Abstract
The effects of vinblastine (VBL) on endothelial cell functions involved in angiogenesis, namely proliferation, chemotaxis, spreading on fibronectin (FN), secretion of matrix-metalloproteinase-2 (MMP-2) and MMP-9, and morphogenesis on Matrigel were tested in vitro, whereas its effects on angiogenesis were studied in vivo by using the chick embryo chorioallantoic membrane (CAM) model. In vitro, at noncytotoxic doses (0.1, 0.25, 0.5, 0.75, and 1 pmol/L), VBL impacted all these functions, except secretion of MMPs, in a dose-dependent fashion. By contrast, proliferation of other primary cells such as fibroblasts and lymphoid tumor cells was not impacted. In vivo, VBL at 0.5, 0.75, and 1 pmol/L again displayed a dose-dependent antiangiogenic activity. Lack of cytotoxicity in vitro and in vivo was shown both morphologically, and also because the antiangiogenic effects were rapidly abolished when VBL was removed. Apoptosis was not induced. At the ultrastructural level, impairment of cell functions in vitro was associated with thin disturbance of the cytoskeleton, in the form of slight depolymerization and accumulation of microfilaments, which was equally reversible. Results suggest that VBL has an antiangiogenic component at very low, noncytotoxic doses, and that antiangiogenesis by VBL could be used to treat a wide spectrum of angiogenesis-dependent diseases, including certain chronic inflammatory diseases, Kaposi's sarcoma, and cancer.
THE VINCA ALKALOID vinblastine (VBL) is a high-charge density cation that binds with high affinity to tubulin, an anionic protein of the cytoskeleton microtubules, and gives rise to a salt-like precipitate. In this way, it prevents their polymerization, and hence the mitotic spindle formation (in which they are involved), and leads to cytotoxicity and cell necrosis.1, 2 VBL is mainly applied for the treatment of hematologic tumors (mainly of lymphoid origin) and some childhood solid tumors.3 There is evidence that VBL treatment results in vascular toxicity in the form of asymptomatic lesions of arterioles, thrombotic microangiopathy, Raynaud's phenomenon, or acute arterial events4, 5 related to cytotoxicity and/or necrosis of endothelium, with exposure of subendothelial matrix and consequent platelet activation, disturbance of the clotting system, and vasculitis.6 VBL's vascular toxicity is also thought to have an indirect cytostatic effect on tumors,2, 6, 7 since their growth is dependent upon angiogenesis.8 It has, in fact, been shown to cause early patchy necrosis of tumor cells closely associated with cytotoxic damage and necrosis of the vascular endothelial cells in the experimental animal.9, 10
This report examines the effects of VBL on human vascular endothelial cells. At very low doses, it strikingly and reversibly affects certain cell functions in vitro strictly correlated with angiogenesis and angiogenesis itself in vivo, without nonspecific cytotoxic or necrotic damage. Very thin, equally reversible disturbances of microfilament polymerization were still evident at the ultrastructural level.
MATERIALS AND METHODS
Source of vinblastine.
VBL (molecular weight = 811.00) as powder (Lilly France SA, Saint Cloud, Paris, France) was solubilized in phosphate-buffered saline (PBS) and diluted stepwise from 0.1 to 1 pmol/L with Dulbecco's modified Eagle's medium (DMEM; GIBCO, Life Technologies Ltd, Paisley, UK).
Cells and preparation of conditioned media.
Human umbilical vein endothelial cells (HUVEC) were prepared and grown as previously described.11 The vein luminal surface was exposed for 20 minutes at 37°C to 0.2% collagenase solution (Sigma Chemical Co, St Louis, MO) in PBS. After centrifugation, the recovered cells were washed in PBS and cultured in Petri dishes precoated with 1% gelatin (Sigma) in M199 medium (GIBCO) containing 10% heat-inactivated fetal calf serum (FCS; GIBCO), 0.02% bovine brain extract, and 0.01% porcine heparin (both from Sigma). The human endothelial-like immortalized cell line EA.hy926, derived from the fusion of HUVEC with the lung carcinoma cell line A549,12was maintained in DMEM containing 10% FCS and 1% glutamine. NIH 3T3 mouse embryo fibroblasts were obtained from the American Tissue Culture Collection (ATCC, Rockville, MD) and cultured in DMEM containing 10% FCS and 1% glutamine before reaching confluence. The Namalwa (Burkitt's lymphoma), LIK (B-cell lymphoblastic leukemia), and CEM (T-cell lymphoblastic leukemia) human cell lines were obtained from ATCC and cultured in RPMI-1640 medium (GIBCO) supplemented with 10% FCS and 1% glutamine.
The conditioned media (CM) of EA.hy926 and NIH 3T3 cells were prepared by incubating subconfluent cells in a T25 flask with 6 mL of serum-free DMEM (SFM) for 24 hours. The supernatant was then collected under sterile conditions, centrifuged sequentially at 1,200 and 12,000 rpm for 10 minutes to eliminate debris, and stored at −20°C. NIH 3T3 CM displays strong angiogenic capability both in vitro and in vivo, because it contains an array of angiogenic factors.13
Proliferation assay.
In the first series of experiments, HUVEC or EA.hy926 cells and NIH 3T3 cells or lymphoid tumor cells (Namalwa, LIK and CEM cells) were plated (2 × 103/well) in 96-well plates (Falcon 3072; Becton Dickinson, Mountain View, CA) in their complete medium containing 10% FCS. After 24 hours, the medium was removed and replaced on days 0 and 2 in quadruplicate by the same medium (positive control), by this medium supplemented with each VBL dose, or by this medium without FCS, ie, starvation (negative control). The cell number was estimated on day 4 by the colorimetric assay of Kueng et al14; cells were fixed for 15 minutes at room temperature with 2.5% glutaraldehyde, stained with 0.1% crystal violet in 20% methanol for 20 minutes, solubilized with 10% acetate, and read at 595 nm in a Microplate Reader 3550 (Bio-Rad Lab, Richmond, CA). The cell number was derived from a calibration curve set up with a known number of cells, and calculated as mean ± 1 standard deviation (SD) per medium.
In the second series, HUVEC or EA.hy926 cells were exposed to control media and VBL doses every 24 hours once, twice, and three times, and counted as above.
Chemotaxis assay.
This was performed in Boyden chambers as previously described.15 HUVEC or EA.hy926 cells pretreated for 24 hours with each VBL dose in complete medium were harvested in trypsin/acetate (0.05/0.02% in PBS), collected by centrifugation, resuspended in DMEM supplemented with 0.1% bovine serum albumin (BSA), and seeded in triplicate for each dose and control in the upper compartment of the chamber (1.2 × 105 cells/400 μL), whereas the lower compartment was filled with 200 μL of the NIH 3T3 CM as chemoattractant (positive control), or with DMEM supplemented with 0.1% BSA in the negative control (to evaluate random migration). The compartments were separated by a polycarbonate filter (12 μm pore/size; Nucleopore, Costar Co, Cambridge, MA) coated with 0.005% gelatin to allow cell adhesion. After incubation for 6 hours in a humidified 5% CO2 atmosphere at 37°C, cells on the upper side of the filter were removed, whereas those that had migrated to the lower side were fixed in absolute ethanol, stained with toluidine blue, counted in 5 to 8 160 immersion fields, and calculated as mean ± 1 SD per filter and per medium.
Adhesion assay on fibronectin (FN).
After 96-well plates were coated with a FN solution (20 μg/mL) at 4°C overnight, HUVEC were plated (5 × 103/well) in triplicate in starvation medium alone (positive control) or containing each VBL dose for 90 minutes at 37°C in 5% CO2 humidified atmosphere, as described.15Cells were fixed with glutharaldeyde 2.5% in PBS at 30 and 90 minutes and their number was calculated as described for the proliferation assay.
Morphogenesis assay on Matrigel.
Unpolymerized Matrigel (17 mg/mL; Collaborative Biomedical Products, Two Oaks, Park Bedford, MA) was placed in the wells (300 μL/well) of a 24-well microtiter plate (1.28 cm2/well) and allowed to polymerize for 1 hour at 37°C. HUVEC or EA.hy926 cells were plated (2 × 105 cells/well) in 1 mL of SFM containing 50% NIH 3T3 CM (positive control), to which VBL doses were added in experimental wells. After 6 hours of incubation17 in a 5% CO2 humidified atmosphere at 37°C, cell growth and three-dimensional organization were observed through a reverted, phase-contrast photomicroscope. Cells plated in SFM alone served as the negative control.
MMP-2 and MMP-9 sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) zymography and activity.
T25 flasks of EA.hy926 cells at 90% confluence were rinsed twice with SFM and incubated for 24 hours in this medium either alone (positive control) or containing each VBL dose. The CM were then collected and the total protein content was measured by absorbance with the Bradford method (Bio-Rad), using BSA as a standard protein.
To visualize the gelatinolytic activity of the secreted MMP-2 and MMP-9, an SDS-PAGE zymography was performed.17 Briefly, aliquots of 5 μg CM proteins were applied to 7.5% SDS-PAGE gels co-polymerized with type A gelatin from porcine skin (Sigma) at a final concentration of 0.6 mg/mL (0.06% wt/vol). After electrophoresis in a Protean II dual lab system (Bio-Rad), the gels were washed in 2.5% Triton 1 × for 1 hour to remove SDS, incubated for 18 hours at 37°C, and stained in 0.1% Coomassie brilliant blue. The gelatinolytic regions were observed as white bands against a blue background. MMP activity was measured by scoring the intensity of bands by computerized image analysis (Apple Computer Inc, Cupertino, CA).
Evaluation of apoptosis of HUVEC.
The fluorescent propidium iodide (PI) stains weakly the subdiploid DNA produced during apoptosis, whereas it stains strongly the diploid DNA of nonapoptotic cells. The percentage of apoptotic HUVEC was thus detected by measurement of weak PI staining, as described elsewhere.18 HUVEC were plated (2 × 106/well) in 78.5 cm2 Petri dishes (Falcon 3003; Becton Dickinson) in complete medium containing 10% FCS. After 24 hours, the medium was removed and replaced on days 0 and 2 by the same medium (negative control) or by this medium containing VBL 0.25 pmol/L or 1 pmol/L, or dexamethasone (Merck Co, Inc, West Point, PA) 1 mmol/L, an apoptogenic drug.19 On day 4, cells harvested in trypsin/acetate (0.05/0.02% in PBS) were treated for 3 hours with 70% ethanol at 4°C, then incubated overnight with 100 μL PI in the presence of RNAse, and tested in a flow cytometry (FACScan, Becton Dickinson) for the magnitude of the subdiploid DNA fluorescent peak (M1 region) in comparison with that of diploid DNA peak (M2 region).
Morphologic study.
HUVEC or EA.hy926 cells were plated (1 × 104/well) in 96-well plates in DMEM supplemented with 10% FCS, allowed to adhere until confluence was reached, and exposed to each VBL dose for 6, 8, and 10 days. A daily morphologic analysis was performed directly with a reverted, phase-contrast photomicroscope, and after the fixation and staining as described in the proliferation assay.
Chorioallantoic membrane (CAM) assay.
Fertilized White Leghorn chicken eggs (10/group) were incubated at 37°C at constant humidity. On incubation day 3, a square window was opened in the shell and 2 to 3 mL of albumen was removed to allow detachment of the developing CAM. The window was sealed with a glass and the eggs were returned to the incubator. On day 8, 1 mm3 gelatin sponges (Gelfoam; Upjohn Co, Kalamazoo, MI) loaded with 3 μL of PBS alone as the negative control, or containing 3 μg (1 mg/mL) of the angiogenic recombinant basic fibroblast growth factor (FGF-2; Pharmacia, Milan, Italy) alone as the positive control20 or together with each VBL dose, were implanted on top of the CAM. The sponge traps the sample and allows slow release of the product. CAM were examined daily until day 12, when the angiogenic response peaks.20 On day 12, blood vessels entering the sponge within the focal plane of the CAM were recognized macroscopically (at 50×), counted by two observers in a double-blind fashion21 under a Zeiss SR stereomicroscope (Zeiss; Oberkochen, Germany), and photographed in ovo with the MC63 Camera system (Zeiss). To better highlight vessels, the CAM were injected into a large allantoic vein with India ink solution, fixed in Serra's fluid, dehydrated in graded ethanols, and rendered transparent in methylbenzoate.22 On day 12, after macroscopic counting, the embryos and their membranes were fixed in ovo in Bouin's fluid. The sponges and the underlying and immediately adjacent CAM portions were removed, embedded in paraffin, sectioned at 8 μm along a plane parallel to the CAM surface, and stained with a 0.5% aqueous solution of toluidine blue (Merck). Angiogenesis was measured by a slightly modified planimetric point count method23: 4 to 6 250× fields covering almost the whole of every third section within 30 serial slides of each sponge per sample were analyzed within a superimposed 144 intersection point square reticulum of 0.125 mm2. Only transversely sectioned microvessels, ie, capillaries and small venules with or without a 3 to 10 μm lumen occupying the intersection points, were counted and calculated as the mean ± 1 SD per section, per CAM, and groups of CAM.
Electron microscopy.
T25 flasks of HUVEC at 90% confluence were maintained for 24 hours in DMEM supplemented with 10% FCS and 1% glutamine alone or containing each VBL dose. The cells were then washed by PBS and fixed in 3% glutaraldehyde in 0.1 mol/L PBS for 3 hours, washed in the same buffer for 12 hours, and post-fixed in 1% osmium tetroxide.23Afterwards, the cells were scraped with a rubber bar, dehydrated in graded ethanols, and embedded in Epon 812. Ultrathin sections were cut with a diamond knife on a LKB ultratome, stained with uranyl acetate followed by lead citrate, and examined in a Zeiss 9A electron microscope.
RESULTS
Inhibition of the angiogenic phenotype of cultured human microvascular endothelial cells by VBL.
The angiogenic phenotype is an ensemble of cell functions, namely proliferation, chemotaxis, adhesion to, and spreading on extracellular matrix constituents, such as FN, morphogenesis and MMP secretion, which are expressed by cells when cultured in optimal growth medium alone or supplemented with angiogenic growth factors.24, 25
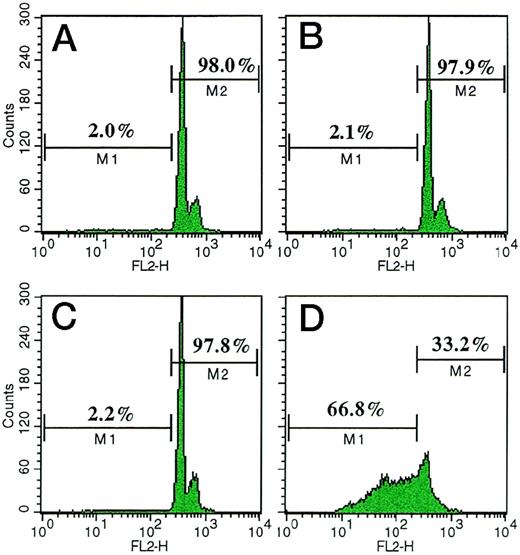
The first series of experiments focused on the effects of VBL on the proliferation of HUVEC and EA.hy926 cells. These cells were exposed on days 0 and 2 to complete medium alone (positive control) or supplemented with each VBL dose, or to starvation SFM (negative control), and their proliferation rate was measured on day 4 by a colorimetric method. Figure 1A shows that VBL was ineffective on HUVEC at 0.1 pmol/L, but significantly inhibited their proliferation at 0.25 pmol/L (−78%, as mean, of the positive control; P < .001 Wilcoxon rank test). This inhibition was progressively enhanced with increasing doses (−92% at 1 pmol/L; P < .05 for the within-sample comparisons by Wilcoxon-Wilcox test). Figure 1B shows that when cells were exposed to control media and VBL every 24 hours for 1 to 3 times, it again gave more evident inhibition of cell proliferation at 0.25 pmol/L, in agreement with the number and overall duration of exposures: compared with their positive control, the three ways of exposure resulted in −20%, −50% and −85% mean inhibition respectively (P < .01, Wilcoxon-Wilcox test). Higher doses inhibited more strongly. Similar results were obtained with EA.hy926 cells (data not shown). The effect was reversible: when the medium containing VBL 0.25 pmol/L or 1 pmol/L was replaced with the medium alone, proliferation resumed within 12 hours and was fully restored within 48 hours and 72 hours, respectively (Fig 1A, insert). Induction of apoptosis was ruled out because by using FACS analysis of HUVEC weakly stained with fluorescent PI for subdiploid DNA, a marker of apoptosis,18 we found that (Fig2) the percentages of weakly fluorescent cells (M1 region) were marginal after exposure to VBL 0.25 pmol/L (2%, Fig 2A) and 1 pmol/L (2.1%, Fig 2B), similar to what was observed in unexposed cells (2.2%, Fig 2C), used as the negative control. By contrast, high percentages of apoptotic HUVEC (66.8%) were produced by dexamethasone 1 mmol/L, an apoptogenic drug19 (Fig 2D), which was used as the positive control. Specularly, the percentages of nonapoptotic HUVEC, ie, cells strongly fluorescent due to diploid DNA (M2 region), were high in cell preparations exposed to VBL 0.25 pmol/L (98%) and 1 pmol/L (97.9%), as in unexposed cells (97.8%), whereas they were much lower (33.2%) in preparations exposed to dexamethasone; apoptotic bodies, nuclear fragmentation, and homogenization, cellular shrinking, or membrane blebbing were not detected, and the inhibitory effect was fully and rapidly reversible.
Effect of VBL on the proliferation of HUVEC. (A) Low-density cultures (2 × 103 cells per 0.32 cm2 well) were incubated on days 0 and 2 in the specific growth medium with FCS 10% (positive control), the starvation medium (negative control), and in the positive control medium containing each dose of VBL. Cells were counted on day 4. Insert: recovery of cell proliferation after removal of VBL. (B) The cells were exposed to VBL every 24 hours for one, two, and three times. Bars represent the mean ± 1 SD in 1 representative experiment out of 5. Significance by the Wilcoxon rank test.
Effect of VBL on the proliferation of HUVEC. (A) Low-density cultures (2 × 103 cells per 0.32 cm2 well) were incubated on days 0 and 2 in the specific growth medium with FCS 10% (positive control), the starvation medium (negative control), and in the positive control medium containing each dose of VBL. Cells were counted on day 4. Insert: recovery of cell proliferation after removal of VBL. (B) The cells were exposed to VBL every 24 hours for one, two, and three times. Bars represent the mean ± 1 SD in 1 representative experiment out of 5. Significance by the Wilcoxon rank test.
FACS analysis of apoptotic HUVEC, ie, cells with subdiploid DNA weakly stained with fluorescent propidium iodide18 (M1 region), in comparison with nonapoptotic HUVEC, ie, strongly stained cells with diploid DNA (M2 region). HUVEC were cultured in (A) complete medium supplemented with VBL 0.25 pmol/L or (B) 1 pmol/L; (C) the medium alone (negative control), or (D) supplemented with dexamethasone 1 mmol/L, an apoptogenic drug19 (positive control). Treatments on days 0 and 2, analysis on day 4. FL2-H = axis of relative fluorescence intensity. One representative experiment out of four.
FACS analysis of apoptotic HUVEC, ie, cells with subdiploid DNA weakly stained with fluorescent propidium iodide18 (M1 region), in comparison with nonapoptotic HUVEC, ie, strongly stained cells with diploid DNA (M2 region). HUVEC were cultured in (A) complete medium supplemented with VBL 0.25 pmol/L or (B) 1 pmol/L; (C) the medium alone (negative control), or (D) supplemented with dexamethasone 1 mmol/L, an apoptogenic drug19 (positive control). Treatments on days 0 and 2, analysis on day 4. FL2-H = axis of relative fluorescence intensity. One representative experiment out of four.
The inhibitory effect of low-dose VBL was restricted to endothelial cells, because neither NIH 3T3 fibroblasts nor Burkitt's lymphoma (Namalwa), B-cell lymphoblastic leukemia (LIK), and T-cell lymphoblastic leukemia (CEM) cell lines displayed reduced proliferation when exposed to 0.25 pmol/L or 1 pmol/L (Table 1).
Proliferation of HUVEC and Other Primary Cells Exposed to Low-Dose Vinblastine
| Vinblastine Dose . | No. of Cells (×103) on Day 4* . | ||||
|---|---|---|---|---|---|
| HUVEC . | NIH 3T3 . | Namalwa . | LIK . | CEM . | |
| 0.25 pmol/L | 8.4 ± 3.2 | 68.2 ± 4.3 | 48.7 ± 4.6 | 37.4 ± 3.7 | 54.5 ± 5.1 |
| 1 pmol/L | 3.5 ± 1.1 | 58.5 ± 5.2 | 41.5 ± 5.8 | 44.9 ± 4.8 | 51.6 ± 4.2 |
| (33.2 ± 5.6) | (51.2 ± 4.7) | (42.3 ± 4.7) | (47.1 ± 6.7) | (59.8 ± 5.8) | |
| [4.1 ± 2.3] | [7.3 ± 2.8] | [11.2 ± 3.4] | [6.4 ± 1.8] | [8.3 ± 3.5] | |
| Vinblastine Dose . | No. of Cells (×103) on Day 4* . | ||||
|---|---|---|---|---|---|
| HUVEC . | NIH 3T3 . | Namalwa . | LIK . | CEM . | |
| 0.25 pmol/L | 8.4 ± 3.2 | 68.2 ± 4.3 | 48.7 ± 4.6 | 37.4 ± 3.7 | 54.5 ± 5.1 |
| 1 pmol/L | 3.5 ± 1.1 | 58.5 ± 5.2 | 41.5 ± 5.8 | 44.9 ± 4.8 | 51.6 ± 4.2 |
| (33.2 ± 5.6) | (51.2 ± 4.7) | (42.3 ± 4.7) | (47.1 ± 6.7) | (59.8 ± 5.8) | |
| [4.1 ± 2.3] | [7.3 ± 2.8] | [11.2 ± 3.4] | [6.4 ± 1.8] | [8.3 ± 3.5] | |
Results are expressed as the mean ± 1 SD in 1 representative experiment of 4 performed. Positive control values are in parentheses. Negative control values are in brackets.
The cells (2 × 103 per 0.32-cm2 well) were exposed to VBL on days 0 and 2 and counted on day 4, as described in the proliferation assay (Fig 1A).
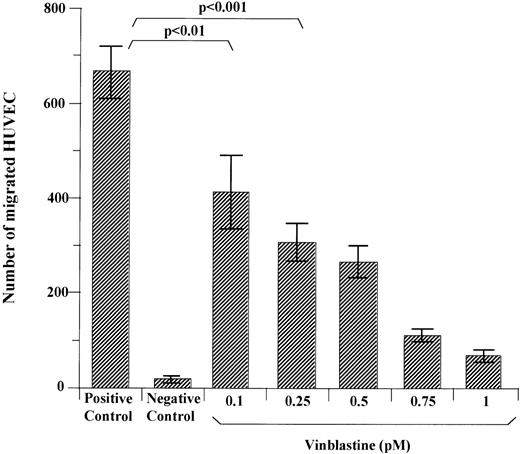
The inhibitory effect of VBL on HUVEC and EA.hy926 cell chemotaxis was assessed in Boyden chambers. Figure 3 shows that when HUVEC were pretreated with each VBL dose and left to migrate towards the NIH 3T3 CM or 0.1% BSA solution (chemoattractant and negative control respectively), the number of migrated cells compared to the positive control was −38% as mean (P < .01, Wilcoxon rank test) and −54% (P < .001) at 0.1 pmol/L and 0.25 pmol/L, respectively, and lowered progressively as the VBL dose increased (P < .01, Wilcoxon-Wilcox test). Chemotaxis of EA.hy926 cells was declined to −40% when 0.1 pmol/L was used (data not shown).
Effect of VBL on chemotaxis of HUVEC. Cells (1.2 × 105) exposed for 24 hours to each VBL dose were seeded in the upper compartment of Boyden chambers, whereas the CM of NIH 3T3 cells was placed as the chemoattractant in the lower compartment. Nonexposed cells were used in the positive and negative controls. The latter were without the chemoattractant. Cells that had migrated to the lower surface of the filter separating the compartments after 6 hours were counted after coding the samples. Bars represent the mean ± 1 SD of the number of migrated cells in 5 to 8 400× fields of the filter per sample in 1 representative experiment out of 5. Significance by the Wilcoxon rank test.
Effect of VBL on chemotaxis of HUVEC. Cells (1.2 × 105) exposed for 24 hours to each VBL dose were seeded in the upper compartment of Boyden chambers, whereas the CM of NIH 3T3 cells was placed as the chemoattractant in the lower compartment. Nonexposed cells were used in the positive and negative controls. The latter were without the chemoattractant. Cells that had migrated to the lower surface of the filter separating the compartments after 6 hours were counted after coding the samples. Bars represent the mean ± 1 SD of the number of migrated cells in 5 to 8 400× fields of the filter per sample in 1 representative experiment out of 5. Significance by the Wilcoxon rank test.
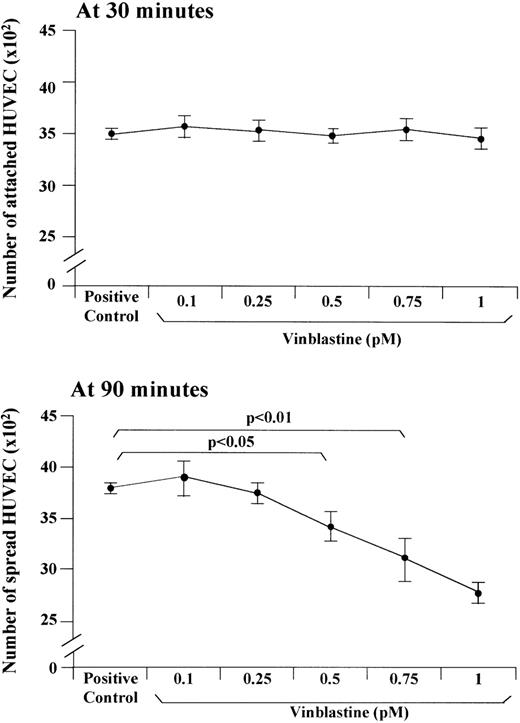
In other experiments, the ability of HUVEC to attach to and spread on FN after treatment with each VBL dose was studied (Fig 4). Attachment evaluated at 30 minutes of treatment was not impacted. By contrast, cell spreading evaluated at 90 minutes was significantly impacted at 0.5 pmol/L (3490 ± 160 spread treated cells v 3800 ± 70 spread control cells,P < .05, Wilcoxon rank test), 0.75 pmol/L (3170 ± 210,P < .01), and 1 pmol/L (2830 ± 80, P < .01) in a dose-dependent fashion (P < .01, Wilcoxon-Wilcox test).
Effect of VBL on the adhesion of HUVEC on the FN substrate. The cells were incubated for 90 minutes in the specific growth medium without FCS (positive control) and in this medium containing each VBL dose. The attachment to and the spreading on FN were assessed at 30 minutes and 90 minutes, respectively. Bars represent the mean ± 1 SD in 1 representative experiment out of 6. Significance by the Wilcoxon rank test.
Effect of VBL on the adhesion of HUVEC on the FN substrate. The cells were incubated for 90 minutes in the specific growth medium without FCS (positive control) and in this medium containing each VBL dose. The attachment to and the spreading on FN were assessed at 30 minutes and 90 minutes, respectively. Bars represent the mean ± 1 SD in 1 representative experiment out of 6. Significance by the Wilcoxon rank test.
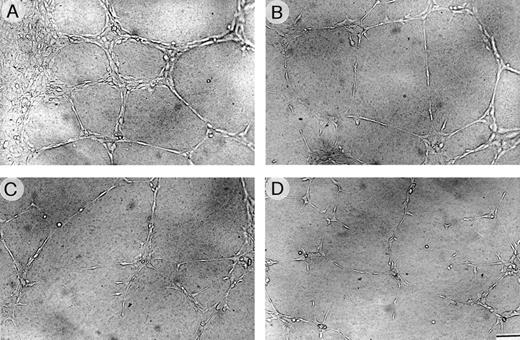
The effect of VBL on vessel morphogenesis of HUVEC and EA.hy926 cells was investigated. Data on HUVEC are presented. Cells seeded on Matrigel in the presence of NIH 3T3 CM spread throughout the Matrigel surface and aligned to form branching, anastomosing tubes with multicentric junctions that formed a closely-knit meshwork of capillary-like structures (Fig 5A). Thin sections perpendicular to the Matrigel surface showed that these capillaries were formed of single or multiple layers of cells, and were both solid or canalized with a narrow lumen (not shown). In contrast, when CM was admixed with VBL at increasing doses, a progressive loss of this picture was observed: 0.5 pmol/L and even more so 1 pmol/L caused most cells to remain spherical and isolated, and few aggregated as small clumps or generated irregular tubes, mostly without lumen (Fig 5B and C). The picture at 1 pmol/L resembled that obtained with the negative control (Fig 5D).
HUVEC morphogenesis on Matrigel. Cells (2 × 105) were plated per 1.25 cm2 well precoated with Matrigel and grown for 6 hours (A) in the specific medium alone (positive control) or containing (B) VBL 0.5 pmol/L, (C) 1 pmol/L, or (D) in the medium without FCS (negative control). Sub (A), cells arranged in branching, reciprocally anastomosing tubes forming a closely knit capillary-like plexus; sub (B) and (C), progressive alterations of the plexus parallel with increasing VBL dose. Bar = 30 μm from (A) to (D).
HUVEC morphogenesis on Matrigel. Cells (2 × 105) were plated per 1.25 cm2 well precoated with Matrigel and grown for 6 hours (A) in the specific medium alone (positive control) or containing (B) VBL 0.5 pmol/L, (C) 1 pmol/L, or (D) in the medium without FCS (negative control). Sub (A), cells arranged in branching, reciprocally anastomosing tubes forming a closely knit capillary-like plexus; sub (B) and (C), progressive alterations of the plexus parallel with increasing VBL dose. Bar = 30 μm from (A) to (D).
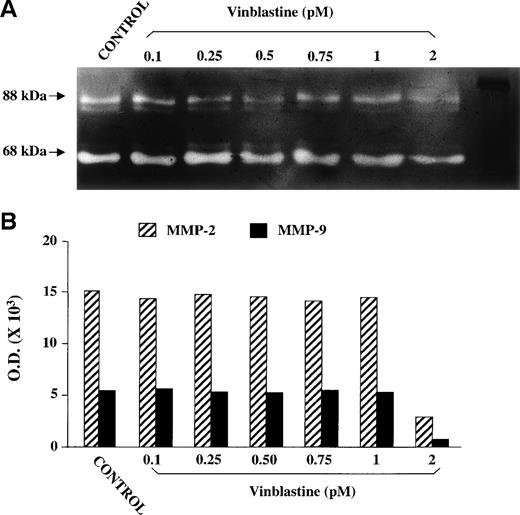
Lastly, SDS-PAGE gelatin zymography of CM of EA.hy926 cells exposed and not exposed for 24 hours to each VBL dose did not show reduced MMP-2 and MMP-9 secretion by exposed cells. The secreted MMP-2 and MMP-9 gave rise to gelatinolytic bands with an apparent Mr of 62 kD and 88 kD respectively (Fig 6A), indicating that both enzymes were present in the CM in their cleaved, activated form.26 The amount of MMP, as evaluated by computerized image analysis of the band intensity (Fig 6B), did not decrease in the CM of VBL-exposed cells, even at 1 pmol/L. A decrease was observed at 2 pmol/L, a cytotoxic dose (see below).
SDS-PAGE gelatin zymography of the CM of EA.hy926 endothelial cells either nonexposed (positive control) or exposed to each VBL dose for 24 hours. (A) White bands against a dark background corresponding to the gelatinolytic areas of the MMP-2 and MMP-9 activity. (B) MMP-2 activity measured by screening band intensity through computerized image analysis.
SDS-PAGE gelatin zymography of the CM of EA.hy926 endothelial cells either nonexposed (positive control) or exposed to each VBL dose for 24 hours. (A) White bands against a dark background corresponding to the gelatinolytic areas of the MMP-2 and MMP-9 activity. (B) MMP-2 activity measured by screening band intensity through computerized image analysis.
VBL in the chick embryo CAM.
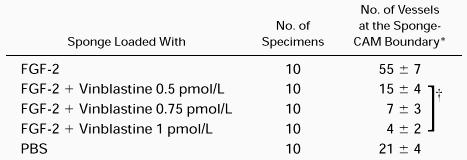
Ten CAM per series were examined macroscopically on incubation day 12. Eight out of 10 (80%) CAM implanted with sponges loaded with the angiogenic factor FGF-2 (positive control) displayed a vasoproliferative response, and in all 10 CAM per series treated with FGF-2 plus VBL 0.5 pmol/L, 0.75 pmol/L, or 1 pmol/L the response was inhibited; 9 of 10 (90%) CAM treated with PBS (negative control) displayed no response. Vessels entering the sponges were macroscopically recognized and counted after their highlighting by India ink injection into a major allantoic vein. CAM implanted with sponges loaded with the FGF-2 showed numerous allantoic vessels converging like spokes towards the sponge, which was completely filled with the ink (Table 2 and Fig 7A). When the sponge was loaded with PBS, physiologic angiogenesis was observed as fewer allantoic vessels arranged partly around the sponge and partly converging towards it (Table 2). By contrast, very few vessels were detectable with 0.5 pmol/L and even fewer with 0.75 pmol/L and 1 pmol/L VBL (Table 2 and Fig 7B).
Chick Embryo CAM-Sponge Assay: Macroscopic Assessment of Vascular Density on Day 12 of Incubation
Macroscopic quantitation was assessed as the number of vessels at 50× at the sponge-CAM boundary.
Results are expressed as the mean ± 1 SD.
P < .01 for the within-sample comparison (Wilcoxon-Wilcoxon test).
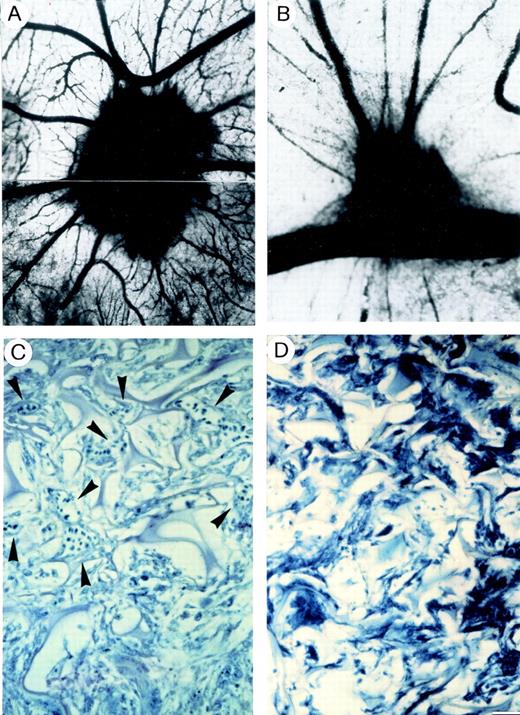
The CAM of a 12-day-old chick embryo incubated for 4 days with a gelatin sponge loaded with (A), (C) the angiogenic basic fibroblast growth factor (FGF-2) or with (B), (D) FGF-2 plus VBL 0.75 pmol/L. Note sub (A) numerous blood vessels converging like spokes toward the sponge after India ink injection, whereas in sub (B) there are very few vessels around the sponge or converging toward it. (C) Histologic section of the sponge at the boundary with the CAM mesenchyme shows several canalized, dilated vessels (arrowheaded) intermingled in a collagenous matrix among the trabeculae. (D) No vessels are observed in the histologic section of the sponge at the boundary. Bar, (A), (B) = 8 mm; (C), (D) = 90 μm.
The CAM of a 12-day-old chick embryo incubated for 4 days with a gelatin sponge loaded with (A), (C) the angiogenic basic fibroblast growth factor (FGF-2) or with (B), (D) FGF-2 plus VBL 0.75 pmol/L. Note sub (A) numerous blood vessels converging like spokes toward the sponge after India ink injection, whereas in sub (B) there are very few vessels around the sponge or converging toward it. (C) Histologic section of the sponge at the boundary with the CAM mesenchyme shows several canalized, dilated vessels (arrowheaded) intermingled in a collagenous matrix among the trabeculae. (D) No vessels are observed in the histologic section of the sponge at the boundary. Bar, (A), (B) = 8 mm; (C), (D) = 90 μm.
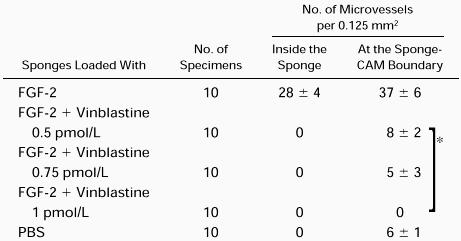
Histologic examination and planimetric vessel counting were also performed (Table 3). FGF-2–loaded sponges displayed a dense collagenous matrix and numerous blood vessels among the sponge trabeculae and at the boundary between the sponge and the CAM mesenchyme (Table 3 and Fig 7C). Vessels pierced the sponge at some points. By contrast, no vessels could be detected inside the PBS–loaded sponges, and fewer were found at the boundary than in the positive control. Sponges loaded with 0.5 pmol/L, 0.75 pmol/L, and 1 pmol/L VBL gave no vessels. The boundaries gave counts overlapping those of the PBS at 0.5 pmol/L, but lower at 0.75 pmol/L, and no vessels at 1 pmol/L (Table 3 and Fig 7D).
Cytotoxicity of VBL on endothelial cells and other primary cells in vitro and in the CAM.
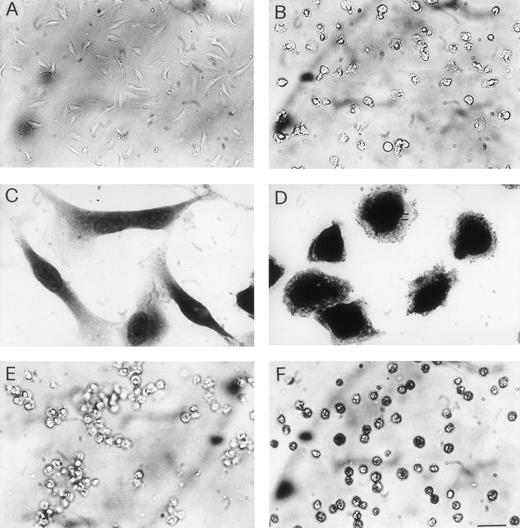
Cells were exposed at day 0 and every 2 days to each VBL dose in specific growth medium, and observed daily for morphologic alterations. At 1 pmol/L, VBL produced signs of cytotoxicity on HUVEC, namely vacuolization, loss of elongated shape, and cytoplasmic swelling in about 60% of cells on day 6. On day 8, most cells displayed these changes, and cell detachment began. At 2 pmol/L, most cells underwent morphologic alterations on day 4 (Fig 8A and B) and cell detachment rapidly occurred afterwards. At 4 pmol/L, these findings were observed on day 2. Similar behavior was displayed by EA.hy926 cells (Fig 8C and D). By contrast, NIH 3T3 cells gave cytoplasmic vacuolization and swelling at 30 pmol/L on day 4 and at 50 pmol/L on day 2. On Namalwa, LIK, and CEM cells cytoplasmic vacuolization and toxic granules were evident with 40 pmol/L on day 4 (Fig 8E and F) and 65 pmol/L on day 2.
Cytotoxicity of VBL. Cells were exposed to VBL in the specific growth medium as described in the proliferation assay (Fig1A): pictures on day 4. (A) HUVEC exposed to 1 pmol/L and (B) 2 pmol/L: note detached cells with loss of elongated shape and cytoplasmic swelling. (C) EA.hy926 cells exposed to 1 pmol/L and (D) 2 pmol/L, fixed in 2.5% glutharaldehyde and stained with May-Grünwald-Giemsa: note signs of toxicity as in HUVEC. (E) Human lymphoblastic leukemia T-cells (CEM cells) exposed to 2 pmol/L and (F) 40 pmol/L: different from (E), note many cells with dusty cytoplasm and some cells with nuclear pyknosis. Bar = 30 μm in (A), (B), (E), (F); 16 μm in (C), (D).
Cytotoxicity of VBL. Cells were exposed to VBL in the specific growth medium as described in the proliferation assay (Fig1A): pictures on day 4. (A) HUVEC exposed to 1 pmol/L and (B) 2 pmol/L: note detached cells with loss of elongated shape and cytoplasmic swelling. (C) EA.hy926 cells exposed to 1 pmol/L and (D) 2 pmol/L, fixed in 2.5% glutharaldehyde and stained with May-Grünwald-Giemsa: note signs of toxicity as in HUVEC. (E) Human lymphoblastic leukemia T-cells (CEM cells) exposed to 2 pmol/L and (F) 40 pmol/L: different from (E), note many cells with dusty cytoplasm and some cells with nuclear pyknosis. Bar = 30 μm in (A), (B), (E), (F); 16 μm in (C), (D).
In the CAM, 1 pmol/L was cytotoxic for endothelial cells and other mononuclear stromal cells at day 16 (antiangiogenesis experiments ended at day 12), when they displayed nuclear pyknosis and cytoplasmic fragmentation and homogenization. VBL at 2 pmol/L and 4 pmol/L was cytotoxic on days 12 and 10, respectively.
Ultrastructural findings.
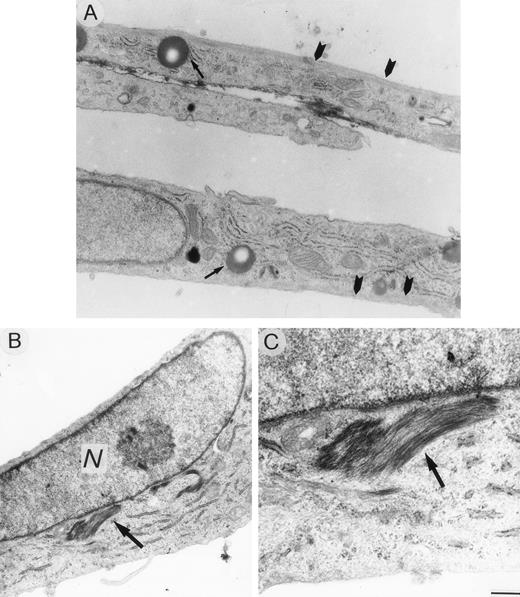
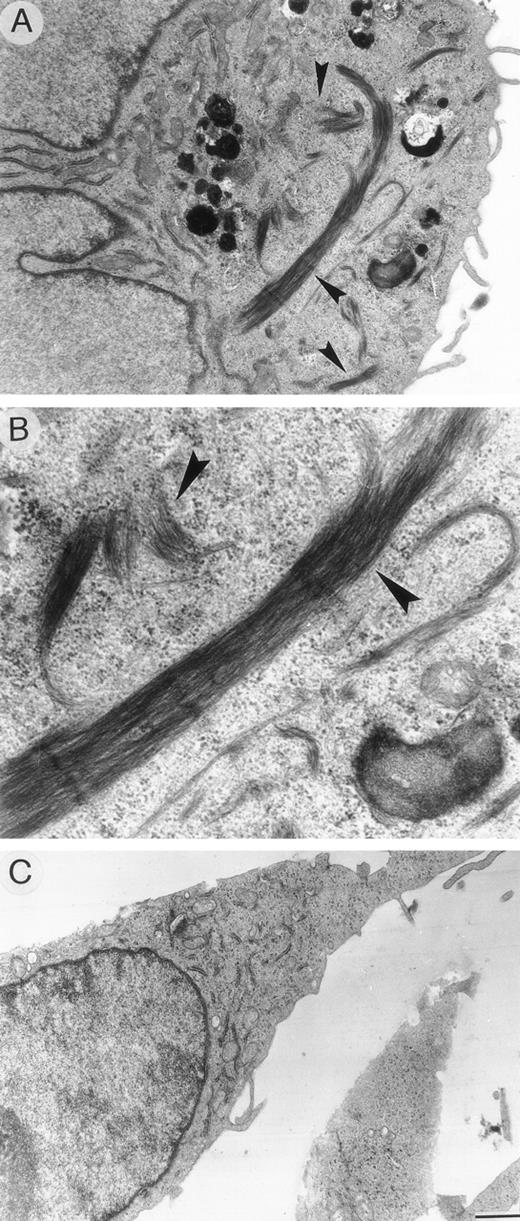
HUVEC cultured in specific medium appeared as elongated cells, containing well-developed organules, lipid droplets, and a network of microtubules and microfilaments extending across the cytoplasm and thickened near the subcortical plasma membrane (Fig 9A). Cells exposed to 0.1 pmol/L VBL for 24 hours displayed very limited lesions of cytoskeleton microfilaments in the form of small perinuclear areas of addensation and depolymerization (Fig 9B and C). These areas were more evident and numerous after exposure at 1 pmol/L, the cells appeared as rounded cells irregularly shaped with numerous bundles of thickened microfilaments (Fig 10A and B). These changes regressed after 12 hours and 24 hours when 0.1 pmol/L and 1 pmol/L, respectively, were removed, and the cells returned to the normal elongated shape (Fig 10C).
Ultrastructural sections of HUVEC under standard culture conditions (A) and after exposure to 0.1 pmol/L VBL (B, C). Cells appear elongated with cytoplasm containing organelles, lipid droplets (arrow) and subcortical microfilaments extending across the cells (arrowheads). (B, C) A bundle of microfilaments (arrow) accumulated to nucleus (N) of a spindle-shaped endothelial cell. Bar (A), (B) = 16.6 μm; (C) = 0.5 μm.
Ultrastructural sections of HUVEC under standard culture conditions (A) and after exposure to 0.1 pmol/L VBL (B, C). Cells appear elongated with cytoplasm containing organelles, lipid droplets (arrow) and subcortical microfilaments extending across the cells (arrowheads). (B, C) A bundle of microfilaments (arrow) accumulated to nucleus (N) of a spindle-shaped endothelial cell. Bar (A), (B) = 16.6 μm; (C) = 0.5 μm.
Ultrathin sections of HUVEC cells exposed to (A, B) 1 pmol/L VBL and (C) 24 hours after removing the VBL-containing medium. (A, B) Round-shaped endothelial cell displaying numerous bundles of thickened microfilaments distributed in the cytoplasm (arrow). (C) Endothelial cell showing normal distribution of cytoskeleton organelles. Bar (A), (C) = 16.6 μm; (B) = 0.5 μm.
Ultrathin sections of HUVEC cells exposed to (A, B) 1 pmol/L VBL and (C) 24 hours after removing the VBL-containing medium. (A, B) Round-shaped endothelial cell displaying numerous bundles of thickened microfilaments distributed in the cytoplasm (arrow). (C) Endothelial cell showing normal distribution of cytoskeleton organelles. Bar (A), (C) = 16.6 μm; (B) = 0.5 μm.
DISCUSSION
Here we show that VBL inhibits certain functions of human microvascular endothelial cells (HUVEC and EA.hy926 cells), namely proliferation, chemotaxis, spreading on FN, and morphogenesis in vitro (though not MMP-2 and MMP-9 secretion), and angiogenesis of the chick embryo CAM in vivo. These effects were directly dose-dependent and obtained at very low doses, namely from 0.1 to 1 pmol/L in vitro, and from 0.5 to 1 pmol/L in vivo. By contrast, 0.25 pmol/L and 1 pmol/L did not impact proliferation of mouse fibroblasts (NIH 3T3 cells) and human lymphoid tumor cells (Namalwa, LIK, and CEM cells).
It has been shown that the VBL uptake into microvascular endothelial cells is already operative at very low doses (5 to 10 nmol/L) in the extracellular milieu.27 The doses we used caused no nonspecific cytotoxicity: 1 pmol/L caused cytoplasmic vacuolization, a loss of elongated cell shape, cytoplasmic swelling, and detachment from FN 2 days after the experiments ended, and 1 pmol/L per CAM gave vascular degeneration and cytoplasmic fragmentation of endothelial cells 4 days afterwards. By contrast, doses of 2 pmol/L or greater were rapidly cytotoxic both in vitro and in vivo. Additional proof of the absence of cytotoxicity in our models was provided by the complete reversal of both in vitro and in vivo inhibition when VBL was removed.
VBL is specifically toxic for cytoskeleton microfilaments and microtubules and hence for the mitotic spindle, which results in metaphase blockage and necrosis of tumor cells.2 These effects have already been shown on human microvascular endothelial cells: HUVEC exposed in vitro to VBL at doses 4 × 105-fold greater than ours (0.4 μmol/L) displayed irreversible signs of cytotoxicity (spherical shape, cytoplasmic vacuolar degeneration, nuclear pyknosis, and disruption of the tight cell-to-cell contacts) within 20 minutes to 2 hours, and 50% survival after only 4 hours, parallel with irreversible accumulation of F-actin microfilaments, other cytoskeleton structures.28 Similar results have been obtained in vitro on porcine aortic endothelial cells exposed to VBL at doses 1 × 106-fold greater (1 μmol/L).29 In vivo, in a Lewis rat10 and different mouse models,9, 30-32 VBL at doses equivalent to those applied in vitro, ie, 7.5 to 10 mg/kg (at or slightly above 50% of the maximum tolerated dose) also provoked diffuse necrosis of vascular endothelial cells, resulting in intratumor vessel collapse, punctuate hemorrhages, reduced overall tumor blood flow and tumor patchy necrosis, in the absence of necrotic damage of endothelial cells and blood flow disturbance in normal tissues.
Angiogenesis in tumors is sustained by rapidly proliferating endothelial cells,33 whose evident sensitivity to the antiproliferative effect of VBL explains the endothelial cell necrosis restricted to tumors in the animal models. We have attained inhibition of functions of human microvascular endothelial cells essential for angiogenesis without cytotoxicity or cell necrosis. Proliferation and chemotaxis are important steps for neovessel sprouting.24Morphogenesis develops by migration, spreading on FN, and the mutual alignment of the endothelial cells to form tubular branching structures that are anastomosed in a capillary-like plexus.34Inhibition of these functions agrees well with that of the in vivo angiogenesis that has been obtained by us in the CAM model, insofar as the same functions are recapitulated by angiogenesis.25Overall data suggest that VBL has an antiangiogenic component when applied at very low, nontoxic doses. The cell functions studied are tightly linked to the integrity of the cytoskeleton, and hence they are typically impaired by VBL as a consequence of its mechanism of action. Accordingly, we have shown at the ultrastructural level that even 0.1 pmol/L VBL produced a thin disturbance of the endothelial cell cytoskeleton in the form of minimal focal depolymerization and accumulation of microfilaments. This was equally reversible, like the impairment of cell functions. VBL at 100 nmol/L to 10 μmol/L has been shown to inhibit other endothelial cell functions associated with cytoskeleton functionality, namely active internalization of the E-selectin ELAM-1 by HUVEC35 and secretion of endothelin-1 by porcine aortic cells.29
The highest antiangiogenic dose applied in vivo was 1 pmol/L per CAM on incubation day 8. Because on this day the CAM-embryo weight was 10 grams,36 the dose evaluated as μg/kg was 0.24 μg/kg, and the equivalent dose in a 70 kg adult subject was 16 μg, which is lower than the daily dose in tumor management (1 mg). VBL could be considered as an antiangiogenic agent along with suramin hexasodium,37 linomide,38 the recombinant human platelet factor 4,39 the fumagillin derivative AGM-1470,40 and Taxol,41 which also inhibit the proliferation and chemotaxis of endothelial cells.
The antiangiogenic activity of low-dose VBL deserves further investigation, alone or together with other antiangiogenic agents42 for the treatment of tumors characterized by vivid angiogenesis, and of other angiogenesis-dependent diseases, such as Kaposi's sarcoma,43 rheumatoid arthritis,44and psoriasis.45
ACKNOWLEDGMENT
The authors thank Prof F. Bussolino (IRCCS, University of Turin Medical School, Turin, Italy), Prof F. Silvestris, and Dr P. Cafforio (DIMO, University of Bari Medical School, Bari, Italy) for helpful advice in performing some experiments.
Supported in part by the Associazione Italiana per la Ricerca sul Cancro (A.I.R.C.) Milan and Ministry of Education (M.U.R.S.T.) Rome (Grant ex 40%, 1998). M.I. and M.M. are recipients of fellowships from the Fondazione Italiana Per la Ricerca sul Cancro (F.I.R.C.) Milan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal