Abstract
Wiskott Aldrich syndrome (WAS) is an X-linked recessive disorder associated with abnormalities in platelets and lymphocytes giving rise to thrombocytopenia and immunodeficiency. WAS is caused by a mutation in the gene encoding the cytoskeletal protein (WASp). Despite its importance, the role of WASp in platelet function is not established. WASp was recently shown to undergo tyrosine phosphorylation in platelets after activation by collagen, suggesting that it may play a selective role in activation by the adhesion molecule. In the present study, we show that WASp is heavily tyrosine phosphorylated by a collagen-related peptide (CRP) that binds to the collagen receptor glycoprotein (GP) VI, but not to the integrin 2β1. Tyrosine phosphorylation of WASp was blocked by Src family kinase inhibitors and reduced by treatment with wortmannin and in patients with X-linked agammaglobulinemia (XLA), a condition caused by a lack of functional expression of Btk. This indicates that Src kinases, phosphatidylinositol 3-kinase (PI 3-kinase), and Btk all contribute to the regulation of tyrosine phosphorylation of WASp. The functional importance of WASp was investigated in 2 WAS brothers who show no detectable expression of WASp. Platelet aggregation and secretion from dense granules induced by CRP and thrombin was slightly enhanced in the WAS platelets relative to controls. Furthermore, there was no apparent difference in morphology in WAS platelets after stimulation by these agonists. These observations suggest that WASp does not play a critical role in intracellular signaling downstream of tyrosine kinase-linked and G protein-coupled receptors in platelets.
WISKOTT ALDRICH syndrome (WAS), resulting from mutations in the WAS protein (WASp), is an X-linked recessive disorder associated with severe thrombocytopenia, eczema, immunodeficiency (recurrent infection), and an increased susceptibility to lymphoid malignancies. The clinical and immunological course of the disease varies from one patient to another. In its most mild form, it is known as X-linked thrombocytopenia and patients have minimal impairment of their immune response.1
WASp is composed of 502 amino acids and has a molecular weight of 64 kD.2 Its expression is limited to hematopoietic cells, where it is found in all lineages and at all stages of development.3 WASp can be divided into a number of protein domains. It has a pleckstrin homology (PH domain) at its N-terminus; a WASp homology (WH) domain, WH1; a G protein binding domain (GBD; also known as CDC42/Rac interactive binding region [CRIB]); a proline-rich domain; a second WH domain, WH2; a verprolin-like sequence; a cofilin homology sequence; and an acidic region. WASp lacks enzymatic activity, and its major role is thought to be as a scaffold or adapter protein.
WASp has been shown to interact with a number of important signaling proteins, although the significance of these interactions is not clear. WASp interacts with the SH3 domain of a number of proteins in vitro, including Btk, Cbl, Fgr, Lyn, and phospholipase Cγ1 (PLCγ1).4-7 However, a reduced number of interactions have been shown to occur in vivo, including binding to the adapters Nck8,9 and Grb210 and to the tyrosine kinases Fyn5 and Btk.7
Mounting evidence suggests that WASp is involved in the regulation of the cytoskeleton downstream of members of the Rho family of small molecular weight G proteins. WASp has been shown to interact directly with Cdc42, a member of the Rho family of G proteins, via its GBD domains.11 The overexpression of WASp leads to formation of extended clusters of WASp-rich particles that are highly enriched in polymerized actin.11 WASp has also been shown to interact in vivo with the cytoskeletal associated proteins PSTPTP,12WIP,13,14 and the Arp2/3 complex.15 WIP has been shown to induce actin polymerization in lymphoid cells through association with the actin binding protein profilin. The Arp2/3 complex, which comprises 7 proteins, is thought to be a major regulator of actin polymerization.16 Other members of the WASp family of proteins, namely N-WASp, WAVE, and the recently discovered Scar proteins, have also been shown to regulate the cytoskeleton.17-19
More than 100 mutations in WASp have been reported. These are found throughout the length of the molecule, although the majority occur in the N-terminal portion covering the PH and WH1 domains; however, there is no specific grouping of mutations to suggest the loss of a particular function of the protein. Instead, a number of reports have shown a correlation between the presence or absence of WASp and the clinical phenotype.20-22 For example, Zhu et al20 determined WASp gene mutations in 48 unrelated WAS families and showed that mutations that permitted WASp expression, albeit at a reduced level, caused mild disease, whereas mutations that resulted in classic WAS were associated with a lack of protein. A more recent study has shown that 8 different mutations resulted in lack of expression of WASp in peripheral mononuclear cells and in B-lymphoblastoid cell lines, at least to a level of less than 0.5% of that found in normal donors, and were associated with classical WAS.22 The lack of protein expression may be the major cause of classic WAS, rather than the expression of a mutated form of WASp.
The absence of WASp results in the alteration of responses in a number of cell types. WASp appears to play an important role in signaling downstream of the T-cell antigen receptor. There is decreased proliferation of WAS T cells in response to antigen challenge,23 and a similar result has been reported in T cells from WASp-deficient mice.24 The role of WASp in B cells is unclear, because both normal and defective responses have been reported. For example, in WASp-knockout mice, B-cell function is normal.24 It has been suggested that the lack of WASp may result in disturbances in cell motility of neutrophils and macrophages, which may contribute to the immunopathology of WAS.25
WAS platelets are characterized by a reduction in cell number (thrombocytopenia) and size. Bleeding disorders such as intestinal intraluminal bleeding are often described in WAS patients.9,26 After splenectomy, platelet number and size return towards normal levels.27 The number of reticulated (young) platelets is also relatively normal in WAS.28 In a recent report, it has been shown that, despite some cytoskeletal defects in WAS megakaryocytes, their ability to produce platelets is not affected.29 Together, these results suggest that the major defect in WAS is increased platelet removal by the spleen rather than impaired production. However, a reduction in density of surface proteins such as GPIIb-IIIa and GPIV is also seen in WAS platelets, suggesting that there may be a defect in development.28
A number of early studies reported defective platelet responses, notably aggregation, in WAS platelets to a number of agonists, including collagen, ADP, adrenaline, and thrombin.30-34However, other studies reported normal platelet aggregation in WAS patients as estimated from changes in optical transmission after the addition of ADP, adrenaline, and collagen.35 These studies were performed in the absence of a detailed understanding of the mechanism of signaling by these agonists and before the genetic basis of WAS was known. This may explain their inconclusive and contradictory nature. It is therefore necessary to reassess the response of WAS-deficient platelets in light of this increased knowledge. For example, a recent study was unable to confirm the defect in aggregation to ADP in WAS platelets, although that to thrombin was reduced.28
Tyrosine phosphorylation of WASp was recently reported in platelets in response to collagen,36 in mast cells stimulated by FcεRI,37 and B cells stimulated through the B-cell antigen receptor.7 These 3 sets of stimuli signal through a similar pathway that involves tyrosine phosphorylation of an immunoreceptor tyrosine-based activation motif (ITAM), the tyrosine kinase Syk, and PLCγ2. The functional consequence of tyrosine phosphorylation of WASp is not known. In the present study, we have investigated the mechanism of tyrosine phosphorylation of WASp in platelets stimulated by the collagen receptor, glycoprotein (GP) VI. In addition to collagen, we have used a collagen-related peptide (CRP) that activates GPVI but is unable to bind the collagen adhesion receptor, the integrin α2β1.38 39 The role of WASp in platelets has been investigated through the study of platelets from 2 WAS brothers with the same genetic defect that results in a lack of detectable expression of the protein.
MATERIALS AND METHODS
Reagents.
A CRP [GCP*(GPP)10GCP*G; single amino acid code P* = hydroxyproline; the monomer is cross-linked through the N- and C-terminals] was cross-linked via cysteine residues as described previously38; CRP was kindly donated by Drs M. Barnes, R.W. Farndale, and G. Knight (Department of Biochemistry, Cambridge University, Cambridge, UK). Collagen (native collagen fibrils from equine tendons) was from Nycomed (Munich, Germany). FcγRII specific monoclonal antibody (MoAb) was purchased from Medarex Inc (Annandale, NJ). Sheep F(ab′)2 raised against mouse IgG (M-1522) and thrombin were purchased from Sigma (Poole, Dorset, UK). Monoclonal antiphosphotyrosine antibody 4G10 and p85 anticortactin polyclonal antibody was purchased from Upstate Biotechnology (TCS Biologicals Ltd, Botolph Claydon, Bucks, UK). GST-Grb2 and GST-PLC-γ2-SH3 fusion proteins were expressed in bacteria as previously described.40,41 GST-Btk-SH3 fusion protein construct was a kind gift from Dr C. Kinnon (Institute of Child Health, University College London, London, UK). WASp monoclonal and polyclonal antibodies were raised as described.42 Annexin V-fluorescein isothiocyanate (FITC) was purchased from Pharmingen (Becton Dickinson, Oxford, UK). PP1 (4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine)43 and PD173956 were kinds gifts from Dr J. Hanke (Pfizer Central Research, Groton, CT) and Dr A.J. Kraker (Parke-Davis, Ann Arbor, MI), respectively. FITC-annexin V was from Becton Dickinson (Oxford, UK). [3H]5-hydroxytryptamine (5-HT) was from New England Nuclear (Herts, UK). Other reagents were from previously described sources or of analar grade.
Platelet preparation and stimulation.
Human platelets were isolated from blood taken on the day of experiment using citrate as anticoagulant. Platelet-rich plasma was collected after centrifugation at 200g for 20 minutes, pooled, and, after the addition of prostacyclin (100 nmol/L), recentrifuged at 1,000g for 10 minutes. The platelet-poor plasma was discarded and the platelet pellet was resuspended in 20 mL of Tyrodes-HEPES buffer (134 mmol/L NaCl, 0.34 mmol/L Na2HPO4, 2.9 mmol/L NaHCO3, 20 mmol/L HEPES, 5 mmol/L glucose, and 1 mmol/L MgCl2, pH 7.3) containing 1 mmol/L EGTA and 10 μmol/L indomethacin, as described in the text. Prostacyclin was added (100 nmol/L) and the platelet suspension was centrifuged for a further 10 minutes. The supernatant was discarded and platelets were resuspended at a concentration of 4 × 108 cells/mL, unless stated (see studies on WAS platelets). Experiments were performed at 37°C in an aggregometer (Chrono-Log Corp, Havertown, PA) with continuous stirring at 1,200 rpm. Stimulation of platelets with CRP, collagen, and thrombin was performed at 37°C for the times shown. Platelets were stimulated via FcγRIIA using MoAb IV.3 (1 μg/mL) for 1 minute and then the cross-linker F(ab′)2 antimouse IgG (30 μg/mL) for 90 seconds.
For studies involving measurement of 5-HT secretion, platelets were labeled in platelet-rich plasma with [3H]5-HT (1 μCi/mL) for 60 minutes. The secretion of [3H]5-HT was measured as previously described.44
Flow cytometry analysis of annexin V binding.
Platelets (2.5 × 106 cells) were stimulated by CRP or thrombin for 3 minutes at room temperature in a volume of 100 μL in Tyrodes-HEPES buffer. Ca2+ (1 mmol/L) was present at every stage. Platelets were incubated with annexin V-FITC for 10 minutes. The final volume was adjusted to 500 μL by the addition of Tyrodes-HEPES buffer and analyzed by flow cytometry using a Becton Dickinson FACScan flow cytometer. Excitation was at 488 nm, with emission measured at 530 nm. Ten thousand events were analyzed per sample. Platelets were gated and results were presented as a percentage of cells positive for annexin V.
Patients.
Blood was taken from 2 brothers with WAS or patients with X-linked agammaglobulinemia (XLA) on the day of the experiment. All patients denied having taken aspirin in the preceding 2 weeks. The work was performed with parental consent and approval of the Central Oxford Research Ethics Committee.
The study on the WAS brothers was completed over a period of 2 years and by the end of this time the boys were 12 and 14 years of age. The oldest was born by Caesarian section (breech presentation) and was found to have bruising, petichiae, and jaundice at birth. He had persistent thrombocytopenia with bruising, but no major bleeding. His infection was a severe episode of bacterial tonsillitis at the age of 18 months for which he was admitted to hospital. He had recurrent otitis media requiring insertion of grommets at 4 years of age. The diagnosis of WAS was made when he was 5 years of age. He began receiving prophylactic cotrimoxazole 1 year later and intravenous immunoglobulin at 7 years of age; he has remained free of bacterial infections since this time. Splenectomy was performed in 1993 for recurrent bruising and epistaxes. His platelet count increased from 20 × 109/L to a resting level of 120 × 109/L. He has had only small infrequent patches of eczema. In view of his family history, his brother had his platelet count measured at birth; this was low, and an initial diagnosis of autosomal recessive congenital thrombocytopenia was made. He had recurrent bruising and minor epistaxes throughout childhood. He developed otitis media and chest infections at 10 months of age. WAS was diagnosed at the same time as for his elder brother. Infections were prevented by prophylactic cotrimoxazole and intravenous Ig as described above. Unlike his older brother, he was referred at 6 years of age for investigation of developmental delay with autistic features and, although no definite diagnosis was made, fragile X syndrome has been ruled out. He also had a splenectomy with similar effect in 1993, after which he had an episode of fever, cervical lymphadenopathy, and a presumptive diagnosis of Epstein-Barr virus infection from which he made a quick recovery. His platelet count is similar to that of his older brother. He remains well and has had only occasional small patches of eczema. The level of expression of WASp in the brothers was measured by Western blotting in peripheral mononuclear cells and in B-lympho- blastoid cell lines. There was no detectable expression. The limit of this assay is 0.5% of that in normal donors. Because of the small vol- ume of blood that could be taken from WAS donors, experiments were performed on a platelet concentration of between 0.7 and 2.0 × 108/mL.
Platelets were taken from 3 different donors with XLA. Although the mutations that give rise to the XLA syndrome have not been identified in these patients, none was found to express Btk in their platelets as measured by Western blotting. We have previously reported that the platelets from all 3 of these donors show impaired activation by collagen and CRP.45
Scanning electron microscopy.
Scanning electron microscopy was performed as described.46Basal or stimulated platelets (300 μL), at a concentration of 5 × 107 platelets/mL, were mixed with an equal volume of 4% glutaraldehyde in 0.15 mol/L NaCl, 50 mmol/L phosphate buffer, pH 7.4 (prewarmed to 37°C). The platelets were collected with gentle suction onto 0.6-μm pore size polycarbonate filters (Whatman, Maidstone, UK) that had been prerinsed with 2% glutaraldehyde in 0.15 mol/L NaCl buffered to pH 7.4 in 50 mmol/L phosphate buffer. Filters were transferred to vials and rinsed once with 0.15 mol/L NaCl and twice with distilled water for removal of glutaraldehyde. Dehydration of filters was accomplished by washing with 10%, 25%, 50%, 75%, 95%, and 100% ethanol. The filters were subjected to critical point drying (on a Polaron CPD7501 critical point drier; Agar Scientific Ltd, Stansted, UK), coated with gold (using a Nanotech Semprep 2 sputter coater; Emitech Ltd, Ashford, UK), and analyzed on a Philips 515 scanner (FEI UK Ltd, Cambridge, UK).
Immunoprecipitation, GST precipitation, and immunoblotting.
Platelets were lysed with an equal volume of lysis buffer (2% NP-40, 300 mmol/L NaCl, 20 mmol/L Tris, 10 mmol/L EDTA, 2 mmol/L Na3VO4, 1 mmol/L phenylmethylsulphonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 μg/mL pepstatin A, pH 7.3). Insoluble cell debris was removed by centrifugation. Cell lysates were precleared with glutathione-agarose or protein A-sepharose for GST precipitation and immunoprecipitation, respectively. For GST precipitation, lysates were incubated with 5 to 10 μg of fusion protein immobilized on agarose. Endogenous WASp was immunoprecipitated using 5 μL of anti-WASp polyclonal antibody. Resulting protein complexes and immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. Immunoblotting was performed using an MoAb to WASp,42 with detection by enhanced chemiluminescence (ECL; Amersham, Bucks, UK).
RESULTS
WASp is tyrosine phosphorylated in CRP-stimulated platelets.
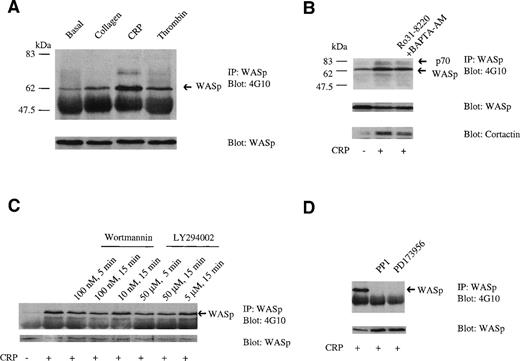
Although collagen has recently been reported to stimulate tyrosine phosphorylation of WASp in platelets, the surface receptor mediating this effect is not known. We have addressed this in the present study using the GPVI-selective ligand CRP that does not bind to the collagen adhesion receptor α2β1.38,39WASp is weakly tyrosine phosphorylated under basal conditions and undergoes a marked increase in phosphorylation after stimulation by CRP (3 μg/mL; Fig 1A). In comparison, collagen (10 μg/mL) and thrombin (1 U/mL) induce a much lower increase in tyrosine phosphorylation. Cross-linking of FcγRIIA induces an increase in tyrosine phosphorylation similar to that induced by collagen (not shown). Oda et al36 reported that collagen induced a greater increase in tyrosine phosphorylation of WASp relative to thrombin, with the latter inducing only marginal phosphorylation. We observed a reproducible increase in phosphorylation of WASp in response to thrombin in all studies, although concentrations of collagen similar to those used by Oda et al36 consistently gave a slightly larger response than that to the protease. The response to collagen was always smaller than that to CRP, which is a more powerful stimulus of protein tyrosine phosphorylation in platelets, eg, Asselin et al.39
WASp is phosphorylated on tyrosine in platelets; effect of inhibitors. (A) Tyrosine phosphorylation of WASp in stimulated platelets. Western blots of WASp immunoprecipitates (10% SDS-PAGE) were probed with antiphosphotyrosine (4G10; upper panel) and, after stripping of the blot, anti-WASp antibodies (lower panel). WASp was tyrosine phosphorylated under basal conditions (lane 1) and underwent an increase in phosphorylation after stimulation by collagen (10 μg/mL) for 90 seconds (lane 2); CRP (3 μg/mL) for 90 seconds (lane 3); and thrombin (1 U/mL) for 30 seconds (lane 4). (B) The effect of inhibition of PLC activity on tyrosine phosphorylation of WASp. Western blots of WASp immunoprecipitates were probed with antiphosphotyrosine (4G10; upper panel) and, after stripping of the blot, anti-WASp (middle panel) and anticortactin (lower panel) antibodies. Basal conditions are shown in lane 1 and platelets stimulated with CRP (3 μg/mL, 90 seconds) are shown in lane 2. No significant effect on tyrosine phosphorylation induced by CRP occurred when platelets were preincubated with 5 μmol/L Ro31-8220 for 5 minutes and 40 μmol/L BAPTA-AM for 5 minutes (lane 3). (C) The effect of PI 3-kinase inhibitors on tyrosine phosphorylation of WASp. (D) The effect of tyrosine kinase inhibitors on phosphorylation of WASp. In (C) and (D), conditions are as in (A), with the exception that the probe for cortactin is not shown. One experiment is shown that is representative of 3.
WASp is phosphorylated on tyrosine in platelets; effect of inhibitors. (A) Tyrosine phosphorylation of WASp in stimulated platelets. Western blots of WASp immunoprecipitates (10% SDS-PAGE) were probed with antiphosphotyrosine (4G10; upper panel) and, after stripping of the blot, anti-WASp antibodies (lower panel). WASp was tyrosine phosphorylated under basal conditions (lane 1) and underwent an increase in phosphorylation after stimulation by collagen (10 μg/mL) for 90 seconds (lane 2); CRP (3 μg/mL) for 90 seconds (lane 3); and thrombin (1 U/mL) for 30 seconds (lane 4). (B) The effect of inhibition of PLC activity on tyrosine phosphorylation of WASp. Western blots of WASp immunoprecipitates were probed with antiphosphotyrosine (4G10; upper panel) and, after stripping of the blot, anti-WASp (middle panel) and anticortactin (lower panel) antibodies. Basal conditions are shown in lane 1 and platelets stimulated with CRP (3 μg/mL, 90 seconds) are shown in lane 2. No significant effect on tyrosine phosphorylation induced by CRP occurred when platelets were preincubated with 5 μmol/L Ro31-8220 for 5 minutes and 40 μmol/L BAPTA-AM for 5 minutes (lane 3). (C) The effect of PI 3-kinase inhibitors on tyrosine phosphorylation of WASp. (D) The effect of tyrosine kinase inhibitors on phosphorylation of WASp. In (C) and (D), conditions are as in (A), with the exception that the probe for cortactin is not shown. One experiment is shown that is representative of 3.
A tyrosine phosphorylated band of 70 kD that was resolved as a doublet in some experiments coimmunoprecipitated with WASp in CRP-stimulated platelets, but was absent in collagen-stimulated samples, possibly because it is a weaker stimulus. This band was shown to contain cortactin by reprobing with a specific antibody (Fig 1B). Immunoprecipitation of cortactin from platelets confirmed that CRP stimulates an increase in tyrosine phosphorylation of cortactin (not shown). A very low level of association of cortactin with WASp was detected under basal conditions that increased upon stimulation by CRP, suggesting that the interaction is dependent on tyrosine phosphorylation of at least 1 of the 2 proteins. This interaction may be similar to that observed between WASp and PSTPIP, because cortactin and PSTPIP show homology in the location and sequence of their SH3 and proline-rich regions.
CRP was used in further biochemical studies in preference to collagen, because it gives a stronger, more reproducible increase in tyrosine phosphorylation of WASp and is also selective to GPVI. CRP induced rapid tyrosine phosphorylation of WASp and this was maintained for up to 600 seconds (not shown). Tyrosine phosphorylation of WASp was detected within 30 seconds, making it one of the earliest proteins to show an increase in phosphorylation upon stimulation by CRP, suggesting that it may play an early role in CRP-induced signaling. A similar time course of tyrosine phosphorylation of WASp by CRP was observed by monitoring its association to GST-PLCγ2-SH3 (see later).
All of the phosphorylation studies were performed in the presence of indomethacin to prevent formation of thromboxanes. The effect of additional inhibitors on tyrosine phosphorylation of WASp by CRP was examined to further investigate the basis of its regulation. Tyrosine phosphorylation of WASp by CRP was not altered significantly in the presence of both Ro 31-8220 and BAPTA-AM, which together prevent the action of the second messengers, 1,2-diacylglycerol/protein kinase C and inositol 1,4,5-trisphosphate/Ca2+, demonstrating that it is independent of activation of phospholipase C (Fig 1B). The ADP scavenger, apyrase, and RGDS, which inhibits activation of the fibrinogen receptor, GPIIb-IIIa, did not alter tyrosine phosphorylation of WASp. Maximally effective concentrations of the 2 structurally distinct inhibitors of phosphatidylinositol 3-kinase (PI 3-kinase), wortmannin and LY294002, gave a partial but incomplete reduction in tyrosine phosphorylation of WASp (Fig 1C). Submaximal concentrations or shorter incubations with wortmannin and LY294002 gave a lower level of inhibition (Fig 1C). In contrast, tyrosine phosphorylation of WASp was completely inhibited under stimulated conditions in the presence of the 2 structurally distinct Src family kinase inhibitors PP1 (10 μmol/L) and PD173956 (10 μmol/L; Fig 1D). It is not clear from this study whether Src kinases play a direct role in phosphorylation of WASp, because they also have a critical early role in signaling by GPVI upstream of phosphorylation of Fc receptor γ-chain.47 48
Oda et al36 reported complete inhibition of WASp phosphorylation by collagen in the presence of wortmannin, which is in contrast to the observation given above that tyrosine phosphorylation of WASp by CRP is only partially reduced in the presence of wortmannin and LY294002. However, we were also unable to confirm the observation of Oda et al36 that tyrosine phosphorylation of WASp induced by collagen is completely inhibited in the presence of wortmannin (n = 4; not shown).
WASp associates with PLCγ2, Btk, and Grb2.
WASp has been shown to bind to the SH3 domains of a number of proteins through its proline-rich region in vitro. In the present study, we show that this can be extended to the SH3 domain of PLC-γ2, the major isoform of PLC-γ in platelets. GST-PLCγ2-SH3, the fusion protein for the SH3 domain of PLC-γ2, associates with 2 major tyrosine phosphorylated bands of 125 and 64 kD and 2 minor bands of 70 and 130 kD in CRP-stimulated platelets (Fig 2A). The tyrosine phosphorylated bands are detected 30 seconds after stimulation with CRP and phosphorylation is maintained for 30 minutes (Fig 2A). The 64-kD protein was shown to contain WASp by immunoblotting. Because a similar level of WASp associates with GST-PLCγ2-SH3 in basal and CRP-stimulated platelets, the interaction is not regulated by tyrosine phosphorylation of WASp.
Association of WASp with Btk, PLCγ2, and Grb2. Lysate from resting or CRP (3 μg/mL)-stimulated platelets were incubated with GST linked to the SH3 domains of Btk (10 μg) and PLCγ2 (5 μg) and full-length Grb2 (10 μg). Proteins were separated on 10% SDS-PAGE and electroblotted to PVDF membranes. Membranes were immunoblotted using the antiphosphotyrosine MoAb 4G10 (upper panel). Membranes were stripped and reprobed with anti-WASp MoAb (lower panel). (A) Time course of WASp association to GST-PLCγ2-SH3. Several tyrosine phosphorylated proteins bind to GST-PLCγ2-SH3 but not to GST (90-second time point shown) from CRP-stimulated platelets. The 64-kD band was shown to contain WASp by stripping the blot and reprobing. (B) WASp association to GST-Btk-SH3. (C) WASp association to GST-Grb2. The gels are representative of 3 to 5 experiments.
Association of WASp with Btk, PLCγ2, and Grb2. Lysate from resting or CRP (3 μg/mL)-stimulated platelets were incubated with GST linked to the SH3 domains of Btk (10 μg) and PLCγ2 (5 μg) and full-length Grb2 (10 μg). Proteins were separated on 10% SDS-PAGE and electroblotted to PVDF membranes. Membranes were immunoblotted using the antiphosphotyrosine MoAb 4G10 (upper panel). Membranes were stripped and reprobed with anti-WASp MoAb (lower panel). (A) Time course of WASp association to GST-PLCγ2-SH3. Several tyrosine phosphorylated proteins bind to GST-PLCγ2-SH3 but not to GST (90-second time point shown) from CRP-stimulated platelets. The 64-kD band was shown to contain WASp by stripping the blot and reprobing. (B) WASp association to GST-Btk-SH3. (C) WASp association to GST-Grb2. The gels are representative of 3 to 5 experiments.
WASp has been reported to bind to the SH3 domains of Btk in vitro6 and to be a substrate for the tyrosine kinase when overexpressed in baby hamster kidney (BHK-21) cells.37 In the present study, we have investigated whether tyrosine phosphorylation of WASp interferes with its ability to interact with the Btk SH3 domain. GST-Btk-SH3 precipitated a tyrosine phosphorylated band of 64 kD in resting platelets that, along with a second band of 75 kD, underwent a marked increase in tyrosine phosphorylation upon stimulation by CRP (Fig 2B). WASp was identified as a component of the 64-kD phosphotyrosyl band by immunoblotting and was present at a similar level in resting and stimulated platelets. The interaction with GST-Btk-SH3 is therefore not altered by tyrosine phosphorylation of WASp.
Oda et al36 recently observed that binding of WASp to GST-Grb2 is reduced in platelets stimulated by collagen. This was also observed in the present study in CRP-stimulated platelets (Fig 2C). The reduction in binding suggests that Grb2 has a decreased affinity for tyrosine phosphorylated WASp. This may reflect an important physiological role of tyrosine phosphorylation of WASp in the platelet. A number of other tyrosine phosphorylated proteins associate with GST-Grb2 in CRP-stimulated platelets, including bands of 120, 75, 53, 36, and 30 kD (Fig 2C).
Regulation of tyrosine phosphorylation of WASp.
WASp has recently been shown to be a potential substrate for the tyrosine kinases Btk and Lyn but not Syk. Coexpression of Btk or Lyn in BHK-21 cells leads to an increase in tyrosine phosphorylation of WASP and both tyrosine kinases coimmunoprecipitated with the cytoskeletal protein, with the interaction with Btk being dependent on its SH3 domain.37
To address whether WASp is a substrate for Btk in platelets, studies were performed on cells from patients with the immunodeficiency syndrome, XLA, caused by mutation of the gene encoding Btk. This approach differs from that of Guinamard et al37 in that the role of an endogenous kinase is investigated rather than an enzyme that has been introduced through transfection and may therefore be present at a nonphysiological level.
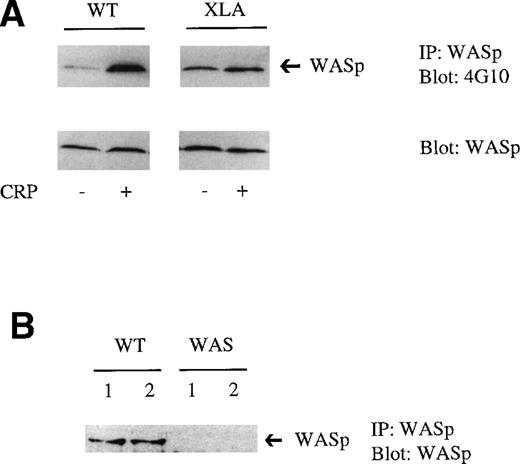
In XLA platelets, tyrosine phosphorylation of WASp was slightly elevated in nonstimulated cells relative to controls. CRP stimulated a small increase in tyrosine phosphorylation of WASp in XLA platelets, although this was considerably weaker than in controls (Fig 3A). This suggests that Btk, and probably a second kinase, lies upstream of WASp phosphorylation in CRP-stimulated platelets, but that phosphorylation under basal conditions is independent of Btk. Candidates for the second kinase include members of the Src family of tyrosine kinases, of which several are expressed in platelets, including Lyn and Fyn, and Tec, another member of the Btk family expressed in platelets.
Tyrosine phosphorylation of WASp in XLA platelets stimulated with CRP. (A) WASp was immunoprecipitated from resting or CRP (3 μg/mL)-stimulated platelets. Proteins were resolved on 10% SDS-PAGE, transferred, and immunoblotted using the antiphosphotyrosine MoAb 4G10 (upper panel). Reprobing with the anti-WASp MoAb confirmed that a similar level of WASp was present in control and XLA platelets (bottom panel). One experiment is shown that is representative of 3. (B) WASp was immunoprecipitated from resting platelets from control (WT) and WAS patients. Proteins were resolved on 10% SDS-PAGE, transferred, and immunoblotted using the WASp MoAb.
Tyrosine phosphorylation of WASp in XLA platelets stimulated with CRP. (A) WASp was immunoprecipitated from resting or CRP (3 μg/mL)-stimulated platelets. Proteins were resolved on 10% SDS-PAGE, transferred, and immunoblotted using the antiphosphotyrosine MoAb 4G10 (upper panel). Reprobing with the anti-WASp MoAb confirmed that a similar level of WASp was present in control and XLA platelets (bottom panel). One experiment is shown that is representative of 3. (B) WASp was immunoprecipitated from resting platelets from control (WT) and WAS patients. Proteins were resolved on 10% SDS-PAGE, transferred, and immunoblotted using the WASp MoAb.
Studies in WAS platelets.
To investigate the role of WASp in platelets after cross-linking of GPVI, a series of studies were performed on 2 brothers who have been shown to have a missense mutation in which valine 75 is substituted by a methionine, resulting in the absence of detectable expression (to a level less than 0.5% of controls) of WASp in B cells.22Neither brother had a detectable level of WASp as measured by Western blotting using a specific antibody (Fig 3B). There was no apparent difference in response between the platelets from the 2 brothers.
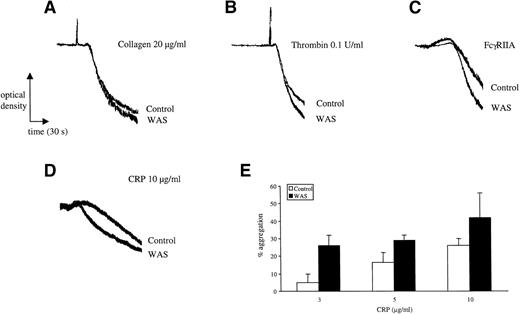
Although a number of studies have been published describing impairment in aggregation in WAS platelets to a number of platelet agonists, including collagen, there is a need for confirmation of these findings, because the majority of these studies were performed before the cloning of WASp and the establishment of the mechanism of platelet activation by the majority of cell surface receptors (see introduction). Platelets were challenged with collagen, CRP, thrombin, and FcγRIIA cross-linking and responses were compared with those of volunteer donors. All platelets were prepared in the same way and suspended at the same concentration. Five separate studies were performed on the 2 sets of WASp-deficient platelets in comparison to platelets from 8 normal donors and also from the boys' mother. The pattern of shape change and aggregation was similar in all cases, although the rate of response to all agonists was slightly increased in the WAS donors for all 4 agonists (Fig 4). Potentiation of aggregation was seen over the length of the concentration response curves for CRP (Fig 4E) and thrombin (not shown).
Aggregation in WAS platelets. Platelets from control and WAS patients were prepared as described in Materials and Methods. Care was taken to ensure that platelets were prepared in exactly the same way and used at the same concentration. The experiments in (A) through (C) were performed on the same day in the absence of indomethacin at a platelet concentration of 2 × 108/mL. Collagen is unable to stimulate aggregation in the presence of indomethacin; similar results were observed for thrombin in the presence of indomethacin. The experiments in (D) and (E) were performed on a separate donor in the presence of indomethacin and at a lower platelet concentration of 0.7 × 108/mL. The mean aggregation over the length of the concentration response curve to collagen was measured. The results are representative of between 2 and 5 separate experiments.
Aggregation in WAS platelets. Platelets from control and WAS patients were prepared as described in Materials and Methods. Care was taken to ensure that platelets were prepared in exactly the same way and used at the same concentration. The experiments in (A) through (C) were performed on the same day in the absence of indomethacin at a platelet concentration of 2 × 108/mL. Collagen is unable to stimulate aggregation in the presence of indomethacin; similar results were observed for thrombin in the presence of indomethacin. The experiments in (D) and (E) were performed on a separate donor in the presence of indomethacin and at a lower platelet concentration of 0.7 × 108/mL. The mean aggregation over the length of the concentration response curve to collagen was measured. The results are representative of between 2 and 5 separate experiments.
Platelet activation is associated with the movement of phosphatidylserine from the inner leaflet of the plasma membrane to the outer layer, forming a catalytic surface to support coagulation reactions (procoagulant activity). Annexin V has high affinity and a strict specificity for aminophospholipids at physiological Ca2+ concentrations, enabling it to be used in flow cytometry for measurement of procoagulant activity when conjugated to FITC. The magnitude of response and concentration response relationships for thrombin and CRP were similar in the control and WAS-platelets (Fig 5A). Similar studies could not be performed with collagen because of the interference of adhesion with flow cytometry.
Aminophospholipid exposure and 5-HT secretion in WAS platelets. Platelets from control and WAS patients were prepared as described in Materials and Methods. Care was taken to ensure that platelets were prepared in exactly the same way and used at the same concentration. Studies were performed on the 2 WAS platelets in comparison with platelets from 2 controls. Data have been pooled and are shown as the mean ± range. (A) Procoagulant activity. Platelets were stimulated with CRP or thrombin for 3 minutes and annexin V binding was measured by flow cytometry as described in Materials and Methods. The number of positive cells is shown in the y-axis; (B) 5-HT secretion. Platelets were stimulated with CRP (90 seconds) or thrombin (30 seconds) and 5-HT secretion was measured as described in Materials and Methods. The results are representative of 3 experiments.
Aminophospholipid exposure and 5-HT secretion in WAS platelets. Platelets from control and WAS patients were prepared as described in Materials and Methods. Care was taken to ensure that platelets were prepared in exactly the same way and used at the same concentration. Studies were performed on the 2 WAS platelets in comparison with platelets from 2 controls. Data have been pooled and are shown as the mean ± range. (A) Procoagulant activity. Platelets were stimulated with CRP or thrombin for 3 minutes and annexin V binding was measured by flow cytometry as described in Materials and Methods. The number of positive cells is shown in the y-axis; (B) 5-HT secretion. Platelets were stimulated with CRP (90 seconds) or thrombin (30 seconds) and 5-HT secretion was measured as described in Materials and Methods. The results are representative of 3 experiments.
WASp-deficient platelets were analyzed for dense granule secretion by prelabeling with [3H]5-HT. The concentration response curve to CRP was similar in control and WAS platelets, although secretion was slightly enhanced in the latter group (Fig 5B). A similar result was seen for the G protein receptor agonist thrombin (Fig 5B and data not shown). WAS platelets were also measured for α-granule secretion by measurement of P-selectin expression by flow cytometry after stimulation with CRP and thrombin. The maximal response to CRP and thrombin was reduced by approximately 40% in the WASp-deficient platelets (not shown), although this may be a consequence of the smaller size of the WAS platelets rather than of a change in reactivity.
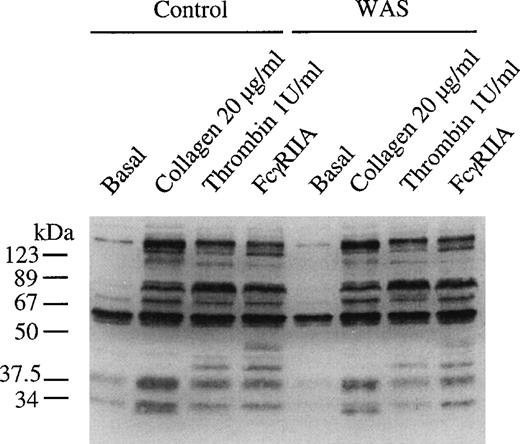
The pattern of increase in protein tyrosine phosphorylation in whole cell lysates was similar in control and WAS platelets challenged with collagen, FcγRIIA, and thrombin (Fig 6). A similar result was seen in platelets stimulated with CRP (not shown). This approach only monitors gross changes in protein tyrosine phosphorylation and could have missed changes in phosphorylation of specific proteins. Although it is beyond the scope of this study to immunoprecipitate all of the proteins that undergo increases in tyrosine phosphorylation upon platelet activation, it is noteworthy that there was no apparent alteration in tyrosine phosphorylation of PLCγ2 in CRP-stimulated platelets (not shown). This result is in contradiction to the observation of Simon et al49 of reduced tyrosine phosphorylation of PLCγ1 in transformed B lymphocytes from patients with WAS.
Tyrosine phosphorylation of total cell protein is not altered in WAS platelets. WASp-deficient platelets and control platelets were incubated in Tyrodes-HEPES buffer and stimulated by the addition of collagen (3 μg/mL) for 90 seconds and thrombin (1 U/mL) for 60 seconds and through cross-linking of FcγRIIA for 90 seconds with 1 μg/mL MoAb IV.3 for 60 seconds, followed by the addition of F(ab′)2 (30 μg/mL). Stimulation was stopped by the addition of equal volume of 2× loading buffer. Proteins were separated by 10% SDS-PAGE, electroblotted onto PVDF membranes, and then immunoblotted using the antiphosphotyrosine MoAb 4G10. The same concentration of platelets (1 × 108/mL) was used in samples from the WAS patients and controls. The gel is representative of 2 experiments.
Tyrosine phosphorylation of total cell protein is not altered in WAS platelets. WASp-deficient platelets and control platelets were incubated in Tyrodes-HEPES buffer and stimulated by the addition of collagen (3 μg/mL) for 90 seconds and thrombin (1 U/mL) for 60 seconds and through cross-linking of FcγRIIA for 90 seconds with 1 μg/mL MoAb IV.3 for 60 seconds, followed by the addition of F(ab′)2 (30 μg/mL). Stimulation was stopped by the addition of equal volume of 2× loading buffer. Proteins were separated by 10% SDS-PAGE, electroblotted onto PVDF membranes, and then immunoblotted using the antiphosphotyrosine MoAb 4G10. The same concentration of platelets (1 × 108/mL) was used in samples from the WAS patients and controls. The gel is representative of 2 experiments.
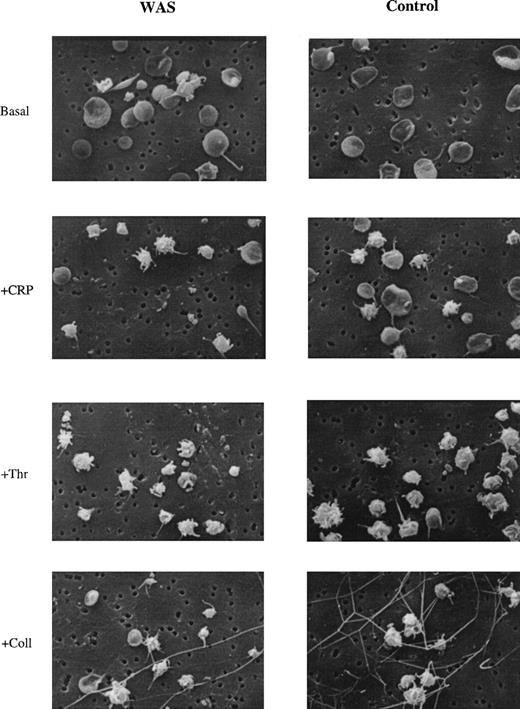
A possible role of WASp in cytoskeletal remodeling was investigated using scanning electron microscopy in platelets that had been stimulated by collagen, CRP, and thrombin. No major morphological differences were observed between normal and WAS platelets, with cells showing characteristic rounding and formation of pseudopodia (Fig 7).
Formation of filopodia in WASp-deficient platelets. The presence of filopodia on control and platelets stimulated with CRP (5 μg/mL), thrombin (1 U/mL), and collagen (30 μg/mL) from WAS and control donors was assessed by scanning electron microscopy (SEM). Original magnification × 5,200. Results are representative of 6 independently analyzed fields.
Formation of filopodia in WASp-deficient platelets. The presence of filopodia on control and platelets stimulated with CRP (5 μg/mL), thrombin (1 U/mL), and collagen (30 μg/mL) from WAS and control donors was assessed by scanning electron microscopy (SEM). Original magnification × 5,200. Results are representative of 6 independently analyzed fields.
DISCUSSION
Collagen activates platelets through a tyrosine kinase-linked pathway that shares many features with signaling by immune receptors. This has lead to the realization that the adhesion molecule is a unique platelet agonist in that it induces activation of platelets through a mechanism distinct from that used by other agonists at sites of damage to the vasculature. In consideration of this, we investigated the ability of collagen and a CRP that is selective to GPVI to stimulate tyrosine phosphorylation of a number of proteins in platelets. We show in this study that WASp undergoes tyrosine phosphorylation after stimulation by CRP to a much greater extent than induced by the G protein-coupled receptor agonist thrombin. Oda et al36 have also recently published that WASp is selectively phosphorylated by collagen in platelets. This observation suggested the possibility that WASp may play a unique role in platelet activation by collagen. The present study was therefore undertaken to investigate the role and regulation of WASp in platelets stimulated by ligation of the collagen receptor GPVI in comparison with results obtained in platelets stimulated by thrombin.
We have characterized the mechanism of regulation of tyrosine phosphorylation of WASp in human platelets stimulated by CRP using a variety of pharmacological inhibitors and by studies on platelets from patients deficient in the tyrosine kinase Btk. We were unable to extend this work to murine platelets from knockout animals to take advantage of these genetically modified cells, because WASp undergoes little or no increase in tyrosine phosphorylation in platelets of this species after stimulation by CRP (not shown). GPVI receptor signaling is believed to be mediated by activation of a Src family kinase, probably Fyn or Lyn, which leads to phosphorylation of the Fc receptor γ-chain.47,48 The observation that the Src family kinase inhibitors PP1 and PD173956 blocked the tyrosine phosphorylation of WASp is consistent with its regulation downstream of GPVI. We observed partial inhibition of tyrosine phosphorylation of WASp in the presence of the PI 3-kinase inhibitors Ly294002 and wortmannin. This is in contrast to the results obtained by Oda et al,36 who observed complete inhibition; the explanation for this is not known. A marked reduction in tyrosine phosphorylation of WASp in XLA platelets was also observed, demonstrating that this event is mediated downstream of Btk and suggesting that WASp is a substrate for the Tec family kinase. Guinamard et al37 and Baba et al7 have also recently shown that WASp is a substrate for Btk downstream of FcεRI and B-cell antigen receptors, respectively, both of which signal via an ITAM. Btk has a PH domain that has high selectivity for the product of PI 3-kinase, phosphatidylinositol 3,4,5-trisphosphate. The inhibition of PI 3-kinase activity has been shown to cause a partial inhibition of Btk activation (Quek and Watson, unpublished observation), suggesting that WASp may be regulated downstream of PI 3-kinase through Btk.
The functional role of WASp in GPVI receptor signaling and platelet function was investigated by studies in 2 WAS brothers who share the same genetic defect. In contrast to earlier results, the present studies did not show impairments in aggregation to a number of agonists, including collagen, CRP, and thrombin. The only differences seen between WASp platelets and those of controls was a slightly increased rate of aggregation and dense granule secretion in response to all agonists. The increase in these responses in the WAS platelets may be analogous to the effect of low concentrations of the actin polymerization inhibitor, cytochalasin. Previous studies have suggested that incubation of platelets with a low dose of cytochalasin before the addition of phorbol ester resulted in the increased secretion of 5-HT.50 Furthermore CRP-, collagen-, and thrombin-induced aggregation was enhanced by a low concentration of cytochalasin (unpublished observation). The pattern of protein tyrosine phosphorylation was similar in WASp-deficient donors in response to GPVI activation, including tyrosine phosphorylation of PLCγ2.
There was also no major difference in overall morphology of the WASp platelets upon stimulation by collagen or thrombin, although a more detailed analysis of the cytoskeletal remodeling may show subtle alterations. The absence of major cytoskeletal defects in WAS platelets may be due to functional redundancy between WASp family proteins. In particular, N-WASp has been shown to be expressed in a number of tissues17 and is capable of inducing filopodia production18 and, like WASp, actin polymerization via an interaction with the Arp2/3 complex.51
In conclusion, this study has shown that, in platelets, WASp is tyrosine phosphorylated downstream of the collagen receptor GPVI, but not the G protein-coupled thrombin receptor via a pathway that involves PI 3-kinase and the tyrosine kinase Btk. However, this study has failed to find evidence for a specific role of WASp in aggregation, dense granule secretion, and shape change, suggesting that tyrosine phosphorylation does not appear to confer a unique role on WASp in GPVI receptor signaling in these responses.
ACKNOWLEDGMENT
The authors are grateful to Nicky Brennan and Nicola Salome-Bentley, specialist immunology nurses, for their considerable help with the patient samples; to Jenny Corrigan (Department of Zoology, University of Oxford) for final preparation and analysis of scanning electron microscopy samples; to Prof Adrian Gear (University of Virginia) for help in setting up the scanning electron microscopy procedure; to Dr Jean-max Pasquet for help with the flow cytometry studies; and to Dr A. Thresher and Prof P. Brickell for useful discussions. The Department of Molecular Immunology, Institute of Child Health, London, kindly performed the initial WAS protein and gene studies.
B.S.G. and J.I.W. contributed equally to this work.
Supported by the Wellcome Trust and British Heart Foundation (BHF). S.P.W. is a BHF Senior Research Scientist. B.S.G. is a Wellcome Prize Student. L.Q. holds a BHF Studentship.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Steve P. Watson, PhD, Department of Pharmacology, University of Oxford, Mansfield Road, Oxford OX1 3QT, UK; e-mail: steve.watson@pharm.ox.ac.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal