Abstract
Erythropoietin (EPO), a major regulator of erythroid progenitor cells, is essential for the survival, proliferation, and differentiation of immature erythroid cells. To gain insight into the molecular mechanism by which EPO functions, we analyzed the activation of Jun N-terminal kinases (JNKs) and extracellular signal-regulated kinases (ERKs) in HCD-57 cells, a murine erythroid progenitor cell line that requires EPO for survival and proliferation. Withdrawal of EPO from the cell culture medium resulted in sustained activation of JNKs plus p38 MAP kinase, and inactivation of ERKs, preceding apoptosis of the cells. Addition of EPO to the EPO-deprived cells caused activation of ERKs accompanied by inactivation of JNKs and p38 MAP kinase and rescued the cells from apoptosis. Phorbol 12-myristate 13-acetate, which activated ERKs by a different mechanism, also suppressed the activation of JNKs and significantly retarded apoptosis of the cells caused by withdrawal of EPO. Furthermore, MEK inhibitor PD98059, which inhibited activation of ERKs, caused activation of JNKs, whereas suppression of JNK expression by antisense oligonucleotides and inhibition of p38 MAP kinase by SB203580 caused attenuation of the apoptosis that occurs upon withdrawal of EPO. Finally, the activation of JNKs and p38 MAP kinase and concurrent inactivation of ERKs upon withdrawal of EPO were also observed in primary human erythroid colony-forming cells. Taken together, the data suggest that activation of ERKs promotes cell survival, whereas activation of JNKs and p38 MAP kinase leads to apoptosis and EPO functions by controlling the dynamic balance between ERKs and JNKs.
ERYTHROPOIETIN (EPO), a glycoprotein of 34 kD produced by the mammalian kidney and liver in response to hypoxia, is critical for survival, proliferation, and differentiation of erythroid cells.1,2 EPO promotes cell viability by preventing apoptosis of erythroid progenitor cells.3,4However, the signal transduction mechanism by which this occurs is still unclear. It is known that EPO transduces its signal through interaction with the EPO receptor by activating multiple signaling pathways, including the Ras/MAP kinase, JNK/p38 MAP kinase, JAK/STAT, and PI-3 kinase signaling cascades.5 Among the various signaling intermediates activated by EPO, extracellular signal-regulated kinases (ERKs; also known as mitogen-activated protein [MAP] kinases) have a crucial role in promoting cell proliferation and differentiation, whereas Jun N-terminal kinases (JNKs; also known as stress-activated protein kinases [SAPKs]) and p38 MAP kinase are thought to be associated with apoptosis.6-9 ERKs, JNKs, and p38 MAP kinase are structurally related, and all of them are activated by phosphorylation of threonine and tyrosine. However, they are activated by very different types of extracellular signals. ERKs are activated by a variety of growth factors and hormones that promote cell proliferation and differentiation. In contrast, JNKs and p38 MAP kinase are activated by various cellular stresses, including inflammatory cytokines, UV light, protein synthesis inhibitors, osmotic, heat and chemical shock, and bacterial endotoxin. All of these stress-related factors also induce apoptosis. Furthermore, activation of JNKs and p38 MAP kinase appears to counteract the activation of ERKs, and the dynamic balance between growth factor-activated ERKs and stress-activated JNK-p38 MAP kinase pathways is thought to be important in determining whether a cell survives or undergoes apoptosis.10 Because withdrawal of EPO leads to apoptosis of erythroid progenitor cells, activation of JNKs and p38 MAP kinase might play a very important role in this process. For this reason, we analyzed here the activation of JNKs, p38 MAP kinase, and ERKs in HCD-57 cells, a murine erythroid progenitor cell line that requires EPO for survival and proliferation, and in human erythroid colony-forming cells. We found that activation of JNKs and p38 MAP kinase plus concurrent inhibition of ERKs, upon withdrawal of EPO, is critical for induction of apoptosis, whereas activation of ERKs accompanied by inhibition of JNKs and p38 MAP kinase is important for promoting cell survival.
MATERIALS AND METHODS
Materials.
Recombinant human EPO was provided by Amgen, Inc (Thousand Oaks, CA). MEK inhibitor PD98059 and rabbit phospho-specific antibodies against JNKs (Thr183/Tyr185), ERKs (Tyr204), MKK3/6 (Ser189/207), and p38 MAP kinase (Thr180/Tyr182) were purchased from New England Biolabs Inc (Beverly, MA). Rabbit polyclonal anti-JNK antibody recognizing both JNK1 and JNK2 and anti-p38 antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-MAPK polyclonal antibody recognized both ERK1 and ERK2 was raised in rabbits as previously described.11 Phorbol 12-myristate 13-acetate (PMA) was purchased from Sigma Chemical Co (St Louis, MO), and p38 MAP kinase inhibitor SB203580 was from Calbiochem (La Jolla, CA).
Cell culture.
HCD-57 cells were maintained at 3 to 5 × 105 cells/mL in Iscove's modified Dulbecco's medium (IMDM) supplemented with 20% heat-inactivated fetal calf serum, 0.2 μmol/L 2-mercaptoethanol, and 2 U/mL recombinant human EPO in a 5% CO2 environment and at 37°C. Human erythroid colony-forming cells were purified from peripheral blood as previously reported.12 The cells were expanded and maintained in IMDM supplemented with 20% fetal calf serum, 1% deionized bovine serum albumin (BSA), 2 U/mL recombinant human EPO, 100 ng/mL recombinant human stem cell factor (SCF), 50 U/mL recombinant human interleukin-3, 10 μg/mL insulin, 10−4 mol/L 2-mercaptoethanol, 500 U/mL penicillin, and 40 μg/mL streptomycin at 37°C in a high humidity 5% CO2, 95% air incubator. Day-8 human erythroid colony-forming cells were collected for study. The purity of the cells was 80% ± 4.6% as determined by plasma clot assays.12The EPO-free media used for starvation of HCD-57 and human erythroid colony-forming cells had the same compositions as the culture media, except that EPO was omitted. For EPO starvation, normal growing cells were spun down and washed twice with the EPO-free medium, followed by further incubation in the same medium. In some cases, EPO (2 U/mL), PMA, PD98059, and SB203580 were added to the starved cells, as detailed in figure legends. For cell viability analyses, 4 cell replicates were counted in the presence of 0.2% trypan blue, with at least 200 cells counted for each sample.
Suppression of JNK1/2 by antisense oligonucleotides.
Antisense oligonucleotide 21861 for mouse JNK1/2 and control oligonucleotide 101125 with a mismatched sequence are both mixed backbone (phosphodiester/phosphorothioate) 2′-methoxyethyl chimera.13 Their sequences were 5′-CGGTAGGCTCGCTTAGCATG-3′ and 5′-TGAGGCGTTAAGACGTTCAA-3′ for the antisense and control oligonucleotides, respectively. The oligonucleotides were introduced into cells by lipofection. Briefly, 0.8 μL of oligonucleotides at a concentration of 1 mmol/L was mixed with 10 μL Lipofectin (1 mg/mL; Life Technologies, Inc, Grand Island, NY) and incubated for 15 minutes at room temperature. The mixtures were then added to HCD-57 cells (∼7.5 × 105/mL) in 2 mL normal culture medium in 6-well plates. After 4.5 hours of incubation at 37°C, the transfection medium was replaced with fresh normal culture medium and cells were further cultured for 40 hours before withdrawal of EPO from medium. After 1 to 4 days of EPO starvation, cells were subjected to apoptosis analyses.
Western blotting analyses.
After the indicated periods of incubation, HCD-57 cells were collected, washed with cold phosphate-buffered saline, and then lysed in buffer A containing 20 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Triton X-100, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerophosphate, 1 mmol/L Na3VO4, 1 μg/mL leupeptin, and 1 mmol/L phenylmethyl sulfonyl fluoride (PMSF). Samples containing 20 μg of total proteins were separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and electrically transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% nonfat dry milk and then probed with various primary antibodies overnight at 4°C. After washing 3 times for 10 minutes each with a washing buffer, the membranes were incubated with horseradish peroxidase-conjugated second antibodies, and the antibody complexes were visualized by using the ECL method (Amersham Life Science Inc, Arlington Heights, IL).
Activity assay of JNKs.
The enzymatic activity of JNKs was determined by using the JNK assay kit from New England Biolabs Inc (Beverly, MA). Briefly, cells were lysed in buffer A as described above. Cell extracts, containing 250 μg total proteins, were incubated overnight at 4°C with the N-terminal c-Jun (1-89) fusion protein bound to glutathione-Sepharose beads. The N-terminal 89 amino acid segment of c-Jun contains a high-affinity binding site for JNKs just N-terminal to the 2 phosphorylation sites, Ser63 and Ser73. It can selectively pull down JNKs from the cell lysates. After washing twice with buffer A and once with a kinase buffer (25 mmol/L Tris, pH 7.5, 5 mmol/L β-glycerolphosphate, 2 mmol/L dithiothreitol [DTT], 0.1 mmol/L Na3VO4, and 10 mmol/L MgCl2) to remove nonspecifically bound proteins, the beads were resuspended in the kinase assay buffer. The kinase reaction was performed by adding 100 μmol/L ΑΤP to the suspension. Phosphorylation of c-Jun was measured by Western blot analyses with a phospho-specific c-Jun antibody that specifically detects Ser63-phosphorylated c-Jun, a site important for c-JUN–dependent transcriptional activity.
Activity assay of p38 MAP kinase.
Cell extracts as obtained above were subjected to immunoprecipitation with regular anti-p38 MAP kinase antibody at 4°C for 4 hours. The beads were washed twice with cell lysis buffer and then twice with kinase buffer (20 mmol/L MOPS, pH 7.2, 2 mmol/L EGTA, 20 mmol/L MgCl2, 0.1 mmol/L sodium orthovanadate, 1 mmol/L DTT, and 0.1% Triton X-100). This was followed by the addition of 2 μg of glutathione-S-transferase (GST)-ATF-2 (1-96) fusion protein (amino terminal domain corresponding to amino acids 1-96 of ATF; Santa Cruz Biotechnology) in 20 μL of kinase buffer supplemented with 30 μmol/L cold ATP and 5 μCi of [γ-32P] ATP. After 20 minutes of incubation at 30°C, the reactions were terminated by mixing with SDS gel sample buffer and boiling. The samples were resolved by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiographed.
Detection of apoptosis of HCD-57 cells.
Apoptosis of HCD-57 cells was analyzed by using the in situ cell death detection kit from Boehringer Mannheim (Indianapolis, IN). The method is essentially based on the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) technique, and it can detect apoptosis at very early stages. In brief, cells were fixed with 4% paraformaldehyde for 30 minutes at room temperature, permeablized with 0.1% Triton X-100/0.1% sodium citrate for 2 minutes on ice, and then labeled with fluorescein dUTP for 1 hour at 37°C. The cells were then analyzed with a FACScan analyzer (Becton Dickinson, San Jose, CA) with standard configuration or centrifuged onto glass slides followed by photography with a fluorescent microscope. For the FACScan analyses, Listmode data were analyzed offline with WinList software (Verity Software House, Inc, Topssham, ME). Baseline apoptosis was set at 3% to 6%, and the experimental effect was measured against this background.
RESULTS
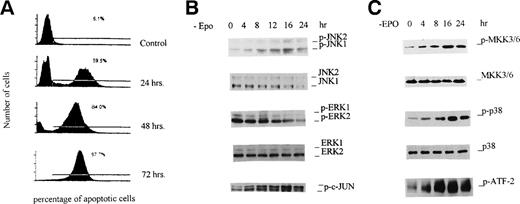
Withdrawal of EPO causes activation of JNKs and p38 MAP kinase preceding apoptosis of HCD-57 cells.
HCD-57 cells require EPO for survival. When cultured in the absence of EPO, the cells ceased proliferating, and by 24 hours of EPO starvation, 60% of the cells were dead due to apoptosis (Fig 1A). After 72 hours, essentially no cells survived. Although this phenomenon has been well documented, the signal transduction initiated by withdrawal of EPO, which leads to apoptosis, is not clear. Because activation of JNKs has been implicated in apoptosis of cells in other systems,10 we analyzed the activity of JNKs in this process. First, we used a phospho-specific antibody that specifically recognizes Thr183/Tyr185 phosphorylated JNKs to determine the activation of the enzymes. As shown in Fig 1B, when HCD-57 cells were cultured in EPO-free medium, sustained and enhanced phosphorylation of JNKs was observed. Increased phosphorylation of JNKs (JNK1 and JNK2) was seen at 4 hours, and it reached a plateau at 16 hours. The magnitude of activation of the JNKs caused by deprivation of EPO was comparable to that seen when HCD-57 cells were exposed to UV light, a well-known stimuli of the JNK pathway (data not shown). After 24 hours, the phosphorylation decreased, which was probably due to the fact that significant cell death occurred. To check whether EPO starvation affects the expression level of JNKs, Western blotting was performed with regular anti-JNK antibody that recognizes both phosphorylated and nonphosphorylated JNKs. As shown in Fig 1B, no significant change occurred in the JNK protein levels. These data indicate that the increased anti–phospho-JNK reactivity is due to increased phosphorylation of protein. Similar experiments demonstrated that EPO starvation caused a decrease in phosphorylation of ERKs, indicating inactivation of ERKs. Because dual phosphorylation of JNKs at Thr183/Tyr185 is essential for kinase activity, phosphorylation at this site is an excellent marker of JNK activity.7-9 To confirm JNK activation, we performed an in vitro kinase assay by using a c-Jun N-terminal fusion protein as a substrate. As expected, the kinase activity of JNKs in cell extracts was consistent with the phosphorylation of JNKs determined by using the phospho-specific antibody (Fig 1B, bottom panel). We further analyzed the activation of p38 MAP kinase and its upstream activator MKK3/6. As expected, withdrawal of EPO also caused activation of both enzymes, as demonstrated by phospho-specific anti-p38 and anti-MKK3/6 antibodies (Fig 1C). For both MKK3/6 and p38 MAP kinase, the activation appeared at 4 hours and peaked at 16 hours. Kinase activity assay of p38 MAP kinase with recombinant ATF-2 as a substrate further verified the results (Fig 1C, bottom panel). Together, the data suggest that apoptosis of HCD-57 cells upon withdrawal of EPO is preceded by sustained activation of JNKs and p38 MAP kinase that might be responsible for the apoptosis.
Withdrawal of EPO produces activation of JNKs and p38 MAP kinase preceding apoptosis of HCD-57 cells. HCD-57 cells (2 × 107/mL) were incubated in EPO-free medium for the indicated periods of time and then either were labeled with fluorescein-dUTP for flow cytometry analyses (A) or were lysed in buffer A for JNK and p38 MAPK activation assays (B and C) as described in Materials and Methods. (A) Flow cytometry analyses of apoptotic HCD-57 cells. The left peak represents normal growing cells, whereas the right peak corresponds to apoptotic cells, and the percentage of cells that undergo apoptosis is indicated. (B and C; top 4 panels) Cell extracts (20 μg) were resolved on 10% SDS-PAGE, transferred to PVDF membranes, and probed with phospho-specific and regular antibodies against ERK1/2, JNK1/2, p38 MAPK, and MKK3/6 as indicated. (B and C; bottom panels) Cell extracts (containing 250 μg of total proteins) were incubated with an N-terminal c-Jun (1-89) fusion protein bound to glutathione Sepharose beads for JNK kinase activity assay or with anti-p38 MAP kinase antibody for p38 MAPK activity assays with ATF-2 as a substrate. Phosphorylation of c-Jun and ATF-2 was determined by Western blotting with the phospho-specific c-Jun antibody and 32P autoradiography, respectively.
Withdrawal of EPO produces activation of JNKs and p38 MAP kinase preceding apoptosis of HCD-57 cells. HCD-57 cells (2 × 107/mL) were incubated in EPO-free medium for the indicated periods of time and then either were labeled with fluorescein-dUTP for flow cytometry analyses (A) or were lysed in buffer A for JNK and p38 MAPK activation assays (B and C) as described in Materials and Methods. (A) Flow cytometry analyses of apoptotic HCD-57 cells. The left peak represents normal growing cells, whereas the right peak corresponds to apoptotic cells, and the percentage of cells that undergo apoptosis is indicated. (B and C; top 4 panels) Cell extracts (20 μg) were resolved on 10% SDS-PAGE, transferred to PVDF membranes, and probed with phospho-specific and regular antibodies against ERK1/2, JNK1/2, p38 MAPK, and MKK3/6 as indicated. (B and C; bottom panels) Cell extracts (containing 250 μg of total proteins) were incubated with an N-terminal c-Jun (1-89) fusion protein bound to glutathione Sepharose beads for JNK kinase activity assay or with anti-p38 MAP kinase antibody for p38 MAPK activity assays with ATF-2 as a substrate. Phosphorylation of c-Jun and ATF-2 was determined by Western blotting with the phospho-specific c-Jun antibody and 32P autoradiography, respectively.
Addition of EPO produces activation of ERKs, inactivation of JNKs and p38 MAP kinase, and prevention of apoptosis.
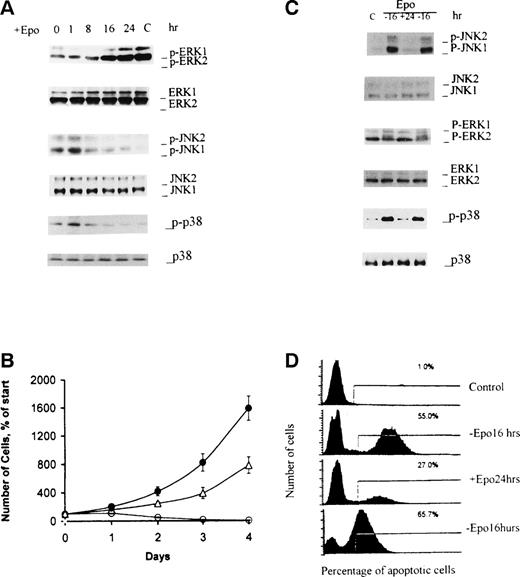
ERKs are major transducers of EPO signaling, and activation of ERKs by EPO has been well documented.14-18 In conjunction with our EPO starvation study described above, in which the cells were EPO-starved but serum-fed, we added EPO back to the EPO-starved cells and analyzed ERKs by using a phospho-specific antibody. Withdrawal of EPO from culture medium caused a significant decrease in the phosphorylation of ERKs, although a basal phosphorylation of ERKs remained even after 16 hours of EPO starvation (see Fig 1B), which might be attributed to the presence of serum. Upon addition of EPO to EPO-starved cells, a marked increase in phosphorylation of ERKs was obtained by 8 to 16 hours after the addition of EPO, and at 24 hours, the level of phosphorylation went back to the level obtained with cells grown in the presence of EPO (Fig 2A, upper panels). Western blotting analyses with regular anti-ERKs antibody showed that the protein levels of ERKs were not affected by the addition of EPO. Our previous study has shown that increased phosphorylation of ERKs parallels an increase in kinase activity of the enzymes, because the phosphorylation is required for activation.18 Therefore, the data indicated activation of ERKs after addition of EPO to EPO-starved cells. It should be noted that this slow but sustained activation of ERKs by EPO is different from the rapid and transient activation of ERKs obtained with serum-starved cells, as reported previously.14-18
The addition of EPO to EPO-deprived cells produces activation of ERKs, inactivation of JNKs and p38 MAPK, and rescue of HCD-57 cells from apoptosis. (A and B) After 4 hours of EPO-starvation, HCD-57 cells were cultured in complete medium containing 2 U/mL EPO for the indicated periods of time. Cells were lysed for Western blot analyses with the indicated antibodies (A) or cell growth was measured as an index of cell viability (B). (•) Cells were cultured in the complete medium with EPO; (▵) cells were EPO-starved for 4 hours and then were cultured in EPO-containing medium; (○) cells were continually incubated in EPO-free medium. (C and D) After 16 hours of EPO-starvation, HCD-57 cells were cultured in complete medium containing 2 U/mL EPO for 24 hours and then placed in EPO-free medium and incubated for another 16 hours. Analyses of JNK1/2, p38 MAPK, and ERK1/2s (C) and assays of apoptosis (D) were performed as described in Fig 1. Lane C in (C) denotes control cells grown in the presence of EPO.
The addition of EPO to EPO-deprived cells produces activation of ERKs, inactivation of JNKs and p38 MAPK, and rescue of HCD-57 cells from apoptosis. (A and B) After 4 hours of EPO-starvation, HCD-57 cells were cultured in complete medium containing 2 U/mL EPO for the indicated periods of time. Cells were lysed for Western blot analyses with the indicated antibodies (A) or cell growth was measured as an index of cell viability (B). (•) Cells were cultured in the complete medium with EPO; (▵) cells were EPO-starved for 4 hours and then were cultured in EPO-containing medium; (○) cells were continually incubated in EPO-free medium. (C and D) After 16 hours of EPO-starvation, HCD-57 cells were cultured in complete medium containing 2 U/mL EPO for 24 hours and then placed in EPO-free medium and incubated for another 16 hours. Analyses of JNK1/2, p38 MAPK, and ERK1/2s (C) and assays of apoptosis (D) were performed as described in Fig 1. Lane C in (C) denotes control cells grown in the presence of EPO.
The addition of EPO to EPO-starved cells also resulted in inactivation of JNKs and p38 MAP kinase, as demonstrated by the decreased phosphorylation of the enzymes (Fig 2A, lower panels). The decrease in phosphorylation of JNKs essentially coincided with the increase in phosphorylation of ERKs. At 16 hours, the phosphorylation of JNKs declined to a basal level equivalent to that observed with cells cultured in EPO-containing medium. Western blotting analysis with regular anti-JNKs and anti-p38 MAP kinase antibodies showed that the levels of the proteins were not affected by addition of EPO. It should be noted that a slight increase in the phosphorylation of JNKs and p38 MAP kinase was observed at 1 hour after the addition of EPO. This might suggest a transient activation of JNKs and p38 MAP kinase by EPO. This is consistent with the results observed with serum-starved cells reported by others.19 The physiological significance of this transient activation of JNKs is not known. The transient nature of the activation makes it differ from the sustained activation of the enzyme caused by withdrawal of EPO and should have a different physiological meaning.
To analyze the consequence of the activation of ERKs and suppression of JNKs and p38 MAP kinase induced by addition of EPO, we measured the viability of cells. As shown in Fig 2B, the addition of EPO resulted in a marked proliferation of the cells. Furthermore, fluorescent flow cytometric analyses demonstrated that apoptosis of cells was inhibited (Fig 2D).
To further show the correlation between activation of ERKs and inactivation of JNKs and p38 MAP kinase, we removed EPO from culture medium 24 hours after readdition of EPO to 16-hour EPO-starved cells (Fig 2C and D). As expected, JNKs and p38 MAP kinase were reactivated, and ERKs were reinactivated. This was accompanied by apoptosis of the cells. Together, these results suggest that EPO promotes proliferation and prevents apoptosis of cells by activating ERKs and suppressing JNKs and p38 MAP kinase, thereby providing further evidence that ERKs and JNKs have distinctly different roles in cell growth.
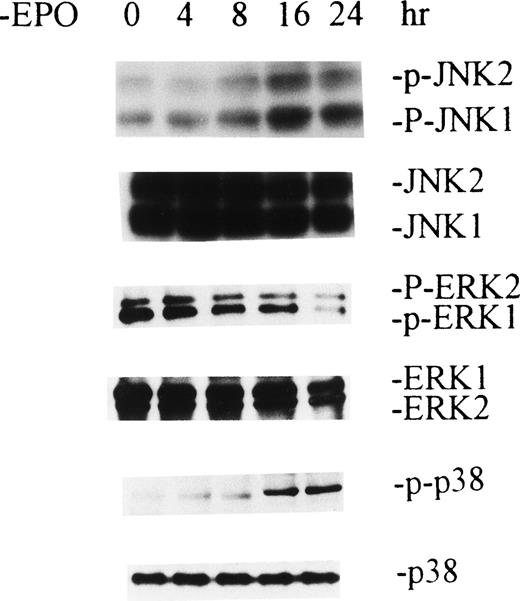
Withdrawal of EPO produces activation of JNKs and p38 MAP kinase and inactivation of ERKs in human erythroid colony-forming cells.
The study described above was performed with HCD-57 cells, an immortal cell line. Because the use of primary erythroid progenitor cells would make the study more physiologically relevant, we used day-8 human erythroid colony-forming cells purified from human peripheral blood according to a well-established method previously described.12 The purity of these cells, which are primarily colony-forming units-erythroid (CFU-E), was 80% ± 4.6% based on the ability of cells to form erythroid colonies in plasma clot assays. The data are shown Fig 3, and as observed with HCD-57 cells, withdrawal of EPO caused activation of JNKs and p38 MAP kinase that reached a plateau at 16 hours. Accompanying this was a decreased phosphorylation of ERKs. These data indicate that withdrawal of EPO also produces activation of JNKs and p38 MAP kinase and inactivation of ERKs in human primary erythroid colony-forming cells.
Withdrawal of EPO produces activation of JNKs and p38 MAPK and inactivation of ERKs in human primary erythroid colony-forming cells. Purifed day-8 human erythroid colony-forming cells, which are mainly CFU-E, were EPO-starved for the indicated periods of time. Cell extractions and Western blot analyses were performed as described in Figs 1 and 2.
Withdrawal of EPO produces activation of JNKs and p38 MAPK and inactivation of ERKs in human primary erythroid colony-forming cells. Purifed day-8 human erythroid colony-forming cells, which are mainly CFU-E, were EPO-starved for the indicated periods of time. Cell extractions and Western blot analyses were performed as described in Figs 1 and 2.
PMA induces activation of ERKs and inhibition of JNKs and retards cell apoptosis.
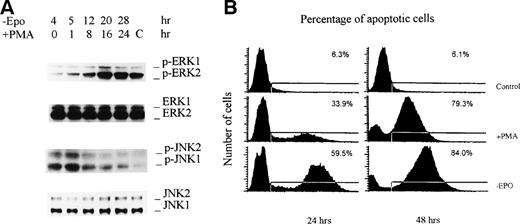
Activation of ERKs coincided with inactivation of JNKs, and vice versa. One is initiated by addition of EPO, whereas the other is caused by withdrawal of EPO. To prove that these 2 events are correlated rather than isolated, we treated EPO-starved cells with a protein kinase C activator, phorbol ester PMA. This notorious activator of ERKs activates ERKs in a Ras-independent manner.6 7 As shown in Fig 4A, when 50 nmol/L PMA was added to 4-hour EPO-starved cells, a slow activation of ERKs was observed with phosphorylation of ERKs, reaching a peak at 16 hours and decreasing thereafter. The level of the maximum activation of ERKs was slightly higher than that obtained with control cells that were grown in the presence of EPO. As observed with the addition of EPO, activation of ERKs was accompanied by the inactivation of JNKs. Phosphorylation of JNKs was reduced to the basal level at 24 hours. Because PMA-induced activation of ERKs and suppression of JNKs does not involve EPO, the data suggest that the ERK and JNK pathways may counteract each other, and this might involve direct participation of the ERKs and JNKs themselves. Additional data suggested that PMA also had a similar effect on activation of p38 MAP kinase (data not shown).
PMA produces activation of ERKs, inactivation of JNKs, and inhibition of cell apoptosis. HCD-57 cells were cultured in an EPO-free medium for 4 hours, followed by the addition of 50 nmol/L PMA. Cells were cultured further for the indicated periods of time before either being lysed for Western blot analyses with the indicated antibodies or analyzed for apoptosis. Lane C denotes control cells grown in the presence of EPO.
PMA produces activation of ERKs, inactivation of JNKs, and inhibition of cell apoptosis. HCD-57 cells were cultured in an EPO-free medium for 4 hours, followed by the addition of 50 nmol/L PMA. Cells were cultured further for the indicated periods of time before either being lysed for Western blot analyses with the indicated antibodies or analyzed for apoptosis. Lane C denotes control cells grown in the presence of EPO.
We further analyzed the effects of PMA on the apoptosis of these cells. As expected, apoptosis of the cells was significantly inhibited at 24 hours of culture by the addition of PMA (Fig 4B). More than 50% inhibition of apoptosis was observed at 24 hours. However, this effect was different from that observed with the addition of EPO. The addition of PMA failed to rescue the cells from apoptosis completely, and significant apoptosis occurred at 48 hours of incubation, even after continued addition of fresh PMA every 24 hours. This correlated with the decreased activation of ERKs after 24 hours of incubation. This might be attributed to desensitization of protein kinase C by prolonged PMA treatment. The data indicate that activation of ERKs participates in the inhibition of apoptosis. Expression of constitutively active ERKs should help to prove this. By showing that activation of ERKs leads to prevention of apoptosis of HCD-57 cells, this study further supports the notion that activation of ERKs has an essential role in the expansion of erythroid progenitor cells, as proposed in our previous studies.18
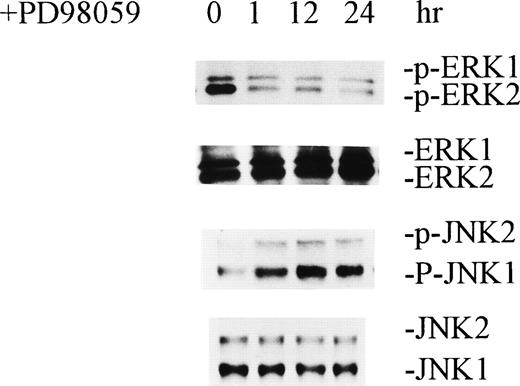
MEK inhibitor PD98059 inhibited activation of ERKs and caused activation of JNKs.
To further define the interplay between ERKs and JNKs, we inhibited activation of ERKs by treating HCD-57 cells with MEK inhibitor PD98059. PD98059 is a potent inhibitor of MEK1, and it also inhibits MEK2. Because MEK1 and MEK2 are directly upstream of ERKs in the MAP kinase activation pathway, inhibition of MEK1/2 results in inhibition of ERKs. As shown in Fig 5, treatment of normal growing HCD-57 cells with 100 μmol/L PD98059 caused significant inhibition of ERKs within 1 hour. Paralleling this was the activation of JNKs as indicated by increased protein phosphorylation of the enzymes. Furthermore, PD98059 also caused a significant inhibition of cell growth and a decrease in the number of viable cells (data not shown). This provides further evidence that inactivation of ERKs causes activation of JNKs.
MEK inhibitor PD98059 inhibited activation of ERKs and caused activation of JNKs. Normal growing HCD-57 cells were incubated with 100 μmol/L PD98059 for the indicated periods of time. Cell extractions and Western blot analyses were performed as described in Figs 1 and 2.
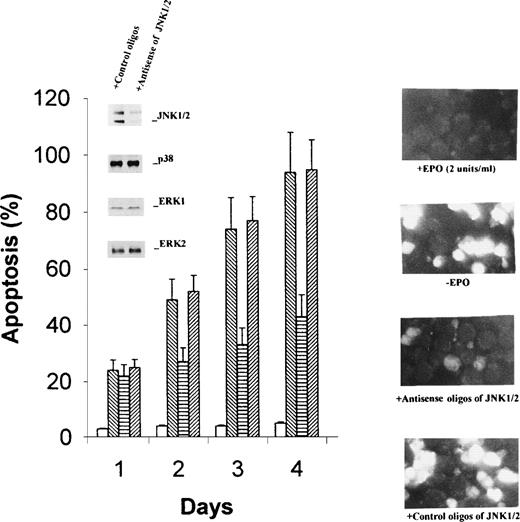
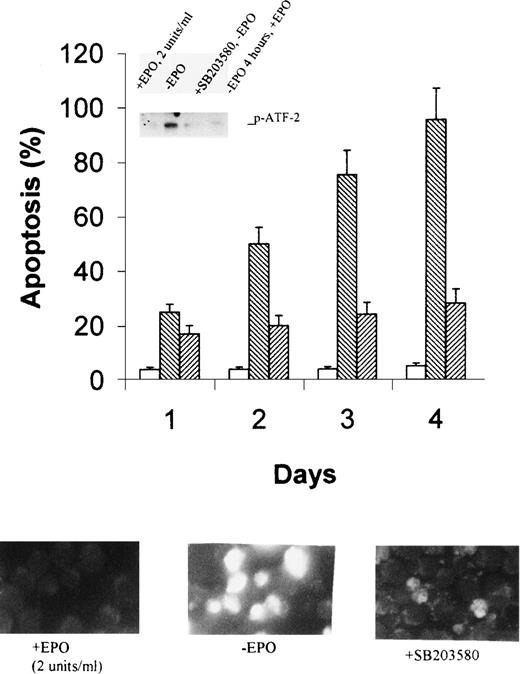
Suppression of JNK1/2 expression and inhibition of p38 MAP kinase by SB203580 retarded apoptosis of cells.
The data described above showed a strong correlation between activation of JNK1/2 and p38 MAP kinase and induction of apoptosis of HCD-57 cells upon EPO withdrawal. To further show that activation of the kinases is responsible for apoptosis of the cells, we inhibited JNK1/2 expression by antisense oligonucleotides and p38 MAP kinase activity by specific inhibitor SB203580. As shown in the inset of Fig 6A, the antisense oligonucleotide of JNK1/2 specifically suppressed expression of JNK1/2, compared with the oligonucleotide with a mismatched sequence, whereas it had no effect on the expression of ERK1/2 and p38 MAP kinase. As expected, suppression of JNK1/2 significantly inhibited apoptosis of HCD-57 cells in the absence of EPO, as shown by flow cytometric assays (Fig 6A) and by fluorescent cell staining (Fig 6B). The effects were most significant after 2 days of EPO starvation and showed 50% inhibition. Similarly, addition of SB203580 totally inactivated p38 MAP kinase (Fig 7A, inset) and caused an even stronger inhibition of apoptosis (Fig 7). These data provide direct evidence suggesting that activation of JNK1/2 and p38 MAP kinase is responsible for apoptosis of HCD57 cells upon withdrawal of EPO.
Antisense oligonucleotides specifically suppress JNK1/2 expression and retard apoptosis of HCD-57 cells in the absence of EPO. Antisense and control oligonucleotides for JNK1/2 were introduced into HCD-57 cells by lipofection as described in Materials and Methods. EPO starvation was started 44 hours after cell transfection. Expression levels of JNK1/2, p38 MAPK, and ERK1/2 were determined by Western blotting analyses 74 hours after EPO starvation (inset in the left panel). Apoptosis was determined by flow cytometric assays after 1 to 4 days of EPO withdrawal (left panel) and by fluorescent cell staining after 2 days (right panel). The photograph was taken with 400× magnification. Open bars and the top photo on right, EPO-containing medium; reverse-slashed bars and the second photo on right, EPO-free medium; horizontal bars and the third photo, EPO-free medium plus antisense oligonucleotide; and slashed bars and the bottom photo on right, EPO-free medium plus control oligonucleotide. Bright cell images indicate apoptosis.
Antisense oligonucleotides specifically suppress JNK1/2 expression and retard apoptosis of HCD-57 cells in the absence of EPO. Antisense and control oligonucleotides for JNK1/2 were introduced into HCD-57 cells by lipofection as described in Materials and Methods. EPO starvation was started 44 hours after cell transfection. Expression levels of JNK1/2, p38 MAPK, and ERK1/2 were determined by Western blotting analyses 74 hours after EPO starvation (inset in the left panel). Apoptosis was determined by flow cytometric assays after 1 to 4 days of EPO withdrawal (left panel) and by fluorescent cell staining after 2 days (right panel). The photograph was taken with 400× magnification. Open bars and the top photo on right, EPO-containing medium; reverse-slashed bars and the second photo on right, EPO-free medium; horizontal bars and the third photo, EPO-free medium plus antisense oligonucleotide; and slashed bars and the bottom photo on right, EPO-free medium plus control oligonucleotide. Bright cell images indicate apoptosis.
SB203580 inhibits p38 MAPK activity and retards apoptosis of HCD-57 cells in the absence of EPO. Cells were pretreated with 10 μmol/L SB203580 for 4 hours in normal growth medium before starvation with EPO-free medium supplemented with the same concentration of the inhibitor. The inset in the top panel shows p38 MAP kinase activity after 2 days of EPO starvation, as determined by using ATF-2 as a substrate. Apoptosis was determined by flow cytometric assays after 1 to 4 days of EPO withdrawal (top panel) and by fluorescent cell staining after 2 days (bottom panel). The photograph was taken with 400× magnification. The stock solution of SB203580 was made in water. Open bars and bottom, left photo, EPO-containing medium; reverse-slashed bars and the bottom, middle photo, EPO-free medium; and slashed bars and the bottom, right photo, EPO- free medium plus 10 μmol/L SB203580.
SB203580 inhibits p38 MAPK activity and retards apoptosis of HCD-57 cells in the absence of EPO. Cells were pretreated with 10 μmol/L SB203580 for 4 hours in normal growth medium before starvation with EPO-free medium supplemented with the same concentration of the inhibitor. The inset in the top panel shows p38 MAP kinase activity after 2 days of EPO starvation, as determined by using ATF-2 as a substrate. Apoptosis was determined by flow cytometric assays after 1 to 4 days of EPO withdrawal (top panel) and by fluorescent cell staining after 2 days (bottom panel). The photograph was taken with 400× magnification. The stock solution of SB203580 was made in water. Open bars and bottom, left photo, EPO-containing medium; reverse-slashed bars and the bottom, middle photo, EPO-free medium; and slashed bars and the bottom, right photo, EPO- free medium plus 10 μmol/L SB203580.
DISCUSSION
The present study has shown a molecular mechanism for the crucial role of EPO in the growth of erythroid progenitor cells. The presence of EPO induces activation of ERKs and suppression of JNKs and p38 MAP kinase, whereas withdrawal of EPO from the cell culture medium causes sustained activation of JNKs and p38 MAP kinase and inactivation of ERKs. Activation of ERKs promotes cell growth, whereas activation of JNKs and p38 MAP kinase is associated with apoptosis. Therefore, EPO appears to promote cell growth and prevent apoptosis by regulating the dynamic balance between ERK and JNK/p38 MAP kinase activities. However, it should be noted that the activation of JNKs and ERKs observed in this study is somewhat different from previous reports. First, we observed a slow but prolonged activation of JNKs and p38 MAP kinase upon withdrawal of EPO, whereas a rapid and transient activation of JNKs was observed after the addition of EPO to serum-starved cells.14-18 The prolonged activation of JNKs correlates with the onset of apoptosis produced by withdrawal of EPO. The rapid and transient activation upon addition of EPO may have a different physiological meaning. In fact, a recent study indicates that activation of p38 MAP kinase and JNKs is required for EPO-induced erythroid differentiation.20 Secondly, we observed a slow and sustained activation of ERKs induced by EPO, whereas previous studies reported a rapid and transient activation. This is probably caused by the different ways in which the cells were starved. In the current study, cells were deprived of EPO but remained in serum containing medium, whereas in the previous studies, the cells were deprived of serum. Serum starvation brings cells to a quiescent state that may produce a different response.
Our study suggests that ERKs counteract JNKs and p38 MAP kinase. Cross-talk between different signal transduction pathways is a basic regulatory mechanism in cells. A well-known example is the downregulation of the ERK activation pathway by cyclic AMP-dependent protein kinase, which can phosphorylate Raf-1, thereby inhibiting Raf-1 activity.21,22 A similar type of cross-talk could take place between the ERK and JNK/p38 MAP kinase pathways. Activation of ERKs by EPO through interaction with the EPO receptor has been extensively studied. One pathway that leads to activation is the classic SHC/Grb2-dependent Ras/Raf-1 pathway, and the other is the SHC/Grb2-independent PI-3 kinase pathways.14-18 Both pathways involve activation of MEK, which then phosphorylates ERKs. How EPO withdrawal triggers the JNK and p38 MAP kinase pathways is not well understood. In the JNK pathway, there is a kinase cascade sequentially involving MEKK1 and MEK-4 (also termed SEK1, MKK4, or JNKK).8 9 MEK-4 directly phosphorylates and activates JNKs. Because the pathways leading to activation of ERKs and JNKs consist of multiple signaling components, most of which are regulated by protein phosphorylation, there are multiple ways for the cross-talk to take place.
Other suggestive evidence for the counteracting effects between the 2 pathways is that one pathway can upregulate the terminating signal of the other pathway. Activated by upstream dual specific kinases, ERKs, JNKs, and p38 MAP kinase are all inactivated by dual-specific protein phosphatases.23,24 It is likely that the ERK and JNK/p38 MAP kinase pathways downregulate each other by turning on the expression of the phosphatases, which will act on the other. MKP-1, an immediate early gene product that is upregulated by stress related factors and growth factor, was identified as the ERK phosphatase,25 although it also acts on JNKs. Enzymes specifically dephosphorylating ERKs or JNKs might exist, but this has not been completely determined.
Apoptosis serves as a major mechanism for the precise regulation of cell numbers and as a defense mechanism to remove unwanted and potentially dangerous cells.26,27 It can be initiated by withdrawal of growth factors and stress conditions, as well as stimulation with tumor necrosis factor or Fas ligand. The apoptosis pathway involves multiple components, and a central element of the pathway is the Bcl-2 family of proteins.28-30 The antiapoptotic members of the family, including Bcl-2 and Bcl-XL, act upstream of the execution caspases, thus preventing their proteolytic processing into active killers. In contrast, the proapoptotic members of the family, such as BAD, form heterodimers with Bcl-2, thereby inhibiting the antiapoptosis activity of the latter. Phosphorylation of the Bcl-2 family proteins has been well documented.31-37 Although phosphorylation of the antiapoptotic members may both augment and suppress activity,31-34 phosphorylation of BAD by protein kinase B (Akt) causes BAD to lose its binding ability to Bcl-2, because the phosphorylated BAD is sequestered in the cytosol by 14-3-3 protein.35-37 Because the Bcl-2 family proteins have multiple phosphorylation sites, it can be postulated that multiple protein kinases are involved in the regulation of these proteins. In fact, phosphorylation of Bcl-2 and Bcl-XL by both ERKs and JNKs has been observed, although the effects on its antiapoptosis activity are not well understood.31-34 This suggests that ERKs and JNKs act upstream of Bcl-2 family proteins. Because certain caspases are able to activate MEKK-1 and Mst1 by specific cleavages,38,39 caspase activation can lead to activation of JNKs, which, in turn, activate additional caspases, comprising a positive feedback loop. Raf-1 provides another link between the MAP kinase activation pathways and the apoptosis pathway. Raf-1, a major component of the ERK activation pathway, is also a major player in prevention of apoptosis. It has been shown that Bcl-2 can target Raf-1 to mitochondria and that active Raf-1 fused with targeting sequences from an outer mitochondrial membrane protein protected cells from apoptosis.40 Finally, MAP kinases are mediators of signal transduction from the cell surface to the nucleus. One nuclear target of these MAP kinase signaling pathways is the transcription factor AP-1 that has been implicated in the induction of apoptosis in cells in response to stress factors and growth factor withdrawal.41A recent study demonstrated that AP1 is necessary for the induction of apoptosis after hormone withdrawal from the EPO-dependent erythroid cell line HCD-57.42 We think that this may be related to activation of JNKs.
Supported by a Veterans Health Administration Merit Review Grant (to S.B.K.), National Institutes of Health (NIH) Grants No. DK-15555 and 2 T32-DK-07186 (to S.B.K.), HL-57393 and CA75218 (to Z.J.Z.), and CA-68485 (to Vanderbilt-Ingram Cancer Center). R.S. is an Ortho Biotech Hematology Fellow.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Sanford B. Krantz, MD, or Zhizhuang Joe Zhao, PhD, 547, MRB II, 2220 Pierce Ave, Nashville, TN 37232-6305; e-mail: joe.zhao@mcmail.vanderbilt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal