAse protection assay. In each pair of samples, there was more mRNA for P-selectin in the HUVECs from term infants compared with the HUVECs from preterm neonates (Fig 7). There was considerable variation in mRNA in these samples that may have been due to the variable period of time between delivery of the placenta and processing of the samples.
P-selectin mRNA is diminished in HUVECs from preterm cords. P-selectin mRNA was quantitated in each sample with an RNAse protection assay (see Materials and Methods) and normalized to GAPDH mRNA in the same sample. The ratio of the relative intensities of P-selectin mRNA compared with GAPDH mRNA is shown in the bar graph with the corresponding lane below. The combined relative densitometric units in the term infants was 4.6× greater than in the preterm infants (P = .016).
P-selectin mRNA is diminished in HUVECs from preterm cords. P-selectin mRNA was quantitated in each sample with an RNAse protection assay (see Materials and Methods) and normalized to GAPDH mRNA in the same sample. The ratio of the relative intensities of P-selectin mRNA compared with GAPDH mRNA is shown in the bar graph with the corresponding lane below. The combined relative densitometric units in the term infants was 4.6× greater than in the preterm infants (P = .016).
DISCUSSION
The etiology of delayed neutrophil transmigration in neonates has been extensively studied. Although there are many studies that have focused on defects in neonatal neutrophils, there are no studies in the literature that address the possibility of defects in neonatal endothelial cells. Using an animal model, we show that leukocytes from adult rats do not transmigrate as efficiently in neonatal rats when compared with adults (Fig 1). This experiment is the first to demonstrate a defect in accumulation of adult leukocytes at an inflamed site in a neonatal host. Our study demonstrates that P-selectin, a rapidly expressed endothelial cell adhesion molecule shown to be essential for both neutrophil rolling early after endothelial cell stimulation15 and neutrophil transmigration in vivo,31 has diminished expression in the newborn rat. Given the complexity of whole animal studies, factors other than decreased expression of endothelial cell adhesion molecules also may be contributing to the delay in transmigration in the neonatal rats in our experiments. For example, newborns have been reported to have decreased levels of interleukin-8 (IL-8),37 granulocyte-macrophage colony stimulating factor,38 and tumor necrosis factor.39 40 Thus, impaired expression of signaling molecules may also contribute to delayed transmigration in neonates in vivo.
We found that P-selectin expression on endothelial cells in humans also increased with maturation. However, there was not a complete absence of P-selectin expression in term infants as in 1-day-old rats. These findings are in accordance with previous reports of decreased P-selectin expression on activated neonatal platelets compared with adult platelets,41 with a particularly low P-selectin expression on the surface of activated platelets from very low birth weight infants.42 In the autopsy specimens used in this study, P-selectin expression on mesenteric endothelial cells was first detected by immunohistochemistry at approximately 27 weeks of gestation. We were not able to determine if the amount of P-selectin was decreased in term infants compared with adults. We attempted to answer this question with endothelial cells obtained from newborn human foreskin compared with human adult dermal endothelial cells. However, these studies were not informative. We found that P-selectin expression was highly dependent on the degree of confluence of the cells. There was as much as a fourfold difference in the amount of P-selectin per milligram of protein in endothelial cells, depending on the degree of confluence (not shown). This made experiments using endothelial cells grown in culture very difficult to interpret with regard to the amount of P-selectin or adhesion and transmigration dependent on P-selectin. However, although cell adhesion and transmigration assays are frequently performed using HUVECs, neutrophils transmigrate across endothelial cells lining postcapillary venules in the systemic circulation.43 Therefore, the immunohistochemical results demonstrating decreased expression of P-selectin on endothelial cells lining postcapillary venules in premature infants (Fig 3) may be more predictive of decreased adhesion and transmigration in vivo.
Our experiments also demonstrate that endothelial cell stores of P-selectin are significantly diminished in preterm human neonates compared with term neonates. This was demonstrated by ELISA (Fig 4A) and by an RNAse protection assay (Fig 7) using HUVECs. A variable that may have influenced the amount of P-selectin in these studies was the administration of glucocorticoids to the mother. Although glucocorticoids are reported to decrease the synthesis of E-selectin and ICAM-1,44,45 they do not decrease the constitutive or induced expression of P-selectin in human endothelial cells.46 We found that exposure to betamethasone actually increased endothelial cell P-selectin. These data indicate that the levels of P-selectin measured in endothelial cells from preterm neonates (Fig 4A) were not due to suppression by exogenous steroids and may have been even lower if they had not been exposed to glucocorticoids in utero.
Subsequent experiments were designed to determine the etiology of decreased P-selectin expression in preterm neonates. Because P-selectin is stored in Weibel-Palade bodies along with vWF, the amount of vWF stored in preterm endothelial cells was measured and found to also be diminished compared with term cells (Fig 4B). Therefore, we postulated that there were fewer Weibel-Palade bodies in endothelial cells from preterm infants. The decreased numbers of Weibel-Palade bodies in endothelial cells was demonstrated by transmission electron microscopy (Figs 5 and 6). These results are similar to those in a previous report in which Kagawa and Fujimoto47 collected HUVECs from artificially aborted fetuses of 12, 19, and 33 weeks of pregnancy and from full-term deliveries. They found that the number and size of Weibel-Palade bodies per HUVEC was decreased from 12 to 19 weeks of gestation and then rapidly increased from 33 weeks to full term.
Without cell storage granules, the half life of P-selectin protein in endothelial cells is likely to be short. Disdier et al48found that, when a P-selectin cDNA was transfected into cells with storage granules and capable of regulated secretion, it was concentrated in storage granules, whereas the fate of P-selectin in cells without storage granules is rapid degradation in lysosomes.49 Green et al50 found that, when P-selectin was transfected into neuroendocrine cells, the fraction of P-selectin that was not targeted to secretory granules was rapidly degraded in lysosomes. These studies indicate that decreased numbers of Weibel-Palade bodies in endothelial cells in vessels of premature infants may result in increased P-selectin degradation and consequent diminished endothelial cell stores and surface expression of P-selectin in response to inflammatory stimuli.
Many cell adhesion molecules, including E-selectin, ICAM-1, VCAM-1, and PECAM-1, are synthesized in the early stages of development.32,51,52 In contrast, the amount of P-selectin message was diminished in preterm endothelial cells compared with cells from term infants, identifying a second mechanism for decreased levels of the protein. The decreased amount of P-selectin mRNA in preterm infants could be the result of decreased transcription, because factors reported to drive P-selectin transcription in humans, such as IL-4,34 may be deficient in neonates.53 mRNA stability has been reported to be altered in mononuclear leukocytes from neonates.54 Therefore, the stability of mRNA for P-selectin, which has a long half life in excess of 12 hours in term HUVECs,34 may be altered in premature endothelial cells.
An alternative explanation for decreased detection of P-selectin in preterm infants is that they may make an isoform of P-selectin not detected by the MoAbs or RNAse protection assay described here. Although the MoAbs we used to detect P-selectin have been reported to recognize human P-selectin precursor proteins and all reported human P-selectin isoforms,55,56 there remains the possibility of an embryonic isoform of P-selectin synthesized by preterm neonates not detected by these antibodies. However, the RNAse protection assay used in these studies protects the entire lectin-like domain of P-selectin that is necessary for adhesion.30 Therefore, an isoform of P-selectin with an alteration in the transcript of this domain may not be able to bind the P-selectin ligand.
Decreased P-selectin protein in endothelial cells from preterm infants compared with term infants is associated with both decreased amount of P-selectin mRNA and fewer P-selectin storage granules in endothelial cells from preterm infants. Consistent with previous studies demonstrating the importance of P-selectin in recruitment of neutrophils into inflamed peritoneum,31,57 we demonstrate that diminished expression of P-selectin in neonatal rats is associated with delayed neutrophil transendothelial cell migration in vivo. The relative deficiency of P-selectin in endothelium of preterm human infants compared with term infants may contribute to the neutrophil targeting defect and the increased susceptibility to infection with increasing prematurity in humans. Increased susceptibility to systemic infection with Streptococcus pneumoniae has been reported in P-selectin–deficient mice.58 Additionally, P-selectin has been reported to participate in various disease states, including some that would affect premature infants, such as intestinal reperfusion injury,26,59,60 a component of necrotizing enterocolitis, and cerebral ischemia and reperfusion injury.61 Therapeutic interventions are currently being designed to block adhesion to P-selectin to prevent reperfusion injury mediated by leukocytes.61-63 Given the relative deficiency of P-selectin in neonatal animals and preterm neonatal humans, the therapeutic targeting of P-selectin would unlikely be efficacious in these patients.
ACKNOWLEDGMENT
The authors thank Donelle Benson, Nancy Chandler, and Deborah Dykstra for excellent technical assistance; Diana Lim for preparation of the figures; Ed Klatt and Cheryl Coffin for the autopsy tissue samples; and Andy Weyrich, Guy Zimmerman, Tom McIntyre, and Steve Prescott for thoughtful discussion. We also thank Rodger McEver for the contribution of antibodies and P-selectin, Steven Albelda for the contribution of antibodies to PECAM-1, and Jerry Roth for purified vWF.
Supported by the Nora Eccles Harrison Foundation, a Special Center of Research in Lung Injury Grant (P50HL50153) sponsored by the National Institutes of Health, a Physician Scientist Award (HL-02726) from the National Institutes of Health (D.E.L.), an Established Award (9640261N) from the American Heart Association (D.E.L.), a Shared Instrumentation Grant (K.H.A.) from the National Institutes of Health (S10-RR 10489), and a Grant-In-Aid (K.H.A.) from the American Heart Association (96014370).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Diane E. Lorant, MD, Department of Pediatrics, Indiana University, Methodist Hospital, 1701 N Senate Blvd, Indianapolis, IN 46202.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal