Abstract
The chemokine stromal-derived factor-1 (SDF-1) is a chemoattractant for CD34+ progenitor cells, in vitro and in vivo. The receptor for SDF-1, CXCR-4, is a 7 transmembrane domain receptor, which is also a coreceptor for human immunodeficiency virus (HIV). Here we show that transformation of hematopoietic cell lines by BCR/ABL significantly impairs their response to SDF-1. Three different hematopoietic cell lines, Ba/F3, 32Dcl3, and Mo7e, were found to express CXCR-4 and to respond to SDF-1 with increased migration in a transwell assay. In contrast, after transformation by the BCR/ABL oncogene, the chemotactic response to SDF-1 was reduced in all 3 lines. This effect was directly due to BCR/ABL, because Ba/F3 cells, in which the expression of BCR/ABL could be regulated by a tetracycline-inducible promoter, also had reduced chemotaxis to SDF-1 when BCR/ABL was induced. The reduced response to SDF-1 was not due to an inability of BCR/ABL-transformed cell lines to migrate in general, as spontaneous motility of BCR/ABL-transformed cells was increased. In mice, injection of SDF-1 into the spleen resulted in a transient accumulation of untransformed Ba/F3 cells, but not Ba/F3.p210BCR/ABL cells administered simultaneously. The mechanism may involve inhibition of CXCR-4 receptor function, because in BCR/ABL-transformed cells, CXCR-4 receptors were expressed on the cell surface, but SDF-1 calcium flux was inhibited. Because SDF-1 and CXCR-4 are felt to be involved in progenitor cell homing to marrow, the abnormality decribed here could contribute to the homing and retention defects typical of immature myeloid cells in chronic myelogenous leukemia.
CHRONIC MYELOGENOUS leukemia (CML) is a myeloproliferative disorder caused by the BCR/ABL fusion oncogene. CML is characterized by premature release of myeloid cells from the marrow and massive accumulation of both mature and immature myeloid cells in blood, spleen, and marrow.1 There is growing evidence to suggest that the interaction of CML cells with the marrow microenvironment is abnormal. Gordon et al2 showed in 1987 that CML progenitor cells had diminished capacity to adhere to stromal cell layers. More recently, it was reported that CML cells have reduced long-term adhesion to the extracellular matrix protein fibronectin, and this impairment was thought to be due to abnormal function of β1 integrins.3,4 p210BCR/ABL is partially localized to the cytoskeleton, and we have previously shown that several cytoskeletal proteins associated with integrin regulation, such as paxillin, FAK, CRKL, and vinculin, are prominent substrates of the BCR/ABL tyrosine kinase.5,6 The functions of these proteins are altered by phosphorylation and/or direct interaction with p210BCR/ABL, presumably contributing to the abnormal integrin function in CML.3
The ability of CML cells to leave the marrow at an immature stage of differentiation, circulate in high numbers in the blood, and accumulate in the spleen indicates that events that normally regulate migration and retention of hematopoietic progenitor cells are also defective. We have previously shown that spontaneous motility of BCR/ABL-transformed cells on fibronectin-coated surfaces is increased, and this prompted us to ask if more specific migration and retention functions of CML progenitor cells might also be abnormal.7
The events controlling homing of CD34+ cells to marrow and the retention of normal immature myeloid cells in the marrow are not well understood. Recently, however, the chemokine stromal-derived factor-1α (SDF-1α), which is produced by marrow stromal cells, has been shown to be a potent chemoattractant for human CD34+ progenitor cells.8 SDF-1α is a mem- ber of the α (CXC) chemokine family and it has homology to interleukin-8 (IL-8) and MIP-1α.9 However, unlike these chemokines, SDF-1α elicits transendothelial chemotaxis of human CD34+ cells both in vitro and in vivo.8,10SDF-1α11 and CXCR-412 13 null mutant mice have profound defects in myeloid and lymphoid development, further suggesting that this chemokine is important in regulating hematopoietic cell migration.
In this report, we provide evidence that BCR/ABL transformation inhibits migration of hematopoietic cells in response to SDF-1α in vitro and in vivo. BCR/ABL did not affect expression of SDF-1α receptors, but directly inhibited signal transduction. This defect may explain some of the homing and retention defects typical of immature myeloid cells in CML.
MATERIALS AND METHODS
Cells and cell culture.
The murine hematopoietic cell lines Ba/F3 and 32Dcl3 (also identified as 32D) were cultured at 37°C with 5% CO2 in RPMI 1640 (Mediatech, Washington, DC) containing 10% (vol/vol) WEHI-3B conditioned medium as a source of interleukin-3 and 10% (vol/vol) fetal calf serum (FCS) (PAA Laboratories Inc, Newport Beach, CA). The human megakaryoblastic cell line Mo7e was maintained in Dulbecco's Modified Eagle's Medium (DMEM; Mediatech), 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF: Genetics Institute, Cambridge, MA), and 20% (vol/vol) FCS at 37°C with 10% CO2. The BCR/ABL-expressing cell lines Ba/F3.p210BCR/ABL, Ba/F3.p185BCR/ABL, 32D.p210BCR/ABL, 32D.p185BCR/ABL , and Mo7e.p210BCR/ABL were generated by transfection with the pGD vector containing the BCR/ABL cDNA, as previously described.14 p185 and p210 are different forms of BCR/ABL. BCR/ABL-containing cells were cultured in RPMI 1640 medium with 10% FCS, but without any source of IL-3 or GM-CSF. A Ba/F3 cell line, TonB210.1, with tetracycline-dependent BCR/ABL expression, was grown in RPMI 1640 medium containing 10% WEHI-conditioned medium and 10% FCS.15 For induction experiments of less than 5 hours, cells were growth factor deprived for 18 hours in RPMI 1640 containing 0.5% bovine serum albumin (BSA). For induction experiments longer than 1 day, cells were grown in RPMI 1640 containing WEHI. The BCR/ABL expression was induced by treatment with doxycycline (2 μg/mL) for 1 day.
Conditioned medium from the bone marrow-derived mouse stromal cell line, MS-5, was used as one source of SDF-1α.8 Once the growing cells reached subconfluence, the medium was replaced with serum-free medium (UltraCULTURE; Biowhittaker, Walkersville, MD), which was collected after 2 days of culture and passed through a 0.45-mm filter. Recombinant, purified human SDF-1α was purchased from R&D Systems (Minneapolis, MN).
Confocal microscopy.
F-actin was visualized in fixed cells (1% paraformaldehyde in phosphate-buffered saline [PBS]) using rhodamine phalloidin (Molecular Probes, Eugene, OR), as described.5 The focal adhesion protein paxillin was visualized using indirect immunofluorescence with the antipaxillin monoclonal antibody clone 5H11 (UBI; Lake Placid, NY), detected by a fluorescein isothiocyanate (FITC)-conjugated goat antimouse antibody. Confocal image analysis was performed using a Zeiss model LSM4 confocal laser scanning microscope (Zeiss, Jena, Germany) equipped with an external argon-krypton laser (488 nm and 568 nm). Optical sections of 512 × 512 pixels were digitally recorded within 2 seconds and 2× line-averaging. Images were printed with a Fujix Pictography 3000 printer (Fuji, Japan) using Adobe Photoshop software (Adobe Systems, Mountain View, CA).
Scanning electron microscopy.
Cells grown in suspension were concentrated to 1 × 105 cells/mL, and the cells were either unstimulated or stimulated with SDF-1α. One drop of such suspension was placed onto a plastic cover slip previously coated with 1% poly-L-lysine (Sigma, St. Louis, MO) in water. The coverslip was then allowed to stand in a small Petri dish at room temperature for 15 to 30 minutes in order for the cells to adhere to the slip. Fixative (1.5% glutaradehyde in 0.01 mol/L phosphate buffer, pH 7.4) was added to the petri dish to cover the slip and the cells were fixed for 1 hour at 40°C. The coverslip was then taken through graded alcohols and dried in a Ladd Critical Point Dryer (Model 28000; Ladd Research Industries, Williston, VT), coated with platinum in a polaron SEM coating system. Samples were examined with a JEOL JSM-35 CF scanning electron microscope at an accelerating voltage of 20 KV.
Calcium flux analysis.
Cells were resuspended and analyzed for calcium flux using fluorescent indicator indo-1 AM (Molecular Probes), at a final concentration of 5 μg/mL at 37°C for 30 minutes. Cells were washed with PBS and starved in RPMI 1640 media containing 10% FCS, 1% penicillin-streptomycin for 10 hours at 5 × 105/mL. After starving in the absence of growth factors, 3 × 106 cells were treated with pertussis toxin (Sigma) at 100 ng/mL. Then the remaining cells were suspended in starvation media at 1 × 107 per mL. Indo-1 AM (1 mg/mL in DMSO) was added to the resuspending cells, making the concentration 1:100, and the cells were placed in the dark for 30 minutes. Afterwards, they were washed and resuspended in starvation media at 5 × 105per mL. Finally, 1 mL of resuspended cells was placed into a FACS analysis tube. Samples were analyzed on an EPICS ELITE (Coulter Corporation, Miami, FL) flow cytometer equipped with an ultraviolet enhanced Argon Ion laser tuned to 351 to 363 nm (10nW output power.) The fluorescent emission of indo-1 AM loaded cells was detected by measuring both the violet bound form (405 nm) and blue/green (525 nm) unbound form of the dye in separate photomultiplier tubes (PMT). A ratio parameter was created using the 405 nm PMT as the denominator and the 525nm PMT as the numerator.
Recombinant SDF-1α was used at a concentration of 1 μg/mL, while ionomycin (Sigma) was used at 5 μg/mL. The tube was then placed back on the cytometer and reanalyzed for 3 minutes to detect any shift in the ratio of 525 nm/405 nm fluorescence. Data was initially analyzed using the Multigraph analysis program that is standard for the instrument. Processed 2 parameter histograms portraying ratio (ordinate) versus time (abscissa) were then subsequently analyzed for degree of responsiveness using the Multitime Software program (Phoenix Flow Systems) and plotted graphically as percent response versus time.
Adhesion assay.
Adhesion of hematopoietic cells (with and without BCR/ABL) was measured on plastic plates that were uncoated, or coated with bovine serum albumin (5μg/mL) or fibronectin (5μg/mL) (Becton Dickinson Labware, Bedford, MA), as described previously.16
Time-lapse video microscopy.
Cells were cultured on fibronectin-coated tissue culture plates (Becton Dickinson Labware) in a temperature and CO2 controlled chamber in their standard growth media and stimulated with either SDF-1α or supernatant from the MS-5 cell line (1:1).7 The cells were examined using an Olympus IX70 inverted microscope (Olympus, Lake Success, NY), Omega temperature control device (Therm-Omega-Tech, Warmington, PA), Optronics Engineering DEI-750 3CCD digital video camera (Optronics, Galeta, CA), and Sony SVT-S3100 time-lapse S-VHS video recorder (Sony, Tokyo, Japan). For image presentation, video images were captured and printed with the Sony Color Video Printer UP-5600MD.
Chemotaxis assay.
Chemotaxis assays were performed using the chemotaxis microplate system (Neuro Probe, Inc, Cabin John, MD). Cells were incubated for 4 hours in IL-3– and GM-CSF–deficient media at 37°C, resuspended in serum-free medium, and rewarmed to 37°C for 20 minutes before loading. UltraCULTURE alone was used as a nega- tive control and SDF-1α was diluted to 100 ng/mL in UltraCULTURE medium. MS-5 supernatant was used undiluted. One × 105 cells (in 25 μL) were allowed to migrate for 3 hours at 37°C, and migrating cells were resuspended in 200 μL of 1% formalin in phosphate-buffered saline (PBS), then counted for 30 seconds on a flow cytometer (Becton Dickinson, Mountain View, CA). The chemotactic index, a measure of the specificity of migration, was determined by dividing: (the number of cells migrating to chemokine)/(the number that migrated to medium alone). For blocking studies, 50 μg/mL of anti–SDF-1α antibody (R&D Systems) was added to the chemokine-containing medium 30 minutes before initiation of chemotaxis.
Detection of CXCR-4 by immunoblotting and immunofluorescent staining.
Immunoblots using the anti–CXCR-4 rabbit polyclonal antibody were performed as previously described.17 For indirect immunofluorescent staining, cells (1 × 106) were washed and resuspended in PBS containing 0.1% bovine serum albumin and 0.1% sodium azide (staining buffer). The cells were incubated for 30 minutes at 4°C with mouse antihuman CXCR-4 (R&D Systems; 0.1 mg/mL final concentration) or rabbit-antimouse-CXCR-4 (1:1000) polyclonal antibody, washed 3 times in staining buffer, and incubated with fluorescein isothiocyanate (FITC)-conjugated goat-antimouse or goat-antirabbit antibody (30 minutes, 4°C). After 3 washes, the stained cells were analyzed by FACS-sort flow cytometry using Cell Quest software (Becton Dickinson).
Reverse transcription-polymerase chain reaction (RT-PCR).
One microgram of RNA was reverse transcribed using a Perkin Elmer RT-PCR kit (Roche Molecular Systems, Inc, Branchburg, NJ). RT reactions were brought to a final volume of 40 μL, and cycling conditions were 25°C × 10 minutes, 42°C × 1 hour, then 99°C × 5 minutes. Primer sequences for SDF-1α are: (5′)GACGGTAAACC- AGTCAG and (3′)ACTGCCCTTGCATCTCCCCAC; mouse CXCR-4: (5′)GGCTGTAGAGCGAGTGTTGCC and (3′)GTAGAGGTTGACA- GTGTAGAT; and mouse β-actin: (5′)TGGAATCCTGTGGCATCCATGAAAC and (3′)TAAAACGCAGC-TCAGTAACAGTCCG. PCR was conducted using 1 to 5 μL of the RT products in a final volume of 25 μL, for 30 cycles (94°C, 1 minute; 54°C, 30 seconds; 72°C, 30 seconds).18
Homing assays.
Ba/F3 and Ba/F3.p210BCR/ABL cells were washed three times in DMEM (Biowhittaker) containing 20 mmol/L HEPES and 1% FCS, pH 7.4. Cells were resuspended at 20 × 106/mL and then incubated for 20 minutes with TRITC (30 μg/mL; Molecular Probes) or Calcein-AM (200 nmol/L; Molecular Probes).19 Labeling was performed for 10 minutes at 37°C and cells were mixed frequently during the incubation. Cells were then spun through an equal volume of fresh FCS or 20% BSA at 1300 rpm for 10 minutes. Viability was checked and cells were rewarmed at 37°C for 15 minutes before injection.
For homing experiments, C57BL/6 mice of both sexes were anesthetized by intraperitoneal injection of physiological saline containing ketamine-HCL (5 mg/mL) and xylazine (1 mg/mL) under Institutional Review Board approved protocols.19 The left carotid artery was cannulated with PE-10 polyethylene tubing to allow injection of cells into the descending aorta. Just before cell injection, a left flank incision was made and the exposed spleen was injected with 50 μL of PBS alone or PBS containing 1 μg of SDF-1α. After suturing the incision, equal amounts of labeled Ba/F3 and Ba/F3.p210BCR/ABL cells were pooled for injection (total volume of 0.5 mL, containing 107 cells). The cells were injected slowly, over a 15 to 20 minute period, and the animals were allowed to awaken. After 3 hours, the animals were sacrificed and spleen, lungs, bone marrow, and peripheral blood (via cardiac puncture) were harvested. Single-cell suspensions were prepared from minced tissues by gently passing them through a 70μ nylon mesh. The single-cell suspensions were separated on a Histopaque-1077 (Sigma) gradient and the cell populations recovered at the gradient interface were washed in DMEM, pelleted and fixed in 4% paraformaldehyde for analysis on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). The residual red and green fluorescent cells present in the catheter after injection were counted to determine the ratio of each cell line in the input population. Between 5 to 10 × 105 cells were analyzed for each tissue and fluorescent cells were quantitated within a gated region whose borders were determined from the injected pool. The percentages of gated cells recovered in each organ were corrected for differences in the percentage of each labeled cell type in the input population.
In vitro methylcellulose colony forming unit (CFU) assay.
Chemotaxis assays were performed using bone marrow (BM) cells harvested from patients with untreated CML or from normal donors with IRB approved protocol. Peripheral blood samples from patients with untreated CML were also tested. Mononuclear cell populations were obtained using Histopaque-1077, and the cells were washed 3 times at 4oC in UltraCULTURE medium. Immunofluorescent staining with monoclonal antibodies to human CD34 (Pharmingen, San Diego, CA) and CXCR-4 (R&D Systems) was done on selected samples. Chemotaxis assays were performed, as described above, using the Corning Costar Transwell assay (5 micron pore) and SDF-1α (100 ng/mL) or UltraCULTURE alone (control). Inserts were loaded with 100 μL containing 5 × 105 viable cells (input population) and the assay was allowed to proceed for 3 hours at 37°C. Separate aliquots of input cells (10, 20, and 60 μL) were also plated in methylcellulose to determine the CFU-GM capacity of the input population.
The migratory cells (output population) were harvested, diluted with UltraCULTURE, spun gently at 900 rpm for 10 minutes and the supernatants were aspirated carefully. The cell pellets were resuspended in 700 μL of a cocktail containing GM-CSF (50 ng/mL). The cocktail was prepared as described.8 An equal volume of methylcellulose was added to the tube, and the cells, GM-CSF cocktail, and methylcellulose were mixed using a 3 mL syringe and 16 gauge needle. Bubbles were allowed to dissipate and the mixture was plated into 35 × 10 mm petri dishes. Colonies were counted after 7 days in culture. Colonies were defined as containing greater than 50 cells and clusters less than 50 cells. The total number of colonies and clusters were scored by a blinded observer in 10 high power fields. The percent CFU-GM were plotted as: (total number of colonies and clusters obtained with the output population)/(the number of colonies and clusters obtained with the input population) × 100. Colony counts obtained with input cells were multiplied by the appropriate correction factor to determine the total CFU-GM capacity of 100 μL (5 × 105) of input cells.
Detection of p70 S6 kinase by immunoblotting.
The murine Ba/F3 and Ba/F3.p210BCR/ABL cell lines were washed in the absence of IL-3 and lysed in lysis buffer (20 mmol/L Tris pH 8.0, 150 mmol/L NaCl, 10% glycerol, 1% NP-40, 0.42% NaF) containing inhibitors (10 μL of 100 μmol/L PMSF, 10 μL of 100 mmol/L Na3VO4, 5 μL aprotinin (Sigma), and 2 μL of 10 mg/mL leupeptin). Whole cell lysates (25 μg) were separated by a 7.5% SDS-PAGE, and electrophoretically transferred to Immobilon PVDF membrane (Millipore, Bedford, MA). The membrane was immunoblotted with the following rabbit polyclonal antibodies: phosphospecific p70 S6 kinase (Thr421/Ser424) antibody (1:1000, rabbit polyclonal; New England Biolabs, Beverly, MA) and S6 kinase antibody (1:1000, rabbit polyclonal; New England Biolabs).
RESULTS
Expression of CXCR-4 receptors in hematopoietic cell lines and their BCR/ABL-transformed counterparts.
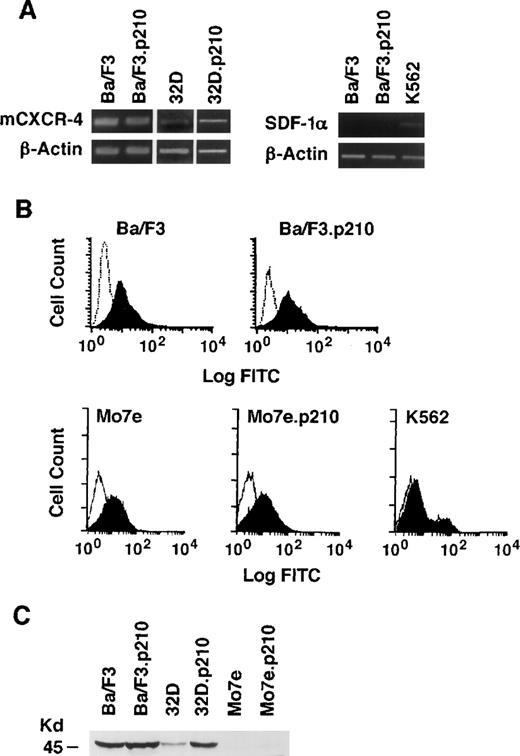
The expression of CXCR-4 and SDF-1α was determined by RT-PCR in the IL-3–dependent murine cell lines Ba/F3 (pre-B) and 32D (myeloid), before and after transformation to growth factor-independence by BCR/ABL (Fig 1A). Expression of CXCR-4 messenger RNA (mRNA) was detected in all cell lines tested. In addition, FACS analysis using a rabbit polyclonal antibody to murine CXCR-4 showed surface expression of CXCR-4 receptors (Fig 1B). Using an antihuman CXCR-4 antibody, surface expression of CXCR-4 was also found to be equivalent in the megakaryoblastic cell lines Mo7e and Mo7e.p210BCR/ABL (Fig 1B). SDF-1α expression was weakly detected in K562 cell lines by RT-PCR but not in the 2 murine cell lines. However, conditioned medium from K562 cells was unable to induce cytoskeletal changes or migration of Ba/F3 cells (data not shown), suggesting that there was no significant secretion of SDF-1α. CXCR-4 was also detected by immunoblotting in 32D and Ba/F3 cells and their BCR/ABL transformed counterparts using an antimurine CXCR-4 antibody (Fig 1C), which has a very low cross-reactivity with human cell lines.
Analysis of SDF-1 mRNA and CXCR-4 expression in murine and human cell lines with and without BCR/ABL. (A) The expression of SDF-1 and CXCR-4 mRNA was evaluated by RT-PCR in the murine pre-B cell line Ba/F3, the murine myeloid cell line 32D, and in their BCR/ABL-transformed counterparts. The human erythroleukemia cell line K562 was also evaluated. Primers for β-actin were used to equalize the amount of RT-products used. (B) Immunostaining with antibodies to murine CXCR-4 was performed with the Ba/F3 and Ba/F3.p210BCR/ABL cell lines (solid histogram). Background staining was determined using a nonspecific isotype matched IgG (clear histogram). Immunostaining with antibodies to human CXCR-4 was performed with the human Mo7e, Mo7e.p210BCR/ABLmegakaryocytic, and K562 cell lines (solid histogram). Background staining (clear histogram) was determined using a nonspecific isotype matched IgG. (C) Expression of murine CXCR-4 by immunoblotting of protein extracts prepared from Ba/F3, 32D, Mo7e, and their BCR/ABL counterparts. The molecular weight marker of 45 kD is shown and CXCR-4 has an approximate molecular weight of 48 kD.
Analysis of SDF-1 mRNA and CXCR-4 expression in murine and human cell lines with and without BCR/ABL. (A) The expression of SDF-1 and CXCR-4 mRNA was evaluated by RT-PCR in the murine pre-B cell line Ba/F3, the murine myeloid cell line 32D, and in their BCR/ABL-transformed counterparts. The human erythroleukemia cell line K562 was also evaluated. Primers for β-actin were used to equalize the amount of RT-products used. (B) Immunostaining with antibodies to murine CXCR-4 was performed with the Ba/F3 and Ba/F3.p210BCR/ABL cell lines (solid histogram). Background staining was determined using a nonspecific isotype matched IgG (clear histogram). Immunostaining with antibodies to human CXCR-4 was performed with the human Mo7e, Mo7e.p210BCR/ABLmegakaryocytic, and K562 cell lines (solid histogram). Background staining (clear histogram) was determined using a nonspecific isotype matched IgG. (C) Expression of murine CXCR-4 by immunoblotting of protein extracts prepared from Ba/F3, 32D, Mo7e, and their BCR/ABL counterparts. The molecular weight marker of 45 kD is shown and CXCR-4 has an approximate molecular weight of 48 kD.
SDF-1α induces actin cytoskeleton changes in untransformed, but not in BCR/ABL-transformed, hematopoietic cell lines.
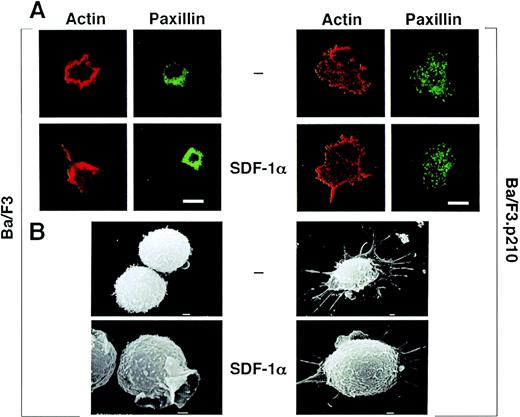
Ba/F3 and Ba/F3.p210BCR/ABL cells were treated with SDF-1α and examined for changes in cytoskeletal morphology and actin content by confocal microscopy. SDF-1α increased the content of F-actin in Ba/F3 cells and also induced a morphological change characterized by polarization, increased lamellipodia, filopodia, and uropod-like structures (Fig 2A). The focal adhesion protein paxillin was detected in lamellipodia upon stimulation with SDF-1α. F-actin and paxillin both form punctate (podosome-like) structures at sites of contact between the cell and the substratum in the BCR/ABL transformed cells, and no changes were appreciated after stimulation by SDF-1α. Similar morphological changes were observed by scanning electron microscopy (Fig 2B).
(A) Actin and paxillin staining, as visualized by confocal microscopy, of untransformed and BCR/ABL-transformed hematopoietic cells in response to SDF-1. Cells were fixed and stained for actin using rhodamine-labeled phalloidin, and for paxillin using indirect immunofluorescent staining with the antipaxillin monoclonal antibody 5H11. Note the difference in shape and staining pattern of actin and paxillin in response to SDF-1 of normal Ba/F3 cells. There is no change in shape or staining pattern in response to SDF-1 for BCR/ABL-transformed Ba/F3 cells. The bar is 10 μm. (B) Scanning electron microscopy of untransformed and BCR/ABL-transformed hematopoietic cells in response to SDF-1. Ba/F3 cells and BCR/ABL-transformed Ba/F3 cells were either unstimulated or stimulated with SDF-1 and scanning electron micrographs were taken. Shown is the ruffling of an untransformed Ba/F3 cell; BCR/ABL containing cells had numerous extensions but there was no change in response to SDF-1. The bar represents 1.0 U.
(A) Actin and paxillin staining, as visualized by confocal microscopy, of untransformed and BCR/ABL-transformed hematopoietic cells in response to SDF-1. Cells were fixed and stained for actin using rhodamine-labeled phalloidin, and for paxillin using indirect immunofluorescent staining with the antipaxillin monoclonal antibody 5H11. Note the difference in shape and staining pattern of actin and paxillin in response to SDF-1 of normal Ba/F3 cells. There is no change in shape or staining pattern in response to SDF-1 for BCR/ABL-transformed Ba/F3 cells. The bar is 10 μm. (B) Scanning electron microscopy of untransformed and BCR/ABL-transformed hematopoietic cells in response to SDF-1. Ba/F3 cells and BCR/ABL-transformed Ba/F3 cells were either unstimulated or stimulated with SDF-1 and scanning electron micrographs were taken. Shown is the ruffling of an untransformed Ba/F3 cell; BCR/ABL containing cells had numerous extensions but there was no change in response to SDF-1. The bar represents 1.0 U.
SDF-1α alters cell motility of untransformed hematopoietic cell lines, but not BCR/ABL-transformed cell lines.
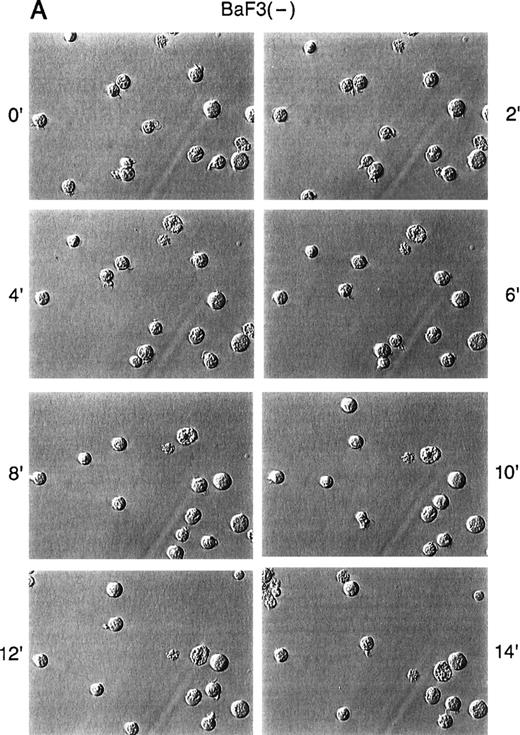
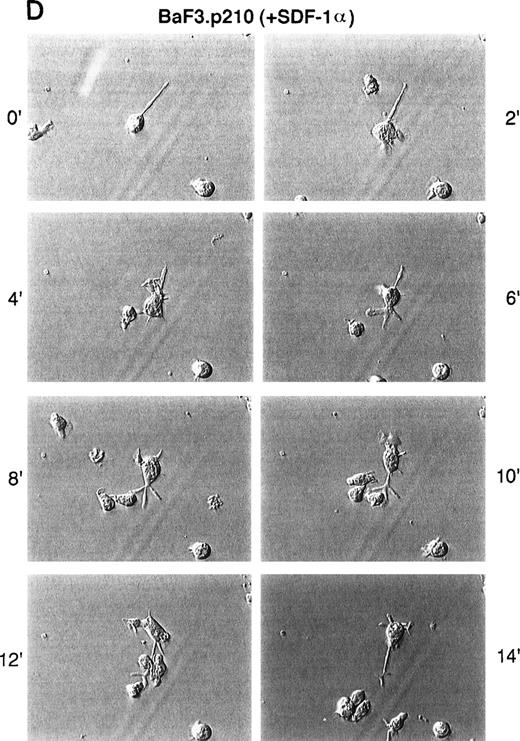
Because there is actin cytoskeleton rearrangement and altered adhesion in response to SDF-1α, we asked if SDF-1α also affects spontaneous in vitro motility of untransformed and BCR/ABL-transformed cell lines. Time-lapse video microscopy (TLVM) showed that unstimulated Ba/F3 cells exhibited a round morphology with little movement on a fibronectin-coated surface (Fig 3). In response to SDF-1α, however, Ba/F3 cells underwent a dramatic increase in spontaneous motility. BCR/ABL-transformed Ba/F3 cells, in contrast, constitutively exhibited a high degree of spontaneous motility in the absence of SDF-1α. In contrast to untransformed cells, these transformed cells did not further increase their spontaneous motility in response to SDF-1α. Similar results were observed comparing 32D and the 32D.p210BCR/ABL cells (data not shown).
Time-lapse video microscopy of untransformed and BCR/ABL transformed cells in response to SDF-1. As described in Materials and Methods, Ba/F3 (A/B) and BCR/ABL-transformed Ba/F3 (C/D) cells were visualized by time-lapse video microscopy, without (A/C) and with (B/D) SDF-1. Ba/F3 cells have increased membrane ruffling, microspikes, and formation of pseudopods in response to SDF-1. In contrast, Ba/F3.p210BCR/ABL cells had enhanced cell motility (as characterized by membrane ruffling, formation of pseudopods, formation of filopodia, and formation of uropod structures) with or without SDF-1.
Time-lapse video microscopy of untransformed and BCR/ABL transformed cells in response to SDF-1. As described in Materials and Methods, Ba/F3 (A/B) and BCR/ABL-transformed Ba/F3 (C/D) cells were visualized by time-lapse video microscopy, without (A/C) and with (B/D) SDF-1. Ba/F3 cells have increased membrane ruffling, microspikes, and formation of pseudopods in response to SDF-1. In contrast, Ba/F3.p210BCR/ABL cells had enhanced cell motility (as characterized by membrane ruffling, formation of pseudopods, formation of filopodia, and formation of uropod structures) with or without SDF-1.
BCR/ABL-transformed cell lines have a reduced chemotactic response to SDF-1α.
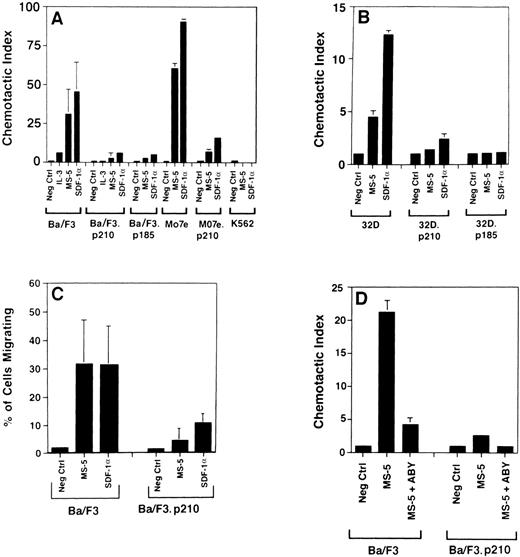
Using a transwell migration assay, the chemotactic index of Ba/F3, 32D, Mo7e cells, and their BCR/ABL-transformed counterparts was determined (Fig 4). Ba/F3 cells have a dramatic chemotactic response to either supernatant harvested from the murine bone marrow-derived stromal cell line MS-5 (as a source of SDF-1α) or recombi- nant SDF-1α, whereas BCR/ABL-transformed cells (both p185 and p210 forms of BCR/ABL) are less responsive. Similar results were obtained when comparing Mo7e cells and Mo7e.p210BCR/ABL cells. Untransformed 32D cells did not have a high chemotactic response to SDF-1α, but again, BCR/ABL-transformed 32D cells had an even lower response. Both the percent migrating cells and the chemotactic index were reduced in BCR/ABL-transformed cells (Fig 4C). Also, using a doxycycline-inducible BCR/ABL cell line, TonB210.1,15 we found that activation of BCR/ABL was associated with a reduced chemotactic response to SDF-1α (Fig 5). We found an average decrease of 2.9-fold in migration of doxycyline-induced BCR/ABL in the TonB210.1 cell line (n = 4). Uninduced TonB210.1 cells have a lower migratory response to SDF-1α compared with Ba/F3 cells, possibly because these cells express low levels of p210BCR/ABL even in the absence of doxycycline induction (data not shown).
Chemotaxis of murine and human cell lines to SDF-1 and MS-5 supernatant. Several cell lines were tested for their ability to migrate in transwell assays in response to SDF-1 (100 ng/mL) and MS-5 supernatant (undiluted). The number of cells that migrated to SDF-1 or MS-5 was divided by the number of background cells (cells that migrated to medium alone) to determine the chemotactic index, for panels A, B, and D. In panel C, the percentage of migrating cells is shown for comparison. In some columns, the standard deviations were too small to be visualized. Similarly, the chemotactic index of transformed K562 cells migrating to MS-5 supernatant and SDF-1 was below 1.0. Standard er- ror bars for the cell lines Ba/F3.p185BCR/ABL, Mo7e, Mo7e.p210BCR/ABL, K562, 32D.p210BCR/ABL, and 32D.p185BCR/ABL are calculated from triplicate values in single migration experiments, whereas the error bars for Ba/F3 and Ba/F3.p210BCR/ABL cells were calculated from data obtained in 6 and 4 migration experiments, respectively. In panel C, Ba/F3 cells transformed with p210 were tested and data from 4 migration experiments (2 per cell line) were pooled.
Chemotaxis of murine and human cell lines to SDF-1 and MS-5 supernatant. Several cell lines were tested for their ability to migrate in transwell assays in response to SDF-1 (100 ng/mL) and MS-5 supernatant (undiluted). The number of cells that migrated to SDF-1 or MS-5 was divided by the number of background cells (cells that migrated to medium alone) to determine the chemotactic index, for panels A, B, and D. In panel C, the percentage of migrating cells is shown for comparison. In some columns, the standard deviations were too small to be visualized. Similarly, the chemotactic index of transformed K562 cells migrating to MS-5 supernatant and SDF-1 was below 1.0. Standard er- ror bars for the cell lines Ba/F3.p185BCR/ABL, Mo7e, Mo7e.p210BCR/ABL, K562, 32D.p210BCR/ABL, and 32D.p185BCR/ABL are calculated from triplicate values in single migration experiments, whereas the error bars for Ba/F3 and Ba/F3.p210BCR/ABL cells were calculated from data obtained in 6 and 4 migration experiments, respectively. In panel C, Ba/F3 cells transformed with p210 were tested and data from 4 migration experiments (2 per cell line) were pooled.
Doxycycline-induced expression of BCR/ABL in TonB210.1 cells impairs chemotaxis to SDF-1. The Ba/F3 cell line, TonB210.1, which expresses BCR/ABL in the presence of doxycycline, was tested (n = 4) for a chemotactic response to medium alone (Neg Ctrl), MS-5 supernatant, and SDF-1 (100 ng/mL) using a transwell assay system. The open bars represent control cells (minus doxycycline) and solid bars represent doxycycline-treated cells. Panel A shows the percentage of migrating cells and panel B shows the chemotactic index.
Doxycycline-induced expression of BCR/ABL in TonB210.1 cells impairs chemotaxis to SDF-1. The Ba/F3 cell line, TonB210.1, which expresses BCR/ABL in the presence of doxycycline, was tested (n = 4) for a chemotactic response to medium alone (Neg Ctrl), MS-5 supernatant, and SDF-1 (100 ng/mL) using a transwell assay system. The open bars represent control cells (minus doxycycline) and solid bars represent doxycycline-treated cells. Panel A shows the percentage of migrating cells and panel B shows the chemotactic index.
To ensure that the response of hematopoietic cells to the MS-5 supernatant was due to SDF-1α, we determined the chemotactic index in the presence of an SDF-1α blocking antibody (Fig 4D). The chemotactic response of Ba/F3 cells to MS-5 supernatant was nearly completely abrogated by a blocking antibody, indicating that the chemotactic response of these cells in the transwell assay was due to the SDF-1α present in the MS-5 supernatant.
When Mo7e and Ba/F3 cell lines were pretreated for 4 hours with pertussis toxin (100 ng/mL), transwell migratory response to SDF-1α was markedly reduced (Mo7e: 47 % v 8 %; Ba/F3: 26% v6%, without or with pertussis toxin, respectively).
Homing of untransformed and BCR/ABL-transformed cells in response to SDF-1α, in vivo.
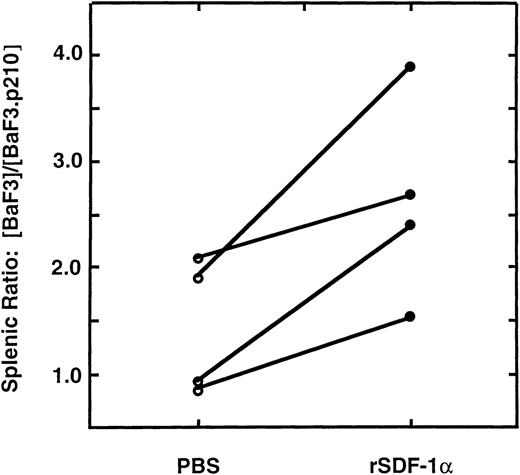
To test the in vivo homing of cells to SDF-1α, we injected SDF-1α (1 μg in 50 μL of PBS) or an equal volume of PBS into a subcapsular site in the spleens of anesthetized C57Bl/6 mice, and then injected Ba/F3 and Ba/F3.p210BCR/ABL cells intra-arterially. The Ba/F3 and Ba/F3.p210BCR/ABL cells were differentially-labeled with the fluorescent dyes, calcein-AM (green) or TRITC (red), respectively. Two-color flow cytometry was then used to quantitate these 2 populations in single-cell suspensions of different organs harvested 3 hours later. Both dye combinations were tested, with similar results found. Equal numbers of labeled wild-type and transformed cells (5 × 106 of each) were mixed and the pool was injected intra-arterially. After 3 hours, the spleen, bone marrow, peripheral blood and lungs were harvested and single-cell suspensions were prepared. There was a significant, 2.6 (± 1.0)-fold increase in the ratio of (Ba/F3: Ba/F3.p210BCR/ABL) cells found in the spleens of recipient mice (n = 4) injected with recombinant SDF-1α (P < .05), compared with mice injected with PBS (Fig6). No significant difference in the ratio of wild-type to BCR/ABL-transformed Ba/F3 cells was found in the peripheral blood, lung, or bone marrow (data not shown). In 2 of 4 experiments, the ratio of Ba/F3 to Ba/F3.p210BCR/ABL cells that homed to the spleen in the presence of PBS alone was greater than 1.0, which may be due to endogenous expression of SDF-1α by splenic stroma.8 Further studies for homing experiments using BCR/ABL and the various mutants of BCR/ABL would be useful.
Enhanced homing of Ba/F3 cells to the spleen in the presence of SDF-1, in vivo. The splenic ratio of differently-labeled, fluorescent Ba/F3 and Ba/F3.p210BCR/ABL cells was determined 3 hours after injection into mice. Recipient mice spleens were injected with PBS (○) or recombinant SDF-1 (1μg; ◍) immediately before infusion of pooled cells. Ba/F3 cells homed more efficiently to the spleen than Ba/F3.p210BCR/ABL cells in the presence of SDF-1 (P < .05). The lines connecting circles indicate paired mice in 4 independent experiments.
Enhanced homing of Ba/F3 cells to the spleen in the presence of SDF-1, in vivo. The splenic ratio of differently-labeled, fluorescent Ba/F3 and Ba/F3.p210BCR/ABL cells was determined 3 hours after injection into mice. Recipient mice spleens were injected with PBS (○) or recombinant SDF-1 (1μg; ◍) immediately before infusion of pooled cells. Ba/F3 cells homed more efficiently to the spleen than Ba/F3.p210BCR/ABL cells in the presence of SDF-1 (P < .05). The lines connecting circles indicate paired mice in 4 independent experiments.
The SDF-1α response of granulocytic and macrophage-colony forming progenitors (CFU-GM) is reduced in CML patient samples.
Using a transwell migration assay, we measured the total CFU-GM capacity of SDF-1α responsive-BM progenitors isolated from 3 normal donors and from 2 patients with untreated CML (Fig 7). Four peripheral blood samples from untreated CML patients were also tested. The results are presented as the percentage of CFU-GM formed by the migrating cell population compared to the total CFU-GM capacity of the input population (see Materials and Methods). The mean corrected ratios (percent migration minus background) obtained were 15.7 ± 3 for normal BM (n = 3), 5.5 for CML BM (n = 2), and 5.4 ± 3 for peripheral blood CML (n = 4) samples.
SDF-1 responsiveness, measured by CFU-GM, is reduced in CML patient samples. Chemotaxis assays were performed with mononuclear cells isolated from normal BM, BM from CML patients, and from peripheral blood samples from CML patients. CFU-GM are plotted as: (total number of colonies and clusters obtained with the migratory population)/(the number of colonies and clusters obtained with the input population) × 100 (see Materials and Methods). The stippled and solid bars represent CFU-GM formed by cells migrating to UltraCULTURE alone (control) or to 100 ng/mL of SDF-1, respectively.
SDF-1 responsiveness, measured by CFU-GM, is reduced in CML patient samples. Chemotaxis assays were performed with mononuclear cells isolated from normal BM, BM from CML patients, and from peripheral blood samples from CML patients. CFU-GM are plotted as: (total number of colonies and clusters obtained with the migratory population)/(the number of colonies and clusters obtained with the input population) × 100 (see Materials and Methods). The stippled and solid bars represent CFU-GM formed by cells migrating to UltraCULTURE alone (control) or to 100 ng/mL of SDF-1, respectively.
Overall, there is a significant, 2.7-fold decrease in the percentage of CFU-GM migrating in response to SDF1α from CML patient samples compared with normal donor BM (P < .01). This correlates well with the altered SDF-1α responses seen in our in vivo homing and in vitro transwell migration assays performed with cell lines. In selected experiments, the mononuclear cells used for chemotaxis were immunofluorescently stained with antibodies to CD34 and CXCR-4. The percentages of double-positive cells in two CML peripheral blood samples were 1.8% and 2.6%, compared with 0.7% in 1 normal BM. Variability between CML samples may reflect differences in expression of BCR/ABL or some other downstream signaling targets of BCR/ABL.
BCR/ABL reduces CXCR-4 signal transduction.
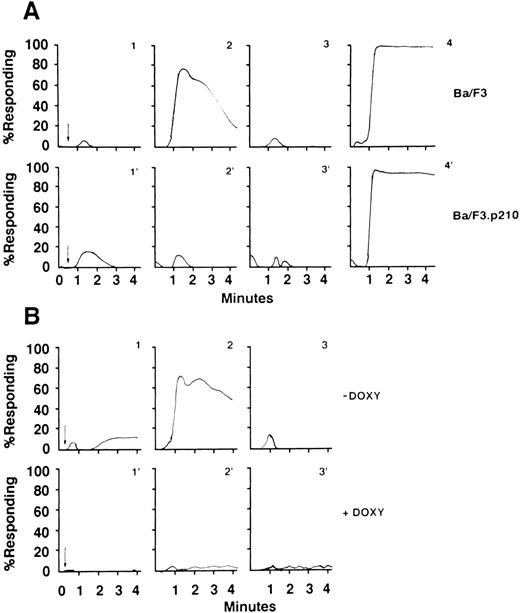
To assess the effects of BCR/ABL on CXCR4 signaling, we examined mobilization of intracellular calcium in response to SDF-1α (100 ng/mL, Fig 8) in hematopoietic cell lines before and after transformation by BCR/ABL. SDF-1α induced a rapid, transient flux of intracellular calcium in wild-type Ba/F3 (panel A, graph 2) and uninduced TonB210.1 cells (panel B, graph 2) but not in BCR/ABL-expressing Ba/F3 cells (panel A, graph 2′) or in the doxycycline-treated TonB210.1 cells (panel B, graph 2′). Positive responses to SDF-1α were blocked by preincubating the cell lines with pertussis toxin (panels A and B, graphs 3). Similar results were obtained in 32D and Mo7e cells before and after BCR/ABL transformation (data not shown). BCR/ABL-transformation of hematopoietic cell lines did not affect expression of CXCR-4 (Fig 1), but reduced SDF-1α–mediated calcium flux in each case.
Calcium flux analysis of untransformed and BCR/ABL-transformed hematopoietic cells in response to SDF-1. Analysis of calcium flux of Ba/F3 and BCR/ABL−transformed Ba/F3 cells, as described in Materials and Methods. Ba/F3, Ba/F3.p210BCR/ABL (panel A, graphs 1 and 1′, respectively), and TonB210.1 (with and without doxycycline, panel B, graphs 1 and 1′) had equal basal levels of calcium fluxes. When SDF-1 (100 ng/mL) was added to Ba/F3 (A, graph 2) and TonB210.1 (without doxycycline; B, graph 2), an increase flux of calcium was observed. Unlike the wild-type Ba/F3 and uninduced TonB210.1, the BCR/ABL-transformed cell line Ba/F3.p210BCR/ABL (A, graph 2′) and activated BCR/ABL TonB210.1 (with doxycycline; B, graph 2′) showed no calcium flux in response to SDF-1. Similar to the baseline levels of calcium fluxes of Ba/F3 and Ton B210.1, cells that were pretreated with pertussis toxin lacked a response to SDF-1 (A, graph 3 and B, graph 3, respectively). As a positive control for the Ba/F3 and Ba/F3.p210BCR/ABL cell lines (A, graphs 4 and 4′, respectively), we measured calcium fluxes in response to ionomycin (5 μg/mL). The arrow in each panel represents the time of addition of SDF-1.
Calcium flux analysis of untransformed and BCR/ABL-transformed hematopoietic cells in response to SDF-1. Analysis of calcium flux of Ba/F3 and BCR/ABL−transformed Ba/F3 cells, as described in Materials and Methods. Ba/F3, Ba/F3.p210BCR/ABL (panel A, graphs 1 and 1′, respectively), and TonB210.1 (with and without doxycycline, panel B, graphs 1 and 1′) had equal basal levels of calcium fluxes. When SDF-1 (100 ng/mL) was added to Ba/F3 (A, graph 2) and TonB210.1 (without doxycycline; B, graph 2), an increase flux of calcium was observed. Unlike the wild-type Ba/F3 and uninduced TonB210.1, the BCR/ABL-transformed cell line Ba/F3.p210BCR/ABL (A, graph 2′) and activated BCR/ABL TonB210.1 (with doxycycline; B, graph 2′) showed no calcium flux in response to SDF-1. Similar to the baseline levels of calcium fluxes of Ba/F3 and Ton B210.1, cells that were pretreated with pertussis toxin lacked a response to SDF-1 (A, graph 3 and B, graph 3, respectively). As a positive control for the Ba/F3 and Ba/F3.p210BCR/ABL cell lines (A, graphs 4 and 4′, respectively), we measured calcium fluxes in response to ionomycin (5 μg/mL). The arrow in each panel represents the time of addition of SDF-1.
We have previously determined that S6 kinase is rapidly phosphorylated in response to SDF-1α in Ba/F3 cells (R. Salgia et al, unpublished observation). As shown in Fig 9, S6 kinase is phosphorylated in response to SDF-1α in Ba/F3 cells and not Ba/F3.p210 cells. Thus, BCR/ABL blocks a very proximal CXCR-4 signaling event, calcium flux, and also blocks a downstream event, phosphorylation of S6 kinase. We have also determined that there is phosphorylation of paxillin in response to SDF-1α in Ba/F3 cells, but not in Ba/F3.p210 cells (data not shown).
Phosphorylation status of p70 S6 kinase in response to SDF-1. Immunoblot analysis of p70 S6 kinase phosphorylation of Ba/F3 and Ba/F3.p210 in response to SDF-1. SDF-1 induces serine 421/threonine 424 phosphorylation of p70 S6 kinase in normal Ba/F3 cells, but not in BCR/ABL-transformed cell lines. The same blot was stripped and probed with anti-S6 kinase showing that the amount of p70 S6 kinase is equivalent in all lanes.
Phosphorylation status of p70 S6 kinase in response to SDF-1. Immunoblot analysis of p70 S6 kinase phosphorylation of Ba/F3 and Ba/F3.p210 in response to SDF-1. SDF-1 induces serine 421/threonine 424 phosphorylation of p70 S6 kinase in normal Ba/F3 cells, but not in BCR/ABL-transformed cell lines. The same blot was stripped and probed with anti-S6 kinase showing that the amount of p70 S6 kinase is equivalent in all lanes.
DISCUSSION
Chronic myelogenous leukemia is caused by the t(9,22)(q34.1;q11.21) translocation that generates the BCR/ABL oncogene.20p210BCR/ABL is an active tyrosine kinase, and this increased kinase activity has been shown to be required for cell transformation. For normal hematopoietic cells, migration of myeloid cells from the marrow to the blood is tightly linked to differentiation, and in the absence of inflammation or infection myeloid cells stay in the marrow until they have fully matured to neutrophils. In contrast, CML myeloid cells circulate in large numbers in the blood at virtually all stages of differentiation, and it is clear that one of the defining characteristics of this illness is the uncoupling of differentiation from the ability to leave the marrow.21 In a variety of model systems, transformation of myeloid cells by BCR/ABL results in abnormal adhesion to the extracellular matrix component fibronectin, growth factor independence, decreased apoptosis, and cytoskeletal abnormalities (not in model systems).1 It is likely that the effects of BCR/ABL on cytoskeletal function, including altered adhesion and increased motility, contribute to early marrow exit and the subsequent myeloproliferation.
Recently, the chemokine SDF-1α has generated considerable interest because of its potential role in progenitor cell homing to the marrow and in regulating marrow egress.22 In vitro, SDF-1α is one of the few known cytokines that has chemotactic activity for hematopoietic progenitor cells and, in vivo, ectopic injection of SDF-1α can temporarily attract immature hematopoietic cells to the injection site.8 Homozygous deletion of the SDF-1α gene results in a lack of myelopoiesis and lymphopoiesis in the developing fetal liver and marrow.11 It is not known if this is due to impaired migration of cells from one developing hematopoietic site to another or to impaired expansion. More recently, it was shown that homozygous deletion of CXCR-4 results in an identical phenotype.12 13 In addition, defects in vascularization, cardiac septal development and cerebellar development were found. It is now believed that CXCR-4 is the primary physiological receptor for SDF-1α. CXCR-4 is expressed in the endothelium of developing blood vessels within the embryo proper, and its absence results in a lack of branching of the mesenteric vessels at day 13.5 of gestation. By day 16.5, hemorrhages and/or areas of congestion were found in the small intestine of mutant embryos. Overall, the available evidence suggests that SDF-1α and CXCR-4 play important roles in establishing and maintaining cellular homeostasis in the marrow, and disruption of this pathway can lead to aberrant homing and/or retention of progenitor cells.
In this study, we show that 3 nontransformed hematopoietic cell lines express CXCR-4 and respond to SDF-1α with increased cell motility, adhesion, and chemotaxis. In contrast, after BCR/ABL-transformation, the response to SDF-1α is significantly diminished. It would be quite interesting to perform the same studies of transwell migration after coating the membranes with other extracellular matrix components as well as transendothelial and trans-stromal migration on the various cells tested. There is decreased calcium flux in response to SDF-1α in BCR/ABL-transformed cells, suggesting that CXCR-4 signaling may be blocked at a very proximal level. The decrease in calcium flux is greater than the inhibition of chemotaxis in BCR/ABL-containing cells. However, the association between calcium flux and chemotaxis is not well understood because there are cells that migrate in response to chemokines without a calcium flux. We show here that distal signaling molecules such as S6 kinase involved in signal transduction by SDF-1α are also affected by BCR/ABL. The results presented here significantly expand the range of cytoskeletal abnormalities already associated with CML.
BCR/ABL is partially localized to the cytoskeleton and phosphorylates several cytoskeletal proteins, including paxillin, CRKL, vinculin, FAK, and tensin. Primary CML cells and cell lines transformed by BCR/ABL have altered adhesion to fibronectin and have abnormal adhesion to stromal cells. These effects are believed to be related to abnormal integrin function, and can be partially reversed by exposure to α-interferon.4 Furthermore, we have shown that CML cells have increased spontaneous motility on fibronectin coated surfaces in vitro, and an increased rate of ruffling and formation of pseudopods.7 The cell motility of CML cells is strikingly increased with an increased adhesiveness to fibronectin. The increased cell motility may aid in the release of progenitors from the stroma. These results also suggest that BCR/ABL has profound effects on the cytoskeleton. In the case of SDF-1α, reduced response may be due to both inhibition of specific signaling from the SDF-1α receptor and more global defects in the cell's ability to regulate cytoskeletal functions.
In contrast to progenitor cells, neutrophils from CML patients have relatively normal function and can clearly accumulate appropriately at sites of inflammation or infection.23 However, the expression of BCR/ABL protein in CML neutrophils is relatively low compared with that in progenitor cells, and the ability to follow a chemotactic gradient of formylated peptides, for example, may represent a different biological event than after a SDF-1α gradient. In this regard, it is interesting that CML neutrophils fail to respond to at least 2 other chemokines, MIP-1α and MCP-1.24 These effects are minimal or absent in CML progenitor cells. MIP-1α–mediated increases in cytosolic calcium levels have also been shown to be abrogated by BCR/ABL.25 MIP-1α binds to a different chemokine receptor than does SDF-1α. MIP-1α null mutant mice, however, do not have viability or bone marrow defects. The results presented show that CXCR-4 is the second chemokine receptor whose function is altered by BCR/ABL.
Recently, Lapidot et al26 presented data that SDF-1α and CXCR-4 were required for bone marrow engraftment by human CD34+ progenitor cells in NOD/SCID mice. They further defined the phenotype of engrafting cells as CD34+/CD38-/low /CXCR-4+, and showed that stem-cell factor induced surface expression of CXCR-4 on CD34+ cells. Integrins such as LFA-1, VLA-4, and VLA-5 were also required for marrow homing.27
Additional studies suggest that SDF-1α is involved in retention of B-lymphoid and granulocytic progenitors within normal mouse marrow.28 Ma et al28 reconstituted irradiated wild-type mice with fetal liver cells from CXCR-4–deficient mice. They found reduced numbers of granulocytes in BM, but elevated and less mature myeloid cells in the periphery. These findings correlate well with the hypothesis that reduced responsiveness to SDF-1α contributes to the release of CML progenitors from the BM. Our results with CML samples are unlikely to be due to diminished proliferative capacity of CFU-GM progenitors, as CML progenitors have the same proliferative capacity as normal progenitors.29
From our data we can generate a hypothesis to explain why CML cells fail to be retained in the marrow, unlike normal cells, and thereby circulate in high numbers in the blood and accumulate in other tissues. SDF-1α is constitutively expressed by bone marrow stroma,30 where it may regulate the adherence of progenitors to stromal layers by activating adhesion molecules.31 Oncogenic transformation by BCR/ABL may then accelerate proliferation, increase spontaneous motility, and reduce long-term adhesiveness to stromal cells by reducing the number, accessibility, or function of adhesion-related molecules, such as CXCR-4. These changes may then aid progenitors in their exit from the marrow as the cellularity increases. We also have preliminary evidence that egress of cells from intact newborn murine femurs can occur, in vitro, under the influence of distally located MS-5 adherent cells (data not shown), suggesting that even in the absence of blood flow, bone marrow cells are capable of releasing themselves from normal intact stroma in response to a chemotactic gradient. In summary, the defect in migration to SDF-1α may represent 1 step in the complex pathway of events that are needed to transform cells by BCR/ABL and result in CML. Also, because at least 2 chemokine receptors are now shown to be inhibited by BCR/ABL, this suggests that BCR/ABL blocks a signaling step common to general chemokine receptors.
ACKNOWLEDGMENT
The authors thank Ms Li Zhang and Dr Yuhui Xu of the Core EM Facility at the Dana-Farber Cancer Institute for their help in scanning electron microscopy. Also, we thank Herb Levine, FACS Core Facility, DFCI for his help in calcium flux assays.
R.S. and E.Q. contributed equally to this work.
Supported by National Institutes of Health Grants No. CA 75348 (R.S.), DK 560654 (J.D.G.), Jose Carreras International Leukemia Foundation fellowship FIJC-95/INT (M.S.), and the Pfizer Scholars Grant for new faculty (E.Q.)
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal