Abstract
Although several mechanisms have been proposed to explain the pathophysiology of severe congenital neutropenia (SCN), the precise defect responsible for SCN remains unknown. We studied the responsiveness of primitive myeloid progenitor cells to hematopoietic factors in 4 patients with SCN. The number of granulocyte-macrophage (GM) colonies formed in patients was decreased in response to granulocyte colony-stimulating factor (G-CSF) in both serum-supplemented and serum-deprived culture. The polymerase chain reaction–single-strand conformational polymorphism analysis of the G-CSF receptor gene showed no variance in structure conformation between the 4 patients and the normal subjects. In patients with SCN, the nonadherent light density bone marrow cells and cells that were purified on the basis of the expression of CD34 and Kit receptor (CD34+/Kit+ cells) showed the reduced response to the combination of steel factor (SF), the ligand for flk2/flt3 (FL), and interleukin-3 (IL-3) with or without G-CSF in serum-deprived culture. Furthermore, when individual CD34+/Kit+ cells from patients were cultured in the presence of SF, FL, and IL-3, with or without G-CSF for 10 days, the number of clones proliferated and the number of cells per each proliferating clone was significantly less than those in normal subjects. These results suggest that primitive myeloid progenitor cells of patients with SCN have defective responsiveness to not only G-CSF, but also the early- or intermediate-acting hematopoietic factors, SF, FL, and IL-3.
CONGENITAL NEUTROPENIAS are a heterogeneous group of disorders. Severe congenital neutropenia (SCN), also known as Kostmann-type neutropenia, is a severe form of neutropenia that is characterized by early onset of chronic life-threatening infections and severe neutropenia in the peripheral blood.1-5 The bone marrow usually shows an arrest of maturation of neutrophil precursors at the promyelocyte or myelocyte stage of differentiation. In most patients with SCN, the treatment with pharmacological doses of recombinant human granulocyte colony-stimulating factor (G-CSF) leads to a significant increase of absolute neutrophil count (ANC) and results in dramatic clinical improvement.3 5-12
G-CSF and its receptor, granulocyte colony-stimulating factor receptor (G-CSFR) are the major regulatory system for the production of neutrophils.13-16 Mice lacking G-CSF have been reported to develop congenital neutropenia.14 Mice deficient in G-CSFR expression have chronic neutropenia and show reduced numbers of marrow progenitors and impaired terminal differentiation of neutrophils.15 However, although numerous studies have been performed on the role of G-CSF and G-CSFR in the pathogenesis of SCN, the underlying pathophysiology of SCN remains unclear. The production of G-CSF by mononuclear leukocytes from SCN patients appears to be normal, and the serum levels of G-CSF are often increased.17-19 In addition, the G-CSFR has been found to be present at normal or increased levels on myeloid cells.19,20 Recently, mutations of the G-CSFR gene have been reported in a subset of patients with SCN and have been determined to contribute to neutropenia.21-26 However, the majority of patients have not shown any mutations of the G-CSFR gene.19,26 27
Bone marrow cells from patients with SCN frequently display a markedly reduced or complete lack of responsiveness to G-CSF in vitro culture.28-30 It has also been shown that this defect of response to G-CSF can be partially restored by the addition of several other hematopoietic factors.29 30 In this study, we examined the responsiveness of purified myeloid progenitor cells to hemtopoietic factors involved in myelopoiesis in 4 patients with SCN. The results show the presence of qualitative and quantitative abnormalities in the proliferation of primitive myeloid progenitor cells from patients with SCN in response to hematopoietic factors including G-CSF.
MATERIALS AND METHODS
Subjects.
Laboratory and hematologic features of the patients with SCN enrolled in this study are presented in Table 1. All patients contracted bacterial infections at the age of less than 1 year and were referred to our hospital. None of the patients had a family history of neutropenia. The complete blood cell count showed a normal total white blood cell count with no neutrophils and normal red blood cell and platelet counts. Bone marrow aspiration showed relative myeloid hypocellularity with maturation arrest at the promyelocyte/myelocyte stage. All patients have received the administration of prophylactic sulfamethoxazole-trimethoprim or G-CSF since the diagnosis was made. Patient 1 continued to have recurrent skin abscesses and chronic gingivitis, and he has been maintained on daily subcutaneous administration of G-CSF for the last 5 years. The 3 patients have received intermittent administration of G-CSF when infections were observed.
Base-Line Laboratory Findings of the Patients Enrolled in the Study
| Patient No. . | Age/ Sex . | White Blood Cells (/μL) . | Percentage of White Blood Cells . | Red Blood Cells (/μL) . | Platelets (/μL) . | |||
|---|---|---|---|---|---|---|---|---|
| Neutrophils . | Lymphocytes . | Monocytes . | Eosinophils . | |||||
| 1 | 11/M | 5,400 | 0 | 69 | 18 | 12 | 480 × 104 | 35 × 104 |
| 2 | 5/M | 6,200 | 0 | 69 | 16 | 15 | 475 × 104 | 27 × 104 |
| 3 | 5/F | 4,800 | 0 | 65 | 23 | 11 | 424 × 104 | 24 × 104 |
| 4 | 7/F | 6,500 | 0 | 71 | 21 | 8 | 444 × 104 | 33 × 104 |
| Patient No. . | Age/ Sex . | White Blood Cells (/μL) . | Percentage of White Blood Cells . | Red Blood Cells (/μL) . | Platelets (/μL) . | |||
|---|---|---|---|---|---|---|---|---|
| Neutrophils . | Lymphocytes . | Monocytes . | Eosinophils . | |||||
| 1 | 11/M | 5,400 | 0 | 69 | 18 | 12 | 480 × 104 | 35 × 104 |
| 2 | 5/M | 6,200 | 0 | 69 | 16 | 15 | 475 × 104 | 27 × 104 |
| 3 | 5/F | 4,800 | 0 | 65 | 23 | 11 | 424 × 104 | 24 × 104 |
| 4 | 7/F | 6,500 | 0 | 71 | 21 | 8 | 444 × 104 | 33 × 104 |
Hematological data show the representative findings before the administration of G-CSF.
Cytokines.
Recombinant human G-CSF, recombinant human interleukin-3 (IL-3) with a specific activity of 1.0 × 108 U/mg and recombinant human steel factor (SF) were supplied by Kirin Brewery Co Ltd (Tokyo, Japan). The recombinant human ligand for flk2/flt3 (FL) was purchased from PeproTech Inc (Rocky Hill, NJ). Unless otherwise specified, the concentrations of factors used were as follows: G-CSF, 100 ng/mL; SF, 100 ng/mL; FL, 100 ng/mL; IL-3, 100 U/mL.
Bone marrow cell separation and purification.
Bone marrow samples were obtained with the informed consent of patients and/or their guardians. Normal bone marrow cells for this study were taken from healthy adult volunteers after obtaining informed consent. Bone marrow samples were diluted with an equal volume of α-modification of Eagle's medium (αMEM; ICN Biomedicals, Inc, Aurora, OH) and centrifuged over Lymphoprep (1.077 g/mL; Nycomed Pharma AS, Oslo, Norway). The light density bone marrow cells (LDBMC) were carefully harvested with a Pasteur pipette, washed 3 times with phosphate-buffered saline (PBS) containing 2% human AB serum (Sigma Chemical Co, St Louis, MO) and 0.1 mg/mL of DNase I (type II-S; Sigma Chemical Co) and resuspended in αMEM containing 10% fetal bovine serum (FBS; ICN Biomedicals, Inc). Cells were incubated in plastic culture flasks (Becton Dickinson Labware, Lincoln Park, NJ) at 37°C for 1 hour to remove adherent cells. Nonadherent cells were used in described purification or cryopreserved by a standard procedure using 10% dimethylsufoxide and stored in liquid nitrogen until use. Cells, fresh or thawed, were washed and resuspended in PBS-human serum-DNase solution containing 0.1% sodium azide for subsequent immunofluorescence staining.
Cell purification was performed according to the methods reported previously with modification.31 Cells (2 × 107/mL) were incubated with fluorescein isothiocyanate (FITC)-labeled monoclonal anti-CD34 antibody (Beckman Coulter, Inc, Fullerton, CA) at a concentration of 2 μg/106 cells for 30 minutes at 4°C. FITC-conjugated mouse IgG1a was used as an isotype control. After the addition of propidium iodide (PI, Sigma Chemical Co) at a concentration of 1 μg/mL, cells were washed twice and resuspended in PBS-human serum-DNase-sodium azide solution. Initial enrichment of CD34+ was performed by setting the FACS Vantage (Becton Dickinson Immunocytometry Systems, San Jose, CA) equipped with a 4-W argon laser to recognize only FITC-positive cells. Low to medium forward scatter and low side scatter, as well as negative PI fluorescence gates were also used. The resulting cell population contained 30% to 50% CD34+ cells. Enriched CD34+ cells were further stained with phycoerythrin (PE)-conjugated anti-c–Kit (Beckman Coulter, Inc) for 30 to 40 minutes at 4°C. After the addition of PI at a concentration of 1 μg/mL, cells were washed twice and resuspended in PBS-human serum-DNase-sodium azide solution. The appropriate isotype controls were used to identify background staining. Forward and orthogonal light scatter signals, as well as specific fluorescence of FITC, PE, and PI excited at 488 nm and 633 nm, were used to establish sort windows. Cells were separated into fractions expressing positive CD34 and c-Kit according to the methods reported previously.31 Data acquisition and analysis was performed with CellQuest software (Becton Dickinson Immunocytometry Systems). Single cell sorting was performed using the Automated Cell Deposition Unit (ACDU) with Clone-Cyt software (Becton Dickinson Immunocytometry Systems).
Clonal cultures.
The clonal cell culture was performed in 35-mm Falcon suspension culture dishes (Becton Dickinson Labware). In the serum-deprived culture, 1 mL of the culture mixture contained purified cells, 1% deionized crystallized bovine serum albumin (BSA, Sigma Chemical Co), 300 μg/mL fully iron-saturated human transferrin (98% pure; Sigma Chemical Co), 10 μg/mL soybean lecithin (Sigma Chemical Co), 6 μg/mL cholesterol (Sigma Chemical Co), 1 × 10−7 mol/L sodium selenite (Sigma Chemical Co), 10 μg/mL insulin (Sigma Chemical Co), 4.5 mmol/L L-glutamin (Sigma Chemical Co), 1.5 mmol/L glycin (Sigma Chemical Co), 1.2% 1,500-centipoise methylcellulose (Shinetsu Chemical, Tokyo, Japan), and designated cytokines.31,32 In the serum-supplemented culture, the crystallized BSA transferrin, lecithin, cholesterol, selenite, insulin, glutamin, and glycin were replaced by 30% FBS and 1% deionized fraction V BSA (Sigma Chemical Co). Cultures were incubated at 37°C in a humidified atmosphere with 5% CO2/95% air. On day 14 of incubation, granulocyte-macrophage (GM) colonies were scored on an inverted microscope using the criteria described previously.33 GM colony contains pure granulocyte colonies consisting of mainly neutrophils and their precursors, and mixed granulocyte-macrophage colonies consisting of mainly neutrophils, macrophages/monocytes, and their precursors. The numbers of colonies represent the mean of triplicate cultures.
Single-cell suspension cultures.
Single cells were cultured in serum-deprived liquid suspension cultures containing SF, FL, and IL-3 with or without G-CSF in 72-well round bottom microtrays (Robbins Scientific, Sunnyvale, CA). Incubation was performed at 37°C in a humidified atmosphere with 5% CO2/95% air for 10 days. All wells were inspected carefully on an inverted microscope 16 to 24 hours after the sorting. We were able to detect 1 cell/well in more than 90% of the wells. We then serially scored the number of cells in each well. In some experiments, some of the proliferated clones were individually picked, centrifuged onto slides using Shandon's Cytospin 2 Centrifuge (Shandon Inc, Pittsburgh, PA), and stained with Wright-Giemsa.
Reverse transcriptase-polymerase chain reaction (PCR) and single-strand conformational polymorphism (SSCP).
Total cellular RNA was extracted from bone marrow mononuclear cells using the guanidinium thiocyanate extraction method. RNA was converted into cDNA by reverse transcriptase. PCR amplification of cDNA was performed accord- ing to the method of Dong et al.21 The primers used were as follows: FW3, 5′-CTGCTGTTGTTAACCTGCCTC-3′ (nucleotides 2075 to 2095, forward); FW4, 5′-CCAAGAGCAGTTTCCACCCAGGCC-3′ (nucleotides 2366 to 2389, forward); RV2, 5′-GTAGATCTTAGTCATGGGTTCATGG-3′ (nucleotides 2750 to 2774, reverse); and RV6, 5′-TCTCAGGGGAGATAGTGGCCC-3′ (nucleotides 2462 to 2481, reverse). The PCR products were applied to electrophoresis in 12% polyaclylamide gel for 3 hours at 30 W at room temperature. After electrophoresis, the bands were visualized by the silver-staining method with a commercially available reagent kit (Daiichi Pure Chemicals, Tokyo, Japan).34
RESULTS
Formation of GM colonies of nonadherent LDBMC in response to G-CSF.
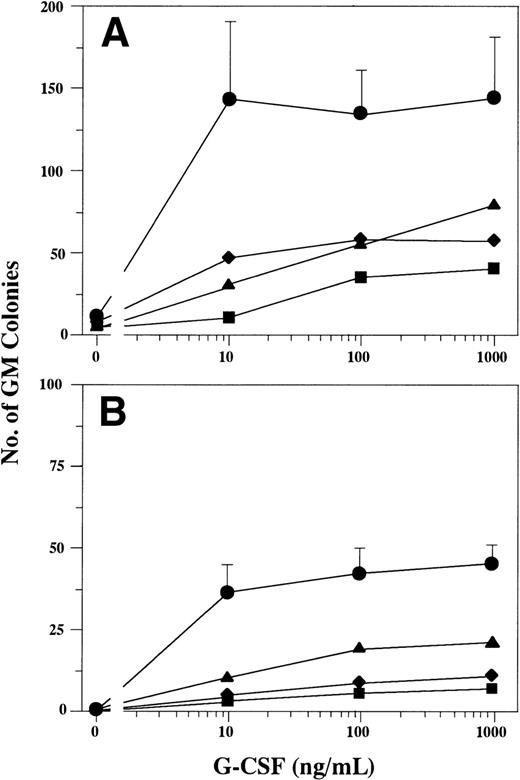
First, we tested the formation of GM colonies of nonadherent LDBMC in response to G-CSF in serum-supplemented and serum-deprived culture. As shown in Fig 1, GM colony formation in patients with SCN markedly decreased relative to that of controls at all concentrations of G-CSF in both serum-supplemented and serum-deprived culture. The difference between the number of GM colonies formed in serum-supplemented and that formed in serum-deprived culture indicates the presence of other factors that cooperate with G-CSF in FBS. The decrease of G-CSF–dependent GM colony formation in serum-deprived culture clearly suggests the defective responsiveness to G-CSF alone in patients with SCN. These observations were consistent with the data reported previously.6,23 28-30
GM colony formation in response to various concentrations of G-CSF. Nonadherent LDBMC (2 × 104 cells) were cultured in a serum-supplemented (A) or serum-deprived (B) conditions containing varying concentrations of G-CSF. Data represent the mean ± standard deviation (SD) of 6 normal subjects (•) and the mean of triplicate cultures of 2 patients with SCN (▪, patient 1; ⧫, patient 2; ▴, patient 3).
GM colony formation in response to various concentrations of G-CSF. Nonadherent LDBMC (2 × 104 cells) were cultured in a serum-supplemented (A) or serum-deprived (B) conditions containing varying concentrations of G-CSF. Data represent the mean ± standard deviation (SD) of 6 normal subjects (•) and the mean of triplicate cultures of 2 patients with SCN (▪, patient 1; ⧫, patient 2; ▴, patient 3).
Analysis of structural conformation of G-CSFR.
Recently, nonsense mutations in the gene encoding the G-CSFR have been described in some patients with SCN.21-26 However, the structure of G-CSFR in the majority of SCN patients is normal.19,26,27 In this study, the structure of the G-CSFR cDNA of patients was analyzed by PCR-SSCP. The cDNA was amplified with primers FW4 and RV6 because the mutations were reported to be located in the same cytoplasmic region of G-CSFR in a majority of patients.26 As shown in Fig 2, SSCP analysis showed that the patterns of morbidity of PCR products of the 4 patients with SCN were indistinguishable from those of the normal subjects. Patterns of morbidity of PCR products amplified with other primers were similarly indistinguishable (data not shown), indicating that there was no SSCP-detected abnormalities in the cytoplasmic domain of G-CSFR in patients with SCN enrolled in this study.
PCR-SSCP analysis of the G-CSFR cytosolic domain. The PCR was performed with primers FW4 and RV6 as described in Materials and Methods. The RT-PCR products from 4 patients (Patients 1 to 4) and normal controls (control) underwent polyacrilamide gel electrophoresis for 3 hours, and the bands were visualized by the silver-staining method.
PCR-SSCP analysis of the G-CSFR cytosolic domain. The PCR was performed with primers FW4 and RV6 as described in Materials and Methods. The RT-PCR products from 4 patients (Patients 1 to 4) and normal controls (control) underwent polyacrilamide gel electrophoresis for 3 hours, and the bands were visualized by the silver-staining method.
GM colony formation of nonadherent LDBMC in response to various hematopoietic factors.
Several hematopoietic factors, including SF, FL, IL-3, and G-CSF, have been reported to be involved in myelopoiesis and classified into 3 different categories.35-38 We next examined the effects of various hematopoietic factors on GM colony formation of nonadherent LDBMC from patients in response to such hematopoietic factors alone or to various combinations of the factors in serum-deprived culture. As presented in Table 2, single factors, such as IL-3 and FL, gave rise to formation of a few GM colonies in both patients with SCN and normal subjects. The various combinations of G-CSF and 1 other factor induced the increase in the number of GM colonies. However, the numbers of GM colonies in SCN patients were less than those in normal subjects. Furthermore, the numbers of GM colonies of LDMNC from SCN patients were less than those from normal subjects in response to SF, FL, or IL-3 with or without G-CSF.
Formation of GM Colonies of Bone Marrow Mononuclear Cells Supported With Various Factors
| Factors . | No. of GM Colonies . | |||||
|---|---|---|---|---|---|---|
| Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Normal Subjects (Mean ± SD, n = 6) . | Normal Subjects (Range, n = 6) . | |
| G-CSF | 4 | 18 | 8 | 6 | 38 ± 14 | 24-48 |
| IL-3 | 2 | 5 | 6 | 7 | 9 ± 4 | 5-15 |
| SF | 1 | 0 | 0 | 0 | 0 ± 1 | 0-1 |
| FL | 4 | 3 | 1 | 1 | 4 ± 5 | 1-12 |
| IL-3, G-CSF | 9 | 26 | 16 | 20 | 50 ± 18 | 28-73 |
| SF, G-CSF | 11 | 24 | 18 | 16 | 39 ± 17 | 25-58 |
| FL, G-CSF | 9 | 26 | 12 | 11 | 33 ± 9 | 20-42 |
| SF, FL, IL-3 | 12 | 36 | 14 | 20 | 51 ± 21 | 32-82 |
| SF, FL, IL-3, G-CSF | 18 | 50 | 36 | 40 | 99 ± 30 | 64-131 |
| Factors . | No. of GM Colonies . | |||||
|---|---|---|---|---|---|---|
| Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Normal Subjects (Mean ± SD, n = 6) . | Normal Subjects (Range, n = 6) . | |
| G-CSF | 4 | 18 | 8 | 6 | 38 ± 14 | 24-48 |
| IL-3 | 2 | 5 | 6 | 7 | 9 ± 4 | 5-15 |
| SF | 1 | 0 | 0 | 0 | 0 ± 1 | 0-1 |
| FL | 4 | 3 | 1 | 1 | 4 ± 5 | 1-12 |
| IL-3, G-CSF | 9 | 26 | 16 | 20 | 50 ± 18 | 28-73 |
| SF, G-CSF | 11 | 24 | 18 | 16 | 39 ± 17 | 25-58 |
| FL, G-CSF | 9 | 26 | 12 | 11 | 33 ± 9 | 20-42 |
| SF, FL, IL-3 | 12 | 36 | 14 | 20 | 51 ± 21 | 32-82 |
| SF, FL, IL-3, G-CSF | 18 | 50 | 36 | 40 | 99 ± 30 | 64-131 |
Cultures were performed in serum-deprived conditions containing 2 × 104 nonadherent LDBMC and designated factors. Data of the patients represent the mean of triplicate cultures.
GM colony formation of CD34+/Kit+ cells in response to various hematopoietic factors.
To further examine the effects of various hematopoietic factors, nonadherent LDBMC were enriched for primitive myeloid progenitors using CD34 antibody and anti-c–Kit receptor antibody because colony-forming unit–GM (CFU-GM) was enriched in CD34+/Kit+fraction. Figure 3 presents a representative flow cytometric analysis of cells that had been enriched for CD34. There was no difference between SCN patients and normal subjects in the expression of CD34 or Kit of nonadherent LDBMC. CD34+/Kit+ cells that were purified from nonadherent LDBMC according to the gate indicated in Fig 3 were cultured in serum-deprived conditions containing various hematopoietic factors. As shown in Table 3, single factors did not effectively support GM colony formation in patients with SCN. Although 2-factor combinations that include G-CSF–induced GM-colony formation in patients, the numbers of GM colonies were less than those in normal subjects. Furthermore, GM colony formation of patients with SCN showed a significant decrease in response to combinations of SF, FL, and IL-3, both with or without G-CSF, compared with that of normal subjects. These findings may suggest the possibility that the primitive myeloid progenitor cells of patients with SCN have defects in response not only to G-CSF, but also to other hematopoietic factors.
Flow cytometric analysis of CD34 and c-Kit expression on bone marrow cells from normal subjects and the patients with SCN. Nonadherent LDBMC enriched for CD34-FITC (see Materials and Methods) were stained by Kit-PE. R indicates the gate for CD34+/Kit+ cells. The figure shows a representative flow cytometric analysis for 2 patients with SCN and 2 normal subjects.
Flow cytometric analysis of CD34 and c-Kit expression on bone marrow cells from normal subjects and the patients with SCN. Nonadherent LDBMC enriched for CD34-FITC (see Materials and Methods) were stained by Kit-PE. R indicates the gate for CD34+/Kit+ cells. The figure shows a representative flow cytometric analysis for 2 patients with SCN and 2 normal subjects.
Formation of GM Colonies of CD34+/c-Kit+ Cells Supported With Various Factors
| Factors . | No. of GM Colonies . | |||||
|---|---|---|---|---|---|---|
| Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Normal Subjects (Mean ± SD, n = 6) . | Normal Subjects (Range, n = 6) . | |
| G-CSF | 2 | 1 | 3 | 2 | 14 ± 8 | 5-22 |
| IL-3 | 1 | 2 | 1 | NT | 11 ± 6 | 4-20 |
| SF | 2 | 0 | 0 | NT | 0 | 0 |
| FL | 1 | 1 | 0 | NT | 2 ± 2 | 2-4 |
| IL-3, G-CSF | 4 | 5 | 20 | 18 | 30 ± 12 | 20-49 |
| SF, G-CSF | 12 | 4 | 17 | 15 | 31 ± 12 | 16-43 |
| FL, G-CSF | 9 | 2 | 17 | 16 | 27 ± 12 | 16-44 |
| SF, FL, IL-3 | 8 | 19 | 25 | 22 | 52 ± 14 | 38-77 |
| SF, FL, IL-3, G-CSF | 22 | 20 | 62 | 65 | 100 ± 30 | 76-164 |
| Factors . | No. of GM Colonies . | |||||
|---|---|---|---|---|---|---|
| Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Normal Subjects (Mean ± SD, n = 6) . | Normal Subjects (Range, n = 6) . | |
| G-CSF | 2 | 1 | 3 | 2 | 14 ± 8 | 5-22 |
| IL-3 | 1 | 2 | 1 | NT | 11 ± 6 | 4-20 |
| SF | 2 | 0 | 0 | NT | 0 | 0 |
| FL | 1 | 1 | 0 | NT | 2 ± 2 | 2-4 |
| IL-3, G-CSF | 4 | 5 | 20 | 18 | 30 ± 12 | 20-49 |
| SF, G-CSF | 12 | 4 | 17 | 15 | 31 ± 12 | 16-43 |
| FL, G-CSF | 9 | 2 | 17 | 16 | 27 ± 12 | 16-44 |
| SF, FL, IL-3 | 8 | 19 | 25 | 22 | 52 ± 14 | 38-77 |
| SF, FL, IL-3, G-CSF | 22 | 20 | 62 | 65 | 100 ± 30 | 76-164 |
Cultures were performed in serum-deprived conditions containing 500 CD34+/c-Kit+ cells and designated factors. Data of the patients represent the mean of triplicate cultures.
Abbreviation: NT, not tested.
Proliferation of individual CD34+/Kit+ cells.
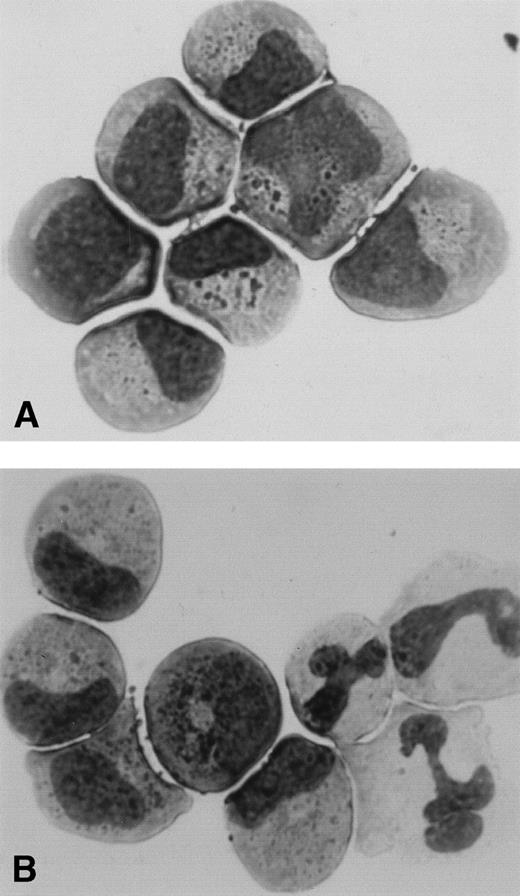
To confirm the direct evidence for proliferation of primitive myeloid progenitors, we sorted the CD34+/Kit+ cells using ACDU and analyzed the proliferation from single cells in response to SF, FL, and IL-3 with or without G-CSF. All wells were inspected carefully by an inverted microscope, and the number of proliferated cells of clones was serially recorded. The number of clones proliferated and the mean number of cells per each proliferated clone after 10 days of culture from 2 patients with SCN and 5 normal subjects are presented in Table 4. The number of clones proliferated and the number of cells per each clone in patients with SCN were significantly less than those in normal subjects irrespective of the presence or absence of G-CSF. This result is consistent with the data showing a decrease in the number of GM colonies formed in methylcellulose. Although the number of the proliferative clones was small in patients with SCN, several clones showed normal proliferation. After 10 days culture, some of the proliferating cells from single CD34+/Kit+ cell were picked, centrifuged onto a slide, and stained. Figure 4 shows the representative cytology of cells from a single CD34+/Kit+ cell of patients with SCN. Single CD34+/Kit+ cells in the presence of SF, FL, and IL-3 developed to the myeloid precursor level (Fig 4A), while the addition of G-CSF to SF, FL, and IL-3 induced the development of mature segmented neutrophils (Fig4B).
Results of Proliferation of Individual CD34+/Kit+ Cells
| . | SF, FL, IL-3 . | SF, FL, IL-3, G-CSF . | ||
|---|---|---|---|---|
| No. of Clones Proliferated . | Mean No.of Cells Per Each Clone . | No. of Clones Proliferated . | Mean No.of Cells Per Each Clone . | |
| Patient 1 | 4 | 125 | 18 | 206 |
| Patient 2 | 6 | 130 | 22 | 250 |
| Control 1 | 28 | 191 | 61 | 609 |
| Control 2 | 20 | 203 | 69 | 828 |
| Control 3 | 15 | 173 | 90 | 770 |
| Control 4 | 15 | 185 | 51 | 780 |
| Control 5 | 28 | 215 | 69 | 828 |
| . | SF, FL, IL-3 . | SF, FL, IL-3, G-CSF . | ||
|---|---|---|---|---|
| No. of Clones Proliferated . | Mean No.of Cells Per Each Clone . | No. of Clones Proliferated . | Mean No.of Cells Per Each Clone . | |
| Patient 1 | 4 | 125 | 18 | 206 |
| Patient 2 | 6 | 130 | 22 | 250 |
| Control 1 | 28 | 191 | 61 | 609 |
| Control 2 | 20 | 203 | 69 | 828 |
| Control 3 | 15 | 173 | 90 | 770 |
| Control 4 | 15 | 185 | 51 | 780 |
| Control 5 | 28 | 215 | 69 | 828 |
Two hundred sixteen CD34+/Kit+ cells were individually seeded in each well of 72-well plates containing serum-deprived media in the presence of SF, FL, and IL-3 with or without G-CSF and cultured for 10 days. Data represent the number of clones proliferated and the mean number of cells per each proliferated clone.
Cytology of cells proliferated from a single CD34+/Kit+ cell of patients with SCN. Single CD34+/Kit+ cells were cultured in the presence of SF, FL, and, IL-3 with (B) or without (A) G-CSF according to the description in Table 4. Some of the proliferating clones were picked, centrifuged onto a slide, and stained with Wright-Giemsa.
Cytology of cells proliferated from a single CD34+/Kit+ cell of patients with SCN. Single CD34+/Kit+ cells were cultured in the presence of SF, FL, and, IL-3 with (B) or without (A) G-CSF according to the description in Table 4. Some of the proliferating clones were picked, centrifuged onto a slide, and stained with Wright-Giemsa.
DISCUSSION
Previously, we and others have shown that the bone marrow cells of patients with SCN showed a reduced response to G-CSF and a partial restoration of defective response to G-CSF by the addition of IL-3, GM-CSF, or SF.29,30 These previous experiments used a serum-supplemented culture with crude bone marrow cells. It is well-known that G-CSF can synergize with early- and/or intermediate-acting cytokines to support the proliferation of neutrophils and their precursors in culture.36,39 The serum in culture is a potential endogenous source of hematopoietic factors that affect the responsiveness of progenitor cells in culture.40 41 As shown in Fig 1, the number of GM colonies supported with G-CSF in serum-supplemented culture was greater than that in serum-deprived culture. In this study, we compared the effects of the combination of the factors on supporting GM colony formation between patients with SCN and normal subjects using a serum-deprived culture system and purified myeloid progenitor cells. The results presented here demonstrated that primitive myeloid progenitor cells from patients with SCN had reduced response to hematopoietic factors involved in myelopoiesis, including G-CSF. This observation was confirmed by the single-cell proliferation studies of CD34+/Kit+ cells in a serum-deprived suspension culture. The number of clones proliferated and the mean number of cells per each proliferated clone of patients in response to SF, FL, IL-3, and G-CSF were significantly reduced when compared with those of normal subjects. These findings directly suggest the presence of quantitative and qualitative abnormalities in the proliferation of primitive myeloid progenitor cells in patients with SCN.
Somatic mutations in the gene for G-CSFR have been identified in some patients with SCN and have been shown to result in a truncation from the C-terminus of the receptor and in an inability of the receptor to transduce the signal upon G-CSF stimulation. However, most patients with SCN have not been found to have mutations in the G-CSFR gene. Recently, Hermans et al42 and McLemore et al43independently generated mice carrying a mutation of the G-CSFR that was roughly identical to the mutation found in patients with SCN and examined the effect of this mutation on granulopoiesis. Although the G-CSFR mutation resulted in the reduced basal neutrophil levels, there was no significant reduction in numbers of neutrophils and their precursors in the bone marrow. Thus, the G-CSFR mutation found in patients with SCN was not sufficient to induce an SCN phenotype in mice. These results suggest that mutations of G-CSFR may not be responsible for the impairment of granulopoiesis present in patients with SCN. In this study, there is no SSCP-detected abnormalities in cytoplasmic domain of G-CSFR. The majority of patients showed reduced response to G-CSF irrespective of the presence or absence of G-CSFR abnormalities. In addition, myeloid progenitor cells of the SCN patients showed reduced response to the combination of SF, FL, and IL-3, as well as to G-CSF alone. Thus, it is likely that abnormalities of molecules involved in common hematopoietic growth factor signal transduction downstream of the receptor might also play a role in the pathogenesis of SCN.
Several hematopoietic factors, including SF, FL, IL-3, GM-CSF, IL-6, and G-CSF, have been shown to be positive regulators of granulopoiesis and to act at different stages of myeloid cell development.13,16,36 Most of these factors support the proliferation of early myeloid progenitor cells. However, G-CSF has the ability to not only stimulate the proliferation, but also potentially induce the terminal maturation of myeloid progenitor cells to neutrophilic granulocytes. In the present single-cell proliferation studies, cytological examination of individual proliferated clones showed that single CD34+/Kit+ cells developed into mature segmented neutrophils when G-CSF was added to SF, FL, and IL-3 in culture. Although the number of responding clones and the kinetic proliferation were less in patients than in normal subjects, some enhancing effect of G-CSF on the proliferation and maturation of progenitor cells in SCN patients was also observed. Therefore, in vitro responsiveness of myeloid progenitor cells to hematopoietic factors in patients with SCN was reduced, but not completely abolished. The CD34+/Kit+ cells of patients had at least a partial capacity to produce the mature neutrophils by addition of G-CSF. These in vitro observations may indicate that most patients with SCN respond favorably to in vivo administration of G-CSF, showing both a significant increase in circulating neutropils and clinical improvement. Furthermore, previous studies of both G-CSF–deficient and G-CSFR–deficient mice suggest the existence of G-CSF or G-CSFR independent of granulopoiesis.14 15
In conclusion, the present study may provide evidence that the bone marrow cells of patients with SCN have abnormal responses not only to G-CSF, but also to early- and intermediate-acting hematopoietic factors involved in myelopoiesis. It appears that the cellular defect in patients with SCN resides downstream of the receptors required for hematopoietic factor-induced myeloid proliferation and maturation.
ACKNOWLEDGMENT
The authors are very grateful to Kirin Brewery Co Ltd (Tokyo, Japan) for providing cytokines.
Supported in part by Grant-in-Aid (to M.K.) for Scientific Research (C) from the Ministry of Education, Science, Sports and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Masao Kobayashi, MD, Department of Child Health, Faculty of Education, Hiroshima University, 1-1-2 Kagamiyama Higashi-Hiroshima, Hiroshima, 739-8523 Japan; e-mail: masa@mcai.med.hiroshima-u.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal